Abstract
Objective
To estimate potential savings associated with the Consumer Reports Best Buy Drugs program, a national educational program that provides consumers with price and effectiveness information on prescription drugs.
Data Sources
National data on 2006 prescription sales and retail prices paid for angiotensin-converting enzyme inhibitors (ACEIs), β-blockers, calcium channel blockers, and 3-hydroxy-3-methylglutaryl coenzyme A (HMG-coA) reductase inhibitors (statins).
Study Design
We converted national data on aggregate unit sales of drugs in the four classes to defined daily doses (DDD) and estimated a range of potential savings from generic and therapeutic substitution.
Principal Findings
We estimated that $2.76 billion, or 7.83 percent of sales, could be saved if use of the drugs recommended by the educational program was increased. The recommended drugs’ prices were 15–65 percent lower per DDD than their therapeutic alternatives. The majority (57.4 percent) of potential savings would be achieved through therapeutic substitution.
Conclusions
Substantial savings can be achieved through greater use of comparatively effective and lower cost drugs recommended by a national consumer education program. However, barriers to dissemination of consumer-oriented drug information must be addressed before savings can be realized.
Keywords: Prescription drugs, costs, consumer, education, evidence-based medicine
Prescription drug expenditures have grown as a share of total health expenditures from 8.1 percent in 1997 to 12.1 percent in 2005, with per person spending doubling over this same period (Catlin et al. 2007; Zuvekas and Cohen 2007). Third-party payers have responded to increased drug spending by implementing tiered formularies that have increased consumer cost-sharing for many drugs (Gibson, Ozminkowski, and Geotzel 2005). Tiered formularies are also highly prevalent among Medicare Part D drug plans (Medicare Payment Advisory Commission 2006). Moreover, a substantial number of Medicare beneficiaries experience periods 4out coverage due to the “donut hole” (Stuart et al. 2005a; Stuart, Simoni-Wastila, and Chancey 2005b; Gellad et al. 2006).
When faced with high out-of-pocket costs for prescription drugs, many patients, particularly the elderly and disabled, skip or take smaller doses of their medications, or stop filling prescriptions (Safran et al. 2002; Piette, Heisler, and Wagner 2004; Safran et al. 2005; Soumerai et al. 2006). As many as 29 percent of disabled Medicare beneficiaries and 13 percent of elderly Medicare beneficiaries have reported such cost-related underuse, and the rates are even higher among individuals with low incomes and/or multiple chronic conditions (Safran et al. 2005; Soumerai et al. 2006). Nonadherence to medication therapy has been shown to lead to negative health outcomes, and greater use of emergency department and inpatient hospital services (Adams, Soumerai, and Ross-Degnan 2001; Artz, Hadsall, and Schondelmeyer 2002; Safran et al. 2002; Maio et al. 2005; Hsu et al. 2006).
Cost-related underuse may be avoided through more cost-effective prescribing practices including greater use of generic and/or therapeutic substitutes (Shrank et al. 2006). For example, one study reported that one-third of the most expensive medications used by Medicare beneficiaries who exceeded their pharmacy benefits in managed care plans had generic equivalents or a lower cost therapeutic alternative (Tseng et al. 2003). However, consumers and physicians often lack accurate information on the prices and quality of prescription drugs required to make more cost-effective choices (Reichert, Simon, and Halm 2000).
In 2004, Consumers Union, a not-for-profit organization and publisher of Consumer Reports, launched the Consumer Reports Best Buy Drugs (CRBBD) program, a national effort to make information on the value of prescription drugs more transparent for consumers (Voelker 2005). The CRBBD program combines information on comparative effectiveness from systematic, evidence-based reviews with information on drug prices to identify “best buy drugs” in several commonly used classes. The program's goal is to reduce consumers’ out-of-pocket expenditures and improve access to medicines. In this paper, we estimate the dollars that would be saved, from a societal perspective, if the CRBBD made up a greater share of sales in four classes of drugs to prevent and treat cardiovascular disease. While several studies have estimated potential savings associated with greater generic substitution (Fischer and Avorn 2004b; Haas et al. 2005), less is known about the potential economic impact of therapeutic substitution in widely used drug classes (Fischer and Avorn 2004a). Understanding the potential economic impact of greater use of lower cost drugs can inform health policy and educational interventions such as CRBBD.
DATA AND METHODS
Identification of Consumer Reports Best Buy Drugs
Consumers Union obtains information on comparative effectiveness from systematic reviews of the clinical evidence compiled by the Drug Effectiveness Review Project (DERP) at Oregon Health and Science University, which houses an Agency for Healthcare Research and Quality (AHRQ)-designated Evidence-Based Practice Center. Since 2003, DERP has conducted evidence-based reviews of 32 drug classes for several state Medicaid programs and other agencies. DERP selects drug classes for review that (1) account for a large share of pharmacy budgets; (2) consist of multiple drugs; (3) feature a substantial amount of off-label use; and (4) have new additions of costly drugs. DERP reviewers consider clinical evidence only and do not take cost or cost-effectiveness studies into account.
The CRBBD program uses the DERP reviews as the basis for selecting “best buy drugs” in each class. A more detailed description of the CRBBD methodology has been published elsewhere (Findlay 2006). Briefly, the CRBBD program chooses drugs that are equal to or better than their competitors in terms of clinical effectiveness, safety, and side effects, yet generally have retail prices that are equal to or lower than those of other drugs in the class. In some cases, the best buy drug(s) are not less expensive. This might be the case if effective, relatively low-cost drugs have undesirable side effects or risks. In addition to the DERP reviews, the CRBBD program relies on other systematic reviews, studies of dosing convenience factors, relevant safety analyses, such as those by the U.S. Food and Drug Administration (FDA), the comparative monthly prices of individual drugs, and peer review.
Drug Classes Selected for Study
Cardiovascular disease remains one of the leading causes of death in the United States (Thom et al. 2006). While pharmacologic treatment of cardiovascular disease and risk factors such as hypertension and hyperlipidemia can significantly reduce morbidity and mortality, evidence suggests that these medicines are underused relative to what treatment guidelines would recommend (Ellis et al. 2004; Rosen et al. 2005; Winkelmayer et al. 2005).
For our analysis, we selected four classes of medication used to treat cardiovascular conditions (angiotensin-converting enzyme inhibitors [ACEIs], β-blockers, calcium channel blockers [CCB], and 3-hydroxy-3-methylglutaryl coenzyme A [HMG-coA] reductase inhibitors [statins]) for several reasons. First, they are commonly used to treat or reduce the risk of a range of chronic cardiovascular conditions. Second, drugs in these classes are intended for long-term use and can result in high annual and lifetime out-of-pocket costs for patients. Third, there is substantial variation in the retail prices of these drugs, so medication choices have significant economic implications for patients depending on the drug chosen and their level of insurance coverage. Fourth, these classes are suitable for this analysis because patients typically take only one from the class. Thus, polypharmacy within a class is not an issue that could complicate our analysis. Finally, with some exceptions noted in the “Analysis” section, it is possible to make reasonable assumptions about therapeutic substitution within these classes (Chobanian et al. 2003).
Sales Data
Aggregate data on pharmaceutical sales were obtained from Wolters Kluwer Health, which collects sales data from a large nationally representative network of pharmacies and pharmacy benefit managers. We collected data from January 1 to December 31, 2006 for every prescription drug in each of the four therapeutic categories. The dataset contains information on each drug's brand and generic name, form (e.g., tablet, capsule), strength, the total number of prescriptions filled, the total units of medication dispensed (number of prescriptions × package size), and total dollar sales. Dollar sales were based on the dispensed prescriptions containing the retail price charged to the consumer (the price faced by cash payers or copay plus plan payment for insured individuals) and vary by payer type (Medicaid, private or cash payer). These sales data do not account for rebates manufacturers provide to payers.
Analysis
We converted medication units sold to defined daily doses (DDDs) using the World Health Organization Centre for Drug Statistics Methodology. DDDs are the assumed average maintenance dose per day for a drug used for its main indication in adults. They provide a fixed unit of measurement independent of price and formulation enabling us to perform comparisons between drugs in the same class with different dosing requirements. We calculated total DDDs for all drugs and computed the average price per DDD for each of the best buy drugs by dividing the total dollar sales by total DDDs.
For our analysis, we made four assumptions about switching behavior: (1) individuals taking the brand name versions of the best buy drugs would switch to their generic equivalents (generic substitution); (2) individuals taking a combination drug (e.g., an ACEI sold in combination with a diuretic) would not switch, and thus we excluded these drugs from our analysis; (3) all individuals taking nonbest buy or “other” drugs in the same class would switch to one of the best buy drugs (therapeutic substitution); and (4) in classes with more than one best buy drug, patients would switch in proportion to the existing market share among the best buy drugs. Therefore, the hypothetical cost of the drugs under the assumption of therapeutic substitution is the product of the DDD units for other drugs and the weighted average best buy drug price. We calculated potential savings associated with patients switching from other drugs in the class to best buy drugs as follows (Fischer and Avorn 2004b):
In order to decompose savings from generic versus therapeutic substitution, we estimated rates of generic use for each multisource drug (i.e., drug with an FDA-approved generic equivalent).
RESULTS
Best Buy Drugs
The CRBBD program identified several best buy drugs in each of these four drug classes selected for study (Table 1). Most but not all of the best buy drugs in the four classes have FDA-approved generic equivalents.
Table 1.
Drugs Identified by Consumer Reports Best Buy Drugs (CRBBD) Program as “Best Buy Drugs”
| ACEIs | β-Blockers* | Calcium Channel Blockers | Statins** |
|---|---|---|---|
| Best buy drugs | |||
| Benazepril (HTN, CKD) | Atenolol (AP, AMI) | Diltiazem CR (HTN, ARR) | Lovastatin (CHOL) |
| Captopril (CHF) | Bisoprolol (CHF) | Diltiazem SR (HTN, ARR) | Atorvastatin (CHOL) |
| Enalapril (HTN, CHF) | Carvedilol (CHF) | Felodipine SR (HTN) | Pravastatin (CHOL) |
| Lisinopril (HTN, AMI) | Metoprolol tartrate (HTN, AP, AMI) | Nifedipine SR (HTN, AP) | Simvastatin (CHOL) |
| Ramipril (DM, CKD) | Metoprolol succinate (CHF) | Verapamil SR (ARR, HTN) | |
| Nadolol (HTN, AP) | |||
| Propranolol (HTN, AP, AMI) | |||
| Others | |||
| Fosinopril | Acebutolol | Amlodipine | Ezetimibe, simvastatin |
| Moexipril | Betaxolol | Isradipine | Fluvastatin |
| Perindopril | Carteolol | Nicardipine | Rosuvastatin |
| Quinapril | Labetalol | Nimodipine | |
| Trandolapril | Penbutolol sulfate | Nisoldipine | |
| Pindolol | |||
| Sotalol | |||
| Timolol | |||
Note: Products that are shaded did not have a generic equivalent as of December 2006.
CRBBD identified bisprolol and metoprolol succinate as the BBDs for mild or moderate heart failure and carvedilol for severe heart failure.
CRBBD identified lovastatin and pravastatin as the BBD for patients who need to lower LDL cholesterol by less than 30%. Simvastatin is recommended for those who need 30% or greater LDL reduction and/or have heart disease, diabetes, or have had a heart attack or have acute coronary syndrome with moderate LDL levels. Atorvastatin is recommended for those who have had a heart attack or have ACS and have highly elevated LDL.
CR, continuous release; SR, sustained release; AMI, postacute myocardial infarction; AP, angina pectoris; ARR, heart rhythm abnormalities; CHF, heart failure; CHOL, hyperlipidemia; CKD, chronic kidney disease; DM, diabetes mellitus; HTN, hypertension.
Total Sales
In 2006, dollar sales for the four classes totaled $35.3 billion or 13.6 percent of total prescription drug sales in the United States. Sales of statins alone reached $20.9 billion in 2006.
Market Share and Prices
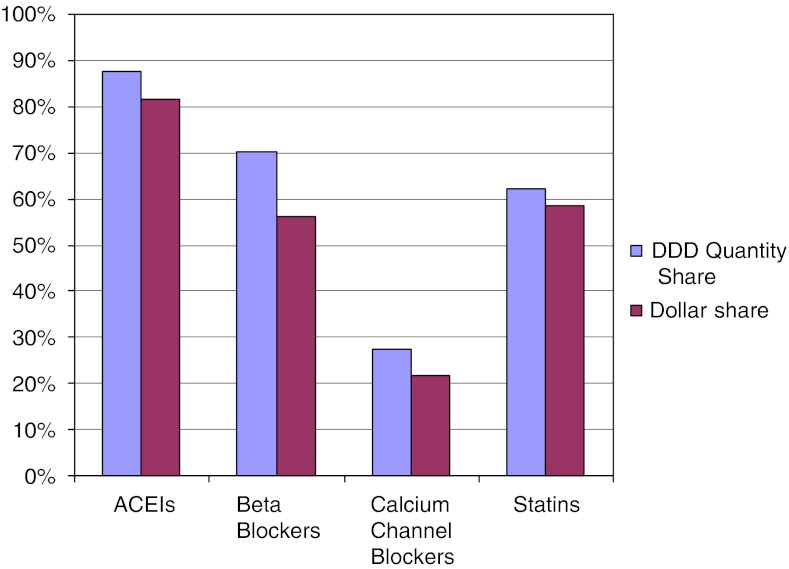
Figure 1 displays the share of DDDs compared with the dollar share of the market held by the best buy drugs and other drugs in each class in 2006. The best buy drugs’ share of DDDs ranged from 27.3 percent in the CCB class to 87.8 percent for ACEIs. In all four classes, the best buy drugs’ dollar share of the market was smaller than the quantity share of DDDs due to the lower relative prices of the best buy drugs. Table 2 shows the average prices per DDD for the drugs in the four classes. The best buy drugs are, on average, 15–65 percent less costly per DDD than their therapeutic alternatives, depending on the class.
Figure 1.
Defined Daily Dose (DDD) Quantity Share and Dollar Share of the Market for Best Buy Drugs by Class, 2006
Table 2.
Retail Prices per DDD for Drugs in Study Classes
| Generic Price ($) | Brand Price ($) | Price Difference (%) | |
|---|---|---|---|
| ACEI | |||
| Benazepril | 0.21 | 0.46 | 121.93 |
| Captopril | 0.72 | 2.71 | 277.73 |
| Enalapril | 0.55 | 1.30 | 135.33 |
| Fosinopril | 0.57 | 1.03 | 81.35 |
| Lisinopril | 0.33 | 0.78 | 135.59 |
| Moexipril | 1.30 | 1.70 | 30.64 |
| Perindopril | NA | 1.33 | |
| Quinapril | 0.56 | 0.86 | 53.39 |
| Ramipril | NA | 0.63 | |
| Trandolapril | NA | 0.88 | |
| β-blockers | |||
| Acebutol | 1.06 | 5.12 | 384.20 |
| Atenolol | 0.66 | 2.46 | 273.73 |
| Betaxolol | 1.66 | 2.40 | 44.33 |
| Bisoprolol fumarate | 1.68 | 3.87 | 130.27 |
| Carteolol | NA | 6.30 | |
| Carvedilol | NA | 5.36 | |
| Labetalol | 1.45 | 3.06 | 110.78 |
| Metoprolol succinate | 4.88 | 2.53 | −48.14 |
| Metoprolol tartrate | 0.76 | 3.23 | 325.98 |
| Nadolol | 2.55 | 8.86 | 248.09 |
| Penbutolol sulfate | NA | 3.76 | |
| Pindolol | 1.41 | 2.68 | 90.65 |
| Propranolol | 1.43 | 3.02 | 110.53 |
| Timolol maleate | 0.80 | 1.30 | 62.87 |
| Calcium channel blockers | |||
| Amlodipine besylate | NA | 1.38 | |
| Diltiazem | 1.46 | 1.70 | 16.60 |
| Felodipine | 1.07 | 1.24 | 15.93 |
| Isradipine | 1.89 | 1.80 | −4.61 |
| Nicardipine | 1.38 | 3.45 | 150.69 |
| Nifedipine | 0.83 | 0.98 | 18.96 |
| Nimodipine | NA | 15.39 | |
| Nisoldipine | NA | 1.97 | |
| Verapamil | 0.92 | 2.52 | 173.99 |
| Statins | |||
| Atorvastatin | NA | 1.43 | |
| Ezetimibe, simvastatin | NA | 1.34 | |
| Fluvastatin | NA | 1.59 | |
| Lovastatin | 1.66 | 2.14 | 29.45 |
| Pravastatin | 1.86 | 1.90 | 2.15 |
| Rosuvastatin | NA | 2.40 | |
| Simvastatin | 1.67 | 2.22 | 33.08 |
Source of price data: Wolters Kluwer Health.
NA, not applicable because no generic equivalent is available.
We found significant variation in the prices of generic equivalents relative to their brand name counterparts. For instance, brand name pravastatin was only 2.2 percent more expensive than its generic equivalent (Table 2). In contrast, some brand name versions of drugs in the β-blocker class had prices that were more than 300 percent higher than their generic equivalents. The significant price differences in the β-blocker class account for the fact that 96.5 percent of the potential savings in this class, or $206.4 million, could be achieved through generic substitution, even though generic penetration in this class was over 70 percent during the study period.
Savings
The estimated savings are presented in Table 3. Maximum potential savings associated with full substitution in the four classes in 2006 is $2.76 billion or 7.8 percent of sales in those classes. Table 3 also presents savings resulting from 25 and 50 percent of substitutions possible. If only 25 percent of potential substitutions were achieved, $689.7 million would have been saved by consumers and payers in these four classes in 2006. Nearly half (45.3 percent) of potential savings comes from the statin class, reflecting their proportion of total spending in these classes. The amount of potential savings varies by class from 4.1 percent of total sales for β-blockers to 19.2 percent of sales for the CCBs. The percentage of sales saved by switching to best buy drugs was highest in the CCB class, where best buy drugs only had a 27.3 percent share of DDDs (Figure 1).
Table 3.
Savings Achieved by Switching to Best Buy Drugs, by Class, in 2006
| ACE Inhibitors | Beta Blockers | Calcium Channel Blockers | Statins | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dollars (in Millions) | % | Dollars (in Millions) | % | Dollars (in Millions) | % | Dollars (in Millions) | % | Dollars (in Millions) | % | |
| Actual sales in 2005 | $3,855.77 | $5,232.08 | $5,282.09 | $20,881.85 | $35,251.79 | |||||
| Savings possible | ||||||||||
| 100% of substitutions achieved | $280.13 | 7.3 | $213.84 | 4.1 | $1,015.16 | 19.2 | $1,249.80 | 6.0 | $2,758.93 | 7.8 |
| 50% of substitutions achieved | $140.07 | 3.6 | $106.92 | 2.0 | $507.58 | 9.6 | $624.90 | 3.0 | $1,379.47 | 3.9 |
| 25% of substitutions achieved | $70.03 | 1.8 | $53.46 | 1.0 | $253.79 | 4.8 | $312.45 | 1.5 | $689.73 | 2.0 |
| Distribution of savings | ||||||||||
| % from generic substitution | 14.9 | 96.5 | 22.5 | 56.0 | 0.0 | |||||
| % from therapeutic substitution | 85.1 | 3.5 | 77.5 | 44.0 | 0.0 | |||||
Generic Penetration
Generic penetration varied widely in the classes we studied. Generic drugs made up over 95 and 70 percent of DDDs for multisource drugs in the ACEI and β-blocker classes, respectively. However, the generic share of DDDs for multisource statins and CCBs was only 54.6 and 56.1 percent, respectively. The proportion of savings from therapeutic (as opposed to generic) substitution ranged from 3.5 percent for β-blockers to 85.1 percent for ACEI depending on the size of the best buy drugs’ market share during the study period, the level of generic use, and the price differences between best buy drugs and other drugs in the class (Table 3).
Conclusion
We set out to estimate the potential savings associated with the drug recommendations in a novel consumer-oriented program of prescription drug education. Our analysis points to significant potential savings for patients and the health care system. If the CRBBD accounted for all drug sales in the four classes we studied in 2006, consumers and payers would have saved a maximum of $2.76 billion dollars or 7.83 percent of total expenditures in these classes.
Public and private payers have instituted a range of policies in recent years to reduce spending on prescription drugs including increased cost-sharing (e.g., tiered or incentive-based formularies) and utilization restrictions such as prior authorization (Huskamp et al. 2003; Fischer et al. 2004; Goldman et al. 2004; Gibson, Ozminkowski, and Geotzel 2005; Hartung, Ketchum, and Haxby 2006; Roughead et al. 2006). Savings resulting from these policies are substantial, but our findings point to potential for further reductions in drug spending. Indeed, studies have shown that many elderly who are under- or uninsured for prescription drugs are prescribed therapeutic agents for which less expensive alternatives are available (Tseng et al. 2003; Fischer and Avorn 2004b). The choice of more costly medicines may relate to clinical factors, but may also be due to the fact that older medicines with generic equivalents are not as heavily promoted by the pharmaceutical industry as newer medicines (Hurwitz and Caves 1988; Iizuka 2004).
We found that the majority of savings in these four classes would be achieved through therapeutic as opposed to generic substitution. The rate of generic use in some of our classes is substantially higher than what recently published estimates indicate (Haas et al. 2005). Payers have become much more aggressive in recent years at requiring patients to fill prescriptions for generic equivalents for multisource drugs (O'Malley et al. 2006). Nearly two-thirds of prescriptions filled in the U.S. in 2006 were for generic drugs (Frank 2007). However, our findings also suggest substantial variability in use of generic equivalents across classes and products. Early experience with the Medicare drug benefit suggests that financial incentives to use generic drugs may outweigh concerns among some elderly and their physicians about the safety and efficacy of generics (Banahan and Kolassa 1997; Hellerstein 1998; Genther and Kreling 2000; Gaither et al. 2001; Saul 2007).
We found evidence of variation in the relative prices of generic drugs compared with their brand name counterparts. Studies have shown that generic drugs are priced 10–70 percent lower than brand name drugs (Frank and Salkever 1997; Suh et al. 2000). It is important to monitor the price differences between generic and brand name drugs in the midst of dramatic changes in pharmaceutical markets. On the one hand, generic manufacturers have more aggressively challenged the patents of brand name drugs, increasing generic entry and price competition (Frank 2007). On the other hand, manufacturers of brand name drugs are increasingly releasing “authorized generics” (generic versions introduced by the original brand manufacturer) in an attempt to maintain revenues.
There are limitations to our analysis, which may limit generalizability. First, medication switches may not be appropriate in some cases due to medication side effect profiles or potential drug interactions. We were interested, however, in gaining an understanding of the maximum economic impact of the program and providing a range of possible savings estimates. Second, our findings are not necessarily generalizeable to other medication classes. As of December 2007, CRBBD had produced consumer reports on 19 of the 32 classes for which DERP has conducted systematic reviews. We made simplifying assumptions in our analysis, which may be difficult to apply to other classes (i.e., it would be difficult to apply the assumption of no polypharmacy to the antidepressant or antipsychotic classes). Thus, while savings could be achieved in other classes through therapeutic substitution, the magnitude of savings will vary by class. Third, we estimated savings in a dynamic market, in which prices and market shares can change dramatically from year to year. Notably two of the statins (simvastatin and pravastatin) became available as generics mid-way through our study period. Savings for 2007 and beyond will be substantially higher due to the availability of multiple generic alternatives in the class (Consumer Reports Best Buy Drugs 2006). Fourth, we cannot take into account any rebates or discounts negotiated by third-party payers. Given that the nature and magnitude of these rebates are not publicly reported, it is unclear how our savings estimates would be affected if rebates and discounts were incorporated. Fifth, because we used aggregate data on total sales in these four pharmacologic classes, we cannot estimate how much of the savings would accrue to patients as opposed to payers. Modest savings that accrue to third-party payers may lower overall health insurance premiums, but are not likely to lead to significant behavior changes among patients. Significant savings that result in lower out-of-pocket spending among consumers could reduce economic barriers to medication adherence.
One important question is how the transition costs associated with medication switches might compare with the savings achieved through increased use of the best buy drugs. For instance, when British Columbia instituted a reference pricing system for ACEIs in 1997, visits to physicians increased slightly among those who switched to lower cost medications. Medication switchers had an 11-percent increase in visits to physicians during the first 2 months following implementation of the policy corresponding to a temporary increase in physician expenditures of approximately $11 per patient just before switching and $13 afterward (Schneeweiss et al. 2002). It is important to note, however, that these were one-time costs as opposed to savings associated with chronic use of medication over a period of several years. A follow-up study estimated that the reference pricing policy resulted in $6.7 million in savings among existing medication users, while expenditures for increased physician claims amounted to only $0.7 million (Schneeweiss et al. 2004). Nevertheless, it is important for efforts like those of the CRBBD program to take these transition costs into consideration.
While the findings we report are theoretical, they are of practical importance given that we assessed the potential impact of an existing program targeting consumers. Some payers have provided price and quality information on hospitals and physicians to consumers in conjunction with consumer-directed health care initiatives, yet little is known about their effects (Ginsburg 2006). In some respects, reporting on the comparative effectiveness of prescription drugs from randomized controlled trials is technically more straightforward than reporting on the quality of care delivered by physicians and hospitals to which patients are not randomly assigned. Programs such as CRBBD may inform broader efforts that encourage consumers to make value-based purchases in health care.
The challenge will be to disseminate the CRBBD information in a way that actually leads to behavior change among consumers and their physicians. Knowing that Internet usage remains low among low-income individuals with high out-of-pocket drug costs who could benefit most from CRBBD-type information (Pew Charitable Trust 2005; Baker, Wagner, and Bundorf 2003), CRBBD works with a number of intermediaries to disseminate the educational material. For instance, Medco, one of the country's largest PBMs, delivers direct mailings of the CRBBD information to its members. One such mailing to just over 1 million users of brand name statins about the availability of low-cost generics was followed by 49,020 members switching to simvastatin or pravastatin (G. Shearer, personal communication).
Prescribing practices are difficult to change. Simple one-time educational interventions aimed at changing physician's behavior are seldom effective (Grimshaw et al. 2001). While strategies such as academic detailing have been shown to alter prescribing in some classes (Spinewine et al. 2007), they require substantial investment and few cost-effectiveness studies of these types of interventions have been conducted (Simon et al. 2007). Very few programs that aim to improve prescribing patterns through consumer education have been evaluated (Fillit et al. 1999; Zorowitz et al. 2005). However, studies of the impact of direct-to-consumer advertising of drugs indicate that physicians are highly responsive to patient requests for medications (Kravitz et al. 2005). Providing consumers with information on prescription drugs in conjunction with financial incentives to use lower cost drugs could increase consumer demand for drugs of high value. Indeed, the design of the new Medicare drug benefit market is predicated upon the notion that seniors will “shop” for plans that offer the lowest out-of-pocket price for their drugs.
Our analysis can provide insights for the return on investment for programs to increase the use of evidence-based medicines. Disseminating this type of information to consumers and assisting them to act on it will be critical if these savings are to be realized. A program that achieved only 5 percent of the potential savings we estimated would pay for itself if it costs <$138 million to implement. By comparison, the average annual expenditure on direct-to-consumer advertising for the top 20 drugs advertised in 2005 was $115 million (Donohue, Cevasco, and Rosenthal 2007).
Acknowledgments
The authors wish to thank Steve Findlay and Gail Shearer of Consumers Union for helpful comments on earlier drafts of this manuscript. We also wish to thank Keith Newsom-Stewart at Consumers Union for valuable assistance with data collection. The authors received grant funding from Consumers Union to evaluate selected aspects of the Consumer Reports Best Buy Drugs program. The Consumer Reports Best Buy Drugs program is supported by grants from the Engelberg Foundation and the National Library of Medicine.
Disclosures: None.
Supplementary material
The following supplementary material for this article is available online: Appendix SA1: Author Matrix.
This material is available as part of the online article from http://www.blackwell-synergy.com/doi/abs/10.1111/j.1475-6773.2008.00858.x (this link will take you to the article abstract).
Please note: Blackwell Publishing is not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Adams AS, Soumerai SB, Ross-Degnan D. Use of Antihypertensive Drugs by Medicare Enrollees: Does Type of Drug Coverage Matter? Health Affairs. 2001;20(1):276–86. doi: 10.1377/hlthaff.20.1.276. [DOI] [PubMed] [Google Scholar]
- Artz MB, Hadsall RS, Schondelmeyer SW. Impact of Generosity Level of Outpatient Prescription Drug Coverage on Prescription Drug Events and Expenditure among Older Persons. American Journal of Public Health. 2002;92(8):1257–63. doi: 10.2105/ajph.92.8.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker L, Wagner TH, Bundorf MK. Use of the Internet and E-mail for Health Care Information: Results from a National Survey. Journal of the American Medical Association. 2003;289(18):2400–6. doi: 10.1001/jama.289.18.2400. [DOI] [PubMed] [Google Scholar]
- Banahan BF, Kolassa EM. A Physicians Survey on Generic Drugs and Substitution of Critical Dose Medications. Archives of Internal Medicine. 1997;157(18):2080–8. [PubMed] [Google Scholar]
- Catlin A, Cowan C, Heffler S, Washington B. National Health Spending in 2005: The Slowdown Continues. Health Affairs. 2007;26(1):142–53. doi: 10.1377/hlthaff.26.1.142. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, Roccella EJ. Joint National Commission on Prevention, Detection, Evaluation and Treatment of High Blood Pressure. National Heart Lung and Blood Institute: National High Blood Pressure Education Program Coordinating Committee. Hypertension. 2003;42(6):1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- Consumer Reports Best Buy Drugs. 2006. [accessed on April 24, 2008]. “The Statin Drugs: Prescription and Price Trends and Potential Cost Savings in Medicare from Increased Use of Lower-Cost Statins”. Available at http://www.consumerreports.org/health/resources/pdf/best-buy-drugs/statins-Rxtrend-FINAL-Feb2007.pdf.
- Donohue JM, Cevasco M, Rosenthal MB. A Decade of Direct-to-Consumer Advertising of Prescription Drugs. New England Journal of Medicine. 2007;357:673–81. doi: 10.1056/NEJMsa070502. [DOI] [PubMed] [Google Scholar]
- Ellis JJ, Erickson SR, Stevenson JG, Bernstein SJ, Stiles RA, Fendrick AM. Suboptimal Statin Adherence and Discontinuation in Primary and Secondary Prevention Populations: Should We Target Patients with the Most to Gain? Journal of General Internal Medicine. 2004;19:638–45. doi: 10.1111/j.1525-1497.2004.30516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillit HM, Futterman R, Orland BI, Chim T, Susnow L, Picariello GP, Scheye EC, Spoeri RK, Roglieri JL, Warburton SW. Polypharmacy Management in Medicare Managed Care: Changes in Prescribing by Primary Care Physicians Resulting from a Program Promoting Medication Reviews. American Journal of Managed Care. 1999;5(5):587–94. [PubMed] [Google Scholar]
- Findlay SD. Bringing the DERP to Consumers: ‘Consumer Reports Best Buy Drugs.’. Health Affairs. 2006;25:W283–6. doi: 10.1377/hlthaff.25.w283. [DOI] [PubMed] [Google Scholar]
- Fischer MA, Avorn J. Economic Implications of Evidence-Based Prescribing for Hypertension: Can Better Care Cost Less? Journal of the American Medical Association. 2004a;291(15):1850–6. doi: 10.1001/jama.291.15.1850. [DOI] [PubMed] [Google Scholar]
- Fischer MA, Avorn J. Potential Savings from Increased Use of Generic Drugs in the Elderly: What the Experience of Medicaid and Other Insurance Programs Means for a Medicare Drug Benefit. Pharmacoepidemiology and Drug Safety. 2004b;13:207–14. doi: 10.1002/pds.872. [DOI] [PubMed] [Google Scholar]
- Fischer MA, Schneeweiss S, Avorn J, Solomon DH. Medicaid Prior-Authorization Programs and the Use of Cyclooxygenase-2 Inhibitors. New England Journal of Medicine. 2004;351(21):2187–94. doi: 10.1056/NEJMsa042770. [DOI] [PubMed] [Google Scholar]
- Frank R. The Ongoing Regulation of Generic Drugs. New England Journal of Medicine. 2007;357(20):1993–6. doi: 10.1056/NEJMp078193. [DOI] [PubMed] [Google Scholar]
- Frank RG, Salkever DS. Generic Entry and the Pricing of Pharmaceuticals. Journal of Economics and Management Strategy. 1997;6(1):75–90. [Google Scholar]
- Gaither CA, Kirking DM, Ascione FJ, Welage LS. Consumers’ Views on Generic Medications. Journal of the American Pharmaceutical Association. 2001;41(5):729–36. doi: 10.1016/s1086-5802(16)31281-5. [DOI] [PubMed] [Google Scholar]
- Gellad WF, Huskamp HA, Phillips KA, Haas JS. How the New Medicare Drug Benefit Could Affect Vulnerable Populations. Health Affairs. 2006;25(1):248–55. doi: 10.1377/hlthaff.25.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genther JM, Kreling DH. Consumer Perceptions of Risk and Required Cost Savings for Generic Prescription Drugs. Journal of the American Pharmaceutical Association. 2000;40(3):378–83. doi: 10.1016/s1086-5802(16)31086-5. [DOI] [PubMed] [Google Scholar]
- Gibson TB, Ozminkowski JR, Geotzel RZ. The Effects of Prescription Drug Cost Sharing: A Review of the Evidence. American Journal of Managed Care. 2005;11(11):730–40. [PubMed] [Google Scholar]
- Ginsburg PB. 2006. “Statement of Paul Ginsburg, President,” Center for Studying Health System Change, Washington, DC.
- Goldman DP, Joyce GE, Escarce J, Pace JE, Solomon MD, Laouri M, Landsman PB, Teutsch SM. Pharmacy Benefits and the Use of Drugs by the Chronically Ill. Journal of the American Medical Association. 2004;291(19):2344–50. doi: 10.1001/jama.291.19.2344. [DOI] [PubMed] [Google Scholar]
- Grimshaw JM, Shirran L, Thomas R, Mowatt G, Fraser C, Bero LA, Grilli R, Harvey E, Oxman A, O'Brien MA. Changing Provider Behavior: An Overview of Systematic Reviews of Interventions. Medical Care. 2001;39(8, suppl 2):II2–45. [PubMed] [Google Scholar]
- Haas JS, Phillips KA, Gerstenberger EP, Seer AC. Potential Savings from Substituting Generic Drugs for Brand-Name Drugs: Medical Expenditure Panel Survey, 1997–2000. Annals of Internal Medicine. 2005;142:891–7. doi: 10.7326/0003-4819-142-11-200506070-00006. [DOI] [PubMed] [Google Scholar]
- Hartung DM, Ketchum KL, Haxby DG. An Evaluation of Oregon's Evidence-Based Practitioner-Managed Prescription Drug Plan. Health Affairs. 2006;25(5):1423–32. doi: 10.1377/hlthaff.25.5.1423. [DOI] [PubMed] [Google Scholar]
- Hellerstein JK. The Importance of the Physician in the Generic Versus Trade-Name Prescription Decision. RAND Journal of Economics. 1998;29(1):108–36. [PubMed] [Google Scholar]
- Hsu J, Price M, Huang J, Brand R, Fung V, Hui R, Fireman B, Newhouse J, Selby J. Unintended Consequences of Caps on Medicare Drug Benefits. New England Journal of Medicine. 2006;354(22):2349–459. doi: 10.1056/NEJMsa054436. [DOI] [PubMed] [Google Scholar]
- Hurwitz MA, Caves RE. Persuasion or Information? Promotion and the Shares of Brand Name and Generic Pharmaceuticals. Journal of Law and Economics. 1988;XXXI:299–320. [Google Scholar]
- Huskamp HA, Deverka PA, Epstein AM, Epstein RS, McGuigan K, Frank RG. The Effect of Incentive-Based Formularies on Prescription-Drug Utilization and Spending. New England Journal of Medicine. 2003;349:2224–32. doi: 10.1056/NEJMsa030954. [DOI] [PubMed] [Google Scholar]
- Iizuka T. What Explains the Use of DTCA of Prescription Drugs? Journal of Industrial Economics. 2004;LII(III):349–79. [Google Scholar]
- Kravitz RL, Epstein RM, Feldman MD, Franz CE, Azari R, Wilkes MS, Hinton L, Franks P, Kravitz RL, Epstein RM, Feldman MD, Franz CE, Azari R, Wilkes MS, Hinton L, Franks P. Influence of Patients’ Requests for Direct-to-Consumer Advertised Antidepressants: A Randomized Controlled Trial. Journal of the American Medical Association. 2005;293(16):1995–2002. doi: 10.1001/jama.293.16.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maio V, Pizzi L, Roumm AR, Clarke J, Goldfarb NI, Nash DB, Chess D. Pharmacy Utilization and the Medicare Modernization Act. Milbank Quarterly. 2005;83(1):101–30. doi: 10.1111/j.0887-378X.2005.00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicare Payment Advisory Commission. Report to the Congress: Increasing the Value of Medicare. 2006. [accessed June 2006]. Available at http://www.medpac.gov/publications/congressional_reports/Jun06_EntireReport.pdf.
- O'Malley AJ, Frank RG, Kaddis A, Rothenberg BM, McNeil BJ. Impact of Alternative Interventions on Changes in Generic Dispensing Rates. Health Services Research. 2006;41(5):1876–94. doi: 10.1111/j.1475-6773.2006.00579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pew Charitable Trust Health Information Online. Pew Internet and American Life Project. Washington, DC: Pew Charitable Trust. Available at http://www.pewinternet.org.
- Piette JD, Heisler M, Wagner TH. Cost-Related Medication Underuse: Do Patients with Chronic Illnesses Tell Their Doctors? Archives of Internal Medicine. 2004;164:1749–55. doi: 10.1001/archinte.164.16.1749. [DOI] [PubMed] [Google Scholar]
- Reichert S, Simon T, Halm E. Physicians’ Attitudes about Prescribing and Knowledge of the Costs of Common Medication. Archives of Internal Medicine. 2000;160:2799–803. doi: 10.1001/archinte.160.18.2799. [DOI] [PubMed] [Google Scholar]
- Rosen AB, Hamel MB, Weinstein MC, Cutler DM, Fendrick AM, Vijan S. Cost-Effectiveness of Full Medicare Coverage of Angiotensin-Converting Enzyme Inhibitors for Beneficiaries with Diabetes. Annals of Internal Medicine. 2005;143:89–99. doi: 10.7326/0003-4819-143-2-200507190-00007. [DOI] [PubMed] [Google Scholar]
- Roughead EE, Zhang F, Ross-Degnan D, Soumerai SB. Differential Effect of Early or Late Implementation of Prior Authorization Policies on the Use of Cox II Inhibitors. Medical Care. 2006;44(4):378–82. doi: 10.1097/01.mlr.0000204056.31664.36. [DOI] [PubMed] [Google Scholar]
- Safran DG, Neuman P, Schoen C, Montgomery JE, Li W, Wilson IB, Kitchman MS, Bowen AE, Rogers WH. Prescription Drug Coverage and Seniors: How Well Are States Closing the Gap? Health Affairs Web Exclusives. 2002:W253–68. doi: 10.1377/hlthaff.w2.253. [DOI] [PubMed] [Google Scholar]
- Safran DG, Newman P, Schoen C, Kitchman MS, Wilson IB, Cooper B, Hong Chang AL, Rogers WH. Prescription Drug Coverage and Seniors: Findings from a 2003 National Survey. Health Affairs Web Exclusives. 2005;(April):W5-152–66. doi: 10.1377/hlthaff.w5.152. [DOI] [PubMed] [Google Scholar]
- Saul S. Strategies to Avoid Medicare's Big Hole. New York: New York Times; 2007. [Google Scholar]
- Schneeweiss S, Dormuth C, Grootendorst P, Soumerai SB, Maclure M. Net Health Plan Savings from Reference Pricing for Angio-Tensing Converting Enzyme Inhibitors in Elderly British Columbia Residents. Medical Care. 2004;42(7):653–60. doi: 10.1097/01.mlr.0000129497.10930.a2. [DOI] [PubMed] [Google Scholar]
- Schneeweiss S, Walker AM, Glynn RJ, Maclure M, Dormuth C, Soumerai SB. Outcomes of Reference Pricing for Angiotensing-Converting Enzyme Inhibitors. New England Journal of Medicine. 2002;346(11):822–9. doi: 10.1056/NEJMsa003087. [DOI] [PubMed] [Google Scholar]
- Shrank WH, Hoang T, Ettner SL, Glassman P, Nair K, DeLapp D, Avorn J, Asch SM. The Implications of Choice: Prescribing Generic or Preferred Pharmaceuticals Improves Medication Adherence for Chronic Conditions. Archives of Internal Medicine. 2006;166:332–7. doi: 10.1001/archinte.166.3.332. [DOI] [PubMed] [Google Scholar]
- Simon SR, Rodriguez HP, Majumdar SR, Kleinman K, Warner C, Salem-Schatz S, Miroshnik I, Soumerai SB, Prosser LA. Economic Analysis of a Randomized Trial of Academic Detailing Interventions to Improve Use of Antihypertensive Medications. Journal of Clinical Hypertension. 2007;9(1):15–20. doi: 10.1111/j.1524-6175.2006.05684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumerai SB, Pierre-Jacques M, Zhang F, Ross-Degnan D, Adams AS, Gurwitz J, Adler G, Safron DG. Cost-Related Medication Nonadherence among Elderly and Disabled Medicare Beneficiaries. Archives of Internal Medicine. 2006;166:1829–35. doi: 10.1001/archinte.166.17.1829. [DOI] [PubMed] [Google Scholar]
- Spinewine A, Schmader K, Barber N, Hughes C, Lapane KL, Hanlon JT. Appropriate Prescribing in Elderly People: How Well Can It Be Measured and Optimised? Lancet. 2007;370:173–84. doi: 10.1016/S0140-6736(07)61091-5. [DOI] [PubMed] [Google Scholar]
- Stuart B, Briesacher BA, Shea DG, Cooper B, Baysac FS, Limcangco R. Riding the Rollercoaster: The Ups and Downs in Out-of-Pocket Spending under the Standard Medicare Drug Benefit. Health Affairs. 2005a;24(4):1022–31. doi: 10.1377/hlthaff.24.4.1022. [DOI] [PubMed] [Google Scholar]
- Stuart B, Simoni-Wastila L, Chancey D. Assessing the Impact of Coverage Gaps in the Medicare Part D Drug Benefit. Health Affairs. 2005b;W5:167–79. doi: 10.1377/hlthaff.w5.167. [DOI] [PubMed] [Google Scholar]
- Suh DC, Manning WG, Schondelmeyer S, Hadsall RS. Effect of Multiple-Source Entry on Price Competition after Patent Expiration in the Pharmaceutical Industry. Health Services Research. 2000;35(2):529–47. [PMC free article] [PubMed] [Google Scholar]
- Thom T, Haase N, Rosamong W, Howard VJ, Rumsfeld J, Manolio T, Zheng Z-J, Flegal K, O’Donnell C, Kittner S, Lloyd-Jones D, Goff DC, Jr, Hong Y. Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P Members of the Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics—2006 Update: A Report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- Tseng C, Brook RH, Keeler E, Mangione CM. Impact of an Annual Dollar Limit or ‘Cap’ on Prescription Drug Benefits for Medicare Patients. Journal of the American Medical Association. 2003;290(2):222–7. doi: 10.1001/jama.290.2.222. [DOI] [PubMed] [Google Scholar]
- Voelker R. Easy-to-Use Drug Reports Help Patients and Physicians Weigh Costs, Benefits. Journal of the American Medical Association. 2005;294(2):165–6. doi: 10.1001/jama.294.2.165. [DOI] [PubMed] [Google Scholar]
- Winkelmayer WC, Fischer MA, Schneeweiss S, Wang PS, Levin R, Avorn J. Underuse of ACE Inhibitors and Angiotensin II Receptor Blockers in Elderly Patients with Diabetes. American Journal of Kidney Diseases. 2005;46(6):1080–7. doi: 10.1053/j.ajkd.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Zorowitz BJ, Stebelsky LA, Muma BK, Romain TM, Peterson EL. Reduction of High-Risk Polypharmacy Drug Combinations in Patients in a Managed Care Setting. Pharmacotherapy. 2005;25(11):1636–45. doi: 10.1592/phco.2005.25.11.1636. [DOI] [PubMed] [Google Scholar]
- Zuvekas SH, Cohen JW. Prescription Drugs and the Changing Concentration of Health Care Expenditures. Health Affairs. 2007;26(1):249–57. doi: 10.1377/hlthaff.26.1.249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.