Abstract
Venous thromboembolic disease is a common cause of mortality and morbidity in patients with cancer. Patients have a 5–6‐fold increase in the risk for a venous thromboembolism (VTE) compared with the general population, increasing to 6–7‐fold for some cancers. Prophylaxis for VTE should be considered whenever additional risk factors intervene. About 10% of patients with an idiopathic VTE will harbour an occult cancer. Half of these can probably be detected after a focused history, examination, routine blood tests and a chest x ray. The remaining cases may be diagnosed with an intensive screening protocol. About 60% of patients diagnosed on screening will have early disease, but we do not know whether screening improves the outcome. Evidence suggests that patients with cancer and a VTE should be treated with low‐molecular‐weight heparin, and treatment continued until the cancer is cured.
The relationship between cancer and venous thromboembolism (VTE) has been recognised for many years. Trousseau1 was the first to describe the relationship between cancer and the development of deep venous thrombosis (DVT) and recurrent superficial phlebitis. Ironically, in a twist of fate not uncommon in the history of medicine, he succumbed to a pulmonary embolism a few years later, thought to be secondary to a gastric cancer diagnosed the year before. It was another 70 years before two British doctors published a case report describing the development of a cancer 2 months after the diagnosis of a DVT, speculating that the occult cancer may have been responsible for the thrombosis.2 Seventy years later, as will become apparent, several important clinical issues remain to be clarified.
This article reviews the role of prophylaxis for venous thrombosis in patients with cancer, asking whether screening for an occult cancer is worthwhile in patients presenting with VTE and briefly discussing treatment for VTE in the presence of an associated cancer. There is still considerable speculation as to the underlying mechanisms behind the relationship between cancer and thrombosis, which is beyond the scope of this review.3
Risk of thrombosis in patients with cancer
Before considering whether prophylaxis in cancer is appropriate, we need to assess the size of the problem. Surprisingly, 140 years after the original observation, the incidence of VTE in patients with cancer is unknown. Two recent case–control studies may give some indication. Heit et al,4 comparing 625 patients presenting with a first VTE with 625 controls, found that patients with cancer were four times more likely to have a VTE than controls (odds ratio (OR) 4 (confidence interval (CI) 1.9 to 8.5)). Bloom et al,5 comparing 3220 patients with a first VTE with 2131 controls, found that patients with cancer were nearly seven times more likely to have a VTE than controls (OR 6.7 (CI 5.2 to 8.6)). The difference between the two studies is probably accounted for by the fact that Heit looked only at patients with a recent diagnosis of cancer, whereas Bloom included patients with any cancer diagnosed over the previous 15 years. For practical purposes, it seems reasonable to suppose that patients with cancer have a 4–5‐fold increase in the risk of VTE compared with those without cancer. Some cancers seem more likely to be associated with VTE than others. In Bloom et al's series,5 haematological tumours were associated with a 28‐fold increase in the risk of VTE, both lung and gastrointestinal tract cancers with a 20‐fold increase, and brain cancer with a sevenfold increase. This risk profile is not generally reflected in other series. Several large cohort studies, linking hospital data with cancer registries, specifically looking at the incidence of cancers developing after an episode of VTE, were able to estimate the relative risk in a wide range of cancers.6,7,8 Although the relative risks for each cancer differed from study to study, cancers of the brain, ovary and pancreas were among those with the highest risk, with standardised risk ratios of 6–7 (table 1). Although these tumours are more likely to be associated with a VTE, tumours such as those of the lung and breast, being considerably more common, will account for more episodes of VTE in absolute terms.
Table 1 Cancers with highest risk of association with venous thromboembolism.
| Baron et al6 n = 61 998 | SIR | Sorenson et al7 n = 26 653 | SIR* | Murchison et al8 n = 77 572 | SIR |
|---|---|---|---|---|---|
| Pancreas | 7.8 | Ovary | 7.9 | Ovary | 7.1 |
| Brain | 7.6 | Liver | 6.3 | Lymphoma | 6.0 and 5.1† |
| Liver | 6.6 | Brain | 5.0 | Kidney | 4.3 |
| Lung | 5.5 | Pancreas | 3.8 | Pancreas | 4.1 |
SIR, standardised incidence ratio.
*SIR related to pulmonary emboli.
†SIR related to Hodgkin's and non‐Hodgkin's lymphoma, respectively.
Cancers that have metastasised may be at increased risk of a VTE, presumably reflecting greater tumour load. In a large population‐based study using the Danish cancer registry, 44% of patients with a known cancer at the time of presentation with a VTE had evidence of metastases, compared with 35% of patients with cancer without VTE.9 In Bloom et al's series,5 patients with metastatic disease were 20 times more likely to have a VTE than those with local disease, and 50 times more likely to have an event than controls without cancer.
Therapeutic interventions and the risk of thrombosis
Surgery
Although early studies suggested that the incidence of VTE was considerably higher in patients having surgery for cancer, more recent studies using logistic regression have shown that other factors such as age and obesity may be at least as or even more relevant.10 Nevertheless, it has been estimated that the incidence of DVT in patients who are not given VTE prophylaxis for cancer surgery is about 30%.11
Chemotherapy
Chemotherapy itself can increase the risk of VTE. In Heit et al's series,4 patients with cancer undergoing chemotherapy had an increased risk of VTE compared with those not undergoing chemotherapy (OR 6.5 (CI 2.1 to 20.2) v 4.1 (CI 1.9 to 8.5)). The association was first described in patients with stage 2 breast cancer.12 Patients undergoing chemotherapy over a 12‐week period had a 6% incidence of VTE. Patients were then randomised to decide whether to continue with treatment for a further 18 weeks or not. At the end of 30 weeks, patients continuing with their chemotherapy had a further 5% incidence of VTE compared with 0% in those who had no further chemotherapy. The same group subsequently found that low‐dose warfarin, initially 1 mg/day, could reduce the relative risk by 85%.13 This increased risk in breast cancer has been confirmed by several other studies and seems to be greater in postmenopausal patients.14 A recent study looked at 3003 patients who had undergone at least one cycle of chemotherapy for a variety of cancers and were followed up for a mean of 2.4 months.15 The overall incidence of VTE was 1.9% (0.8%/month), but this varied according to the cancer site. The incidence was highest in upper gastrointestinal cancers (2.3%/month), followed by lung cancer (1.1%/month) and lymphoma (1.1%/month). Interestingly, multivariate analysis showed that the incidence was markedly higher in patients with a platelet count >350 000, 3.98% (1.66%/month), compared with those whose platelet counts were <200 000, 1.25% (0.52%/month). It would seem that the newer non‐cytotoxic agents, such as the matrix metalloproteinase inhibitors and the angiogenesis inhibitors, are also associated with an increased risk of VTE when used in combination with chemotherapy.16
Hormonal therapy
Hormonal therapy is known to increase the risk of VTE in patients with breast cancer. A study of 705 postmenopausal patients randomised to tamoxifen, or tamoxifen and chemotherapy, resulted in an incidence of VTE of 1.4% and 9.6%, respectively.17 These results have been confirmed and show that the risk seems to be considerably lower in premenopausal patients, with incidences of 0.8% and 2.8%, respectively.18 A systematic review has shown a 2–3‐fold increase in the risk of VTE using either tamoxifen or raloxifine alone, equivalent to the risk of hormone‐replacement therapy in women generally.19 The review found a lower but still significant risk with the newer aromatase inhibitor anastrozole (2.1% in patients on anastrozole v 3.5% in patients on tamoxifen).20 The risk is markedly increased if tamoxifen is combined with chemotherapy. This review calculates a relative risk of 3–8 for VTE in patients on chemotherapy and tamoxifen compared with tamoxifen alone, 3–5 compared with chemotherapy alone, and 20 compared with patients on placebo or observation alone. The review confirms that the risk of VTE increases with age and is greater in postmenopausal women. Currently, evidence available is insufficient to show whether the newer hormonal agents are less likely to be associated with VTE when combined with chemotherapy.
Long‐term intravenous catheters
Long‐term indwelling catheters used in chemotherapy are known to be associated with local thrombosis and pulmonary embolism. Small early studies, using positive venography as an end point, showed a high incidence of local thrombosis of 36–66%, which was dramatically reduced using low‐dose warfarin or low‐molecular‐weight heparin (LMWH) to 9.5% and 8%, respectively.21,22 More recent studies have shown a much lower rate of thrombosis, presumably owing to a combination of improvements in catheter technology, insertion techniques and overall maintenance. Although a recent large prospective study has shown a high incidence of complications, particularly infection, the rate of clinical thrombosis was low, at only 3.45% (1.1 per 1000 catheter days).23 Low‐dose warfarin made no difference to the incidence of clinical thrombosis in two further recent studies, and the thrombosis rate was <5%.24,25 The thrombosis rate was much higher in a study in which the patients were screened by venography, presumably reflecting the greater sensitivity of venography over clinical presentation, but again, LMWH made no significant difference to the outcome, with thrombosis occurring in 14% patients on LMWH and in 18% on placebo.26 As a result of these studies, the most recent American College of Chest Physicians guidelines do not recommend thrombosis prophylaxis for long‐term indwelling catheters.27
Prophylaxis for VTE in patients with cancer
Although cancer is a major risk factor for VTE (OR 4–5, or even as high as 7–8 for some cancers), this in itself is not sufficiently high to warrant starting prophylaxis. On the other hand, the doctor should have a low threshold for considering the diagnosis of a pulmonary embolus if the patient deteriorates unexpectedly, and should certainly consider prophylaxis if any other risk factor intervenes.
Immobilisation
Immobilisation, a known risk factor for VTE in patients, seems to pose an even greater risk in patients with cancer. One study has shown that of patients immobilised in hospital, those with cancer are almost twice as likely to die of a pulmonary embolism as those with benign disease (14% v 8% confirmed by autopsy).28 Thus, prophylaxis should be considered for all hospitalised patients with cancer, unless they are terminally ill.
Surgery
Although there is a 30% incidence of DVT in patients who undergo surgery for cancer, LMWH for 10 days after abdominal surgery can reduce this to 15%.29 Some evidence suggests that more prolonged prophylaxis could reduce the incidence further. In one study, the incidence of venographically detected DVT was reduced from 12% in those treated for 6–10 days postoperatively to 4.8% in those treated for 3 weeks after discharge.30 Patients undergoing abdominal or pelvic surgery for cancer should receive DVT prophylaxis, with LMWH at a dose appropriate for patients at high risk. Ideally, prophylaxis should continue for a month postoperatively. Alternative methods of prophylaxis, particularly pneumatic compression and graded elastic stockings, are probably ineffective in this setting.12
Chemotherapy
Chemotherapy is known to increase the risk of VTE, although in the past this has not been widely recognised by oncologists.31 It is not known whether this is a property of any particular single chemotherapeutic agent or the result of combining agents, but it does seem to be a feature of a wide range of treatment regimens. Before prophylaxis can be recommended for patients on chemotherapy, more studies will be required to define the size of the problem and the treatments and cancers most likely to benefit from it.
Metastatic cancer
Two studies have shown an increased risk of thromboembolic disease in patients with metastatic cancer5,9; the second study found a 20‐fold increase in the incidence of VTE compared with patients with localised disease. If these findings can be confirmed, then patients with known metastatic disease would certainly be candidates for prophylaxis.
Summary
Venous thrombosis is common in patients with cancer, and pulmonary emboli are probably an important cause of premature death and morbidity in this group. Although death from a pulmonary embolus may at one time have been accepted as an unfortunate pre‐terminal event, this approach is no longer acceptable. In view of the considerable resources available for cancer management, it is perhaps suprising that prevention of this common and potentially fatal complication has not received more attention. Unfortunately, there is currently little evidence to guide management, and more studies are required to assess the scale of the problem and the need for active prophylaxis (box 1).
Box 1: Key points
Patients with cancer have a 4–5‐fold increase in their risk of venous thromboembolism (VTE).
Cancers of the ovary, brain and pancreas are among those with the highest risk of VTE, but cancers of the breast and lung, being the most common, will account for a higher proportion in absolute terms.
The risk of VTE is much higher in patients with metastatic disease.
Surgery for cancer is associated with a 30% incidence of VTE.
Chemotherapy for patients with cancer is an independent risk factor for VTE and may be associated with an incidence of up to 2.2%/month of chemotherapy.
Tamoxifen is associated with a 2–3‐fold increase in the risk of VTE, increasing to a 20‐fold increase when combined with chemotherapy. Data regarding the risks of the newer hormonal treatments are insufficient.
Prophylaxis for VTE in patients with cancer should always be considered when other risk factors intervene, particularly hospitalisation or surgery, and may be appropriate for all patients with metastatic disease.
Screening for cancer
It is known that a proportion of patients presenting with a VTE will harbour an occult cancer, which raises the question of whether all patients presenting with a VTE should undergo some form of screening for cancer. The answer will depend on just how common cancer is in this group, the sensitivities of the tests used to detect the cancer and the efficacy of the treatments available, and whether they can affect the outcome. These factors have to be balanced against the acceptability of the tests and the anxiety induced by the screening process.
Risk of cancer in patients with VTE
Three large retrospective cohort studies of patients presenting with a VTE found a 4–9% incidence of cancer over a follow‐up period of 2 years.6,7,8 The standardised incidence ratios ranged from 1.2 to 4.4. The highest risk of cancer occurred in the first 12 months of follow‐up. In two studies, the risk remained raised for 2 years after VTE, whereas in the third, the risk persisted for 10 years. The standardised incidence ratio was highest in the <65 age group, but because the incidence of cancer increases with age, the absolute risk was higher in the >65 age group.
Several much smaller case–control studies found a higher incidence of cancer, 4.7–12% over a 6–12‐month follow‐up, in patients who had no obvious risk factors for a VTE.32,33,34 This compared with an incidence of 1.5–2% in patients who presented with a recognised risk factor. In two small studies, patients with no risk factors underwent extensive investigation for an occult cancer, including biochemistry, blood count, tumour markers and abdominal/pelvic computed tomography or ultrasound scanning.35,36 The incidence of cancer was high at 22–25%. In common with a larger case–control study,37 abnormalities in the history, examination, simple blood tests or chest x ray led to further detailed investigations in 64–100% of patients who were subsequently diagnosed with cancer. In practice, these routine measures would have led to more focused investigations, bypassing the need for intensive screening in most of the patients. These studies were small, associated with an unusually high incidence of cancer, and gave no indication as to whether early diagnosis of the cancers would have resulted in reduced mortality. Following on from these studies, there has been a general consensus that intensive screening would be inappropriate, at least until properly designed trials could show improved outcomes.38,39
Which screening tests?
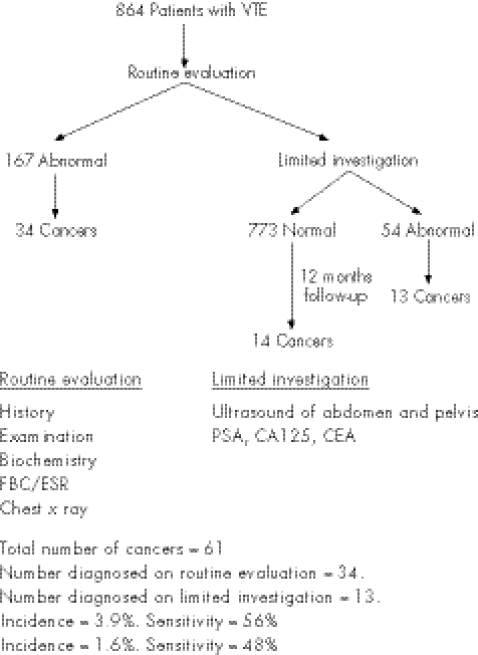
A recent large study by Monreal et al40 has looked at two intensities of screening, and gives a slightly clearer picture of the incidence of occult cancers and of the diagnostic yield of a screening programme (fig 1). Patients underwent a “routine evaluation”, consisting of history, examination, including a vaginal, rectal and breast examination, biochemistry, full blood count, erythrocyte sedimentation rate and a chest x ray. Any abnormalities were followed up vigorously. So, for example, a patient complaining of weight loss would undergo upper and lower gastrointestinal endoscopy and a computed tomography scan of the abdomen. If no abnormalities were found after routine evaluation, they would proceed to a “limited investigation”, consisting of ultrasound of the abdomen and pelvis, measurement of prostate‐specific antigen and tumour markers CA125 and CEA. In all, 864 patients were studied for 12 months. After the routine evaluation, 167 patients required further investigations; 34 (20%) patients were found to have an underlying cancer. All the remaining patients (830) then underwent a “limited investigation”. Abnormalities were found in 54 patients and cancer was confirmed in 13 (24%). During the 12‐month follow‐up, cancer became clinically evident in a further 14 patients. Thus, “routine evaluation” led to the detection of 56% of all the occult cancers, somewhat less than that found in the smaller studies. The incidence of cancer diagnosed on “routine evaluation” was 3.9%, and the sensitivity of this evaluation was 56%. The “limited investigation” uncovered half of the remaining tumours, giving it a sensitivity of 48% for an incidence of 1.6%. Could the search for occult cancers using this scheme be clinically justified? A total of 9 of the 34 (26%) cancers discovered on “routine evaluation” and 8 of the 13 (62%) cases diagnosed by “limited investigation” were early‐stage T1 or T2 tumours, and thus may have been amenable to radical treatment. Considerable resources were expended for a relatively low incidence of cancer. Unfortunately, the study included both patients with recognised risk factors and those with idiopathic VTE, and investigations would undoubtedly have been more productive if confined to those patients without risk factors. So, for example, the incidence of cancer in patients <70 years with an underlying risk factor was only 1.8%, whereas in patients >70 years without an underlying risk factor, the incidence of cancer was 9.3%. A screening programme for a population with an almost 10% risk of an occult cancer might be justified, as, in a high proportion of those diagnosed, curative treatment seems to be feasible. Given the resource implications, however, this would still need to be shown in a randomised control trial.
Figure 1 Screening for cancer.40 CEA, carcinoembryonic antigen; ESR, erythrocyte sedimentation rate; FBC, full blood count; PSA, prostate‐specific antigen; VTE, venous thromboembolism.
Does screening affect the outcome?
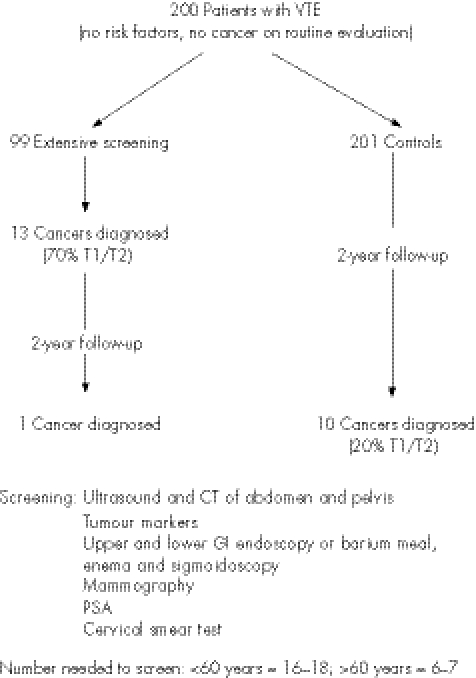
Piccioli et al41 planned the definitive randomised study, sufficiently powered to establish whether a screening programme could improve the outcomes (fig 2). Unfortunately, the project could not be completed. Patients with their first ever VTE, no recognised risk factors and no evidence of cancer on routine evaluation (as performed in previous study40) were randomised to extensive screening or a control group. Screening included ultrasound and computed tomography scanning of the abdomen and pelvis, tumour markers, gastroscopy or double‐contrast barium meal, colonoscopy or sigmoidoscopy and barium enema, sputum cytology, mammography, a cervical smear test and prostate‐specific antigen. A total of 500 patients were to be recruited per group, from over 40 participating centres. However, only 200 patients in total were recruited over 5 years. The incidence of cancer in this group was 12%. Thirteen cancers were detected by screening tests in the screened group, with one further cancer becoming evident over the 2‐year follow‐up. Ten cancers were diagnosed in the control group, and 9 of 13 (70%) cancers in the screened group were deemed early stage compared with 2 of 10 (20%) in the control group. During the course of the study, cancer‐related mortality was 2% in the screened group and 4% in the control group. The number needed to be screened to detect one cancer was age related, at 16–18 in the <60 age group and at 6–7 in the >60 age group. Intensive investigation could have been reduced to a computed tomography scan of the chest, abdomen and pelvis, as this would have detected all the tumours diagnosed on screening—that is, 93% of occult tumours. The study was inadequately powered because many of the centres were unable to obtain ethical approval for the study. To avoid patient anxiety, permission to enter the study was sought only if the patient was allocated to the screening arm, which was deemed unethical by some institutions. In other units, it was considered unethical either not to screen the patients or to expose the patients to extensive invasive tests. Furthermore, as the study progressed, doctors were increasingly likely to start screening patients randomised to the control group.
Figure 2 Screening for cancer.41 CT, computed tomography; GI, gastrointestinal; PSA, prostate‐specific antigen; VTE, venous thromboembolism.
Box 2: Key points
The percentage incidence of an occult cancer in patients with their first idiopathic venous thromboembolism (VTE) is about 10%.
A thorough focused history, examination, routine blood tests and a chest x ray can help in detecting around half of the occult cancers.
Computed tomography of the chest, abdomen and pelvis may help in detecting 90% of occult tumours not detected on preliminary investigations.
No study has yet shown a survival advantage from screening for occult cancer in patients with an idiopathic VTE, although as many as 70% of cancers detected by screening may potentially be curable.
Unfortunately, this underpowered study cannot confirm an improvement in outcome related to screening, and given the difficulties encountered, it seems unlikely that this question will be answered in the foreseeable future.
Screening for cancer: a summary
Where does this leave the clinician? We now have a better understanding of the true incidence of occult cancer in patients presenting with an idiopathic VTE, and there is some evidence, albeit incomplete, to help us decide on whether or not to screen our patients.
The incidence of occult cancer
In the large cohort studies, the incidence of occult cancer was 4–9%. Patients were excluded if their records suggested that they had undergone recent surgery, but these studies could not have been as tightly controlled for possible risk factors as the case–control studies, and hence may have underestimated the true incidence. On the other hand, they were very large, involving tens of thousands of patients. Smaller case–control studies gave an incidence of 5–12%, whereas the incidence in the small early screening studies was 22–25%. Monreal et al40 presents the largest well‐controlled case series and found an overall incidence of 5.6% (18/320 of patients with idiopathic VTE), ranging from 3.1% in the <70 age group to 9.8% in the >70 age group. Analysis of Piciolli's study shows an incidence of 16%, which includes those patients diagnosed by the routine evaluation, but who were not included in the final analysis. The age range was identical to that of Monreal's group. In both studies, the routine evaluation led to the diagnosis in just over half of all the occult cancers. The true incidence seems to depend on the populations studied and increases dramatically with age, but for a general population perhaps a reasonable estimate would be around 10%.
Box 3: Key points
Low‐molecular‐weight heparin is associated with a lower recurrence rate than warfarin in the treatment of venous thromboembolism (VTE) in patients with cancer.
Treatment of a VTE should continue for as long as the cancer is active—that is, until the cancer is cured.
Key references
Lip GYH, Chin BSP, Blann AD. Cancer and the prothrombotic state. Lancet Oncol 2002;3:27–34.
Geerts WH, Pineo GF, Heit JA, et al. The prevention of venous thromboembolism. The seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 2004;126(Suppl):311s–37s.
Bergqvist D, Agnelli G, Cohen AT, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med 2002;346:975–80.
Piccioli A, Lensing AWA, Prins MH, et al. Extensive screening for occult malignant disease in venous thromboembolism: a prospective randomized clinical trial. J Thromb Haemost 2004;2:884–9.
Lee AY, Levine MN, Baker RI, et al. Low molecular‐weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003;349:146–53.
To screen
Some clinicians may take the view that in the face of a 10% incidence, and the likelihood that 70% of the cancers are potentially curable, screening is fully justified. Confining intensive investigations to patients >60 years of age is likely to be the most cost‐effective option, and whole‐body computed tomography scanning would be the screening tool of choice, detecting most of the occult cancers not detected on routine evaluation.
Or not to screen
Most doctors, certainly in the UK, who have to deal with hard‐pressed radiology departments, will want to wait until convincing evidence of benefit is available before committing scarce recourses to an intensive screening programme. In the meantime, they should attempt an evaluation consisting of a focused history, examination, routine blood tests and a chest x ray, and, importantly, followup any suspicious results, as evidence suggests that this will detect >50% of occult cancers (box 2).
Treatment of VTE in patients with cancer
Patients with cancer who have a VTE are at greater risk of recurrent VTE both while on anticoagulants42,43 and after treatment is completed,44 compared with other patients. Treatment with LMWH for the duration of therapy, as opposed to traditional management with warfarin, is associated with a markedly lower rate of recurrence, 9% v 17%,45 and is now the recommended treatment for this group of patients in the USA.46 Such a regimen may or may not be acceptable or convenient for an individual patient or their carers, in which case warfarin can be used, with the option of converting to LMWH if there is any evidence of recurrent disease. Treatment should be continued for as long as the cancer is “active”—that is, until the patient has undergone radical treatment, with a view to complete cure. Several studies have shown that the incidence of haemorrhage is greater in anticoagulated patients with cancer,47 and this risk may not be related to the international normalised ratio.48 This increase has not been evident in all studies,46 and from the practical point of view, if warfarin is to be used, patients should be anticoagulated as normal (box 3).
Conclusion
It has been reasoned that the ability of a tumour to manipulate the thrombotic pathway may benefit tumour growth and its ability to metastasise. By interfering with these processes, it might be possible to prolong survival. Several studies have suggested just such an effect using anticoagulants, specifically LMWHs,49,50 and raise the tantalising prospect of an additional mode of treatment for cancer.
Abbreviations
DVT - deep venous thrombosis
LMWH - low‐molecular‐weight heparin
VTE - venous thromboembolism
Self‐Assessment Questions (True (T)/False (F)); Answers at end of references
Most VTEs occurring in patients with cancer are associated with cancers of the ovary, pancreas or brain.
Patients with indwelling central lines for delivering chemotherapy should routinely receive anticoagulant prophylaxis.
The new aromatase inhibitor anastrozole is associated with a negligible risk of VTE.
Low‐molecular‐weight heparin prophylaxis should be given for 6–10 days postoperatively.
The incidence of occult cancers in patients with non‐idiopathic VTE is <3%.
Screening for occult cancers in patients with idiopathic VTE has been shown to improve survival.
Treatment for VTE with warfarin, in patients with cancer is associated with a 17% recurrence rate.
Low‐molecular‐weight heparin has been shown to improve survival in some patients with cancer.
Answers
(1) F (2) F (3) F (4) F (5) T (6) F (7) T (8) T
Footnotes
Competing interests: None.
References
- 1.Trousseau A. Phlegmasia alba dolens. In: Lectures on clinical medicine delivered at the Hotel‐Dieu, Paris, 5th edn. [translated by Cormack JR]. London: New Sydenham Society, 1872281–295.
- 2.Illtyd James T, Matheson N. Thrombo‐phlebitis in cancer. Practitioner 1935134683–684. [Google Scholar]
- 3.Lip G Y H, Chin B S P, Blann A D. Cancer and the prothrombotic state. Lancet Oncol 2002327–34. [DOI] [PubMed] [Google Scholar]
- 4.Heit J A, Silverstein M D, Mohr D N.et al Risk factors for deep vein thrombosis and pulmonary embolism. Arch Intern Med 2000160809–815. [DOI] [PubMed] [Google Scholar]
- 5.Bloom J W, Doggen C J M, Osanto S.et al Malignancies, prothrombotic mutations and the risk of venous thrombosis. JAMA 2005293715–722. [DOI] [PubMed] [Google Scholar]
- 6.Baron J A, Gridley G, Weiderpass E.et al Venous thromboembolism and cancer. Lancet 19983511077–1080. [DOI] [PubMed] [Google Scholar]
- 7.Sorenson H T, Mellemkjaer L, Steffensen F H.et al The risk of a diagnosis of cancer after primary deep vein thrombosis or pulmonary embolism. N Engl J Med 19983381169–1173. [DOI] [PubMed] [Google Scholar]
- 8.Murchison J T, Wylie L, Stockton D L. Excess risk of cancer in patients with primary venous thromboembolism: a national, population‐based cohort study. Br J Cancer 20049192–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorensen H T, Mellemkjaer L, Olsen J H.et al Prognosis of cancers associated with venous thromboembolism. N Engl J Med 20003431846–1850. [DOI] [PubMed] [Google Scholar]
- 10.Gallus A S. Prevention of post‐operative deep leg vein thrombosis in patients with cancer. Thrombos Haemost 199778126–132. [PubMed] [Google Scholar]
- 11.Geerts W H, Heit J A, Clagett G P.et al Prevention of venous thromboembolism. Chest 2001119(Suppl 1)132s–75s. [DOI] [PubMed] [Google Scholar]
- 12.Levine M N, Ghent M, Hirsh J.et al The thrombogenic effect of anticancer drug therapy in women with stage 11 breast cancer. N Engl J Med 1988318404–407. [DOI] [PubMed] [Google Scholar]
- 13.Levine M N, Hirsh J, Ghent M.et al Double blind randomised trial of a very low dose warfarin for prevention of thromboembolism in stage IV breast cancer. Lancet 1994343886–889. [DOI] [PubMed] [Google Scholar]
- 14.Levine M N. Prevention of thrombotic disorders in cancer patients undergoing chemotherapy. Thromb Haemost 199778133–136. [PubMed] [Google Scholar]
- 15.Khorana A A, Francis C W, Culakovana E.et al Risk factors for chemotherapy‐associated venous thromboembolism in a prospective observational study. Cancer 20051042822–2829. [DOI] [PubMed] [Google Scholar]
- 16.Caine G J, Lip G Y H. Thromboembolism associated with new anti‐cancer treatment strategies in combination with conventional chemotherapy: new drugs, old risks? Thromb Haemost 200390567–569. [PubMed] [Google Scholar]
- 17.Pritchard K I, Paterson A H G, Paul N A.et al Increased thromboembolic complications with concurrent tamoxifen and chemotherapy in a randomised trial of adjuvant therapy for women with breast cancer. J Clin Oncol 1996142731–2737. [DOI] [PubMed] [Google Scholar]
- 18.Saphener T, Tormeg D C, Gray R. Venous and arterial thrombosis in patients who received adjuvant chemotherapy for breast cancer. J Clin Oncol 19919286–294. [DOI] [PubMed] [Google Scholar]
- 19.Deitcher S R, Gomes M P V. The risk of venous thromboembolic disease associated with adjuvant hormone therapy for breast carcinoma. Cancer 2004101439–449. [DOI] [PubMed] [Google Scholar]
- 20.ATAC (Arimidex Tamoxifen Alone or in Combination) Trialist Group Anastozole alone or in combination with Tamoxifen versus Tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet 20023592131–2139. [DOI] [PubMed] [Google Scholar]
- 21.Bern M N, Lokich J J, Wallach S R.et al Very low doses of warfarin can prevent thrombosis in central vein catheters: a randomised prospective trial. Ann Intern Med 1990112423–428. [DOI] [PubMed] [Google Scholar]
- 22.Monreal M, Alastrue A, Rull M.et al Upper extremity deep vein thrombosis in cancer patients with venous access devices–prophylaxis with a low molecular weight heparin (Fragmin). Thromb Haemost 199675251–253. [PubMed] [Google Scholar]
- 23.Walshe L J, Malak S F, Eagan J.et al Complication rates among cancer patients with peripherally inserted central catheters. J Clin Oncol 2002203267–3281. [DOI] [PubMed] [Google Scholar]
- 24.Couban S, Goodyear M, Burnell M.et al Randomised placebo‐controlled study of low dose warfarin for the prevention of central venous catheter‐associated thrombosis in patients with cancer. J Clin Oncol 2005234063–4069. [DOI] [PubMed] [Google Scholar]
- 25.Heaton D C, Han D Y, Inder A. Minidose warfarin as prophylaxis for central vein catheter thrombosis. Intern Med J 20023284–88. [PubMed] [Google Scholar]
- 26.Verso M, Agnelli G, Bertoglio S.et al Enoxaparin for the prevention of venous thromboembolism associated with central vein catheter: a double blind, placebo‐controlled, randomised study in cancer patients. J Clin Oncol 2005234057–4062. [DOI] [PubMed] [Google Scholar]
- 27.Geerts W H, Pineo G F, Heit J A.et al The prevention of venous thromboembolism. The seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 2004126(Suppl)311s–37s. [DOI] [PubMed] [Google Scholar]
- 28.Shen V S, Pollack K E W. Fatal pulmonary embolism in cancer patients: is heparin prophylaxis justified? South Med J 198073841–843. [DOI] [PubMed] [Google Scholar]
- 29.ENOXACAN Study Group Efficacy and safety of enoxaparin versus unfractionated heparin for prevention of deep vein thrombosis in elective cancer surgery: a double‐blind randomised multicentre trial with venographic assessment. Br J Surg 1997841099–1103. [PubMed] [Google Scholar]
- 30.Bergqvist D, Agnelli G, Cohen A T.et al Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med 2002346975–980. [DOI] [PubMed] [Google Scholar]
- 31.Kirwan C C, Nath E, Byrne G J.et al Prophylaxis for venous thromboembolism during treatment for cancer: questionnaire survey. BMJ 2003327597–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prandoni P, Lensing A W A, Buller H R.et al Deep vein thrombosis and the incidence of subsequent symptomatic cancer. N Engl J Med 19923271128–1133. [DOI] [PubMed] [Google Scholar]
- 33.Cornuz J, Pearson S D, Creager M A.et al Importance of findings on initial evaluation for cancer in patients with symptomatic idiopathic deep venous thrombosis. Ann Intern Med 1996125785–793. [DOI] [PubMed] [Google Scholar]
- 34.Hettiarachchi R J K, Lok J, Prins M H.et al Undiagnosed malignancy in patients with deep vein thrombosis. Cancer 199883180–185. [DOI] [PubMed] [Google Scholar]
- 35.Monreal M, Lafoz E, Casals A.et al Occult cancer in patients with deep venous thrombosis. Cancer 199167541–545. [DOI] [PubMed] [Google Scholar]
- 36.Bastounis E A, Karayiannakis, Makri GG, et al The incidence of occult cancer in patients with deep venous thrombosis: a prospective study. J Int Med 1996239153–156. [DOI] [PubMed] [Google Scholar]
- 37.Nordstrom M, Lindblad B, Anderson H.et al Deep venous thrombosis and occult malignancy: an epidemiological study. BMJ 1994308891–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fennerty A. Screening for cancer in venous thromboembolic disease. BMJ 2001323704–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prins M H, Hettiarachchi R J K, Lensing A W.et al Newly diagnosed malignancy in patients with venous thromboembolism. Search or wait and see? Thromb Haemost 199778121–125. [PubMed] [Google Scholar]
- 40.Monreal M, Lensing A W A, Prins M H.et al Screening for occult cancer in patients with acute deep vein thrombosis or pulmonary embolism. J Thromb Haemost 20042876–881. [DOI] [PubMed] [Google Scholar]
- 41.Piccioli A, Lensing A W A, Prins M H.et al Extensive screening for occult malignant disease in venous thromboembolism: a prospective randomized clinical trial. J Thromb Haemost 20042884–889. [DOI] [PubMed] [Google Scholar]
- 42.Prandoni P, Lensing A W A, Cogo A.et al The long‐term clinical course of acute deep venous thrombosis. Ann Intern Med 19961251–7. [DOI] [PubMed] [Google Scholar]
- 43.Levitan N, Dowlati A, Remick S C.et al Rates of recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Medicine 199978285–291. [DOI] [PubMed] [Google Scholar]
- 44.Levine M N, Hirsh J, Gent M.et al Optimum duration of oral anticoagulant therapy: a randomised trial comparing four weeks with three months of warfarin in patients with proximal deep vein thrombosis. Thromb Haemost 199574606–611. [PubMed] [Google Scholar]
- 45.Lee A Y, Levine M N, Baker R I.et al Low molecular‐weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003349146–153. [DOI] [PubMed] [Google Scholar]
- 46.Buller H R, Agnelli G, Hull R D.et al Antithrombotic therapy for venous thromboembolic disease: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 2004126(Suppl)401s–28s. [DOI] [PubMed] [Google Scholar]
- 47.Levine M N, Raskob G, Beyth R J.et al Hemorrhagic complications of anticoagulant treatment: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 2004126(Suppl)287s–310s. [DOI] [PubMed] [Google Scholar]
- 48.Palareti G, Legnani C, Lee A.et al A comparison of the safety and efficacy of oral anticoagulation for the treatment of venous thromboembolic disease in patients with or without malignancy. Thromb Haemost 200084805–810. [PubMed] [Google Scholar]
- 49.Kakkar A K, Levine M N, KadziolaZ, et al Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: the Fragmin Advanced Malignancy Outcome Study (FAMOUS). J Clin Oncol 2004221944–1948. [DOI] [PubMed] [Google Scholar]
- 50.Lee A Y Y, Rickles F R, Julian J A.et al Randomised comparison of low molecular weight heparin and coumarin derivatives on the survival of patients with cancer and venous thromboembolism. J Clin Oncol 2005232123–2129. [DOI] [PubMed] [Google Scholar]