Abstract
Objectives
Male circumcision is associated with reduced risk of HIV infection. This may be partly because of a protective effect of circumcision on other sexually transmitted infections (STI), especially those causing genital ulcers, but evidence for such protection is unclear. Our objective was to conduct a systematic review and meta‐analyses of the associations between male circumcision and infection with herpes simplex virus type 2 (HSV‐2), Treponema pallidum, or Haemophilus ducreyi.
Methods
Electronic databases (1950–2004) were searched using keywords and text terms for herpes simplex, syphilis, chancroid, ulcerative sexually transmitted diseases, or their causative agents, in conjunction with terms to identify epidemiological studies. References of key articles were hand searched, and data were extracted using standardised forms. Random effects models were used to summarise relative risk (RR) where appropriate.
Results
26 articles met the inclusion criteria. Most syphilis studies reported a substantially reduced risk among circumcised men (summary RR = 0.67, 95% confidence interval (CI) 0.54 to 0.83), although there was significant between study heterogeneity (p = 0.01). The reduced risk of HSV‐2 infection was of borderline statistical significance (summary RR = 0.88, 95% CI 0.77 to 1.01). Circumcised men were at lower risk of chancroid in six of seven studies (individual study RRs: 0.12 to 1.11).
Conclusions
This first systematic review of male circumcision and ulcerative STI strongly indicates that circumcised men are at lower risk of chancroid and syphilis. There is less association with HSV‐2. Potential male circumcision interventions to reduce HIV in high risk populations may provide additional benefit by protecting against other STI.
Keywords: male circumcision, syphilis, chancroid, herpes simplex, systematic review
Male circumcision is one of the oldest surgical procedures and is almost universal among Muslim and Jewish men and in some ethnic groups in sub‐Saharan Africa. In the late 19th century, lower disease prevalence among Jews in the United Kingdom was ascribed to circumcision, which became increasingly common in English speaking industrialised countries as physicians advocated it for preventing a range of conditions.1 By the mid‐20th century, neonatal male circumcision was routine in many parts of the United States and New Zealand, and was common in Australia2 and Canada.3 Rates in these countries subsequently fell as medical organisations found no clear medical indication for neonatal circumcision.4
The potential association between male circumcision and sexually transmitted diseases (STDs) was first reported in 1855, in a study where 61% of non‐Jewish compared with 19% of Jewish STD patients had syphilis.5 Later studies supported this finding, reporting higher than expected proportions of uncircumcised men among case series of genital herpes,6 syphilis,3,7 chancroid,7,8,9 and gonorrhoea.3,7 Similar observations were reported at a meeting of traditional healers in South Africa in 199210 and in a study of circumcision practices in Mwanza.11
In 1998, Moses et al12 reviewed the evidence for male circumcision as a preventive health measure and concluded that circumcised men had a lower risk of diseases such as chancroid, syphilis, and genital herpes. However, in 1999 another review found little evidence of an association with these infections,13 and the American Academy of Pediatrics concluded that the evidence was “complex and conflicting.”4
In contrast, there is clear evidence that circumcised men are at significantly lower risk of acquiring HIV infection,14,15,16 probably because the inner surface of the foreskin contains numerous Langerhan's cells and CD4+ T lymphocytes (primary HIV‐1 target cells),17 and because of the warm, moist environment under the foreskin.18,19 The latter could also facilitate infection with other sexually transmitted pathogens. The protective effect of circumcision on HIV is especially strong among populations more highly exposed to sexually transmitted infections (STIs), suggesting that part of the effect on HIV may be mediated via protection against other STIs that facilitate HIV transmission.14
The aim of this paper was to review systematically the evidence for an association between male circumcision and infection with ulcerative STIs, herpes simplex virus type 2 (HSV‐2), Treponeum pallidum, and Haemophilus ducreyi (the causative agents of syphilis and chancroid).
Methods
Study selection
We searched PubMed and Embase for papers published in any language between 1950 and April 2004. In PubMed, search terms for the outcomes of interest included the exploded MeSH terms “herpes simplex,” “syphilis,” “chancroid,” “Herpesvirus2, Human,” “Treponema pallidum,” “Haemophilus ducreyi,” and “sexually transmitted diseases” (the latter combined with the MeSH term “ulcer”) and the free text terms “genital herpes,” “HSV2,” “HSV‐2,” “syphilis,” “chancroid,” “chancre,” or “ducreyi.” We did not include circumcision as a search term to minimise ascertainment bias, as authors may be more likely to mention circumcision in the abstract if they found an association. Instead, we searched for articles with the outcomes of interest plus any of the MeSH terms “epidemiologic studies,” “seroepidemiologic studies,” “risk factors,” “odds ratio,” “prevalence,” “incidence,” “risk,” or “multivariate analysis,” or the free text terms “prevalence” or “incidence.” Similar terms were used for searching Embase. We checked the reference lists of all relevant papers, and of previously published reviews of circumcision and STIs.12,13 Additional information was sought where necessary from authors.
Each identified abstract was reviewed independently by two authors (SM, HW). We were interested in the effect of circumcision on acquisition of infection rather than on clinical disease because, for example, circumcision may plausibly protect against HSV‐2 infection but is unlikely to affect the risk of recurrences of genital herpes once infected. Further, there is potential for selection bias in studies of clinically diagnosed disease, as circumcised men may be more likely to notice and seek treatment for infections with relatively painless ulcers (such as syphilis), resulting in a possible underestimate of any true effect. Hence, studies were restricted in the first instance to those with the selected outcomes based on serological evidence of infection, not disease. We excluded studies among women; studies from countries where overall prevalence of circumcision is either extremely high or very low (<5% or >95% prevalence) or where more than 99% of the population is Muslim (as these were unlikely to be informative), case series of genital ulcer disease (GUD) patients or HIV positive individuals, and studies of syphilis without confirmatory treponemal tests, as non‐treponemal tests tend to have poor specificity.20
The effect of male circumcision on STIs among STD clinic attenders is subject to selection bias. Comparing circumcision rates among men with one STI versus another could underestimate any true effect if circumcision protects against several STIs (as has been suggested), because the comparison group may have a lower circumcision rate than the background population.12 To minimise such selection biases, we limited the meta‐analyses to studies in which the comparison groups were asymptomatic clinic attenders or seronegative individuals.
Studies whose abstracts indicated analysis of risk factors for either HSV‐2 seropositivity or past/recent infection with syphilis or chancroid were eligible for full text searching, as were HIV risk factor studies that mentioned male circumcision, as these could have also included data on circumcision and other STIs. Study populations that appeared in more than one publication were included only once, choosing the publication with the more informative study design or that controlled most fully for confounders.
Data extraction
For each study, we extracted the following data using a standardised sheet: authors, country, year(s) of study, study design, proportion circumcised, method of ascertaining circumcision status, proportion with STI of interest, method of STI diagnosis, HIV prevalence, statistical methods used, crude and adjusted risk ratios, and other quality issues (participation rates, loss to follow up, confounders adjusted for). In studies where circumcision was assessed by both self report and genital examination, the latter was used. Where possible, we re‐analysed data to compare STI risk in uncircumcised men with those circumcised before reported age at first sex. Circumcision in the United States, Peru, and Australia was assumed to have occurred neonatally.
Statistical methods
Effect sizes (relative risk, RR) were estimated with rate ratios for cohort studies, prevalence ratios or odds ratios for cross sectional studies, and odds ratios for case‐control studies. Where the RR was not presented but raw data were available, the RR and 95% confidence interval (CI) were calculated. The “best” effect estimate (adjusted RR where available, otherwise unadjusted RR) was included in a random effects meta‐analysis.21 This calculates a weighted average log RR, with weights inversely proportional to the sum of the “within study” and “between study” variance. Sensitivity analyses were carried out restricting meta‐analyses to studies that (1) adjusted for confounding by age and at least one measure of sexual behaviour; (2) ascertained circumcision status by examination; (3) (for syphilis studies) estimated lifetime infection with syphilis (initial screening with treponemal tests) rather than recent/active infection; (4) allowed us to estimate whether circumcision had occurred before sexual debut (and therefore before infection) for all participants—that is, studies where men are circumcised neonatally, or where reported age at circumcision and age at sexual debut are given; (5) were in populations of heterosexual men. Publication bias was assessed with funnel plots and Begg's test22 for correlation between the effect estimates and their variances. Statistical and graphical analyses were carried out using Stata 8.2.23
Results
Results of search strategy
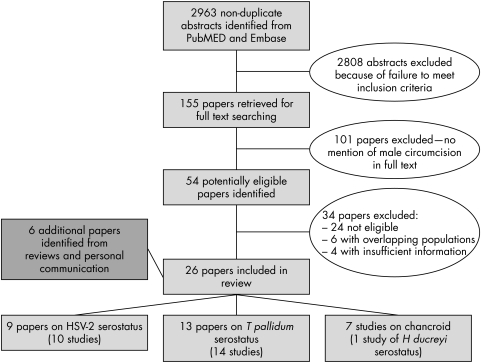
In total, 2963 non‐duplicate articles were identified from database searches, of which 155 were eligible for full text searching (fig 1). Of these, 54 included male circumcision in the text and data were extracted. Six authors publishing relevant but insufficient data provided further analyses which enabled inclusion of their studies in the review, including three further eligible papers,24,25,26 which were in press at the time of our search. Twenty four papers were excluded because they were not eligible after data extraction, six were excluded because their study populations overlapped with other papers in the review,27,28,29,30,31,32 and four contained insufficient information to be included.33,34,35,36 Only one study with serological evidence of past infection with Haemophilus ducreyi was identified,37 and so all studies of chancroid diagnosed clinically or microbiologically are presented.7,38,39,40,41,42 A total of 26 papers incorporating 28 studies were included in the systematic review. Three papers included both HSV‐2 and syphilis as outcomes.25,26,43
Figure 1 Flow chart of study selection for inclusion in the systematic review.
Association of male circumcision and HSV‐2 seropositivity
Ten eligible studies of HSV‐2 seropositivity were identified; eight from Africa, one from India, and one from the United States.24,25,26,43,44,45,46,47,48 Six studies were among men at generally low risk for STIs (general populations, outpatients) and four were among men at higher risk of STIs (bar workers, truck drivers, and STD clinic attenders). Participation rates ranged from 43%24 to 100%46 in the seven studies that reported these, and were greater than 70% in four studies. Loss to follow up was 25% and 36% in the two cohort studies.24,26
Circumcised men were at lower risk of HSV‐2 seropositivity than uncircumcised men on univariable analysis in six studies (table 1), and the association was statistically significant (p<0.05) in three of these.24,25,45
Table 1 Summary of studies of the association of male circumcision and HSV‐2 serostatus.
| First author | Design | Location, date‐of study | Study population | Study‐size | HSV‐2 | Circumcised | Assessment of‐circumcision | Crude RR* ‐(95% CI) | Adjusted RR* ‐(95% CI) | Adjusted for |
|---|---|---|---|---|---|---|---|---|---|---|
| Auvert48 | Cross sectional | Carltonville, South Africa 1999 | Young adults aged 14–24 years | 676 | 15% | 3% | Self report | 1.20 (0.48 to 2.96) | – | |
| Gottlieb24 | Cohort | Five cities, USA 1993–6 | STD clinic | 1120 | 9% | 71%† | Clinical examination | 0.88 (0.5 to 1.4) | 1.0 (0.6–1.6) | Age, race, city, HSV1 status, condom use with occasional partner |
| Gray25‡ | Nested case‐control | Rakai, Uganda 1994–8 | General | 674 | 70% | 18%† | Self report | 0.82 (0.68 to 0.99) | 0.81 (0.67 to 0.97) | Age, marital status, condom use, number of lifetime partners |
| Kapiga47 | Cross sectional | Moshi, Tanzania 2000 | Bar workers | 206 | 29% | 95% | Clinical examination | 1.07 (0.40 to 2.88) | 0.56 (0.13 to 2.5) | Age only |
| Lavreys43 | Cross sectional | Mombasa, Kenya 1993–7 | Trucking employees | 113 | 46% | 57% | Clinical examination | 1.18 (0.78 to 1.79) | to | |
| Obasi44 | Cross sectional | Mwanza, Tanzania 1992–3 | General | 133 | 23% | 23% | Self report | 0.68 (0.28 to 1.62) | 0.39 (0.1 to 1.52) | Age, residence, mobility, marital status, lifetime partners, TPHA status |
| Reynolds26 | Cohort | Pune, India 1993–2000 | STD clinic attenders | 2298 | 14% | 8%† | Clinical examination | 0.89 (0.48 to 1.53) | 0.91 (0.51 to 1.64) | Age, religion, education, living with family, year, marital status, number of sex partners, number of female sex worker partners, condom use, tattoos, medical injections |
| Suligoi46 | Cross sectional | Garoua, Cameroon 1997–8 | Outpatients | 82 | 24% | 91%† | Self report | 0.84 (0.24 to 2.90) | – | |
| Weiss45 | Cross sectional | Kisumu, Kenya 1997 | General | 583 | 35% | 27%† | Clinical examination | 0.65 (0.47 to 0.90) | 0.73 (0.47 to 1.13) | Age, marital status, ethnic group and number of lifetime partners. |
| Weiss45 | Cross sectional | Ndola, Zambia 1997 | General | 607 | 36% | 9%† | Clinical examination | 1.20 (0.81 to 1.77) | 1.04 (0.74 to 1.44) | Age, marital status and number of lifetime partners. |
*Rate ratio in references 24, 26. Odds ratio in references 45, 47; Prevalence ratio in references 25, 44, 45, 46, 48.
†Circumcision before sexual debut.
‡Nested HIV incident case‐control study of HIV seroconvertors and matched seronegative controls.
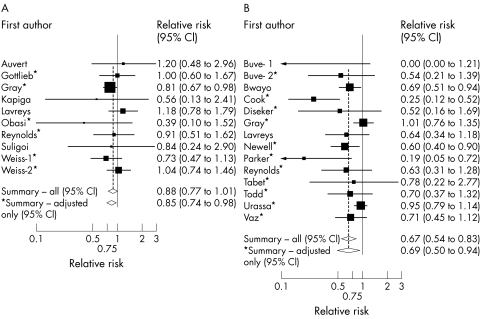
Seven studies included a RR with some adjustment for confounding.24,25,26,44,45,46,47 These all adjusted for age, and all but one47 adjusted for several other potential confounders including sociodemographic factors, sexual behaviour, and other risk factors (table 1). The “best” estimate RRs ranged from 0.39 to 1.20, and the random effects summary RR was 0.88 (CI 0.77 to 1.01; p value for homogeneity = 0.57; fig 2A).
Figure 2 Relative risk (RR) of circumcision status with (A) HSV‐2 and (B) syphilis seropositivity. The square and horizontal line corresponds to the RR and 95% CI for each study. The area of the square reflects the weight of each study. The summary RRs are shown by the diamonds.
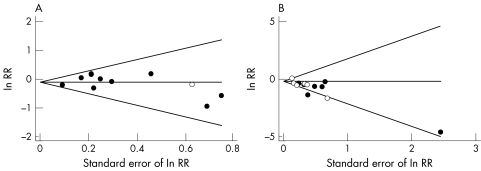
Results were similar when restricted to studies that adjusted for age and at least one measure of sexual behaviour (summary RR = 0.85, CI 0.74 to 0.98), or those where circumcision occurred before first sexual intercourse (summary RR = 0.86, CI 0.74 to 0.99). The effect of circumcision was less protective among the six studies,24,26,43,45,47 which assessed circumcision by genital examination compared with those which asked for self report25,44,46,48 (summary RR = 0.97, CI 0.80 to 1.17 versus RR = 0.81, CI 0.68 to 0.98), although this difference was not statistically significant (p = 0.18). Restricting analyses to studies that used a rate ratio or prevalence ratio (because HSV‐2 infection is common in these populations, and so the OR may not closely estimate the RR) made little difference (summary RR = 0.89, CI 0.78 to 1.02). There was little evidence of publication bias (p = 0.72; fig 3A).
Figure 3 Funnel plots to detect publication bias in the meta‐analysis of circumcision with (A) HSV‐2 and (B) syphilis seropositivity. The horizontal line indicates the summary log RR, and guidelines to assist in visualising the funnel are plotted at the 95% pseudo‐confidence limits for this estimate.
Six studies examined the effect of male circumcision on both HIV and HSV‐2.25,26,43,44,45 Among these studies, the magnitude of association between circumcision and HIV (summary RR = 0.34; CI 0.18 to 0.62) was about twice that for HSV‐2 (summary RR = 0.69; CI 0.46 to 1.03).
Association of male circumcision and syphilis seropositivity
Fourteen studies examined the association between male circumcision and serological evidence of syphilis infection (table 2), from sub‐Saharan Africa (nine studies), the United States (two studies), Australia, India, and Peru. The outcome was lifetime infection (initial TPHA screening) in six studies,43,49,50,51,52,53 and more recent infection (initial RPR screening with TPHA confirmation) in the remainder.25,26,53,54,55,56,57,58 Prevalence ranged from 2–3% among STD clinic attenders from the United States to 25% for past syphilis among truck drivers from Kenya. Participation rates ranged from 43% to 86% in the seven studies with available information, and were more than 70% in three studies. Loss to follow up in the cohort studies was 26% and 28%, respectively.26,55
Table 2 Summary of studies of the association of male circumcision and risk of syphilis infection.
| First author | Design | Location, date of ‐study | Study ‐population | Outcome* | Study size | Seropositive | Circumcised | Assessment of ‐circumcision | Crude RR† ‐(95% CI) | Adjusted RR† ‐(95% CI) | Adjusted for |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Buve53 | Cross sectional | Kisumu, Kenya 1997 | General | RPR TPHA | 580 | 3% | 27%‡ | Clinical examination | 0 ‐(0 to 1.21) | – | |
| Buve53 | Cross sectional | Ndola, Zambia 1997 | General | TPPA | 593 | 17% | 9%‡ | Clinical examination | 0.63 (0.25–1.63) | 0.54 ‐(0.21 to 1.41) | Age, marital status and number of lifetime partners |
| Bwayo52 | Cross sectional | 25 k from Nairobi 1989–92 | Truck drivers | TPHA | 570 | 25% | 80% | Not stated | 0.69 ‐(0.51 to 0.94) | – | |
| Cook54 | Cross sectional | King County, US 1988 | STD clinic attenders | RPR TPHA | 985 | 3% | 79%‡ | Clinical examination | 0.16 ‐(0.08 to 0.31) | 0.25 ‐(0.12 to 0.53) | Age, race, number of sexual partners in last month, place of residence, other STI |
| Diseker55 | Cohort | US cities 1993–6 | STD clinic attenders | RPR TPHA | 865 | 3% | 74% | Clinical examination | 0.53 ‐(0.15 to 1.85) | 0.52 ‐(0.16 to 1.74) | Age, race/ethnicity and study location |
| Gray25 | Cross sectional | Rakai, Uganda 1994–8 | General | TRUST TPHA | 5072 | 10% | 12%‡ | Self report | 1.00 ‐(0.76 to 1.32) | 1.01 ‐(0.76 to 1.35) | Age, marital status, condom use and number of sexual partners |
| Lavreys43 | Cross sectional | Mombasa, Kenya 1993–7 | Truck drivers | TPHA | 746 | 8% | 87%‡ | Clinical examination | 0.64 ‐(0.34 to 1.18) | to | |
| Newell57 | Cross sectional | Mwanza, Tanzania 1990–1 | General | RPR TPHA | 1996 | 8% | 32% | Self report | 0.74 ‐(0.52 to 1.05) | 0.6 ‐(0.4 to 0.9) | Age, residence, marital status, no. sex partners in past 5 years, travel to Mwanza town in past 2 years |
| Parker51 | Cross sectional | Perth, Australia 1981 | STD clinic attenders | TPHA | 1319 | 2% | 55% | Clinical examination | 0.20 ‐(0.06 to 0.74) | 0.19 ‐(0.05 to 0.73) | Age only |
| Reynolds26 | Cohort | Pune, India 1993–2000 | STD clinic attenders | RPR TPHA | 2298 | 7% | 8%‡ | Clinical examination | 0.74 ‐(0.38 to 1.46) | 0.63 ‐(0.31 to 1.28) | Religion, education, living with family, year, age, marital status, number of sex partners, contact with sex workers, condom use, tattoos, medical injections |
| Tabet56 | Cross sectional | Lima, Peru 1996 | Men who have sex with men | VDRL TPHA | 440 | 16% | 8%‡ | Not stated | 0.46 ‐(0.14 to 1.53) | 0.78 ‐(0.22 to 2.77) | Education level, HIV serostatus, sexual identity, history of rectal discharge |
| Todd50 | Nested case‐control | Mwanza, Tanzania 1991–6 | General | TPHA | 482 | 14%§ | 25% | Self report | 0.54 ‐(0.34 to 0.86) | 0.70 ‐(0.37 to 1.32) | Age, community, education, occupation, living away from community in past 2 years, perceived STD risk |
| Urassa49 | 2 cross sectional; 1 case‐control | Mwanza, Tanzania 1990–5 | General and factory workers | TPHA | 4984 | 18% | 18–47% | Clinical examination & self report | 0.87 ‐(0.76 to 1.00) | 0.95 ‐(0.79 to 1.15) | Age, area of residence, education, ethnicity, occupation, religion |
| Vaz58 | Cross sectional | Maputo, Mozambique 1990–1 | Prisoners | RPR FTA | 1284 | 8% | 36% | Self report | 0.68 ‐(0.45 to 1.03) | 0.71 ‐(0.45 to 1.11) | History of genital ulcer, captivity by RENAMO during the civil war |
*RPR, rapid plasma regain test, TPHA, Treponema pallidum haemagglutination assay; TPPA, Treponema pallidum particle agglutination; TRUST, toludidine red unheated serum test; VDRL, Venereal Disease Research Laboratory Slide Test; FTA, fluorescent treponemal antibody absorbed (FTA‐ABS) test
†Rate ratio in reference 26; prevalence ratio in references 43, 52, 53; odds ratio in references 25, 49, 50, 51, 54, 55, 56, 57, 58.
‡Circumcision before sexual debut.
§Baseline prevalence of TPHA in the study population.
Eleven studies included some adjustment for potential confounders (table 2). The “best” estimates varied from zero to 1.01, and five showed statistically significant reduced risk. The random effects summary RR was 0.67 (CI 0.54 to 0.83; fig 2B), but with evidence of between study heterogeneity (p = 0.01). The summary RR was little altered when analyses were restricted to studies that assessed circumcision by genital examination, studies among heterosexual men, or studies that included some adjustment for confounding (summary RR = 0.69, CI 0.50 to 0.94), but the effect was stronger among men for whom circumcision occurred before first sexual intercourse (RR = 0.53, CI 0.34 to 0.83; p for effect modification compared with later circumcision = 0.15;25,26,43,51,53,54,55,56). The association among studies of lifetime infection (initial TPHA screening) was similar to that overall, although there was less heterogeneity (p = 0.08).
The funnel plot was asymmetrical (fig 3B) with the two largest studies finding the least protective effects25,49 (p value for Begg's test = 0.10).
Association of male circumcision and chancroid
Seven studies examined the association between male circumcision and chancroid (table 3). Three were from Kenya and the remainder from the United States, United Kingdom, and the US and Australian military.7,30,38,39,40,41,42 Six of seven studies found a reduced risk of chancroid among circumcised men, and this was statistically significant in four studies (table 3).7,38,41,42 No meta‐analysis for the chancroid studies was carried out because (1) the definition and ascertainment of the outcome varied between studies, and (2) the comparison groups varied considerably and some included men with other STIs (mainly urethritis) against which circumcision may also be protective.12,13
Table 3 Summary of studies of the association between male circumcision and chancroid.
| First author | Design | Location, ‐date of ‐study | Study ‐population | Outcome,‐ cases | Outcome, ‐comparison | Study ‐size | Circumcised | Assessment of ‐circumcision | Crude RR ‐(95% CI) | Adjusted RR‐(95% CI) | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rakwar30 | Cross sectional | Mombasa, Kenya 1993 | Trucking company employees | H ducreyi seropositive | H ducreyi seronegative | 501 | 87% | Clinical examination | 1.22 ‐(0.8 to 2.0) | 1.11 ‐(0.5 to 2.1) | Adjusted for age, marital status, history of CSW contact, history of alcohol intake, travel |
| Hand7 | Case control | New York State 1945 | US Naval Hospital | Clinically diagnosed chancroid | Asymptomatic controls | 1529 | 22% | Clinical examination | 0.04 ‐(0.02 to 0.09) | 0.13 ‐(0.06 to 0.29) | Adjusted for race |
| Lloyd39 | Cross sectional | London 1932 | STD clinic attenders | Clinically diagnosed chancroid | Asymptomatic controls | 110 | 23% | Clinical examination | 0.40 ‐(0.05 to 3.43) | – | |
| Barile38 | Case‐control | US military, Japan 1962 | US servicemen | Penile lesions | Asymptomatic controls | 82 | 43% | Clinical examination | 0.04 ‐(0.01 to 0.16) | – | 21/35 lesions had a definitive diagnosis, and 8 were H ducreyi |
| Nasio40 | Case‐control | Nairobi, Kenya 1993 | STD clinic attenders | Culture positive H ducreyi ulcer | Urethritis | 660 | 89% | Clinical examination | 0.59 ‐(0.32 to 1.09) | 0.66 ‐(0.35 to 1.24) | Among HIV negative men only. |
| Cameron41 | Cohort | Nairobi, Kenya 1985 | STD clinic attenders | Genital ulcer disease* | Urethritis | 293 | 73% | Clinical examination | – | 0.62 ‐(0.50–0.76) | |
| Hart42 | Cross sectional | Australian military 1970 | STD clinic attenders | Clinically diagnosed chancroid | Other STD clinic attenders | 1970 | 57% | Clinical examination | 0.21 ‐(0.14 to 0.29) | – | Comparison group included 52% no aetiology, 3% with herpes, 15% gonorrhoea, 14% urethritis |
*Of the 150 GUD cases, 89% of ulcers were clinically diagnosed as chancroid, and 50% of these were culture positive for H ducreyi. The remaining ulcers were diagnosed as syphilis (4%), genital herpes (5%), and LGV (2%). Of the 316 urethritis patients, 213 (67%) were culture positive for N gonorrhoeae.
The single study with a serological outcome found no association with circumcision (table 3).37 Three early studies that compared chancroid patients (diagnosed by clinical diagnosis or microbiology) or penile ulcer patients with asymptomatic controls found that circumcised men were at much lower risk (RR from 0.04 to 0.40).7,38,39 Two more recent studies compared H ducreyi culture positive patients40 or GUD patients (89% clinically diagnosed as having chancroid)41 with urethritis patients. Each found a slightly weaker association than those with asymptomatic controls. The five studies that reported response rates were retrospective record based studies, and so had 100% responses.7,38,39,40,42
Discussion
Review of findings
Our results suggest that male circumcision is associated with a reduced risk of ulcerative STIs, especially syphilis and chancroid. All included studies are observational, and their limitations need consideration. However, as discussed below, many potential biases tend to underestimate an association, indicating that the true association may be stronger than the summary RRs presented.
Most included studies assessed circumcision status through a clinical examination, but longitudinal studies from Tanzania11 and the United States55 have indicated substantial misclassification of status even by examination. However, ascertainment of circumcision status generally occurred before knowledge of serological status and so misclassification is likely to be non‐differential, which might underestimate any association.
The selected outcomes in this review were primarily based on serological evidence of infection, not disease. However, serological studies are also subject to misclassification. For example, there are concerns about the validity of commercial HSV‐2 assays in samples from African populations59 and about the validity of serological tests for H ducreyi. We identified only one study of H ducreyi serostatus, which used an ELISA developed from lipo‐oligosacharide. This test has high sensitivity and specificity for detecting H ducreyi antibodies in patients with culture proved chancroid,37,60 but is not commonly used. Further, the specificity for this test was assessed in a Canadian population and, as for HSV‐2 assays, it is not clear that results can be extrapolated to other populations. In addition, misclassification of serostatus is likely to be non‐differential with respect to circumcision status, and will thus underestimate any association.
The summary RR for syphilis should be interpreted cautiously, as there was significant heterogeneity between studies. Five of the nine studies of recent or active syphilis screened initially with a non‐trepenomal test, confirming seropositives using a treponemal test. This means that some controls may have been TPHA seropositive, and this non‐differential misclassification of outcome may underestimate any protective effect of circumcision. However, the summary RR was similar for past infection as for recent/active infection. The single study among homosexual men found a relatively weak effect (table 2) as might be expected because circumcision status is irrelevant to T pallidum infection for the receptive partner.
Some of the heterogeneity was caused by the null effect seen in two large population based African studies,25,49 compared with a highly protective association among STD clinic attenders in the United States and Australia.51,54 One likely explanation for this heterogeneity is age at circumcision. In studies from the United States, Peru, and Australia, almost all men are likely to have been circumcised in infancy or early childhood.54 In contrast, the median age at circumcision was 16 and 17 respectively in the studies from South Africa,48 and Mwanza, Tanzania.11 For cross sectional and case‐control studies in populations which tend to circumcise at puberty or later, some men are likely to have become infected with an STI (especially HSV‐2 which has high incidence among youth) before becoming circumcised. This would tend to underestimate any protective effect of male circumcision. We minimised this bias where possible25,45,48,53 by excluding individuals who were circumcised after first sexual intercourse or after the age of 11. However, this information was not available for all studies, including those from Mwanza where many men are circumcised in their late teens or early twenties.11 The largest syphilis study from Mwanza49 pooled results from three studies and thus contributed more weight to the meta‐analysis than if the three studies had been analysed individually.
The participation rates in several studies were low. If participation was differential with respect to circumcision status and STIs, this could either overestimate or underestimate the association, although this seems unlikely.
Prevalence of male circumcision varies with ethnicity, and different ethnic groups may also differ with respect to sexual behaviours. Residual confounding may therefore have affected the results. However, sensitivity analyses showed that adjustment for confounding had little effect on the results for either HSV‐2 or syphilis.
There was little evidence of publication bias for studies of HSV‐2. For syphilis, there was some indication that smaller studies tended to find larger associations. The asymmetry of the funnel plot (fig 3B) is partly because of the influence of the paper by Buve et al,53 where there were no cases of syphilis among the circumcised men. The objective of this paper was to look generally at risk factors among both men and women for gonorrhoea, chlamydia and syphilis, and so publication bias as the result of the association of circumcision and syphilis is implausible. Most of the included studies did not have circumcision as the primary exposure of interest. However, the other two studies with a large association51,54 did have as their main hypothesis the relation between circumcision and STIs, and may have been susceptible to publication bias.
As many of the above potential biases would tend to bias our summary RR towards the null, our results may be a conservative estimate of a true protective association of male circumcision and STIs.
Biological rationale for association
There are clear biological reasons why circumcision may protect against both bacterial and viral STIs. The warm, moist area under the foreskin may provide a suitable location in which the pathogens can replicate. Further, uncircumcised men may be at increased risk as the result of entry of pathogens through the inner surface of the foreskin and frenulum, or through micro‐abrasions occurring during intercourse. The physical location of ulcers may also affect the role of circumcision on infection. For example, chancroid lesions frequently occur on the external and internal surfaces of the foreskin9,61,62 and circumcision may therefore be more protective against chancroid than against syphilis and herpes, where lesions tend to be found more widely on the male genitalia.
Previous studies have found a strongly reduced risk of HIV among circumcised men.14,15,16 In contrast, we found a weak association with HSV‐2 infection. This difference may result from different mechanisms of infection for the two viruses. The inner and outer epithelia of the foreskin are composed mainly of keratinocytes, and the inner mucosal layer is rich in Langerhans cells18 and CD4+ T helper lymphocytes, especially during infection.17 HSV replicates largely in the epithelial cells but also infects Langerhans and other dendritic cells and both stimulates and inhibits their immune function.63 Circumcision results in a smaller surface area for infection, but also fewer immune cells to respond against HSV. HSV‐2 is shed more widely from the female genital tract than HIV, and there are several portals of entry in female‐male transmission besides the foreskin. The role of the foreskin on HSV‐2 infection may thus be relatively minor.
Key messages
Results support the common belief that circumcised men are at lower risk of syphilis and chancroid
Evidence for a protective effect of male circumcision on HSV‐2 infection is weak and contrasts with a strong, consistent effect seen against HIV infection
If male circumcision is promoted as an HIV prevention measure in the future, an added benefit would be protection against ulcerative STI
In contrast, HIV does not infect the epithelial cells but infects CD4+ lymphocytes, macrophages and some dendritic cells.64 HIV also binds passively to the surface of dendritic cells that, upon migration to lymph nodes, deliver the virus to susceptible CD4+ T cells.65 Circumcision may thus reduce risk of HIV infection in two ways. Firstly, absence of a foreskin may directly decrease the risk of HIV infection by removing a rich source of CD4+ T cells and Langerhans cells. Secondly, if the foreskin provides a niche for ulcerative STIs, those lesions may afford greater accessibility of HIV to local macrophages and lymphocytes by destroying the integrity of the mucosa and by provoking an immune response.
Implications of these results
Results from the first randomised controlled trial of male circumcision have shown a strongly protective effect on HIV incidence among South African men.16 Two further trials are under way in Uganda and Kenya. If these trials also show a clear effect of male circumcision on HIV, it may be introduced as an HIV prevention measure in populations at high HIV risk. Our results indicate that such an intervention in high risk populations could also provide a direct benefit in reducing risk of STIs (which themselves carry a substantial public health burden), as well as indirect protection against HIV by lowering STI prevalence. Our results will also be useful for ongoing modelling studies of the spread of HIV in populations.
Acknowledgements
We thank the following for contributing information and additional data analysis for this review: Bertran Auvert (INSERM U88, Paris, France), Anne Buve (ITM Antwerp, Belgium); Sami Gottlieb (CDC, Atlanta, Georgia USA); Ron Gray, Xianbin Lin, Michael Chen (Bloomberg School of Public Health, Baltimore, USA); Katherine Thomas, King Holmes, Jorge Sanchez, Javier Lama (University of Washington, Seattle, USA); Jim Todd (LSHTM, London, UK). We also thank Laura Rodrigues and Robin Weiss for helpful comments on the manuscript.
Contributors
All authors participated in the study planning and design; HAW, SM, and ST conducted the systematic review and data extraction; HAW conducted the statistical analysis and was the lead writer; and all authors provided detailed comments on the paper.
Abbreviations
CI - confidence interval
FTA‐ABS - fluorescent treponemal antibody absorbed test
GUD - genital ulcer disease
HSV - herpes simplex virus
LGV - lymphogranuloma venereum
RPR - rapid plasma regain test, RR, relative risk
STD - sexually transmitted diseases
STI - sexually transmitted infections
TPHA - Treponema pallidum haemagglutination assay
TPPA - Treponema pallidum particle agglutination
TRUST - toludidine red unheated serum test
VDRL - Venereal Disease Research Laboratory Slide Test
References
- 1.Remondino P C.History of circumcision from the earliest times to the present Popular edition (unabridged), ed. Philadelphia, London: The FA Davis Co, 1891
- 2.Fredman R M. Neonatal circumcision: a general practitioner survey. Med J Aust 19691117–120. [DOI] [PubMed] [Google Scholar]
- 3.Wilson R A. Circumcision and venereal disease. Can Med Assoc J 19475654–56. [PMC free article] [PubMed] [Google Scholar]
- 4.American Academy of Pediatrics Circumcision policy statement. Task Force on Circumcision. Pediatrics 1999103686–693. [PubMed] [Google Scholar]
- 5.Hutchinson J. On the influence of circumcision in preventing syphilis. Med Times Gazette 185532542–543. [Google Scholar]
- 6.Parker J D, Banatvala J E. Herpes genitalis; clinical and virological studies. Br J Vener Dis 196743212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hand E A. Circumcision and venereal disease. Arch Dermatol Syphilol 194960341–346. [DOI] [PubMed] [Google Scholar]
- 8.Asin J. Chancroid; a report of 1,402 cases. Am J Syph Gonorrhea Vener Dis 195236483–487. [PubMed] [Google Scholar]
- 9.Hammond G W, Slutchuk M, Scatliff J.et al Epidemiologic, clinical, laboratory, and therapeutic features of an urban outbreak of chancroid in North America. Rev Infect Dis 19802867–879. [DOI] [PubMed] [Google Scholar]
- 10.Green E C, Zokwe B, Dupre J D. Indigenous African healers promote male circumcision for prevention of sexually transmitted diseases. Trop Doc 1993182–183. [DOI] [PubMed]
- 11.Nnko S, Washija R, Urassa M.et al Dynamics of male circumcision practices in northwest Tanzania. Sex Transm Dis 200128214–218. [DOI] [PubMed] [Google Scholar]
- 12.Moses S, Bailey R C, Ronald A R. Male circumcision: assessment of health benefits and risks. Sex Transm Infect 199874368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Howe R S. Does circumcision influence sexually transmitted diseases? : a literature review, BJU Int 199983(Suppl 1)52–62. [DOI] [PubMed] [Google Scholar]
- 14.Weiss H A, Quigley M A, Hayes R J. Male circumcision and risk of HIV infection in sub‐Saharan Africa: a systematic review and meta‐analysis. AIDS 2000142361–2370. [DOI] [PubMed] [Google Scholar]
- 15.Siegfried N, Muller M, Volmink J.et al Male circumcision for prevention of heterosexual acquisition of HIV in men. Cochrane Database Syst Rev 2003CD003362. [DOI] [PubMed]
- 16.Auvert B, Puren A, Taljaard D.et al Impact of male circumcision on the female‐to‐male transmission of HIV (Abstract TuOa0402). 3rd IAS Conference on HIV Pathogenesis and Treatment. Rio De Janeiro 2005
- 17.Patterson B K, Landay A, Siegel J N.et al Susceptibility to human immunodeficiency virus‐1 infection of human foreskin and cervical tissue grown in explant culture. Am J Pathol 2002161867–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szabo R, Short R V. How does male circumcision protect against HIV infection? BMJ 20003201592–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCoombe S G, Cameron P U, Short R V. p. 2004.
- 20.Peeling R W, Ye H. Diagnostic tools for preventing and managing maternal and congenital syphilis: an overview. Bull World Health Organ 200482439–446. [PMC free article] [PubMed] [Google Scholar]
- 21.DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 19867177–188. [DOI] [PubMed] [Google Scholar]
- 22.Begg C B, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994501088–1101. [PubMed] [Google Scholar]
- 23. Intercooled Stata 8.2 for Windows. College Station, TX 2004
- 24.Gottlieb S L, Douglas J M, Jr, Foster M.et al Incidence of herpes simplex virus type 2 infection in 5 sexually transmitted disease (STD) clinics and the effect of HIV/STD risk‐reduction counseling. J Infect Dis 20041901059–1067. [DOI] [PubMed] [Google Scholar]
- 25.Gray R, Azire J, Serwadda D.et al Male circumcision and the risk of sexually transmitted infections and HIV in Rakai, Uganda. AIDS 2004182428–2430. [PubMed] [Google Scholar]
- 26.Reynolds S J, Shepherd M E, Risbud A R.et al Male circumcision and risk of HIV‐1 and other sexually transmitted infections in India. Lancet 20043631039–1040. [DOI] [PubMed] [Google Scholar]
- 27.Barongo L R, Borgdorff M W, Newell J N.et al Intake of a cohort study of urban factory workers in northwest Tanzania. Risk factors for HIV‐1 infection. Trop Geogr Med 199446157–162. [PubMed] [Google Scholar]
- 28.Grosskurth H, Mosha F, Todd J.et al Impact of improved treatment of sexually transmitted diseases on HIV infection in rural Tanzania: randomised controlled trial. Lancet 1995346530–536. [DOI] [PubMed] [Google Scholar]
- 29.Kelly R, Kiwanuka N, Wawer M J.et al Age of male circumcision and risk of prevalent HIV infection in rural Uganda. AIDS 199913399–405. [DOI] [PubMed] [Google Scholar]
- 30.Rakwar J, Lavreys L, Thompson M L.et al Cofactors for the acquisition of HIV‐1 among heterosexual men: prospective cohort study of trucking company workers in Kenya. AIDS 199913607–614. [DOI] [PubMed] [Google Scholar]
- 31.Simonsen J N, Cameron D W, Gakinya M N.et al Human immunodeficiency virus infection among men with sexually transmitted diseases. Experience from a center in Africa. N Engl J Med 1988319274–278. [DOI] [PubMed] [Google Scholar]
- 32.Gottlieb S L, Douglas J M, Jr, Schmid D S.et al Seroprevalence and correlates of herpes simplex virus type 2 infection in five sexually transmitted‐disease clinics. J Infect Dis 20021861381–1389. [DOI] [PubMed] [Google Scholar]
- 33.Le Bacq F, Mason P R, Gwanzura L.et al HIV and other sexually transmitted diseases at a rural hospital in Zimbabwe. Genitourin Med 199369352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manjunath J V, Thappa D M, Jaisankar T J. Sexually transmitted diseases and sexual lifestyles of long‐distance truck drivers: a clinico‐epidemiologic study in south India. Int J STD AIDS 200213612–617. [DOI] [PubMed] [Google Scholar]
- 35.Rodrigues J J, Mehendale S M, Shepherd M E.et al Risk factors for HIV infection in people attending clinics for sexually transmitted diseases in India. BMJ 1995311283–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sutcliffe S, Taha T E, Kumwenda N I.et al HIV‐1 prevalence and herpes simplex virus 2, hepatitis C virus, and hepatitis B virus infections among male workers at a sugar estate in Malawi. J Acquir Immune Defic Syndr 20023190–97. [DOI] [PubMed] [Google Scholar]
- 37.Rakwar J, Jackson D, Maclean I.et al Antibody to Haemophilus ducreyi among trucking company workers in Kenya. Sex Transm Dis 199724267–271. [DOI] [PubMed] [Google Scholar]
- 38.Barile M F, Blumberg J M, Kraul C W.et al Penile lesions among US Armed Forces personnel in Japan. The prevalence of herpes simplex and the role of pleuropneumonia‐like organisms. Arch Dermatol 196286273–281. [DOI] [PubMed] [Google Scholar]
- 39.Lloyd V E, Lloyd N L. Circumcision and syphilis. BMJ 1934144–146. [DOI] [PMC free article] [PubMed]
- 40.Nasio J M, Nagelkerke N J, Mwatha A.et al Genital ulcer disease among STD clinic attenders in Nairobi: association with HIV‐1 and circumcision status. Int J STD AIDS 19967410–414. [DOI] [PubMed] [Google Scholar]
- 41.Cameron D W, Simonsen J N, D'Costa L J.et al Female to male transmission of human immunodeficiency virus type 1: risk factors for seroconversion in men. Lancet 19892403–407. [DOI] [PubMed] [Google Scholar]
- 42.Hart G. Venereal disease in a war environment: incidence and management. Med J Aust 19751808–810. [DOI] [PubMed] [Google Scholar]
- 43.Lavreys L, Rakwar J P, Thompson M L.et al Effect of circumcision on incidence of human immunodeficiency virus type 1 and other sexually transmitted diseases: a prospective cohort study of trucking company employees in Kenya. J Infect Dis 1999180330–336. [DOI] [PubMed] [Google Scholar]
- 44.Obasi A, Mosha F, Quigley M.et al Antibody to herpes simplex virus type 2 as a marker of sexual risk behavior in rural Tanzania. J Infect Dis 199917916–24. [DOI] [PubMed] [Google Scholar]
- 45.Weiss H A, Buve A, Robinson N J.et al The epidemiology of HSV‐2 infection and its association with HIV infection in four urban African populations. AIDS 200115(Suppl 4)S97–108. [DOI] [PubMed] [Google Scholar]
- 46.Suligoi B, Tchamgmena O, Sarmati L.et al Prevalence and risk factors for herpes simplex virus type 2 infection among adolescents and adults in northern Cameroon. Sex Transm Dis 200128690–693. [DOI] [PubMed] [Google Scholar]
- 47.Kapiga S H, Sam N E, Shao J F.et al Herpes simplex virus type 2 infection among bar and hotel workers in northern Tanzania: prevalence and risk factors. Sex Transm Dis 200330187–192. [DOI] [PubMed] [Google Scholar]
- 48.Auvert B, Ballard R, Campbell C.et al HIV infection among youth in a South African mining town is associated with herpes simplex virus‐2 seropositivity and sexual behaviour. AIDS 200115885–898. [DOI] [PubMed] [Google Scholar]
- 49.Urassa M, Todd J, Boerma J T.et al Male circumcision and susceptibility to HIV infection among men in Tanzania. AIDS 19971173–80. [DOI] [PubMed] [Google Scholar]
- 50.Todd J, Munguti K, Grosskurth H.et al Risk factors for active syphilis and TPHA seroconversion in a rural African population. Sex Transm Infect 20017737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parker S W, Stewart A J, Wren M N.et al Circumcision and sexually transmissible disease. Med J Aust 19832288–290. [DOI] [PubMed] [Google Scholar]
- 52.Bwayo J, Plummer F, Omari M.et al Human immunodeficiency virus infection in long‐distance truck drivers in east Africa. Arch Intern Med 19941541391–1396. [PubMed] [Google Scholar]
- 53.Buve A, Weiss H A, Laga M.et al The epidemiology of gonorrhoea, chlamydial infection and syphilis in four African cities. AIDS 200115(Suppl 4)S79–S88. [DOI] [PubMed] [Google Scholar]
- 54.Cook L S, Koutsky L A, Holmes K K. Circumcision and sexually transmitted diseases. Am J Public Health 199484197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diseker R A, 3rd, Peterman T A, Kamb M L.et al ircumcision and STD in the United States: cross sectional and cohort analyses. Sex Transm Infect 200076474–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tabet S, Sanchez J, Lama J.et al HIV, syphilis and heterosexual bridging among Peruvian men who have sex with men. AIDS 2002161271–1277. [DOI] [PubMed] [Google Scholar]
- 57.Newell J, Senkoro K, Mosha F.et al A population‐based study of syphilis and sexually transmitted disease syndromes in north‐western Tanzania. 2. Risk factors and health seeking behaviour. Genitourin Med 199369421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vaz R G, Gloyd S, Folgosa E.et al Syphilis and HIV infection among prisoners in Maputo, Mozambique. Int J STD AIDS 1995642–46. [DOI] [PubMed] [Google Scholar]
- 59.Van Dyck E, Buve A, Weiss H A.et al Performance of commercially available enzyme immunoassays for detection of antibodies against herpes simplex virus type 2 in African populations. J Clin Microbiol 2004422961–2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alfa M J, Olson N, Degagne P.et al Humoral immune response of humans to lipooligosaccharide and outer membrane proteins of Haemophilus ducreyi. J Infect Dis 19931671206–1210. [DOI] [PubMed] [Google Scholar]
- 61.Kerber R E, Rowe C E, Gilbert K R. Treatment of chancroid. A comparison of tetracycline and sulfisoxazole. Arch Dermatol 1969100604–607. [PubMed] [Google Scholar]
- 62.Nsanze H, Fast M V, D'Costa L J.et al Genital ulcers in Kenya. Clinical and laboratory study. Br J Vener Dis 198157378–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pollara G, Speidel K, Samady L.et al Herpes simplex virus infection of dendritic cells: balance among activation, inhibition, and immunity. J Infect Dis 2003187165–178. [DOI] [PubMed] [Google Scholar]
- 64.Popov S, Chenine A L, Gruber A.et al Long‐term productive human immunodeficiency virus infection of CD1a‐sorted myeloid dendritic cells. J Virol 200579602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Geijtenbeek T B, Kwon D S, Torensma R.et al DC‐SIGN, a dendritic cell‐specific HIV‐1‐binding protein that enhances trans‐infection of T cells. Cell 2000100587–597. [DOI] [PubMed] [Google Scholar]