Abstract
Daily in the UK, frontline medical and paramedical staff are required to manage patients with “collapse ?cause”. This universal colloquialism refers to patients who have had an abrupt loss of postural tone. Some of these patients would have had a “blackout” or a transient loss of consciousness (T‐LOC). The three most important causes of T‐LOC are syncope, epilepsy and psychogenic blackouts. Determining the correct cause is an important challenge; if the initial clinical diagnosis is wrong, investigations may be misdirected, and the final diagnosis and treatment incorrect. Syncope is much more common than epilepsy and may present with symptoms akin to the latter. This fact is not well appreciated and often leads to misdiagnosis. This article deals with the clinical features of the three main causes of blackouts, the value of investigations in arriving at a diagnosis and the problem of misdiagnosis. Pathways for managing patients presenting with blackouts are suggested.
Keywords: blackouts, syncope, epilepsy, psychogenic blackouts, misdiagnosis
Every day in the UK, many medical and paramedical staff providing acute care are called upon to manage patients with “collapse ?cause”. This universal colloquialism refers to those who have had an abrupt loss of postural tone. Patients become unaware of, or unresponsive to, their surroundings because of several reasons, but for the purpose of this article, we will consider those who have briefly lost consciousness, and refer to this transient loss of consciousness as T‐LOC. Patients often refer to T‐LOC as a “blackout”. There are several causes of T‐LOC; determining the correct cause is an important challenge. If the initial clinical diagnosis is wrong, investigations may be misdirected, and the final diagnosis and treatment incorrect.
T‐LOC is common, affecting as many as 50% of people at some stage of life. It causes 3% of casualty attendances and 1% of admissions to hospital.1 Reasons why examining the clinical evidence and arriving at a correct diagnosis is challenging in patients with T‐LOC include the inability of many patients to help with an accurate description, the lack of or unreliability of witnesses, and the recovery of the patient and normality of vital signs by the time they are examined. Subsequent tests are usually normal, both bedside and laboratory, when the patient is examined, and is by then asymptomatic. The clinical history is often the only useful or unequivocal information. Patients may be able to offer only a recollection of their symptoms immediately before and after a blackout (but may later notice other features, eg, urinary incontinence, a bitten tongue). Some patients, particularly elderly people, may deny T‐LOC because of retrograde amnesia. This is seen in 30% of patients aged ⩾65 years who collapse.2 However, in such patients, it is important to determine whether the collapse is due to a fall. Falls may be a cause of “collapse ?cause”; however, by definition,3 they are not synonymous with T‐LOC. Typically, falls are due to factors such as arthritis, unsteadiness of gait, postural hypotension due to drugs and attempting to ambulate without good support. Measures aimed at falls may not help those collapsing due to T‐LOC.
T‐LOC has three main underlying mechanisms:
Transient global impairment of cerebral perfusion (ie, syncope, often resulting from a sudden change in heart rate, heart rhythm or blood pressure)
An excessive and inappropriate discharge from cortical neurones (ie, epilepsy)
Apparent T‐LOC due to psychogenic causes.
Syncope is overall much more common than epilepsy.4,5 It is not widely recognised that a witness will commonly report limb twitching, rolling of the eyes and urinary incontinence as features of syncope. It is often assumed that these features denote epilepsy,6 but convulsive movements occur in syncope when cerebral neurones are irritated by anoxia, due to lack of oxygenated blood perfusing the brain. Most commonly, this results in myoclonic jerking of the limbs, hence the term “convulsive syncope”. Failure to understand these common features of convulsive syncope may lead to many wrong diagnoses, unnecessary administration of antiepileptic drugs, and considerable human and financial cost.5 Syncope can therefore produce an apparent “seizure”, but this should not be taken to mean that the convulsive effects of cerebral hypoperfusion are epileptic.
Box 1: Definitions
T‐LOC: transient loss of consciousness
Collapse: abrupt loss of postural tone, with or without T‐LOC
Syncope: T‐LOC due to global impairment of cerebral perfusion causing collapse
Epilepsy: an excessive asynchronous discharge of cortical neurones, leading to a clinical event
Psychogenic blackouts: a cause of apparent T‐LOC without evidence of epilepsy or syncope, or other organic disease
Fall: an event whereby a person comes to rest on the ground or another lower level with or without loss of consciousness
Although the clinical history can be misleading, over‐reliance on bedside or laboratory testing may also cause confusion. All patients should undergo a thorough physical examination and electrocardiography (ECG) (and echocardiography (Echo)) if physical examination is not completely normal)7 to exclude structural heart disease (SHD). However, it is usual for patients with T‐LOC to be asymptomatic between episodes, and investigations between episodes are usually normal.8
This article will describe the definitions, clinical presentation, physical findings and investigations of T‐LOC (blackouts). We highlight the specific problem of the misdiagnosis of epilepsy, and suggest care pathways that can be used to assess, diagnose and manage patients with blackouts, thus helping to reduce a misdiagnosis.
Definitions
Syncope is derived from the Greek “syn” meaning “with” and “kopto” meaning “I cut” or “I interrupt”. The European Society of Cardiology defines syncope as “a transient, self‐limited loss of consciousness, usually leading to collapse. The onset of syncope is relatively rapid, and the subsequent recovery is spontaneous, complete, and usually prompt. The underlying mechanism is transient global cerebral hypoperfusion.”7 Reflex syncope, the most common cause of syncope, has many synonyms—for example, vasovagal, neurocardiogenic, vasodepressor, neurally mediated hypotension and bradycardia syndrome, emotional fainting, pallid breath‐holding spells, pallid infantile syncope, reflex anoxic seizure, reflex asystolic syncope and malignant vasovagal syncope. All these terms imply the same process. Syncope may be caused by the triggering of inappropriate reflex hypotension, with a variable degree of bradycardia, or even transient asystole; hence the much simpler term, reflex syncope. The exact trigger of the afferent arc of this reflex is unknown, but the efferent arc, which arises in the brain stem vasomotor and cardioinhibitory centres, results in an abrupt withdrawal of sympathetic outflow to resistance vessels (in particular, skeletal muscle arterioles) and abrupt vagal hypertonia.9
The International League Against Epilepsy10 and the International Bureau of Epilepsy define an epileptic seizure as a transient occurrence of signs and/or symptoms due to abnormal excessive or asynchronous neuronal activity in the brain.
They go on to define epilepsy as a “disorder of the brain characterised by an enduring predisposition to generate epileptic seizures and by the neurobiological, cognitive, psychological and social consequences of this condition”.10 Thereby, a single epileptic seizure that a person experiences may never recur, and the diagnosis of epilepsy should be reserved for those patients with recurrent epileptic seizures.
Blackouts are also seen in patients in whom there is no evident organic cause. These phenomena have also been given many names, including pseudoseizure, psychogenic seizure, non‐epileptic seizure, non‐epileptic attacks, hysterical epilepsy, hysteroepilepsy, hysterical seizures, conversion fits, pseudoattacks, doxogenic seizures and paroxysmal somatoform disorder. These episodes can be described as “unintentional paroxysms of altered sensation, movement, perception, or emotion that clinically resemble epileptic seizures but are not accompanied by epileptiform neurophysiological changes”.11 Cardiologists tend to use the term “psychogenic blackouts” when referring to this group of patients, whereas neurologists usually prefer “psychogenic non‐epileptic attacks”.
Epidemiology
Syncope is common across all age groups, with nearly 1 in 2 people experiencing a blackout in their lifetime.2 The Framingham Study reported a 10.5% incidence of at least one syncopal event over a 17‐year period.12 The incidence of syncope does not remain constant, but increases sharply at age >70 years, with further increases in women aged >80 years. Among elderly people confined to long‐term care, the annual incidence of syncope is reported to be as high as 6%.13 Population‐based studies have shown that among all the causes of syncope, reflex syncope is the most common mechanism.7
Epilepsy, on the other hand, while being the most common chronic disabling neurological condition in the UK, is much less common than syncope. Available data indicate an annual incidence of 40–70 episodes per 100 000 people in developed countries, with a lifetime prevalence of 5–10 per 1000. The incidence is high in childhood, decreases in adulthood and rises again in older people.14 Roughly 30 000 new cases of epilepsy are seen every year in the UK, with a prevalence of 0.7–1.0%5 (up to 600 000 people).
A psychogenic blackout, or psychogenic non‐epileptic attack disorder (NEAD), often coexists with epilepsy, and can be encountered in as many as 20% of patients with epilepsy.15,16,17 The incidence is reported to range from 3 to 5 per 100 000 people.18,19,20 Most patients are women, begin to have symptoms in their late teens or early 20s, and as many as 80% have a history of medically unexplained symptoms.17 Other described risk factors for NEAD are depression and personality disorder,21,22 poor coping strategies, acute stress or loss,23 sexual and physical abuse,24,25 previous head injury26,27,28 and asthma.29
Recurrences
Recurrences occur approximately in 1 in 3 patients with syncope, with most recurrences occurring in the first 2 years after the onset of symptoms.30 Although recurrences themselves are not shown to be associated with increased mortality or sudden death rates, morbidity among such patients is higher and their quality of life is lower.7 More than 1 in 10 patients with recurrent syncope experience fractures and soft‐tissue injuries directly as a result of collapse,31 and functional impairment was found to be similar to chronic illnesses such as rheumatoid arthritis, low‐back pain and psychiatric disorders.32 Also, recurrent syncope is negatively correlated with overall perception of health.33
Of all the patients diagnosed with epilepsy, one third of patients have <1 seizure per year, one third have 1–12 seizures per year, and the remainder have >1 seizure per month.34 Remission, or being free of seizures for 5 years, either with or without treatment, is seen in 70% of adults and children. Early and long‐term remission can be predicted by the number of seizures in the first 6 months after presentation.5
Patients with NEAD have considerable disability, but early diagnosis and psychotherapeutic intervention have been shown to reduce undue hospital attendance and avoid unnecessary anticonvulsant treatment.35 Ettinger et al36 followed up 43 patients with videoelectroencephalogram‐documented diagnosis of non‐epileptic seizures, and found that only one in five patients was episode free at 6–9 months of follow‐up. Roughly half of the patients studied had improved, 20% of patients reported no change in their symptom frequency and about 10% reported an increase in frequency of episodes. Patients currently having many friends, and good relationships with friends as a child, were more likely to be episode free. Not surprisingly, patients with pending litigation were less likely to experience a reduction in frequency of their symptoms. Psychiatric comorbidities, including personality disorders, were associated with a poor long‐term outcome.15
Prognosis
The prognosis of syncope depends on the underlying cause (box 2). SHD (eg, symptomatic aortic stenosis, hypertrophic cardiomyopathy and arrhythmogenic right ventricular dysplasia, old myocardial infarction) greatly increases the risk of death in patients with syncope.7 Patients with advanced heart failure, New York Heart Association Class III–IV, mean ejection fraction of 20% and syncope were found to have a 45% risk of sudden death at 1 year when compared with those without syncope.37 Similarly, patients with syncope caused by ventricular tachycardia in the setting of severe left ventricular dysfunction have a very poor prognosis.38 In contrast, patients with reflex syncope, age <45 years, no SHD, a normal electrocardiogram, supraventricular tachycardia or sick sinus syndrome have a relatively good prognosis. Patients with syncope due to an unknown cause—that is, those with a benign cause as well as those with an undiagnosed cardiac cause—have an intermediate risk of increased mortality and sudden cardiac death.7
Box 2: Causes of syncope
Syncope is a cardiovascular disorder. Causes can thus be divided into the following:
Cardiac causes
Due to underlying structural heart disease
Aortic stenosis
Hypertrophic cardiomyopathy
Arrhythmogenic right ventricular dysplasia
Severe ischaemic left ventricular dysfunction
Some forms of congenital heart defect
Left atrial myxoma
Pulmonary embolism
Due to an arrhythmia
Tachyarrhythmias—for example, ventricular tachycardia, ventricular fibrillation, polymorphic ventricular tachycardia, Brugada syndrome, long‐QT syndrome, pre‐excited atrial fibrillation, supraventricular tachycardia
Bradycardias—for example, atrioventricular block, sinus node disease
Vascular causes
Reflex causes
Vasovagal syncope
Carotid sinus hypersensitivity
Situational causes
Cough syncope
Micturition syncope, etc
Postural causes
Orthostatic hypotension
Postural orthostatic tachycardia syndrome
The UK National General Practice Study of Epilepsy39 was a prospective, population‐based study on patients with newly diagnosed epilepsy, in which a cohort of 792 patients was followed up for a median duration of 11.8 years. The standardised mortality for this group of patients was twice that of the normal population. The increase in mortality was most noticeable in the first few years after diagnosis. Subgroups that were associated with a higher risk of death were patients with acute symptomatic epilepsy, generalised tonic–clonic seizures and in whom epilepsy was due to congenital neurological defects. Similar findings, of increased mortality, were mirrored in a Dutch cohort of patients with epilepsy followed up for a mean of 28 years.40 Unlike patients with newly diagnosed epilepsy, in whom most deaths are due to the underlying cause (eg, tumour, vascular disease), in patients with chronic epilepsy, the cause is sudden unexpected death in epilepsy (SUDEP).41 SUDEP is defined as “sudden, unexpected, witnessed or unwitnessed, nontraumatic and nondrowning death in individuals with epilepsy, with or without evidence for a seizure, and excluding documented status epilepticus, in which post‐mortem examination does not reveal a toxicological or anatomical cause for death”.42 Young age, generalised tonic–clonic seizures, uncontrolled epilepsy, learning disability, seizures occurring during sleep and poor adherence to antiepileptic drug regimen are risk factors for SUDEP. It is estimated that approximately 500 lives are lost per year in the UK due to SUDEP.43
Box 3: Common abnormalities on electrocardiograms in patients with arrhythmic syncope
Bifasicular block (left‐bundle or right‐bundle branch block combined with left anterior or left posterior fasicular block)
Other intraventricular conduction abnormalities (QRS duration ⩾0.12 s)
Sinus bradycardia <40 beats/min
Sinoatrial block or sinus pauses >3 s in the absence of negatively chronotropic drugs
Mobitz second‐degree or third‐degree atrioventricular block
Alternating left‐bundle and right‐bundle branch block
Ventricular tachycardia
Long‐QT syndrome
Brugada syndrome
Wolff–Parkinson–White syndrome
Negative T waves in right precordial leads, and ε waves suggestive of arrhythmogenic right ventricular dysplasia
Q waves suggesting myocardial infarction
Clinical features
The yield from bedside and laboratory examinations in patients with blackouts is shown to be low. Typically, when simple examinations are unhelpful, more sophisticated, high‐tech investigations have a very low yield at a very high cost.8 Attention has to be focused on obtaining the best possible history from patients and eye witnesses. T‐LOC is a story (often told over and over again). Every story has a beginning, a middle and an end. The clinician should make a detailed record of events before, during and after a blackout. A good history provides clues to whether a blackout is due to syncope or epilepsy, and may also help in differentiating various causes of syncope or epilepsy syndromes.7 One meta‐analysis of six studies showed that a good history, physical examination and a 12‐lead ECG made a diagnosis of syncope in roughly 45% of patients presenting with T‐LOC.42a In the Syncope Symptom Study,44 all patients presenting with T‐LOC completed a questionnaire. Points were allocated for their presenting symptoms. It was reported that 94% of patients were correctly diagnosed on the basis of their presenting symptoms alone. Epilepsy was said to have been diagnosed with 94% sensitivity and specificity. However, the obvious limitation of the study was that it used circular logic. Clinical features were not proved for a particular cause of T‐LOC in advance. However, given the unhelpfulness of tests, the importance of gaining experience with detailed, consistent, history taking cannot be overemphasised.
T‐LOC precipitated by fear, severe pain, emotional distress, instrumentation or prolonged standing suggests reflex syncope. A prodrome of nausea, vomiting, sweating, or feeling of cold and tiredness may suggest reflex syncope. Brief myoclonic jerking of limbs starting after LOC, which itself is of short duration, may also be seen in convulsive syncope. Facial pallor is particularly important during T‐LOC. Blood drains away from the skin with diversion to skeletal muscle when the arterioles open with a loss of sympathetic tone. Often patients are so pale that witnesses say “I thought he/she was dead”, and they are described as being “as white as a sheet”. Nausea and vomiting after the event are suggestive of reflex syncope,45 and presumably represent reflex vagal hypertonia. Syncope occurring during or immediately after urination, defecation, coughing or swallowing is called “situational syncope”. These situations are associated with stimulation of autonomic reflexes, such as the gag reflex, with manoeuvres that raise intrathoracic pressure and reduce venous return during standing (in men, often when getting up to go to the bathroom in the middle of the night), or with both. Patients experiencing T‐LOC in the setting of definite SHD, or during (not after) exercise, may indicate that syncope is due to a malignant tachyarrhythmia. Arrhythmias might also cause T‐LOC while the person is supine, sometimes when preceded by palpitation. In patients with a family history of sudden cardiac death, an inherited cardiomyopathy, such as hypertrophic cardiomyopathy, or a primary electrical heart disease, such as the long‐QT or Brugada syndrome, arrhythmic syncope must be considered very likely, and every effort made to prove the diagnosis, because the risk of death is high.7
In contrast, a diagnosis of epilepsy is more likely when there is a history of an aura, such as unusual or distinctive smell before the event. Genuine tonic–clonic movements that are prolonged and coincident with the onset of T‐LOC, rather than limb twitching (myoclonic jerks), are more suggestive of generalised epilepsy. A turn of the head at onset of lateralised clonic movements, or clear automatisms, such as chewing or lip smacking, suggest focal epilepsy. Lateral tongue biting, a suffused or cyanosed face, prolonged post‐T‐LOC confusion, or headache and aching muscles suggest generalised epilepsy, as does a family history of epilepsy.5 Epilepsy is far more likely if there is a history of brain injury,45 and it is also common in conjunction with cerebral birth trauma or hypoxia, severe learning disabilities and autism.5,46
Psychogenic blackouts (NEAD) usually present as15 dissociative seizures, factitious disorders (eg, panic disorder) or other psychiatric disorders.47 A dissociative seizure is by far the most common imitator of epilepsy. Prominent motor features that wax and wane throughout a blackout, long duration of blackouts (>2 min) and, on recovery, evidence that the patient is able to recall events for a period of unresponsiveness, poor response to antiepileptic drugs and the presence of risk factors (eg, previous unexplained medical symptoms, a psychiatric history and a history of childhood traumatic experiences) favour a diagnosis of dissociative seizure or a psychogenic cause of blackouts.15 Interestingly, about 1 in 10 patients with NEAD presents in apparent status epilepticus.48
Investigations: the importance of the ECG
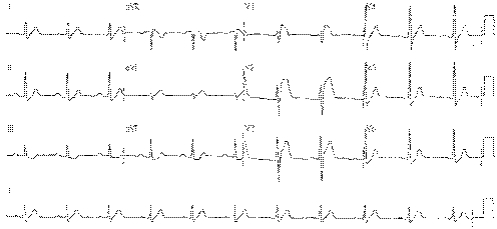
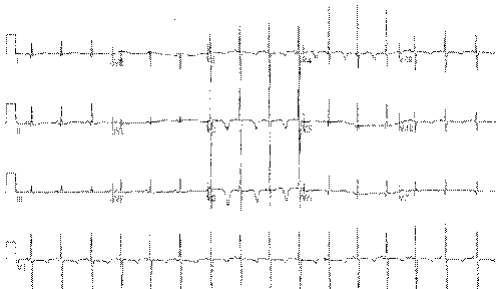
Reflex syncope is the most common cause of syncope, and usually there is no SHD. By the time a patient has been transported to hospital after such an episode, a 12‐lead ECG is usually normal.7 However, although the ECG may be normal, this is an important negative finding. Only about 4% of patients seen in UK neurology outpatient settings undergo an ECG, and important findings, although rare, may be crucial. We have seen a small but considerable number of patients with primary electrical diseases of the heart who have been misdiagnosed, leading to near tragedy. Figure 1 shows the ECG of a 35‐year‐old man who was misdiagnosed with an acute coronary syndrome after being admitted with T‐LOC. “Failed thrombolysis” led to transfer for coronary angioplasty. His coronary arteries were normal. Neurological review for epilepsy suggested syncope. Eventually, he was diagnosed with the Brugada syndrome and received an implantable cardioverter defibrillator. He has since had three successful device discharges for ventricular fibrillation. Figure 2 shows the ECG of a 3‐year‐old girl with blackouts who was treated unsuccessfully for epilepsy. An ECG was finally carried out and it showed long‐QT syndrome (type III). She continued to black out on β‐blockers, and received an implantable cardioverter defibrillator. Since then, episodes of non‐sustained polymorphic ventricular tachycardia are stored in the device memory. An ECG must be carried out in each and every patient with blackouts. An abnormal ECG (see box 3) is an independent predictor of cardiac syncope or increased mortality.7 The most common diagnoses encountered in the accident and emergency department in patients with arrhythmic syncope are ventricular tachycardia and bradycardias.
Figure 1 An electrocardiogram of a 35‐year‐old man with transient loss of consciousness who was misdiagnosed as having an acute coronary syndrome. The appearances are typical of the Brugada syndrome.
Figure 2 An electrocardiogram (ECG) of a 3‐year‐old girl misdiagnosed and treated for epilepsy. The ECG shows long‐QT type III syndrome.
Tilt‐table testing
Kenny et al49 introduced tilt‐table testing in unexplained syncope. Although it is widely accepted, its value in the management of T‐LOC is uncertain.
Standing shifts 1000 ml of blood volume into the venous capacitance vessels of the lower legs. After standing continuously for about 10 min, increased fluid volume in the legs causes a rise in capillary pressure. This causes egress of tissue fluid into the interstitial space, with further depletion of the intravascular volume. This in turn leads to a decrease in venous return and stroke volume, and the activation of compensatory mechanisms (vasoconstriction of the resistance vessels in the splanchnic, musculocutaneous and renal vascular beds) to prevent a fall in blood pressure. During the initial phase, changes are due to adjustment of sympathetic tone. Later adjustments arise from recruitment of the humoral limb of the neuroendocrine system.50 Failure of these compensatory mechanisms is thought to play a part in patients with reflex syncope and form the basis of the tilt‐table test. Tilt‐positive patients have a gradual asymptomatic fall in mean arterial blood pressure after being tilted to 60°, before the sudden collapse that reproduces syncope, whereas the mean arterial blood pressure of tilt‐negative patients gradually increases over 45 min.51 Tilt‐positive patients also have exaggerated reflex responses to gravitational stress simulated by lower‐body negative pressure while they are supine,52 but attenuated reflex responses to gravitational stress when they are tilted to 60°.53 Tilt‐positive patients are definitely different from tilt‐negative patients, at least on the day of their particular tilt response. However, tilt responses are not consistent within individual patients,54,55 are poorly reproducible56,57 and depend on the pre‐test likelihood of a positive or negative tilt result.58
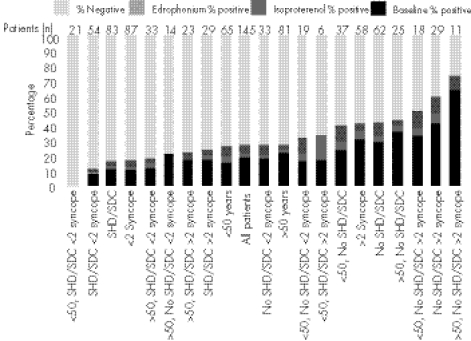
Figure 3 shows the results of tilt testing in unselected patients with T‐LOC. All comers had a low overall yield, akin to the lowest yield reported elsewhere.58 Older patients with recurrent blackouts and no SHD had a high yield similar to the highest yield reported.59 This suggests that the clinical characteristics of patients tilted are the clue to their response, and that once again, a good history of blackouts is as useful as any test. Also, by inference, tilt‐table testing is unlikely to discriminate effectively between unselected patients with unexplained blackouts—that is, it may not be helpful as a discriminative tool in all comers with T‐LOC.
Figure 3 Results of tilting in 145 patients with transient loss of consciousness (T‐LOC). Overall positivity in all comers was about 20%. Patients who were older, had no structural heart disease (SHD) and who had recurrent T‐LOC (right side of chart) had a higher rate of positive test. Patients who were young, had SHD (usually congenital or corrected congenital heart disease) and fewer episodes had no positive tests. The pre‐test likelihood of a positive or a negative result was determined by clinical features predicted the outcome of the test. Adding drug provocation (isoprenaline and edrophonium first, in randomised fashion) after 45 min of baseline tilt achieved only a modest increase in yield. Drug provocation may increase the sensitivity of the test, but at the cost of decreasing specificity.58 SDC, sudden diminished consciousness.
There is one important exception to this rule. In patients with suspected psychogenic blackouts, tilt‐table testing is legitimate and very useful if accompanied by simultaneous recording of ECG, phasic blood pressure and EEG. Patients with psychogenic blackouts are suggestible to black out during tilt, even at a pre‐suggested time. However, the ECG, blood pressure and EEG may remain quite normal throughout.60 This is useful in making a diagnosis.
The European Society of Cardiology recommends that tilt‐table testing be carried out for diagnostic purposes in patients with a single syncopal episode in high‐risk settings or in people who have recurrent syncopal episodes in the absence of organic heart disease.7 When the history is typically of reflex syncope, tilt‐table testing adds little to the overall diagnosis and management of the condition. Debate continues over the value of this test in assessing patients with recurrent unexplained falls, recurrent presyncope or dizziness.7 The value of the tilt‐table test in unselected patients with blackouts may be limited.
Implantable loop recorder
ECG monitoring is an important tool in the examination of patients presenting with T‐LOC. However, to rely on this investigation to confirm or rule out an arrhythmia as the cause of symptoms, it is important to have a correlation between symptoms and ECG, which is difficult to achieve61 and is rarely achieved by conventional Holter monitoring.62,63,64,65 Indeed, there is a good chance that random monitoring without the recurrence of typical symptoms may lead to a false reassurance of normality in the absence of arrhythmia, or a false assumption of arrhythmic syncope when an incidental arrhythmia is found, but in the absence of symptoms.7
Holter monitoring, for 1–7 days, is commonly undertaken in patients with T‐LOC and has a few apparent advantages. It is non‐invasive, with beat‐to‐beat ECG recording, reasonable quality and low recording costs. However, the diagnostic yield is around 1% for symptom–ECG correlation.7 Most patients with T‐LOC have infrequent symptoms and a small pre‐test likelihood of blackout symptom–ECG correlation during recording. This makes Holter monitoring costly, rarely useful and potentially misleading. Prolonged periods of Holter monitoring are precluded because of poor compliance and skin irritation, which is common. External event recorders, to be applied to the chest during a blackout, have a slightly higher yield,66 but are impractical, owing to infrequent symptoms67 and the inability of the patient to use the recorder successfully during a blackout.
The implantable loop recorder (ILR) is implanted on the left side of the chest by means of a minor surgical procedure carried out under local anaesthesia. This has perceived disadvantages because it is invasive and has high unit costs. The ILR costs about £1500 per treatment, whereas a 24‐h ECG costs about £70 per tape. However, an ILR battery lasts between 18 and 24 months. It is always capable of monitoring during that time. It has the ability to record a high‐fidelity ECG during an episode of blackout using a retrospective loop memory. This allows ILR activation after consciousness is restored. The ILR gives a high symptom–ECG correlation of about 88% at 6 months.68 The diagnostic yield is far higher than that of Holter monitoring, and thus it is definitely more cost effective. In the Manchester Heart Centre (Manchester UK), Holter monitoring is 4–5 times as costly per symptom/ECG correlation as the ILR in patients who have had blackouts. The value of ILR in patients with unexplained syncope, after conventional investigations had failed, was highlighted in a recent publication by Brignole et al.69 Patients aged ⩾65 years in this study, when compared with those aged <65 years, had a higher syncope recurrence rate and a considerably higher incidence of arrhythmias as a cause of their symptoms that were appropriately detected and treated by the ILR. Gradually, we anticipate that the ILR will supplant other investigations in patients with blackouts, because of a much greater symptom–ECG correlation and a much greater cost effectiveness. The ISSUE II study confirms this.70
EEG and neuroimaging
Similar to syncope, the diagnosis of epilepsy is essentially clinical. In most cases, it is possible for medical practitioners with specialised training, experience and hence expertise in epilepsy to arrive at a diagnosis on the basis of the history and examination of the affected people, and information obtained from eye witnesses.5 Once a clinical diagnosis is made, further investigations, either in the form of an EEG or brain imaging, are justified to make a full classification of epilepsy and the epilepsy syndromes. It is important to recognise the limitations of these examinations in establishing a diagnosis of epilepsy. The standard EEG has a variable sensitivity and specificity, and over‐reliance on this investigation to make a diagnosis is likely to lead to misdiagnosis because of the number of false positives.5,43,71 If a standard EEG is unhelpful, long‐term video or ambulatory EEG may be helpful.
Both magnetic resonance imaging (MRI) and computed tomography can identify structural abnormalities in the brain that cause certain epilepsies. Because of its higher sensitivity and specificity, MRI is the preferred method in patients with structural abnormalities. This investigation is particularly important in those children who present with epilepsy before the age of 2 years or in adulthood, in patients of any age whose history and examination suggest a focal onset of epilepsy, and in the minority of patients in whom seizures continue despite first‐line treatment.5,72 Computed tomography can be used to aid management if facilities for an MRI are not available, or in an acute situation, to decide whether seizures are a result of an acute neurological lesion or illness.
Which patients benefit from hospitalisation?
Blackouts are the sixth most common reason for admission of adults aged >65 years to acute medical hospital beds.7 The average length of stay for such patients is 5–17 days, and hospitalisation accounts for 74% of the cost of investigating syncope.73 Currently, the strategies for assessment of a patient with a blackout vary widely among doctors and among hospitals. Some authors have evaluated the effect of the introduction of in‐hospital protocols.74,75 However, a large number of inappropriate admissions and investigations still occur, increasing the cost per diagnosis.
Hospitalisation is strongly recommended when the cause of syncope is likely to be prognostically relevant. Patients with blackouts due to an acute myocardial infarction, acute pulmonary embolism, high‐grade atrioventricular block, long‐QT syndrome, Brugada syndrome, WPW syndrome with atrial fibrillation and rapid ventricular response, ECG abnormalities suggestive of an arrhythmic cause of syncope, symptoms occurring during exercise or causing severe injury, and patients with a family history of sudden death, benefit from hospitalisation for either diagnosis or treatment.
Patients with isolated or infrequent blackouts, who have no evidence of SHD and any worrisome physical injury, and have a normal resting ECG and a history typical of reflex syncope, can safely be evaluated on an outpatient basis as the long‐term prognosis in such patients is favourable.
The misdiagnosis of epilepsy
The misdiagnosis of epilepsy is common.5,76,77,78,79,80,81,82 It affects 20–30% of adults and as many as 40% of children.5,83,84,85 As epilepsy affects as many as 600 000 patients in the UK, a misdiagnosis may affect as many as 180 000 people.76,77,78,79 Misdiagnosis of epilepsy in the UK may be compounded by poor access to neurological assessment, and the diagnosis of epilepsy by generalists in primary and secondary care.78 Reflex syncope affects as many as 50% of the people, but epilepsy affects only 1–2%. Hence, many misdiagnoses of epilepsy may probably occur because of syncope, possibly because of misinterpretation of the appearances and meaning of convulsive syncope by witnesses and medical attendants. Indeed, reports indicate that misdiagnosed epilepsy is due to syncope with several mechanisms.79
A misdiagnosis of epilepsy is costly and damages the lives of patients, families and the wider National Health Service. Direct costs are incurred as a result of utilisation of resources, including medical costs. These include treatment in primary care, hospital care, drugs, investigations and non‐medical costs, such as community and residential care. Indirect costs include decreased or lost productivity due to underemployment or unemployment, psychological problems due to stigmatisation by society, and transport problems due to driving restrictions. A misdiagnosis leads many patients to receive antiepileptic drugs inappropriately, not only proving ineffective in controlling their symptoms but also exposing them to side effects of the drugs. If prescribed to pregnant women, they can have serious adverse consequences for the unborn child.86 In addition, patients do not receive the appropriate treatment for the condition they actually have. The economic burden to the National Health Services and to social care is substantial. The direct costs are estimated at £24.3–82.9 million, with the total cost in the range of £130–189 million.5 However, this may be a considerable underestimate. Further concern is regarding the issue of SUDEP. Patients with poorly controlled epilepsy have at least a ninefold increased risk of sudden death,5,87 and the mechanism remains uncertain, other than that it is usually preceded by collapse. A low level of even the simplest cardiological screening, by ECG, in patients with blackouts in UK neurological clinics, raises concerns that some cases of SUDEP may actually be due to unsuspected cardiac disease and arrhythmic syncope.
That the presenting clinical features of epilepsy can be mimicked by syncope6 is clearly not widely appreciated. It is probably assumed that a patient presenting with a convulsive blackout and abnormal movements has epilepsy, and so the history taken may be too superficial, and the features described may too readily be attributed to a primary brain dysfunction rather than to a secondary effect of cerebral hypoperfusion. In attempting to confirm this clinical impression, doctors may turn to laboratory tests. Again, it is poorly appreciated that interictal EEG/(s) cannot reliably confirm or rule out the diagnosis of epilepsy.88 Although an EEG can show specific epileptiform discharges in many patients with epilepsy,89 similar discharges can be seen in 10% of patients having undergone intracranial surgery and in 3% of patients with psychiatric disorders who do not have epilepsy.90 It is also poorly appreciated that normal phenomena, artefacts and non‐specific abnormalities, occurring in many from the general population, are open to misinterpretation and therefore contribute to false‐positive results.91 The incidence of apparent abnormalities on EEG varies with the population studied, and can be particularly high in patients attending hospital clinics, who have other medical disorders, making them particularly prone to misdiagnosis. Epilepsy is a clinical diagnosis, and laboratory tests such as EEG are used by neurologists to define the epilepsy syndrome of a patient, not to cement a diagnosis in unselected patients with blackouts.
Driving regulations
The need for the driver of a motor vehicle to notify the Driver Vehicle and Licensing Agency (DVLA) and the duration of the ban from driving depend on the underlying cause of collapse and the type of vehicle the driver is licensed to drive. The purpose of the ban is to reduce the risk of an injury resulting from an accident to either the driver or other road users. To achieve this aim, the DVLA has tried to risk stratify patients with blackouts on the basis of clinical features and investigations.92 The longest and most severe ban is for patients with suspected or proved epilepsy, as this disorder is the most common cause of collapse at the wheel of the car, while driving. Car or motorcycle drivers are not permitted to drive for at least 1 year after an episode of epilepsy. Patients with blackouts who have an abnormal ECG, clinical evidence of SHD, sustained an injury as a result of their blackouts, symptoms occurring at the wheel (while driving), while sitting or lying, or with more than one episode in 6 months are considered to be at high risk. The DVLA needs to be notified whether any high‐risk features are present, and driving may be banned for up to 6 months. On the other hand, no driving restrictions apply and the DVLA need not be notified if blackouts are due to simple faints—that is, there are definite provocational factors, associated with prodromal symptoms and unlikely to occur while sitting or lying. If no cause of blackouts is found despite extensive investigations, the patient is not permitted to drive for 6 months.
Conclusion
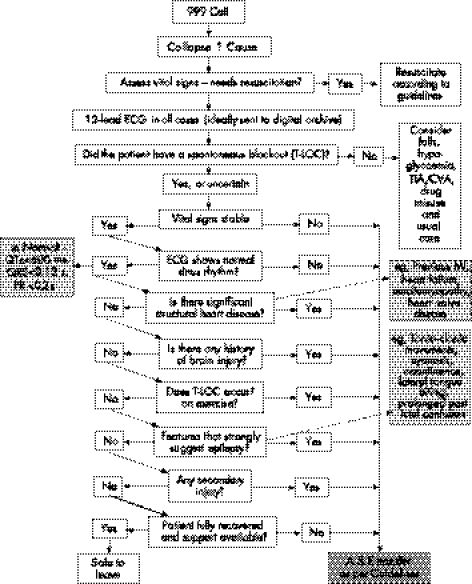
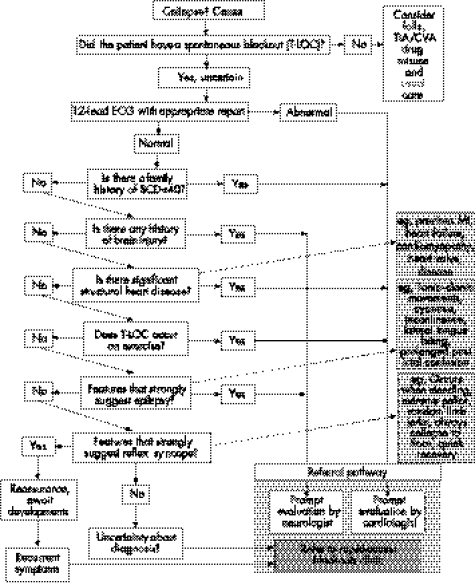
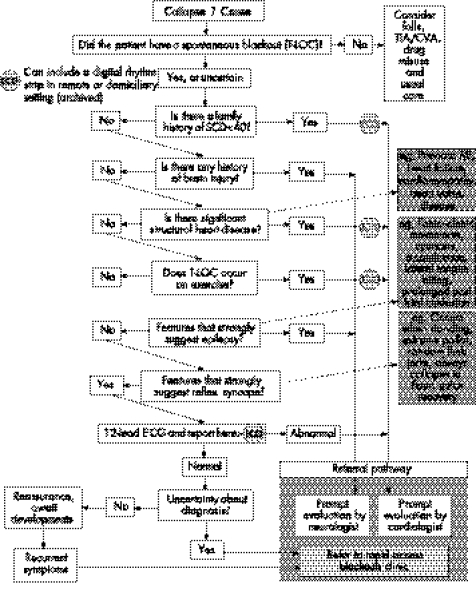
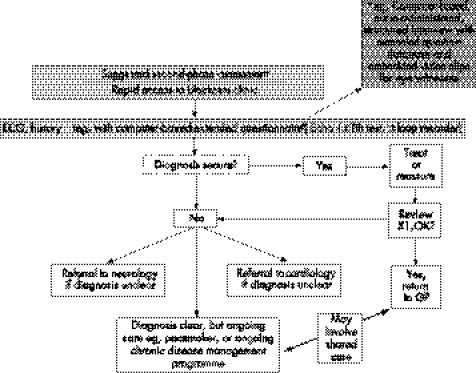
Syncope is far more prevalent than epilepsy in the general population. Similar clinical features, especially between convulsive syncope and generalised epilepsy, mean that the misdiagnosis of epilepsy is common. After work on the NSF for Arrhythmias and Sudden Cardiac Death, the Department of Health, UK, has approved care pathways (figs 4–7) to assist medical or paramedical people at the first and subsequent points of contact with a blackout patient.93 These pathways may help to tackle the common problem of a misdiagnosis of epilepsy.
Figure 4 Point of care: ambulance service. A&E, accident and emergency; CVA, cardiovascular attack; ECG, electrocardiography; MI, myocardial infarction; TIA, transient ischaemic attack; T‐LOC, transient loss of consciousness.
Figure 5 Point of care: accident and emergency department. CVA, cardiovascular attack; ECG, electrocardiography; MI, myocardial infarction; SCD, sudden cardiac death; TIA, transient ischaemic attack; T‐LOC, transient loss of consciousness.
Figure 6 Point of care: general practitioner and out of hospital. CVA, cardiovascular attack; ECG, electrocardiography; MI, myocardial infarction; SCD, sudden cardiac death; TIA, transient ischaemic attack; T‐LOC, transient loss of consciousness.
Figure 7 Point of care: rapid access to clinics for patients with blackouts. CVA, cardiovascular attack; ECG, electrocardiography; GP, general practitioner; MI, myocardial infarction; TIA, transient ischaemic attack; T‐LOC, transient loss of consciousness.
Key References
The Task Force on Syncope, European Society of Cardiology. Guidelines on management (diagnosis and treatment) of syncope—update 2004. Europace 2004;6:467–537.
Stokes T, Shaw EJ, Juarez‐Garcia A, et al. Clinical guidelines and evidence review for the epilepsies: diagnosis and management in adults and children in primary and secondary care. London: Royal College of General Practitioners, 2004.
Fitzpatrick A. Ambulatory electrocardiographic (AECG) monitoring for evaluation of syncope. In: Benditt DG, Blanc JJ, Brignole M, Sutton B, eds. The evaluation and treatment of syncope. A handbook of clinical practice. New York: Blackwell, 2003: 63–70.
Department of Health Expert Reference Groups for the National Service Framework for Arrhythmias. National Service Framework for Coronary Heart Disease. Arrhythmias and sudden cardiac death: implementation. http://www.dh.gov.uk/PolicyAndGuidance/HealthAndSocialCareTopics/CoronaryHeartDisease
Zaidi A, Clough P, Cooper P, et al. Misdiagnosis of epilepsy: many seizure like attacks have a cardiovascular cause. J Am Coll Cardiol 2000;36:181–4.
Self‐assessment questions (True (T)/False (F)); answers at the end of references
1. Regarding the epidemiology of blackouts:
Nearly one in two people experience a syncopal event in their lifetime.
The incidence of syncope decreases in elderly people.
Reflex syncope is the most common cause of syncope.
The prevalence of epilepsy in the UK is approximately 0.7–1.0%.
Psychogenic blackouts often coexist in patients with epilepsy.
Quality of life is the same in patients with or without syncope.
Well‐controlled epilepsy is a risk factor for SUDEP.
2. The following clinical features are suggestive of reflex syncope:
History of aura.
Lateral tongue biting.
Prolonged post‐T‐LOC confusion.
T‐LOC precipitated by prolonged standing or instrumentation.
Facial pallor during T‐LOC.
Brief myoclonic jerks of limbs starting after the loss of consciousness.
3. Regarding investigations of blackouts:
The 12‐lead electrocardiogram (ECG) is often normal in patients with reflex syncope.
An abnormal resting 12‐lead ECG makes an arrhythmic cause of syncope more likely.
The prognosis of syncope is better in patients with underlying structural heart disease.
Left ventricular dysfunction is a marker of sudden cardiac death.
Holter monitoring yields a high ECG–symptom correlation.
The results of the tilt table test are highly reproducible.
Drug provocation during tilt table testing increases the specificity of the test.
The implantable loop recorder (ILR) has no role in the investigation of elderly patients with blackouts.
A combined EEG and tilt‐table test is valuable in evaluating patients with psychogenic blackouts.
The battery life of an ILR is approximately 6 months.
Abbreviations
DVLA - Driver Vehicle and Licensing Agency
ECG - electrocardiography
EEG - electroechocardiography
ILR - implantable loop recorder
MRI - magnetic resonance imaging
NEAD - non‐epileptic attack disorder
SHD - structural heart disease
SUDEP - sudden unexpected death in epilepsy
T‐LOC - transient loss of consciousness
Answers
(1) (A) T (B) F (C) T (D) T (E) T (F) F (G) F (2) (A) F (B) F (C) F (D) T (E) T (F) T (3) (A) T (B) T (C) F (D) T (E) F (F) F (G) F (H) T (I) T (J) F
Footnotes
Competing interests: None.
References
- 1.Silverstein M D, Singer D E, Mulley A E.et al Patients with syncope admitted to medical intensive care units. JAMA 19822481185–1218. [PubMed] [Google Scholar]
- 2.Benditt D G, Blanc J J, Brignole M, Sutton B. eds. The evaluation and treatment of syncope. A handbook of clinical practice. New York: Blackwell, 2003
- 3.National Institute for Clinical Excellence Clinical practice guideline for the assessment and prevention of falls in older people. London: NICE, 2004
- 4.Petkar S, Jackson M, Fitzpatrick A. Management of blackouts and misdiagnosis of epilepsy and falls. Clin Med 20055514–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stokes T, Shaw E J, Juarez‐Garcia A.et alClinical guidelines and evidence review for the epilepsies: diagnosis and management in adults and children in primary and secondary care. London: Royal College of General Practitioners, 2004
- 6.Lempert T, Bauer M, Schimdt D. Syncope: a videometric analysis of 56 episodes of transient cerebral hypoxia. Ann Neurol 199436233–237. [DOI] [PubMed] [Google Scholar]
- 7.The Task Force on Syncope, European Society of Cardiology Guidelines on management (diagnosis and treatment) of syncope—update 2004. Europace 20046467–537. [DOI] [PubMed] [Google Scholar]
- 8.Kapoor W N, Karpf M, Weant S.et al A prospective evaluation and follow‐up of patients with syncope. N Engl J Med 1983309197–203. [DOI] [PubMed] [Google Scholar]
- 9.Benditt D G, Ermis C, Lu Fei Head‐up tilt table testing. In: Zipes, Jalife, eds. Cardiac electrophysiology. From cell to Bedside. Philadelphia: Saunders 2004
- 10.Fisher R S, Boas W, Blume W.et al Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 200546470. [DOI] [PubMed] [Google Scholar]
- 11.Gumnit R J, Gates J R. Psychogenic seizures. Epilepsia 198627(suppl 2)S124–S129. [DOI] [PubMed] [Google Scholar]
- 12.Soteriades E S, Evans J C, Larson M G.et al Incidence and prognosis of syncope. N Engl J Med 2002347878–885. [DOI] [PubMed] [Google Scholar]
- 13.Lipsitz L A, Pluchino F C, Wei J Y.et al Syncope in an elderly institutionalized population: prevalence, incidence and associated risk. Q J Med 19855545–54. [PubMed] [Google Scholar]
- 14.Sander J W, Hart Y M, Johnson A L.et al National general practice study of epilepsy: newly diagnosed epileptic seizures in a general population. Lancet 19903361267–1271. [DOI] [PubMed] [Google Scholar]
- 15.Mellers J D C. The approach to patients with ‘non‐epileptic seizures'. Postgrad Med J 200581498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith P E M. If it's not epilepsy …. J Neurol Neurosurg Psychiatry 2001709–14.11118240 [Google Scholar]
- 17.Hadjikoutis S, Smith P E M. Approach to the patient with epilepsy in the outpatient department. Postgrad Med J 200581442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sigurdardottir K R. Incidence of psychogenic seizures in adults: a population based study in Iceland. Epilepsia 199839749–752. [DOI] [PubMed] [Google Scholar]
- 19.Szaflarski J P, Ficker D M, Cahill W T.et al Four year incidence of psychogenic non epileptic seizures in adults in Hamilton County, OH. Neurology 200055156–163. [DOI] [PubMed] [Google Scholar]
- 20.Krumholz A, Niedermeyer E. Psychogenic seizures: a clinical study with follow up data. Neurology 198333498–502. [DOI] [PubMed] [Google Scholar]
- 21.Vanderzant C W, Giordani B, Berent S.et al Personality of patients with psuedoseizures. Neurology 198636664–668. [DOI] [PubMed] [Google Scholar]
- 22.Bowman E S, Markand O N. Psychodynamics and psychiatric diagnoses of psuedoseizure subjects. Am J Psychiatry 199615357–63. [DOI] [PubMed] [Google Scholar]
- 23.Krumholz A. Nonepileptic seizures: diagnosis and management. Neurology 199953(Suppl 2)S76–S83. [PubMed] [Google Scholar]
- 24.Alper K, Devinsky O, Perrine K.et al Nonepileptic seizures and childhood sexual and physical abuse. Neurology 1993431950–1953. [DOI] [PubMed] [Google Scholar]
- 25.Betts T, Boden S. Diagnosis, management and prognosis of a group of 128 patients with non epileptic attack disorder. Part II. Previous childhood sexual abuse in the etiology of these disorders. Seizure 1992127–32. [DOI] [PubMed] [Google Scholar]
- 26.Pakalnis A, Paolicchi J. Psychogenic seizures after head injury in children. J Child Neurol 20001578–80. [DOI] [PubMed] [Google Scholar]
- 27.Barry E, Krumholz A, Bergey G K.et al Nonepileptic posttraumatic seizures. Epilepsia 199839427–431. [DOI] [PubMed] [Google Scholar]
- 28.Westbrook L E, Devinsky O, Geocadin R. Nonepileptic seizures after head injury. Epilepsia 199839978–982. [DOI] [PubMed] [Google Scholar]
- 29.de Wet C J, Mellers J D C, Gardner W N.et al Psuedoseizures and asthma. J Neurol Neurosurg Psychiatry 200374639–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kapoor W. Evaluation and outcome of patients with syncope. Medicine 199069169–175. [DOI] [PubMed] [Google Scholar]
- 31.Kapoor W, Petersen J, Wieand H S.et al Diagnostic and prognostic implications of recurrences in patients with syncope. Am J Med 198783700–708. [DOI] [PubMed] [Google Scholar]
- 32.Linzer M, Pontinen M, Gold D T.et al Impairment of physical and psychosocial function in recurrent syncope. J Clin Epidemiol 1991441037–1043. [DOI] [PubMed] [Google Scholar]
- 33.Rose M S, Koshman M L, Spreng S.et al The relationship between health related quality of life and frequency of spells in patients with syncope. J Clin Epidemiol 2000351209–1216. [DOI] [PubMed] [Google Scholar]
- 34.Brown S, Betts T, Crawford P.et al Epilepsy needs revisited: a revised epilepsy needs document for the UK. Seizure 19987435–436. [DOI] [PubMed] [Google Scholar]
- 35.Martin R C, Gilliam F G, Kilgore M.et al Improved health care resource utilization following video‐EEG‐confirmed diagnosis of nonepileptic psychogenic seizures. Seizure 19987385–390. [DOI] [PubMed] [Google Scholar]
- 36.Ettinger A B, Dhoon A, Weisbrot D M.et al Predictive factors for outcome of nonepileptic seizures after diagnosis. J Neuropsychiatr Clin Neurosci 199911458–463. [DOI] [PubMed] [Google Scholar]
- 37.Middlekauff H, Stevenson W, Stevenson L.et al Syncope in advanced heart failure: high risk of sudden death regardless of origin of syncope. J Am Coll Cardiol 199321110–116. [DOI] [PubMed] [Google Scholar]
- 38.Middlekauff H R, Stevenson W G, Saxon L A. Prognosis after syncope: impact of left ventricular function. Am Heart J 199325121–127. [DOI] [PubMed] [Google Scholar]
- 39.Lhatoo S D, Johnson A L, Goodridge D M.et al Mortality in epilepsy in the first 11 to 14 years after diagnosis: multivariate analysis of a long term, prospective, population‐based cohort. Ann Neurol 200149336–344. [PubMed] [Google Scholar]
- 40.Shackelton D P, Westendorp R G J, Kasteleijn‐Nolst Trenite D G A.et al Mortality in patients with epilepsy: 40 years of follow up in a Dutch cohort study. J Neurol Neurosurg Psychiatry 199966636–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nashef L, Fish D R, Sander J W.et al Incidence of unexpected death in an adult outpatient cohort with epilepsy at a tertiary referral centre. J Neurol Neurosurg Psychiatry 199558462–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nashef L. Sudden unexpected death in epilepsy: terminology and definitions. Epilepsia 199738S6–S8. [DOI] [PubMed] [Google Scholar]
- 42a.Linzer M, Yang E H, Estes III M.et al Diagnosing syncope. Part I: value of history physical examination, and electrocardiography, Ann Intern Med 1997126989–996. [DOI] [PubMed] [Google Scholar]
- 43.Hanna N J, Black M, Sander J W.et alEpilepsy‐death in the shadows. London: The Stationery Office, 2002
- 44.Sheldon R, Rose S, Ritchie D.et al Historical criteria that distinguish syncope from seizures. J Am Coll Cardiol 200240142–148. [DOI] [PubMed] [Google Scholar]
- 45.Blanc J J, Benditt D G. Syncope: definition, classification, and multiple potential causes. In: Benditt DG, Blanc JJ, Brignole M, Sutton B, eds. The evaluation and treatment of syncope. A handbook of clinical practice. New York: Blackwell, 2003
- 46.Deb S. Epidemiology and treatment of epilepsy in patients who are mentally retarded. CNS Drugs 200013117–128. [Google Scholar]
- 47.Alper K, Devinsky O, Perrine K.et al Psychiatric classification of nonconversion nonepileptic seizures. Arch Neurol 199552199–201. [DOI] [PubMed] [Google Scholar]
- 48.Meierkord H, Will B, Fish D.et al The clinical features and prognosis of psuedoseizures diagnosed using video‐EEG telemetry. Neurology 1991411643–1646. [DOI] [PubMed] [Google Scholar]
- 49.Kenny R A, Ingram A, Bayliss J.et al Head‐up tilt: a useful test for investigating unexplained syncope. Lancet 198684941352–1355. [DOI] [PubMed] [Google Scholar]
- 50.Smit A A J, Halliwill J R, Low P A.et al Topical review. Pathophysiological basis of orthostatic hypotension in autonomic failure. J Physiol 19995191–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fitzpatrick A P, Sutton R. Blood pressure and heart rate during positive and negative 60° head up tilt testing. Eur J Pacing Electrophysiol 1991125–30. [Google Scholar]
- 52.Sneddon J F, Counihan P J, Bashir Y.et al Assessment of autonomic function in patients with neurally mediated syncope: augmented cardiopulmonary and baroreceptor responses to graded orthostatic stress. J Am Coll Cardiol 1993211193–1198. [DOI] [PubMed] [Google Scholar]
- 53.Sneddon J F, Counihan P J, Bashir Y.et al Impaired immediate vasoconstrictor response in patients with recurrent neurally mediated syncope. Am J Cardiol 19937172–76. [DOI] [PubMed] [Google Scholar]
- 54.de Buitleir M, Grogan E W, Jr, Picone M F.et al Immediate reproducibility of the tilt‐table test in adults with unexplained syncope. Am J Cardiol 199371304–307. [DOI] [PubMed] [Google Scholar]
- 55.Grubb B P, Wolfe D, Temesy‐Armos P.et al Reproducibility of head upright tilt table test results in patients with syncope. Pacing Clin Electrophysiol 1992151477–1481. [DOI] [PubMed] [Google Scholar]
- 56.Brooks R, Ruskin J N, Powell A C.et al Prospective evaluation of day‐to‐day reproducibility of upright tilt‐table testing in unexplained syncope. Am J Cardiol 1993711289–1292. [DOI] [PubMed] [Google Scholar]
- 57.Omar A R, Ng K S, Ng W L.et al Reproducibility of tilt‐table test result in patients with malignant neurocardiogenic syncope. Intern Med J 200434504–506. [DOI] [PubMed] [Google Scholar]
- 58.Fitzpatrick A P, Lee R J, Epstein L M.et al Effect of patient characteristics on the yield of prolonged head‐up tilt testing and the additional yield of drug provocation. Heart 199676406–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fitzpatrick A P, Sutton R. Tilting towards a diagnosis in unexplained syncope. Lancet 1989i658–660. [DOI] [PubMed] [Google Scholar]
- 60.Petersen M E V, Williams T R, Sutton R. Psychogenic syncope diagnosed by prolonged head‐up tilt testing. Q J Med 199588209–213. [PubMed] [Google Scholar]
- 61.Fitzpatrick A. Ambulatory electrocardiographic (AECG) monitoring for evaluation of syncope. In: Benditt DG, Blanc JJ, Brignole M, Sutton B, eds. The evaluation and treatment of syncope. A handbook of clinical practice. New York: Blackwell, 2003
- 62.Kapoor W N. Evaluation and management of the patient with syncope. JAMA 19922682553–2560. [PubMed] [Google Scholar]
- 63.Gibson T C, Heitzman M R. Diagnostic efficacy of 24 hour electrocardiographic monitoring for syncope. Am J Cardiol 1984531013–1017. [DOI] [PubMed] [Google Scholar]
- 64.DiMarco J P, Philbrick J T. Use of electrocardiographic (Holter) monitoring. Ann Intern Med 199011353–68. [DOI] [PubMed] [Google Scholar]
- 65.Bass E B, Curtiss E J, Arena V C.et al The duration of Holter monitoring in patients with syncope: is 24 hours enough? Arch Intern Med 19901501073–1078. [PubMed] [Google Scholar]
- 66.Linzer M, Pritchett E L C, Pontinen M.et al Incremental diagnostic yield of loop eletrocardiographic recorders in unexplained syncope. Am J Cardiol 199066214–219. [DOI] [PubMed] [Google Scholar]
- 67.Schuchert A, Maas C, Kretzschmar C.et al Diagnostic yield of external loop recorders in patients with recurrent syncope and negative tilt table test. Pacing Clin Electrophysiol 2003261837–1840. [DOI] [PubMed] [Google Scholar]
- 68.Krahn A, Klein G J, Norris C.et al The etiology of syncope in patients with negative tilt table and electrophysiologic testing. Circulation 1995921819–1826. [DOI] [PubMed] [Google Scholar]
- 69.Brignole M, Menozzi C, Maggi R.et al The usage and diagnostic yield of the implantable loop‐recorder in detection of the mechanism of syncope and in guiding effective antiarrhythmic therapy in older people. Europace 20057273–279. [DOI] [PubMed] [Google Scholar]
- 70.Brignole M, Sutton R, Menozzi C.et al Early application of an implantable loop recorder allows effective specific therapy in patients with recurrent suspected neurally mediated syncope. Eur Heart J 2006271085–1092. [DOI] [PubMed] [Google Scholar]
- 71.Fowle A J, Binnie C D. Uses and abuses of the EEG in epilepsy. Epilepsia 200041(suppl 3)S10–S18. [DOI] [PubMed] [Google Scholar]
- 72.Ross S D, Estok R, Chopra S.et al Management of newly diagnosed patients with epilepsy: a systematic review of the literature. Evidence Report/Technology Assessment No 39 (Contract 290‐97‐0016 to MetaWorks Inc) AHRQ Publication No 01‐E038 Rockville, MD: Agency for Healthcare Research and Quality. Sept 2001 [PMC free article] [PubMed]
- 73.Kenny R A, O'Shea D, Walker H F. Impact of a dedicated syncope and falls facility for older adults on emergency beds. Age Ageing 200231272–275. [DOI] [PubMed] [Google Scholar]
- 74.Ammirati F, Colvichhi F, Santini M. Diagnosing syncope in clinical practice. Implementation of a simplified diagnostic algorithm in a multicentre prospective trial—the OESIL 2 study (Osservatorio Epidemiologico della Sincope nel Lazio). Eur Heart J 200021935–940. [DOI] [PubMed] [Google Scholar]
- 75.Farwell D J, Sulke A N. Does the use of a syncope diagnostic protocol improve the investigation and management of syncope? Heart 20049052–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chadwick D, Smith D. The misdiagnosis of epilepsy. BMJ 2002324495–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scheepers B, Clough P, Pickles C. The misdiagnosis of epilepsy: findings of a population study. Seizure 19987403–406. [DOI] [PubMed] [Google Scholar]
- 78.Smith D, Defalla B A, Chadwick D W. The misdiagnosis of epilepsy and the management of refractory epilepsy in a specialist clinic. Q J Med 19999215–23. [DOI] [PubMed] [Google Scholar]
- 79.Zaidi A, Clough P, Cooper P.et al Misdiagnosis of epilepsy: many seizure like attacks have a cardiovascular cause. J Am Coll Cardiol 200036181–184. [DOI] [PubMed] [Google Scholar]
- 80.King M A, Newton M R, Jackson G D.et al Epileptology of the first seizure presentation: a clinical, electroencephalographic, and magnetic resonance imaging study of 300 consecutive patients. Lancet 19983521007–1011. [DOI] [PubMed] [Google Scholar]
- 81.Grubb B P, Gerard G, Roush K.et al Differentiation of convulsive syncope and epilepsy with head‐up tilt testing. Ann Intern Med 1991115871–876. [DOI] [PubMed] [Google Scholar]
- 82.Linzer M, Grubb B P, Ho S.et al Cardiovascular causes of loss of consciousness in patients with presumed epilepsy: a cause of the increased sudden death rate in people with epilepsy? Am J Med 199496146–154. [DOI] [PubMed] [Google Scholar]
- 83.Eiris‐Punal J, Rodriguez‐Nunez A, Fernandez‐Martinez N.et al Usefulness of the head‐upright tilt test for distinguishing syncope and epilepsy in children. Epilepsia 200142709–713. [DOI] [PubMed] [Google Scholar]
- 84.Pacia S V. The prolonged QT syndrome presenting as epilepsy: a report of two cases and literature review. Neurology 1994441408–1410. [DOI] [PubMed] [Google Scholar]
- 85.Garson A, Jr, Dick M, Fournier A.et al The long QT syndrome in children. An international study of 287 patients. Circulation 1993871866–1872. [DOI] [PubMed] [Google Scholar]
- 86.Arpino C, Brescianini S, Robert E.et al Teratogenic effects of antiepileptic drugs: use of an International Database on Malformations and Drug Exposure (MADRE). Epilepsia 2000411436–1443. [DOI] [PubMed] [Google Scholar]
- 87.Hirsch L J, Hauser W A. Can sudden unexplained death in epilepsy be prevented? Lancet 20043642157–2158. [DOI] [PubMed] [Google Scholar]
- 88.Smith D, Bartolo R, Pickles R M.et al Requests for electroencephalography in a district general hospital: retrospective and prospective audit. BMJ 2001322954–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ajmone‐Marsan C, Zivin L S. Factors related to the occurrence of typical paroxysmal abnormalities in the EEG records of epileptic patients. Epilepsia 197011361–381. [DOI] [PubMed] [Google Scholar]
- 90.Bridgers S L. Epileptiform abnormalities discovered on electroencephalographic screening of psychiatric in‐patients. Arch Neurol 198744312–316. [DOI] [PubMed] [Google Scholar]
- 91.Gregory R P, Oates T, Merry R T G. Electroencephalogram epileptiform abnormalities in candidates for aircrew training. Electroencephalogr Clin Neurophysiol 19938675–77. [DOI] [PubMed] [Google Scholar]
- 92.Anon http://www.dvla.gov.uk/at_a_glance (accessed 1 Apr 2006)
- 93.Department of Health Expert Reference Groups for the National Service Framework for Arrhythmias National Service Framework for Coronary Heart Disease. Arrhythmias and sudden cardiac death: implementation, http://www.dh.gov.uk/PolicyAndGuidance/HealthAndSocialCareTopics/CoronaryHeartDisease (accessed 30 Aug 2006)