Abstract
Plant cells can respond qualitatively and quantitatively to a wide range of environmental signals. Ca2+ is used as an intracellular signal for volume regulation in response to external osmotic changes. We show here that the spatiotemporal patterns of hypo-osmotically induced Ca2+ signals vary dramatically with stimulus strength in embryonic cells of the marine alga Fucus. Biphasic or multiphasic Ca2+ signals reflect Ca2+ elevations in distinct cellular domains. These propagate via elemental Ca2+ release in nuclear or peripheral regions that are rich in endoplasmic reticulum. Cell volume regulation specifically requires Ca2+ elevation in apical peripheral regions, whereas an altered cell division rate occurs only in response to stimuli that cause Ca2+ elevation in nuclear regions.
Keywords: Fucus; Ca2+ waves; Ca2+ sparks; inositol 1,4,5-trisphosphate; volume regulation
Plant growth and development in a fluctuating environment requires the ability to sense and respond, both qualitatively and quantitatively, to different stimuli. Ca2+ serves multiple roles as a second messenger (1), although the spatiotemporal patterns of Ca2+ signals, their mechanisms of propagation, and the ways in which specificity of response is encoded into appropriate downstream responses are poorly understood (1, 2). Both the vacuole (3, 4) and endoplasmic reticulum (ER) (5) have been implicated in the generation of physiologically significant Ca2+ signals in plants, although their relative roles in different signal-response pathways are uncertain. Whereas different magnitudes and temporal patterns of Ca2+ signal have been correlated with altered gene expression in animals and plants (6–9), the spatial patterns of environmentally induced Ca2+ signals underlying short-term physiological responses or longer-term regulation of gene expression remain poorly resolved. Moreover, the relative importance of nuclear (10) and cytoplasmic Ca2+ signals has not been studied in detail in any plant or algal cell type.
Transient elevations of Ca2+ following hypo-osmotic treatments have been reported in animals, yeast, algae, and higher plants (11–14). Both Ca2+ influx and intracellular release are involved in the generation of hypo-osmotic Ca2+ signals (11–14) that can vary in magnitude according to stimulus strength, although their spatial characteristics have not been characterized. Whereas propagating Ca2+ signals have been reported in plant cells in response to inositol 1,4,5-trisphosphate [Ins(1,4,5)P3] (15) and electrical stimulation (16), the Fucus embryo provides one of the only examples among plants or algae of a Ca2+ wave as an essential signal arising in direct response to a physiological stimulus (13). Fucoid algae inhabit the intertidal zone and experience regular and dramatic changes in their external osmotic environment. They have thus evolved physiological mechanisms for regulation of cell volume and provide an ideal system for studying the mechanism of physiological Ca2+ signal propagation in plant cells.
Locally elevated Ca2+ is required in the growth zone of several polarized plant cell types (11–14, 17, 18). Two-celled Fucus embryos comprise a polarized growing rhizoid cell and a thallus cell. In their natural intertidal environment, they experience dramatic fluctuations in external osmotic conditions varying between extreme hypo- and hypersalinity. Hypo-osmotic treatment causes transient elevation of Ca2+ that is also initiated in the apical rhizoid region (13). Thus, osmotic Ca2+ signals are generated in the same cell region as the Ca2+ gradient involved in regulation of polarized growth, and it is not clear how responses of growth and osmoregulation to these Ca2+ signals are integrated.
To determine the spatiotemporal patterns of Ca2+ signals in response to different magnitudes of the same stimulus, we subjected two-celled Fucus embryos to varying degrees of hypo-osmotic shock. In addition, we compared downstream responses to Ca2+ signals that were generated in specific cell regions.
Materials and Methods
Plant Material and Dye Injection.
Fucus serratus embryos were cultured to the two-celled stage in seawater (SW) for 24 h in unidirectional white light at 17°C as described (19, 20). Rhizoid cells were pressure microinjected with 10–50 μM concentrations of dye prepared in artificial cytoplasm solution (19, 20). Dyes used included fura-2-dextran (10 kDa), Calcium Green-dextran (10 kDa), or a mixture of Calcium Green-dextran and Texas Red-dextrans (10 kDa) (Molecular Probes).
Ca2+ Imaging.
Fluorescence of injected cells was monitored at least 3 h after injection and recovery in SW. Photometric measurements of Ca2+ were made on fura-2-dextran-injected rhizoid cells (19, 20). For confocal imaging, zygotes were imaged after >3 h with a Bio-Rad (Hemel Hempstead, Hertfordshire, U.K.) 1024 confocal laser scanning microscope equipped with a Nikon ×40 (numerical aperture = 1.3) oil immersion lens and an argon/krypton laser. Cells injected with Calcium Green-dextran were imaged at 488 nm excitation and 522 nm emission, and those injected with Calcium Green/Texas Red-dextrans were excited simultaneously with 488 and 568 nm laser lines and emission monitored at 522 nm (for Calcium Green) and 605 nm (for Texas Red). Dark background was subtracted at each wavelength before image acquisition. In all cases, cell autofluorescence accounted for less than 10% of the minimum dye fluorescence values. Fluorescence sections (normally 20 lines × 512 pixels; 100 ms per section) and single confocal line scans (2 ms per line) of Calcium Green fluorescence, along the longitudinal axis of the rhizoid of injected zygotes were obtained with Bio-Rad time course software before and during hypo-osmotic Ca2+ signal generation.
Localized Ins(1,4,5)P3 Photorelease.
Rhizoid cells were microinjected with a mixture of Calcium Green-dextran and Texas Red-dextran (10 kDa, final intracellular concentrations of 10–50 μM) and caged Ins(1,4,5)P3 (10 μM, Calbiochem). Localized photorelease of Ins(1,4,5)P3 was achieved with a UV-pulsed nitrogen laser (10-ns pulses, 20 Hz, 1-s exposure; VSL 337; Laser Science, Cambridge, MA) delivered and focused to an adjustable spot size via a beam expander and adjustable focusing lens in the fluorescence port, delivery optics, and microscope objective. Laser intensity was adjusted with an adjustable diaphragm on the delivery optics to achieve photorelease. Calcium Green and Texas Red fluorescence were monitored simultaneously as described above.
Image Analysis.
After acquisition, Calcium Green images were ratioed sequentially against an initial pretreatment image to give relative changes in dye fluorescence (F/Fo). The ratioed scans were spatially averaged with a 3 × 3 pixel window by using Kinetic Imaging (Liverpool, U.K.) Lucida software which calculates the average of the fluorescence values of a 3 × 3 pixel box and assigns the value to the pixel at the center of the box. Ratioed scans were displayed sequentially to form an image with the position along the rhizoid on the x axis and time on the y axis. For dual dye-injected zygotes, Calcium Green images were ratioed against the corresponding Texas Red image. Ca2+ concentrations were calculated from Calcium Green/Texas Red ratio values assuming elevation to saturating ratio values at the peak of the transient (13) and assuming a resting [Ca2+] of 139 nM measured in studies with fura-2 (13).
Line scans were displayed sequentially so that each image represents 1 s (512 lines). Three-dimensional plots were generated from these images by using Kinetic Imaging (Liverpool, U.K.) Lucida software. Statistical analysis of means, standard deviations, and variance of pixel values in a chosen area was performed before sequential ratio generation. The size and shape of the area analyzed (normally 20 × 20–50 pixels) was chosen to minimize variance of the Ca2+ signal because of the temporal increase in fluorescence intensity at the wave front. The occurrence of elemental events was identified by the increased variance/mean ratio of the calcium green fluorescence signal compared with the variance/mean ratio of a corresponding Ca-independent signal. Individual events were identified as having a fluorescence pixel value of >2 SD of background fluorescence intensity after 3 × 3 averaging.
Cell Division.
Hypo-osmotic shocks of 15-min duration were administered to 24-h embryos at the two-cell stage. Cell numbers were counted after a further 12 h. Individual cells were visualized in confocal sections of embryos after incubation for 10 min with 1 μM fluorescent membrane dye FM1–43 (Molecular Probes).
Results and Discussion
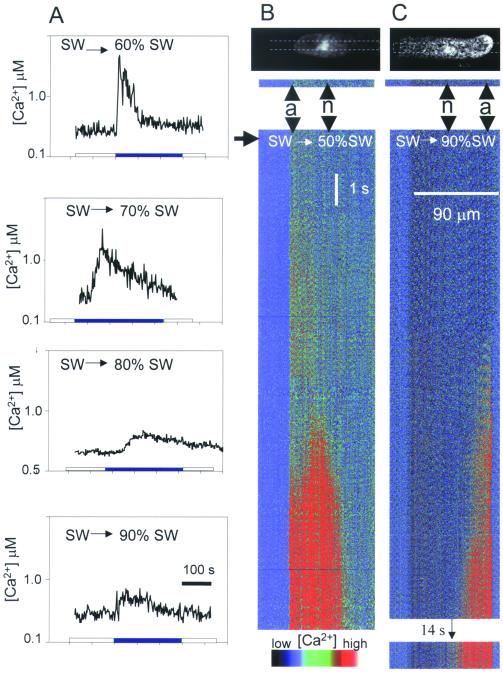
The magnitude, duration, and pattern of hypo-osmotically induced cytosolic Ca2+ transients in the rhizoid cell strikingly depend on the magnitude of the stimulus (Fig. 1). Severe shocks (e.g., from 100% SW to 50% or 60% SW) induced an almost immediate rapid elevation of Ca2+ that declined within 100 s (Fig. 1A). This was characterized by an initial small transient elevation at the rhizoid apex that was followed, after 4–5 s, by an explosive Ca2+ increase initiating in the nuclear region that propagated bidirectionally at approximately 20 μm/s (80% of cells; Fig. 1B). This Ca2+ elevation was not excluded from the nucleoplasm. In the remaining 20% of cells, Ca2+ was seen to elevate in the nuclear region either before or simultaneously with the apical increase (Fig. 3B). In contrast to severe shocks, milder hypo-osmotic shocks (e.g., 100% SW to 90% SW) elicited Ca2+ elevations with a delayed onset (up to 60 s; Fig. 1 A and C) but which were significantly more prolonged. These were restricted to the rhizoid apex and did not propagate as Ca2+ waves.
Figure 1.
Patterns of cytosolic Ca2+ signals in response to different degrees of hypo-osmotic shock. (A) Ratio photometric recordings of average global cell Ca2+ monitored with fura-2-dextran (13) in rhizoid cells in response to transfer from 100% SW to 60% (n = 7), 70% (n = 20), 80% (n = 20), or 90% (n = 3) SW. (B and C) Sequential plots of confocal sections (shown on the Calcium Green fluorescence images at the top of each plot) showing the relative increase in Calcium Green-dextran fluorescence in a rhizoid cell in response to transfer from 100% SW to 50% (B; n = 26) or 90% (C; n = 7) SW. Arrow indicates onset of osmotic treatment; a = cell apex; n = nuclear region. Confocal sections indicated were compiled sequentially to produce the pseudocolor temporal plots below each image.
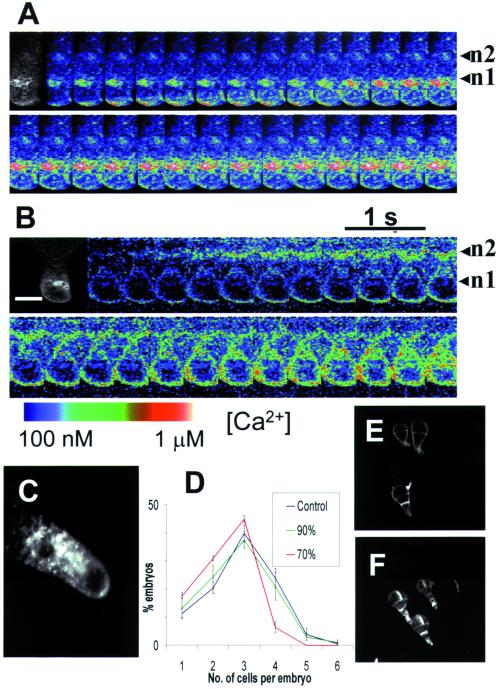
Figure 3.
Patterns of Ca2+ elevation following hypo-osmotic treatment in dividing cells. (A) Calcium Green to Texas Red ratio images during the onset of a hypo-osmotically induced Ca2+ wave (100% SW to 50% SW) shows an initial elevation of Ca2+ in the rhizoid apex which declines before the onset of Ca2+ elevation arising in the apical nucleus region (n = 40). (B) A variation in this pattern was evident in a minority of cells where Ca2+ elevations were observed to arise in subapical nucleus simultaneous with the apical Ca2+ elevation (n = 10). (C) Confocal sections of ER stained with 1.0 μM DiOC6 (Molecular Probes) for 10 min show ER distributed in the rhizoid apex and perinuclear regions. (D) The number of cell divisions was significantly reduced following more severe hypo-osmotic shocks (e.g., 100% SW to 70% SW) that cause elevated nuclear Ca2+. (E and F) Polarized rhizoid growth was unaffected by hypo-osmotic treatments up to 50% SW (E) compared with control untreated embryos (F). (Bar = 15 μm.)
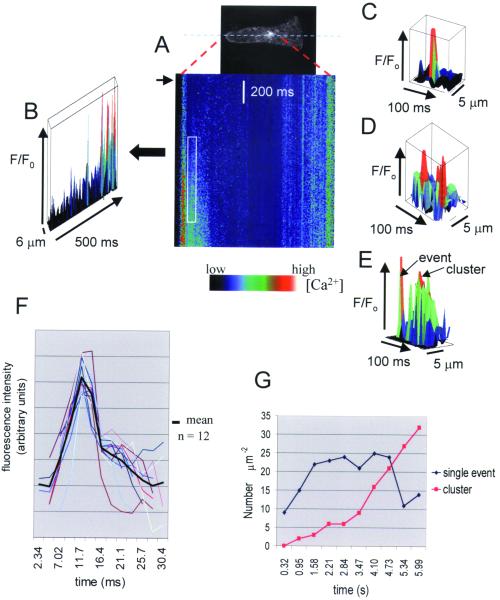
Repetitive line scans along a longitudinal transect during the onset of hypo-osmotically induced Ca2+ waves revealed nonuniform Ca2+ elevations in highly discrete domains in both the rhizoid apex and perinuclear regions (Fig. 2 A and B). Individual events were spatially confined and lasted for 15–30 ms (mean = 24.9 ± 1.2, n = 29; Fig. 2 C–F), characterized by a rapid onset (duration 7–8 ms, mean = 7.2 ± 0.25, n = 25) and a slower decline (duration 17–20 ms; mean = 19.9 ± 1.4, n = 25). We estimate from the relative increase in Ca2+ dye fluorescence during unitary Ca2+ elevations that the amplitude of the events represents Ca2+ levels up to 200–300 nM above resting levels. However, analysis of event amplitude and frequency is complicated by the fact that not all events detected in a defined area necessarily originated in the region of interest or the plane of focus (21). There was no significant difference in the duration of apical and subapical events (25.0 ± 1.7 ms; n = 9 and 24.9 ± 1.5 ms; n = 14, respectively). During wave onset, the frequency of identifiable single events with amplitudes > 2 SD above the mean background level increased initially but declined as events became more clustered as the wave progressed (Fig. 2 E and G). In areas of sustained elevation, it was more difficult to discern individual events because of the increase in background Ca2+. Unitary Ca2+ elevations were often observed to occur repetitively in the same domain (Fig. 2D) spatially separated by regions with fewer events and significantly lower variance to mean ratios of pixel intensity (two-tailed 1% significance level Wilcoxon test; n1 = 11, n2 = 11). Simultaneous line scans of Calcium Green and Texas Red fluorescence gave variance to mean ratios of pixel intensity at propagating wave fronts ranging from 3.1 to 10 (mean = 4.34, n = 8) for Calcium Green and from 0.77 to 2.17 (mean = 1.09, n = 8) for Texas Red fluorescence. There was no change in fluorescence of the Texas Red signal during wave progression (not shown). The significantly higher spatial variance of the Ca2+-dependent fluorescence signal confirms that Ca2+ elevation occurred by increased frequency of spatially discrete elevations.
Figure 2.
Elemental Ca2+ elevations during Ca2+ wave propagation in a rhizoid cell. (A) Single-line confocal scans of Calcium Green fluorescence along the longitudinal axis of the cell during the initiation of Ca2+ waves are displayed sequentially to show the relative change in fluorescence following hypo-osmotic treatment [to 50% SW (arrow); n = 25.] The three-dimensional plot in B shows a nonuniform increase in Ca2+ during the onset of the Ca2+ wave in the rhizoid apex. In this plot, time increases from left to right whereas in the line-scan image (A), time increases from top to bottom. Elemental Ca2+ elevations in perinuclear regions (C) were frequently observed to arise repetitively at the same location (D). Events either occurred individually or appeared to cluster into more prolonged elevations (E). The data in A–E represent pixel values at least two SD of the background of a quiescent adjacent area (50 × 25 pixels). (F) Temporal profiles (colored plots) and averaged plot through elemental Ca2+ elevations show a rapid increase followed by a more gradual decline (n = 12). (G) The number of discrete elevations with values > 2 SD of the adjacent background at the rhizoid apex monitored over 630-ms time intervals increased initially during the first 1.5 s of wave propagation and then declined with the appearance of more prolonged elevations.
In rhizoid cells that had undergone nuclear division but not partition wall formation (Fig. 3), hypo-osmotically induced Ca2+ waves were observed to propagate from either of the two nuclear regions (Fig. 3). Most cells showed an initial transient elevation in the rhizoid apex followed by elevation in the apical nucleus (n1) spreading toward the subapical nucleus (n2) (Fig. 3A). However, in a minority of cells (20%), Ca2+ increased in the region of subapical nucleus either before, or simultaneous with, elevation at the rhizoid apex (Fig. 3B), followed by elevation in apical nucleus. Thus, the elevation of Ca2+ in the nuclear regions is not simply the result of a single propagating wave initiating at the rhizoid apex. The pattern of apical and perinuclear Ca2+ elevations correlated well with the observed distribution of ER (Fig. 3C). ER was prominent in the perinuclear region and at the cell apex but was only sparsely distributed in a chloroplast-rich region (not shown) 5–10 μM behind the rhizoid apex.
To determine the respective roles of Ca2+ signals in the apical and perinuclear regions, we monitored changes in rhizoid cell volume and rates of cell division following treatments that caused Ca2+ elevations in these regions. The cell division rate was influenced by different degrees of hypo-osmotic shock. Significantly fewer two-cell embryos subjected to hypo-osmotic treatments (from 100% SW into 70% SW or less) that caused nuclear Ca2+ signals, had progressed to the four-cell stage after 12 h compared with nonshocked embryos or those given treatments (from 100% SW into 90% SW) where Ca2+ elevations were restricted to the rhizoid apex (Fig. 3D). The response to more severe shocks was specific to cell division because rhizoid growth was unaffected by the hypo-osmotic treatment (Fig. 3 E and F).
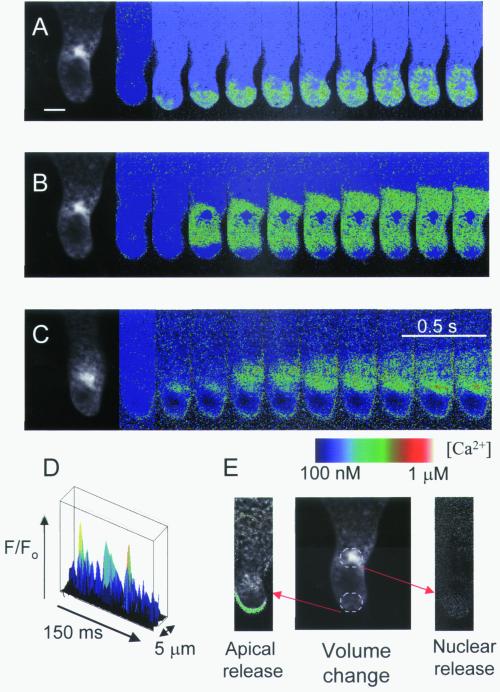
Ca2+ elevations could be elicited in selected regions of the rhizoid cell by localized flash photolysis of caged Ins(1,4,5)P3 (Fig. 4). This suggests a role for Ins(1,4,5)P3 in the generation of Ca2+ signals in this system but does not provide direct proof that Ins(1,4,5)P3-generated Ca2+ signals underlie responses to osmotic treatments. Photorelease of Ins(1,4,5)P3 in the rhizoid apex caused Ca2+ elevations that were restricted to this region (Fig. 4A). Release of Ins(1,4,5)P3 in the nuclear region produced Ca2+ elevations that propagated bidirectionally through the perinuclear region (Fig. 4B) but did not propagate to the extreme rhizoid apex (Fig. 4 B and C). In most cells (80%), intranuclear Ca2+ remained essentially unchanged in response to Ins(1,4,5)P3 photorelease. However, in a minority of cells (20%), Ins(1,4,5)P3-induced Ca2+ elevation was also observed to occur within the nucleoplasm (Fig. 4C). Thus, in contrast to the osmotically induced nuclear Ca2+ signal that was not excluded from the nucleus, the Ins(1,4,5)P3-induced Ca2+ elevation did not necessarily permeate the nucleoplasm, suggesting the involvement of factors additional to cytosolic Ins(1,4,5)P3-induced Ca2+ release during hypo-osmotic signaling. The nuclear envelope may thus form a barrier to diffusion of Ca2+ or Ins(1,4,5)P3 released in the perinuclear cytoplasm. Altered permeability of the nuclear envelope during the cell cycle (22, 23) could account for differences in nuclear Ca2+ observed in response to Ins(1,4,5)P3-induced Ca2+ elevations.
Figure 4.
Patterns of Ca2+ elevation in response to localized photorelease of caged Ins(1,4,5)P3. (A) Photorelease of Ins(1,4,5)P3 from a 10-μm diameter region at the rhizoid apex (E) caused Ca2+ elevation that propagated in a subapical direction but was generally (60% of cells, n = 23) restricted to the apical 40 μm. (B) Ins(1,4,5)P3 release in the nuclear region induced a Ca2+ wave that propagated bidirectionally and did not permeate the nucleoplasm (8 out of 14 cells). (C) In a further six cells, Ca2+ release in the nuclear region gave an initial Ca2+ increase in the nucleoplasm followed by perinuclear Ca2+ waves. (D) Elemental Ca2+ elevations (>2 SD above the mean background) could be observed during propagation of Ins(1,4,5)P3-induced Ca2+ waves in apical and subapical (not shown) regions. (E) Representative volume decrease in response to nuclear or apical Ins(1,4,5)P3-induced Ca2+ elevations. The green area indicates volume decrease in a representative cell after release of caged Ins (1,4,5)P3 in the apical rhizoid regions (n = 5). No volume decrease could be observed following Ins(1,4,5)P3 release in nuclear regions. (Bar = 15 μm.)
Line scans of Ca2+ elevations in response to photorelease of Ins(1,4,5)P3 revealed the presence of unitary Ca2+ elevations at the wave front (Fig. 4D). Ins(1,4,5)P3 events and osmotically induced events did not differ significantly in amplitude, spatial dimensions (diameter at half-maximum amplitude: 2.11 ± 0.1 μm, n = 13 and 2.23 ± 0.1 μm, n = 13, respectively), rise rate/fall rate ratio (2.36, n = 10 and 2.41, n = 15, respectively), and duration (27.7 ± 1.2 ms, n = 10 and 24.9 ± 1.2 ms, n = 10, respectively). Comparison of cell volume before and after generation of Ins(1,4,5)P3-induced Ca2+ transients showed that Ca2+ elevations at the rhizoid apex, but not those in the nuclear regions, resulted in cell volume decrease (Fig. 4E).
The occurrence of different Ca2+ signaling patterns in clearly defined cellular domains in response to a graded stimulus provides new insights into the generation of specificity of responses. The Ca2+ signal at the rhizoid apex is essential for turgor and volume regulation (13) and probably involves the regulation of ion channels that allow osmoregulatory ion efflux (24). Polarized growth requires localized Ca2+ elevation, transduced via Ca2+/calmodulin (25, 26), regulation of targeted exocytosis (27), and a functional cytoskeleton (28). Studies with the Mn2+ quench technique revealed a role for Ca2+ influx in the generation of Ca2+ signals in the rhizoid apex (13). In the present study, the detection of elemental Ca2+ elevations within the cortical cytoplasm invokes an additional contribution from intracellular Ca2+ release in regulation of Ca2+ in this region. We also show here that nuclear Ca2+ signals elicited in response to more severe hypo-osmotic treatment regulate a different set of downstream responses. Thus, alterations in the cell division rate occurred only when there was a Ca2+ elevation in the nuclear region. This has parallels with yeast where hypo-osmotic Ca2+ transients monitored with aequorin luminescence correlate with inhibition of G2-M transition via activation of the mitogen-activated protein kinase MPK1 and calcineurin (29).
Whereas it is clear that the vacuole can serve as a Ca2+ source during intracellular signaling in plants (3, 4), this demonstration of propagating Ca2+ waves in ER-rich regions containing no significant vacuoles indicates the involvement of ER Ca2+ stores in these signals. In pollen tubes, discrepancy between the position of major vacuoles and the location of Ca2+ signals, elicited by photorelease of caged Ins(1,4,5)P3, also suggested the involvement of nonvacuolar Ca2+ stores (30). The present study provides evidence that Ca2+ elevations propagate as unitary increases that have not been reported in nonanimal cells and which meet the criteria for elemental Ca2+ release from single channels or groups of channels in the ER (31–37). Moreover, the demarcation of osmotic Ca2+ signals to specific domains according to the strength of the stimulus indicates that Ca2+ release may be regulated differently in apical and perinuclear regions. This could reflect differences in the spatial distribution of Ins(1,4,5)P3 receptor channels, functional characteristics of Ins(1,4,5)P3 receptors, or specific regulatory factors in different regions. This may explain the occurrence of both graded volume regulation and qualitatively different longer-term responses arising from varying degrees of osmotic stimuli.
Acknowledgments
This work was supported by the Biotechnology and Biological Sciences Research Council, U.K. (N.F.H.M. and C.B.) and the Natural Environment Research Council, U.K. (C.B.). A University of Wales William Roberts Ph.D. studentship was awarded to H.G.
Abbreviations
- ER
endoplasmic reticulum
- Ins(1,4,5)P3
inositol 1,4,5-trisphosphate
- SW
seawater
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.020516397.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.020516397
References
- 1.Sanders D, Brownlee C, Harper J F. Plant Cell. 1999;11:691–706. doi: 10.1105/tpc.11.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McAinsh M R, Hetherington A M. Trends Plant Sci. 1998;3:32–35. [Google Scholar]
- 3.Allen G J, Muir S R, Sanders D. Science. 1995;268:735–737. doi: 10.1126/science.7732384. [DOI] [PubMed] [Google Scholar]
- 4.Allen G J, Schroeder J I. Trends Plant Sci. 1998;3:123–125. [Google Scholar]
- 5.Muir S R, Sanders D. Plant Physiol. 1997;114:1511–1521. doi: 10.1104/pp.114.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knight H, Trewavas A J, Knight M R. Plant J. 1997;12:1067–1078. doi: 10.1046/j.1365-313x.1997.12051067.x. [DOI] [PubMed] [Google Scholar]
- 7.Dolmetsch R E, Lewis R S, Goodnow C C, Healy J I. Nature (London) 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 8.Gu X, Spitzer N C. Nature (London) 1995;375:784–787. doi: 10.1038/375784a0. [DOI] [PubMed] [Google Scholar]
- 9.Li W H, Llopis J, Whitney M, Zlokamik G, Tsien R Y. Nature (London) 1998;392:936–941. doi: 10.1038/31965. [DOI] [PubMed] [Google Scholar]
- 10.Ehrhardt D W, Wais R, Long S R. Cell. 1996;85:673–681. doi: 10.1016/s0092-8674(00)81234-9. [DOI] [PubMed] [Google Scholar]
- 11.Altamirano J, Brodwick M S, Alvarez-Leefmans F J. J Gen Physiol. 1998;112:145–160. doi: 10.1085/jgp.112.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batiza A F, Schulz T, Masson P H. J Biol Chem. 1996;271:23357–23362. doi: 10.1074/jbc.271.38.23357. [DOI] [PubMed] [Google Scholar]
- 13.Taylor A R, Manison N F H, Fernandez-Cruz C, Wood J, Brownlee C. Plant Cell. 1996;8:2015–2031. doi: 10.1105/tpc.8.11.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi K, Isobe M, Knight M R, Trewavas A J, Muto S. Plant Physiol. 1997;113:587–594. doi: 10.1104/pp.113.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franklin-Tong V E, Drobak B K, Allan A C, Watkins P A C, Trewavas A J. Plant Cell. 1996;8:1305–1321. doi: 10.1105/tpc.8.8.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grabov A, Blatt M R. Planta. 1997;201:84–95. [Google Scholar]
- 17.Rathore K S, Cork R J, Robinson K R. Dev Biol. 1991;148:612–619. doi: 10.1016/0012-1606(91)90278-b. [DOI] [PubMed] [Google Scholar]
- 18.Miller D D, Callaham D A, Gross D J, Hepler P K. J Cell Sci. 1992;101:7–12. [Google Scholar]
- 19.Roberts S K, Gillot I, Brownlee C. Development (Cambridge, UK) 1994;120:155–163. [Google Scholar]
- 20.Taylor A R, Brownlee C. Plant Physiol. 1992;99:1686–1688. doi: 10.1104/pp.99.4.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pratusevich V R, Balke C W. Biophys J. 1996;71:2942–2957. doi: 10.1016/S0006-3495(96)79525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feldherr C M, Akin D. J Cell Biol. 1990;111:1–8. doi: 10.1083/jcb.111.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lipp P, Thomas D, Berridge M J, Bootman M D. EMBO J. 1997;16:7166–7173. doi: 10.1093/emboj/16.23.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brownlee C, Goddard H, Hetherington A M, Peake L-A. J Exp Bot. 1999;50:S1001–S1011. [Google Scholar]
- 25.Love J, Brownlee C, Trewavas A J. Plant Physiol. 1997;115:249–261. doi: 10.1104/pp.115.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson K R. Planta. 1996;198:378–384. [Google Scholar]
- 27.Shaw S L, Quatrano R S. Development (Cambridge, UK) 1996;122:2623–2630. doi: 10.1242/dev.122.9.2623. [DOI] [PubMed] [Google Scholar]
- 28.Kropf D L. Plant Cell. 1997;9:1011–1020. doi: 10.1105/tpc.9.7.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mizunuma M, Hirata D, Miyahara K, Tsuchiya E, Miyakawa T. Nature (London) 1998;392:303–306. doi: 10.1038/32695. [DOI] [PubMed] [Google Scholar]
- 30.FranklinTong V E, Drobak B K, Allan A C, Watkins P A C, Trewavas A J. Plant Cell. 1996;8:1305–1321. doi: 10.1105/tpc.8.8.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein M G, Cheng H, Santana L F, Jiang Y H, Lederer W J, Schneider M F. Nature (London) 1996;379:455–458. doi: 10.1038/379455a0. [DOI] [PubMed] [Google Scholar]
- 32.Song L-S, Stern M D, Lakatta E G, Cheng H. J Physiol (London) 1997;505:665–675. doi: 10.1111/j.1469-7793.1997.665ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bootman M D, Berridge M J. Cell. 1995;83:675–678. doi: 10.1016/0092-8674(95)90179-5. [DOI] [PubMed] [Google Scholar]
- 34.Lipp P, Niggli E. J Physiol (London) 1996;492:31–38. doi: 10.1113/jphysiol.1996.sp021286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niggli E. Annu Rev Physiol. 1999;61:311–335. doi: 10.1146/annurev.physiol.61.1.311. [DOI] [PubMed] [Google Scholar]
- 36.Shirokova N, Rios E. J Physiol (London) 1997;502:3–11. doi: 10.1111/j.1469-7793.1997.003bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parker I, Yao Y. Proc R Soc London Ser B. 1991;246:269–274. doi: 10.1098/rspb.1991.0154. [DOI] [PubMed] [Google Scholar]