Abstract
Chagas disease is the clinical condition triggered by infection with the protozoan Trypanosoma cruzi. The infection is transmitted by triatomine insects while blood feeding on a human host. Field studies predict that one third of an estimated 18 million T cruzi‐infected humans in Latin America will die of Chagas disease. Acute infections are usually asymptomatic, but the ensuing chronic T cruzi infections have been associated with high ratios of morbidity and mortality: Chagas heart disease leads to unexpected death in 37.5% of patients, 58% develop heart failure and die and megacolon or megaoesophagus has been associated with death in 4.5%. The pathogenesis of Chagas disease appears to be related to a parasite‐induced mutation of the vertebrate genome. Currently, treatment is unsatisfactory.
American trypanosomiasis is an enzootic infection caused by the kinetoplastid flagellate Trypanosoma cruzi. The spread of the enzootics coincides with the distribution of the triatomine vectors from parallel 42°N in the southern US to 42°S in Patagonia, Argentina.1 Fossil remnants have suggested that humans arrived on the American continent about 50 000 years ago. It may be supposed that the triatomines involved in the life‐cycle of T cruzi, eventually transmitted the infection to Amerindians approximately 9000 years ago, probably when triatomines adapted to human dwellings in Chile and Peru, adjacent to the Atacama desert.2 Paleoparasitological studies showed that 97 (40.6%) of 238 mummies yielded molecular data consistent with T cruzi infection. It is reasonable to suppose that American trypanosomiasis was a serious burden to Amerindians in pre‐Columbian days. It is now known that T cruzi infection was readily transmitted to European and African settlers living close to triatomine wild‐life ecotopes in the American continents.
Chagas disease is the clinical condition associated with T cruzi infection, described in the pioneering work of Dr Carlos Chagas, a Brazilian physician who worked at the Oswaldo Cruz Institute, Rio de Janeiro.3 He described the dividing epimastigote and the metacyclic trypomastigote forms of T cruzi in the fore‐gut and the hind‐gut, respectively, of a Panstrongylus megistus captured in a human hut in the hinterland of Minas Gerais State, Brazil, in 1909. Next, he reported that he had found trypomastigotes in the blood of a mammal, a domestic cat. Serendipitously, he investigated a 1‐year‐old child with fever of unknown origin and showed T cruzi trypomastigotes in a peripheral blood smear by direct microscopic examination.4
T cruzi colonisation of the human body
Insect vectors of T cruzi infection
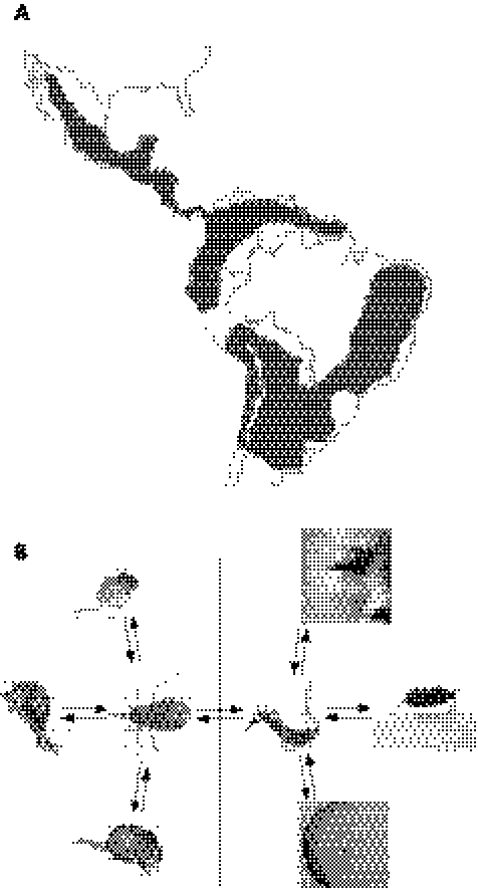
At least 40 triatomine species can harbour T cruzi, thus they are all potential transmitters of infection.5 The Triatoma sp, which adapted to the rural peri‐domicile areas and domiciles of dry ecosystems of Central and South America,6 are the main transmitters of the infection (fig 1A, B). These species have been implicated in 10–12 million cases of Chagas disease in humans. Next in epidemiological importance are the Rhodnius sp, dwelling in humid tropical climates, and the ubiquitous Panstrongylus sp.1,5 After having a blood meal, the triatomine becomes engorged and the infective metacyclic trypomastigotes are passed in the faeces. The parasitic form enters the body when the individual scratches the skin wound inflicted by the insect's mouth‐part, or it may enter the body through permissive mucosa or conjunctival membranes. On entry into the body, the trypomastigotes (fig 2) that invade tissue histiocytes survive the acidic parasitophorous vacuoles and leave the hostile environment to freely enter the host‐cell cytoplasm. The port of entry of the infection in the skin or in the eye conjunctiva is clinically seen as an indurate, chronic inflammatory process typical of a delayed‐type hypersensitivity reaction. The flagellates round up into amastigotes that undergo many cycles of multiplication by binary fission. The cell fills with amastigotes (fig 3), which will differentiate into mobile trypomastigotes (fig 4) that burst out reaching the blood stream ready to infect other cells of the body. Early papers have shown a direct relationship between host cell membrane receptor density and parasite load in tissues.7,8 In fact, every cell in the human body with the exception of neurones could be affected by T cruzi colonisation in vivo. T cruzi infection persists in the human body for life.
Figure 1 Chagas disease and lifecycle of Trypanosoma cruzi. (A) Geographical extent of human Chagas disease in Central and South America. Dark areas indicate regions showing high ratios of insect‐vector‐transmitted T cruzi infections. Adapted from Silveira.6 (B) Sylvatic and peri‐domicile lifecycles of T cruzi in early mammalian hosts and in humans. Haematophagous bugs contaminated with the parasite can be ingested by or feed upon opossum, armadillo and rat (left). Alternatively, the triatomine can feed on man to initiate the peri‐domestic cycle of Chagas disease (right). Note the ports of entry of the parasites into the human body as evidenced by Romaña's sign (top) and chagoma (bottom).
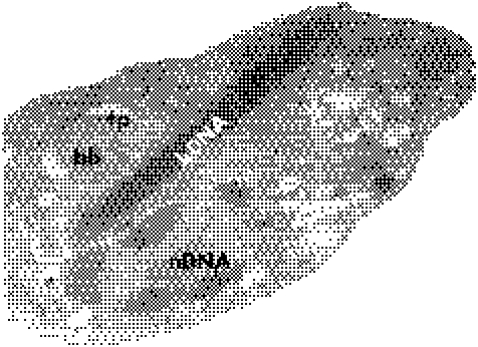
Figure 2 Electron microscopy of the Trypanosoma cruzi amastigote. Note the nucleus (nDNA), the kinetoplast (kDNA), the basal body of the flagellum (bb) and the flagellar pocket (fp). Magnification ×4800.
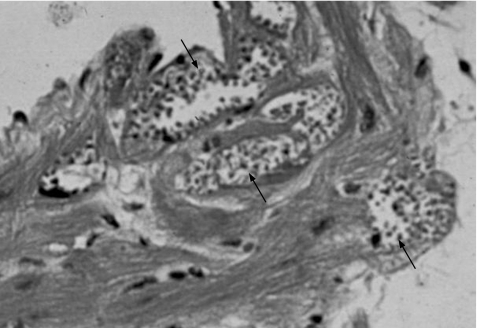
Figure 3 Dividing forms of Trypanosoma cruzi in cells. Multiple heart cells are filled with amastigote forms of the parasite (arrows).
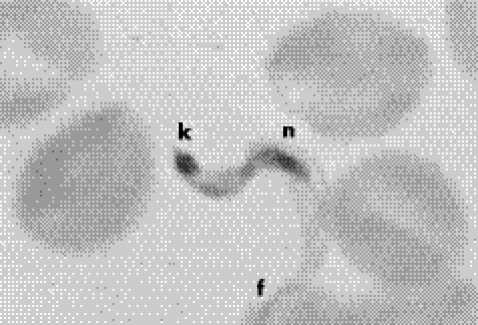
Figure 4 Trypanosoma cruzi trypomastigote blood form showing its nucleus (n), kinetoplast (k) and the flagellum (f). Giemsa stain, ×1000.
Blood transfusion
T cruzi infection can be readily transmitted by blood transfusion.9,10 The migration of people infected by T cruzi poses a threat to those countries where this insect‐transmitted disease does not occur. Chagas disease has become a potential problem associated with migration from endemic areas to the US, Canada, Eastern Europe, Australia and Japan.11 About 8 (3%) of 250 patients from Central America were found to be seropositive in Brooklyn and Queens in New York City (CA Santos‐Buch, unpublished observation), and a similar proportion was found among Nicaraguan immigrants in Washington, DC.12 Appropriate selection of blood donors, the use of sensitive screening tests and the application of a mandatory quality assurance system have maintained the safety of blood banks in most Latin American countries.13 The addition of cresyl violet to donor blood while in storage in Buenos Aires blood banks was effective in preventing the transmission of T cruzi. The prevention of transfusion‐associated protozoan infections depends mainly on selection of donors.14 Additionally, the use of more sensitive and accurate advanced molecular diagnostic tests has greatly reduced the risk of acquisition of blood‐borne Chagas disease.15
Congenital transmission of T cruzi has been reported in Latin American countries where Chagas disease is prevalent in those of reproductive age. Congenital transmission ranges from 2.5%16 in northeastern Brazil to 9.5% in Bolivia.17 Additionally, T cruzi can be transmitted by organ transplant, by laboratory accidents and by accidental self‐inoculation by hospital personel.
Transmission
Protozoan Trypanosoma cruzi is the causal agent.
The disease is transmitted by blood‐feeding triatomine bugs.
Blood transfusion is the second most important mode of transmission.
Placental transmission of Chagas disease can occur from the mother to child. Transmission by organ transplant and by accident is not unusual.
Diagnosis
Direct demonstration of the parasite in a living mammalian host can be achieved only in the acute phase, by microscopic observation of a fresh blood smear having the motile parasites amid red cells. Chronic infections require diagnostic procedures that rely on parasite amplification before they can be detected by microscopy. Xenodiagnosis consists of allowing a parasite‐free triatomine to have a blood meal from the clinically suspected candidate and examining the bug's excrements 30 and 60 days later for the presence of the parasite.18 A parallel diagnostic attempt can be made by inoculating citrate‐treated blood in axenic culture medium followed by a microscopic search for the parasite at set times. The sensitivity of both methods varies with the ability of a low number of parasites to multiply in fresh medium and requires long periods of time. The immediate detection of chronic Chagas disease relies on the indirect demonstration of specific anti‐T cruzi antibodies in patient serum or on nucleic acid‐based tests.18 High‐fidelity tests19,20 to detect specific antibodies include indirect haemagglutination, indirect immunofluorescence and the enzyme‐linked immunosorbant assay. The sensitivity of individual tests ranges from 96.5% to 100% and their specificity from 87% to 98.9%. However, cross‐reactive antibodies to T cruzi‐specific antigens can be present in the serum of some patients with other kinetoplastid infections, with some intracellular parasite or bacterial diseases (eg, malaria, toxoplasmosis, paracoccidioidomycosis, tuberculosis, leprosy, syphilis and pemphygus) and with some autoimmune conditions (eg, rheumatoid arthritis, systemic lupus erythematosus, etc), albeit at much low titres.21,22 Differential diagnosis can be achieved by immunoblot assays.23 Diagnoses can be confirmed by the use of nucleic acid tests such as polymerase chain reaction amplification with specific primer sets followed by hybridisation with internal probes.24,25,26 In view of the major caveats leading to false‐negatives and false‐positives, it has been agreed that at least two immunological tests are required to confirm a diagnosis of Chagas disease.20,27
Diagnosis
Acute infection: parasite detection in blood smears and immunglobulin (Ig)M antibody tests.
Chronic infection: xenodiagnosis, haemoculture and IgG immunological assays.
Two consistently positive immunological tests are required.
Nucleic acid tests are required to confirm diagnosis in some cases.
Epidemiology and control
The unquestionable benefits of major insecticide‐based control programmes28 showing abrupt drops of T cruzi infection in young populations need to be emphasised. It is expected that reported success will be reflected in low death rates three decades after dislodgment of domestic Triatoma infestans. The progress achieved thus far provides a window of opportunity before the triatomine develops resistance to the widespread and indiscriminate use of pyrethroid insecticides.29 More measures for control aimed at a sustainable reduction in prevalence of Chagas disease in the tropical rain‐forest ecosystems of Central and South America have been envisaged30,31,32,33. Above all, control cannot be satisfactorily achieved by means other than social development and improvement of human living conditions. Particularly, interventions to prevent infection by T cruzi and improve children's health mean poor housing has to be improved.34
However, given the large pool of primary hosts for this enzootic disease, complete control of T cruzi arthropod vector transmission to the human population seems daunting. Demographic change in Latin America has been marked over the past four decades. For example, in the 1960s, 75% of the population lived in rural areas, whereas at present 81% live in metropolitan areas. As a consequence of the rural exodus, millions of people with Chagas disease live in the largest cities.35 The rural exodus appears to be related to the migration of people with Chagas disease to the cities for jobs and education, and thus the disease has spread to more socioeconomic classes. The spread of Chagas disease in urban areas needs to be reviewed separately on the basis of active vector‐transmitted T cruzi infection in populations inhabiting the outskirts of major cities in many Latin American countries.36,37,38,39,40 Furthermore, localised transmission of acute Chagas disease has been associated with the oral route of contamination.41,42,43,44,45 Overall, Chagas disease in urban areas has been associated directly with about 1 death in 10 among people aged between 25 and 64 years.46
Effective prophylaxis against T cruzi appears to be a formidable task as there are elusive biological challenges. An effective immunoprophylaxis against intracellular infection is unattainable because specific, concomitant, steady‐state immunity poses a somewhat effective barrier against parasite circulation in the blood, but does not eliminate parasites that colonise non‐phagocytic muscle cells, allowing the protozoa to persist throughout its host's life.1 Thus, vaccination against Chagas disease is not feasible with the currently available biotechnologies.9,29 On the other hand, chemotherapy for T cruzi infection may be ideal because it would curtail parasitism in reservoir hosts, diminishing peridomestic insect vector dissemination. Anti‐trypanosome nitroderivatives that suppress blood parasitism show severe toxicity, requiring careful prescription.27,47,48,49,50,51,52,53
T cruzi infections occur in a circumscribed region of the world that represents a relatively small market. Accordingly, pharmaceutical companies have been reluctant to invest resources for the development of vaccines and drugs to control and treat Chagas disease.
Epidemiology and prophylaxis
Chagas is the most lethal endemic infectious disease in the western hemisphere.
Pyrethroid insecticides eliminate triatomines in peridomiciles and domiciles.
Bed nets and shields prevent the bug's invasion of human domiciles.
Improvement of housing conditions.
Social and economic development.
Education, information and communication.
Excluding candidate blood donors through epidemiological history.
Effective chemotherapy would diminish transmission in households.
Clinical manifestations
Acute Chagas disease
Acute cases of Chagas disease are characterised by the presence of the parasite in the patient's blood, thus allowing the demonstration of T cruzi trypomastigotes by direct microscopic examination of a fresh blood smear. Often, the acute phase of the infection is not perceived by the patient; 95% of the cases are asymptomatic. In the remaining symptomatic cases, the clinical manifestations of acute Chagas disease are fever, malaise, muscle and joint pains, somnolence, cramps and diarrhoea, oedema, respiratory disturbances, cyanosis and comma. Considering that <5% of patients with acute symptoms die,54 it can be estimated that mortality in this phase of the infection is between 1:2500 and 1:5000. Death in the acute phase of the disease is caused by myocarditis or meningoencephalitis, with attendant complications such as bronchopneumonia.55 The acute phase of T cruzi infection usually goes into remission spontaneously and the infection enters the chronic stage within 3–4 months of its onset.
Chronic Chagas disease
Acute cases with or without symptoms of T cruzi infection lead to the chronic stages, which can present in any age group.56 Interestingly, two thirds of 18 million people harbouring chronic T cruzi infection do not show any detectable clinical manifestation of Chagas disease. People considered to be in the indeterminate chronic phase of the infection do not die of Chagas disease. About one third of the chronically infected human population develop clinical manifestations of the disease.56 The symptomatic disease affects the heart in 94.5% of cases; these patients are considered to have chronic Chagas heart disease. Heart insufficiency is related to the cause of death in 58% of patients, whereas arrhythmias have been associated with unexpected deaths in 36.5%. The remaining 4.5% of patients with chronic Chagas infection show mega syndromes, a disease state that involves the oesophagus (megaoesophagus) and the colon (megacolon).57 Chronic Chagas heart disease affects both sexes equally, frequently at between 30 and 45 years of age. In the group of patients showing progressive changes on electrocardiogram, unexpected death can occur in 37.5% of cases. Importantly, 58% of the patients with chronic Chagas heart disease show ominous signs of heart insufficiency and frequently die 7 months to 2 years after the onset of symptoms.56 People with Chagas disease with cardiac or gastrointestinal manifestations of the disease may also show widespread lesions involving the sympathetic and parasympathetic peripheral nervous systems. These chagasic nervous system lesions specifically explain the physiopathological condition of the heart and the mega syndromes observed at the bedside.58,59
Chagas disease and AIDS
The natural course of T cruzi infection can be modified by coinfections. Chagas disease and HIV mutually affect each other.60 HIV infection may lead to reactivation of T cruzi infection.61 The disease in some T cruzi‐HIV‐infected patients may have a long silent clinical course, whereas in others it may present with meningoencephalitis or myocarditis. Therefore, these coinfections should be investigated in endemic and non‐endemic regions of the world.62
Clinical course
Acute infections usually subside spontaneously.
Chronic infections are asymptomatic in two thirds of the human population.
Chronic Chagas disease affects mostly the heart and the digestive tract.
Arrhythmias and congestive heart failure are ominous signs of the disease.
Megaoesophagus and megacolon cause dysphagia and constipation, respectively.
Pathogenesis
Two theories have been set forth to explain the pathogenesis of the lesions in Chagas disease. The parasite persistence theory postulates that Chagas lesions could be a direct consequence of the mechanical rupture of the parasitised host cell and subsequent inflammation. The autoimmune theory ascribes Chagas lesions to the rejection of parasite‐free target cells by immune lymphocytes. Here we present data showing that these theories are not mutually exclusive.
Parasite persistence
Most observers agree that parasite persistence in the human host may lead to the rupture of colonised cells in the acute phase of the infection. The microscopic finding of parasite‐free target cell lyses by immune effector lymphocytes has cast doubt on whether mechanical rupture is the main feature of the pathogenesis of acute disease. The difficulty in establishing a direct relationship between the intracellular presence of parasitic T cruzi forms and concomitant tissue destruction can be clearly shown in endemic areas of Chagas disease, where approximately one third of the infected human population succumb to Chagas disease. Moreover, 80% of patients with Chagas disease who died of the disease did not show amastigote nests of the parasite in histopathological sections of the heart. Furthermore, a lack of physical proximity between the parasite nest and the inflammatory lesions was clearly shown among chronic Chagas hearts with intracellular amastigotes. There are, however, several questions that remain unanswered: (1) Why are acute infections usually asymptomatic and remit spontaneously? (2) If severe pathology is present in every patient with Chagas disease, is it also present in the cryptic T cruzi infection? (3) Why do patients with cryptic T cruzi infection have high rates of morbidity and mortality?
Answers to these questions have been sought in longitudinal field studies—for example, among 190 adults positive for antibody tests for T cruzi infection, 56 patients had parasitaemias detected by xenodiagnoses, but the remaining 134 patients with Chagas disease did not. In this series, progressive Chagas heart lesions were shown in 30% of the patients with detected parasitaemias, but in 28.8% of the patients there was no detectable parasitaemia.63 These findings have suggested that the severity of heart lesions and the evolution of the disease may not be associated with the prevalence of parasitaemias in patients with chronic Chagas disease.
Autoimmunity
Experimental data have shown that autoimmunity is an important component of the pathogenesis of Chagas disease.64 The observed rejection of target heart fibres by the immune system mononuclear effector cells (fig 5) appears to favour autoimmunity. The accelerated rejection of embryonic rabbit cells by immune lymphocytes derived from chronically T cruzi‐infected rabbits has been considered evidence of autoimmunity in Chagas disease.64,65 Immune factors and possible mechanisms triggering the lesions of Chagas disease have stirred discussions on the role of T cruzi antigens in the pathogenesis of the disease, such as crossreactivity and molecular mimicry.66 A lot more information on this subject can be found in previous review papers.67,68 We believe that the roles played by parasite persistence in the body and by autoimmunity are essential to the pathogenesis of Chagas disease.
The observation of progressive Chagas heart disease in T cruzi‐infected rabbits that had been treated with anti‐trypanosome nitroderivatives generated an unavoidable question: what could be sustaining those destructive lesions in the heart cells of treated rabbits? We sought an answer to this question by examining the hypothesis that horizontal DNA transfer from the parasite to the mammal host (a mutation) may explain the autoimmune lesions of Chagas disease.
Horizontal DNA transfer
Horizontal transfer of T cruzi kinetoplast DNA (kDNA) minicircles to the genome of mammal hosts has been detected.68,69,70,71,72 Furthermore, a truncated kDNA sequence was found in the genome of a T cruzi‐infected baboon (Papyo hamadryas), which flanked the host DNA (GenBank accession number DQ241812). Southern blots of genomic DNA from the heart, skeletal muscle and intestine of a chronic Chagas rabbit hybridised with a kDNA probe. Interestingly, the 2.2 kb kDNA band formed with the rabbit genome DNA showed a configuration different from the typical 1.4 kb band formed with the parasite DNA. These specific bands did not hybridise with other parasite nuclear DNA internal probes.25,73 The sequencing showed that both ends of the kDNA were flanked by rabbit DNA (GenBank accession numbers AF400668, AF399841 and AF415293) and that the kDNA integrated in direct CACCAACC repeats within the rabbit genome. The host DNA, flanking the kDNA, showed homology with the clone LBNL‐125D4, which is a LINE‐1 retrotransposon, loaded with short SINE repeats interspersed in the vertebrate genome.74 An open reading frame that began in the rabbit DNA extended into the integrated kDNA. DataBank analysis revealed a transcript potentially coding the chimera antigen r45 (GenBank accession number AAR24603.1). These observations have suggested that persisting T cruzi infections can be a cumulative source of parasite‐induced kDNA mutations. We suggest that the newly formed open reading frames translating chimera proteins trigger the pathology of Chagas disease.
kDNA inheritance
When germ cells of an 18‐month‐old boy were found colonised by T cruzi amastigotes, we predicted that the parasite could invade stem cells (ARL Teixeira, unpublished observation). The permissiveness of stem cell lines to T cruzi infection was an indication that genital crest cells could acquire kDNA mutations during the differentiation stage from the 4th to the 8th day of gestation. In fact, kDNA integration in the offspring of chronically T cruzi‐infected rabbits was shown. Genomic DNA extracted from various body tissues showed that the experimental animals were only kDNA positive by nucleic acid tests. The cloning and sequencing of kDNA‐positive samples showed minicircle sequences integrated in the genome of the rabbits (GenBank accession numbers AY488498–AY488503). To determine whether a live infection is required for integration, kDNA minicircle sequences were inoculated intravenously in T cruzi‐infected rabbits. Minicircle sequences were polymerase chain reaction amplified only during the following 3 weeks, thus showing that living infection is required for sustained vertical transfers of kDNA mutations. Hallmark chagasic histopathological condition (minimal rejection units) was found in the heart, skeletal muscles and parasympathetic nervous system of the offspring. In the control group, no lesions were found in the tissues of kDNA‐negative rabbit offspring.
Birds are refractory to T cruzi infection,1 and thus represent a “clean” model to study the vertical transfer of kDNA mutations. Therefore, experiments were conducted in Gallus gallus because T cruzi infections could be established early in embryonic life. kDNA mutations were shown to be present in 24% of the chickens which hatched from T cruzi‐infected eggs. The mutated chickens yielded sperm and unfertile ova, whose DNA contained kDNA amplification products. Crossing of kDNA‐positive hens with roosters resulted in kDNA‐mutated chickens in all instances. Sequencing showed integration of kDNA in a retrotransposon (CR‐1) at chromosome 4 (GenBank accession number AY237306). Interestingly, some growing kDNA‐positive chickens developed muscle weakness whereas others showed signs of heart failure. When these birds died, they showed the typical minimal rejection units of Chagas disease found in rabbits and in humans. The results therefore strongly suggest that the pathology observed in FO, F1 and F2 kDNA‐mutated birds is parasite‐independent. Typically, the lesions in the heart (fig 5), skeletal muscle and the parasympathetic ganglia (fig 6) of kDNA‐mutated birds were comprised of minimal rejection unit lesions (fig 7) with features similar to those seen in the human host. We believe that kDNA integration in the vertebrate‐host genome has the potential to trigger an autoimmune response that results in host‐tissue lesions, and explains the pathogenesis and variable clinical manifestations of Chagas disease.1
Figure 5 Myocarditis in a kinetoplast DNA‐mutated chicken. A typical feature of the Chagas lesion is the immune system mononuclear cell adherence and lyses of parasite‐free target heart myofibres. Haematoxylin and eosin (H&E) stain, ×200.
Figure 6 Ganglionitis and neuronal cell lyses (arrows) in a kinetoplast DNA‐mutated chicken. Haematoxylin and eosin (H&E) stain, ×200.
Figure 7 The “minimal rejection unit” in the heart of a kinetoplast DNA‐mutated chicken. Parasite‐free target cells are lysed by activated immune lymphocytes. Haematoxylin and eosin (H&E) stain, ×400.
Pathogenesis
Parasite persistence cannot be excluded.
Autoimmunity appears to explain parasite‐free target cell destruction.
Mechanism triggering autoimmunity needs further clarification.
The horizontal transfer of T cruzi kDNA has been detected.
Parasite‐refractory chickens show kDNA mutations and evident pathology.
Chagasic condition is present in F1 and F2 kDNA‐mutated chicken.
New genes coding chimera proteins may trigger autoimmunity.
Investigation of the cause of autoimmunity will be a daunting task.
Histopathological study
Acute Chagas disease
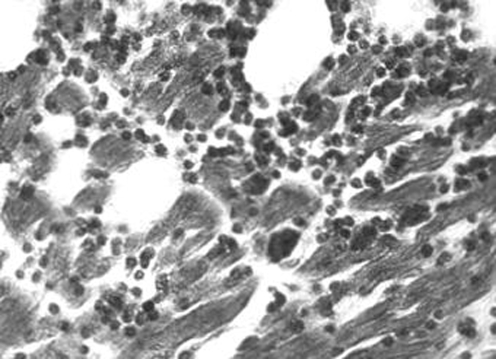
T cruzi can parasitise any nucleated cell in the human body. Therefore, connective tissue, striated and smooth muscles, germinal cells, and phagocytic mononuclear cells can be intensely parasitised. Also, epithelial cells of the liver, kidneys, thyroid, pancreas and skin are permissive to T cruzi. Schwann cells of the peripheral nerve fibres, ganglia and glial cells (usually astrocytes) of the brain are parasitised, but neurones are spared. Often, amastigote parasitic forms that colonise muscle cells become inaccessible to the immune system effector cells and, therefore, can hibernate in those niches for an unknown period of time. Grossly, the heart in humans with acute disease increases in size and becomes dilated, flabby and congested. The lymph nodes near the emergence of the aorta and the pulmonary artery become engorged. In the epicardial surface, the coronary and the lymphatic vessels are dilated. These gross findings predict the presence of intense inflammatory infiltrates in the heart. Microscopically, muscle fibres and occasionally histiocytes show pseudocysts (cysts without a wall) formed by intracellular T cruzi amastigotes. However, a conspicuous finding in the acute phase of the infection is that of inflammatory mononuclear cell infiltrates and parasite‐free host cell lyses (fig 8). These inflammatory cells, mainly macrophages and activated lymphocytes, invade muscle structures and the parasympathetic and sympathetic ganglia in the body. The lysis of parasite‐free neurones is associated with adherence of the immune system mononuclear cells to the target cell outer plasma membrane. Electron microscopic studies of tissue lesions have shown inflammatory infiltrates in association with glial cell elements. Heart cell destruction by immune lymphocytes and depopulation of the neurones are hallmarks of the pathology of acute‐phase Chagas disease. Although the pathology of the acute phase shows the presence of scattered parasites in the heart and nervous system, its main components are the inflammatory infiltrates and target‐cell lyses. Sometimes the immune system mononuclear cells palisade the perforating meningeal blood vessels in Virchow‐Robin spaces. In the brain, T cruzi amastigotes are more frequently found in astrocytes. These lesions are characteristic of meningoencephalitis.
Figure 8 Electron microscopy of the heart of a patient who died of acute Chagas disease. Notice lymphocytes (L) invading the cytoplasm of a cardiac fibre (FC), adhering to the microfilaments (Mi) and concomitant lyses (courtesy of Dr Washington Luiz Tafuri, Faculdade de Medicina da Universidade Federal de Minas Gerais). Magnification ×7000.
Indeterminate phase
During the indeterminate phase of chronic T cruzi infections, patients do not show clinic manifestations related to heart or digestive tract lesions.75 People who were in the indeterminate phase of the infection and died of fatal accidents had no heart lesions or megaoesophagus or megacolon. Nonetheless, the indeterminate phase of the infection was established by the direct demonstration of the parasite or by phenotype and genotype markers of the cryptic infection. Microscopically, biopsies of 20 patients with these cryptic infections showed discrete, very low intensity inflammatory heart lesions.76
Chronic Chagas disease
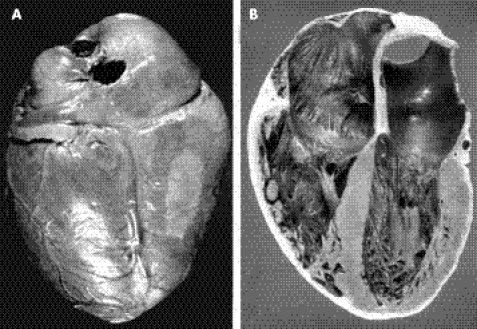
The size of the heart of a patient with congestive heart failure is markedly increased and often reaches twice the normal size (fig 9). A typical gross finding of chronic Chagas heart disease is apex effacement of the left ventricle or aneurysm formation. Whenever either of these occurs, a thrombus appears in different stages of organisation. The other site of thrombus formation is the huge right atrium of the Chagas heart. Thrombi at these locations can travel to the brain and the lungs, and are frequent causes of death.
Figure 9 Gross pathology of chronic Chagas heart disease. (A) Posterior surface of a large heart showing whitish soldier's patch and dilated coronary vessels. (B) Internal view showing dilation of the right and left chambers of the heart.
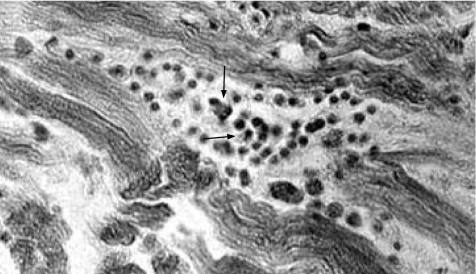
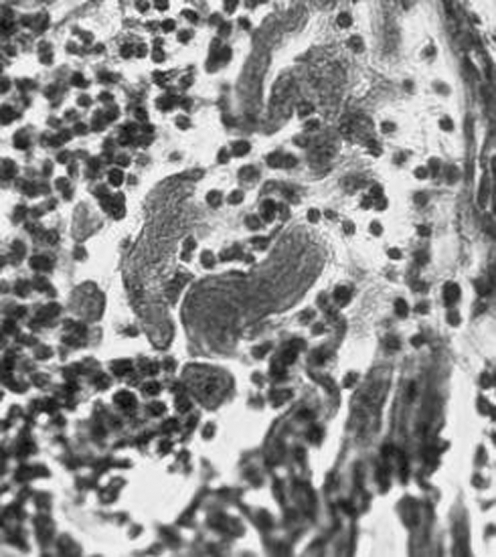
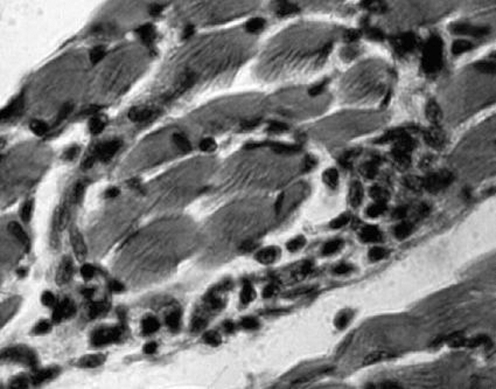
The main microscopic findings always present in a patient with chronic Chagas heart disease are inflammatory infiltrates and target heart cell lyses (fig 10). These inflammatory infiltrates are formed by macrophages, and small and large lymphocytes that migrate from the dilated lymphatic vessels to the epicardial surfaces. These immune system mononuclear cells invade the heart and produce lyses of parasite‐free target heart cells. The finding of T cruzi amastigote nests in heart cells can be detected microscopically in 10–20% of chronic cases. Importantly, in these cases, the parasite's intracellular nests are more often found in areas of the myocardium free of inflammatory infiltrates. Consistently, nucleic acid tests show the remnants of parasite DNA in the affected tissues of all chronically infected patients.27 Therefore, the hallmark of the microscopic pathology in Chagas heart disease is the destruction of the target heart cells by immune effector lymphocytes. This microscopic feature defines a minimal rejection unit (fig 11). The confluence of numerous rejection units signifies chronic Chagas myocarditis. The destroyed heart fibres are gradually replaced by fibrous scars as the inflammation subsides. Inflammatory cells infiltrate the heart conducting system in the same manner as the contractile myocardium. The intensity of the auto‐destructive process varies from one site to another in the heart; whereas some lesions are fresh, others evolve into fibrous scars as the inflammatory process fades away. With ageing, fibrous scars accumulate in the myocardium and can be found in every heart chamber, particularly in the left ventricle during late stage insufficiency.
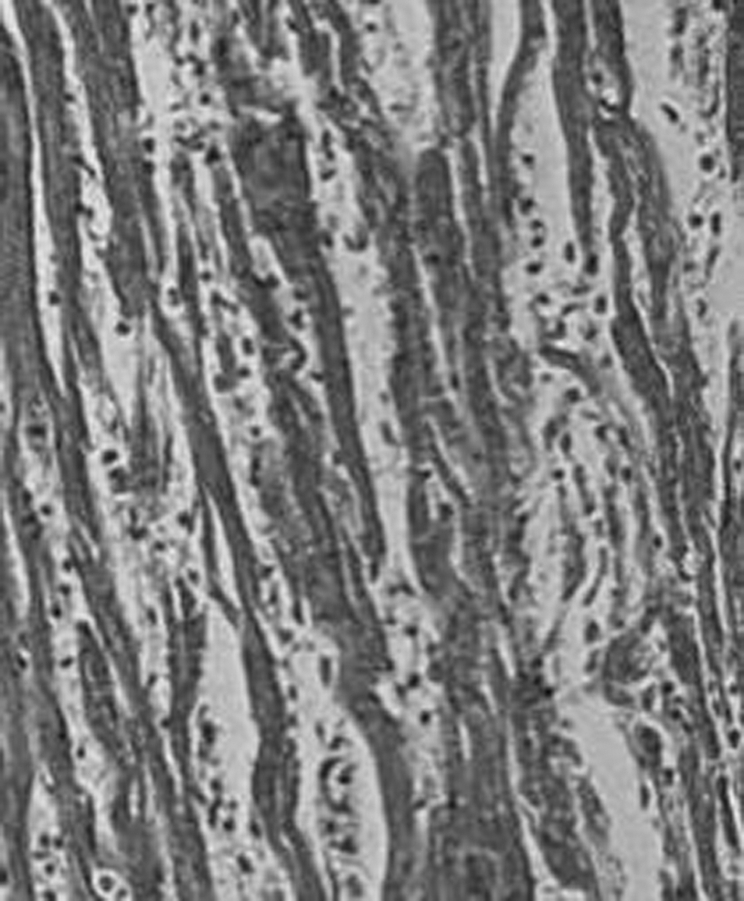
Figure 10 Myocarditis in the heart of a patient with Chagas disease. Infiltration and adherence of mononuclear cells to target myofibres and lyses. Haematoxylin and eosin (H&E) stain, ×200.
Figure 11 The “minimal rejection unit” in the skeletal muscle of a patient with Chagas disease. Note the immune system mononuclear cell palisade of the target cell sarcolemma and concomitant lyses of the myofibres in the absence of parasite colonies in situ.
Histochemical analysis of the sympathetic and parasympathetic nerve endings of patients with chronic Chagas disease has shown a low quantity of catecholamines and deficient acetylcholinesterase activity. Both findings are consistent with progressive autonomic denervation.77 Furthermore, the autonomous intracardiac nervous system in every Chagas heart shows typical lesions with varying degrees of intensity. The inflammatory infiltrates are evident in the parasympathetic ganglia and in the sympathetic nerve endings in the myocardium. Ganglion cells and peri‐ganglion areas are associated with mononuclear cell infiltrates and proliferation of glial cells. Inflammatory cells palisade the neurones, which show degeneration and lysis. Neuronal lysis with degeneration and depopulation of neurones are the typical findings of Chagas heart disease.
Megaoesophagus and megacolon consist of gross dilation of the hollow viscera and thickening of their walls. Such lesions are associated with loss of coordination of the motility of these digestive tube segments.78,79,80,81,82,83 These changes stem from inflammatory infiltrates of the internal and external layers of smooth muscle cells of the digestive tract wall. However, inflammatory lesions particularly affect the parasympathetic neurones within the walls (fig 12). Neuronal cell depopulation occurs randomly in all segments of the digestive tract, but this is clinically more often evident in the oesophagus and the colon. Usually, a mega syndrome manifests when neurone loss reaches 55% of the units in the intramural parasympathetic ganglia. In some patients, neuronal depopulation can average 76.5% as compared with control neurone counts.80 The use of nucleic acid tests has shown the persistence of remnants of T cruzi DNA in tissue samples taken from mega lesions.83 Although the parasite persists in tissues showing mega syndromes, the literature does not document colonisation of neurones by T cruzi. As neurotoxin release by the parasite has not been shown, it is presumed that neuronolysis and depopulation are not associated with parasitism of these target cells. In contrast, neuronolysis has been associated with adherence of immune system mononuclear cells, as described for a minimal rejection unit. These observations suggest the rejection unit is the common denominator of Chagas disease pathology.
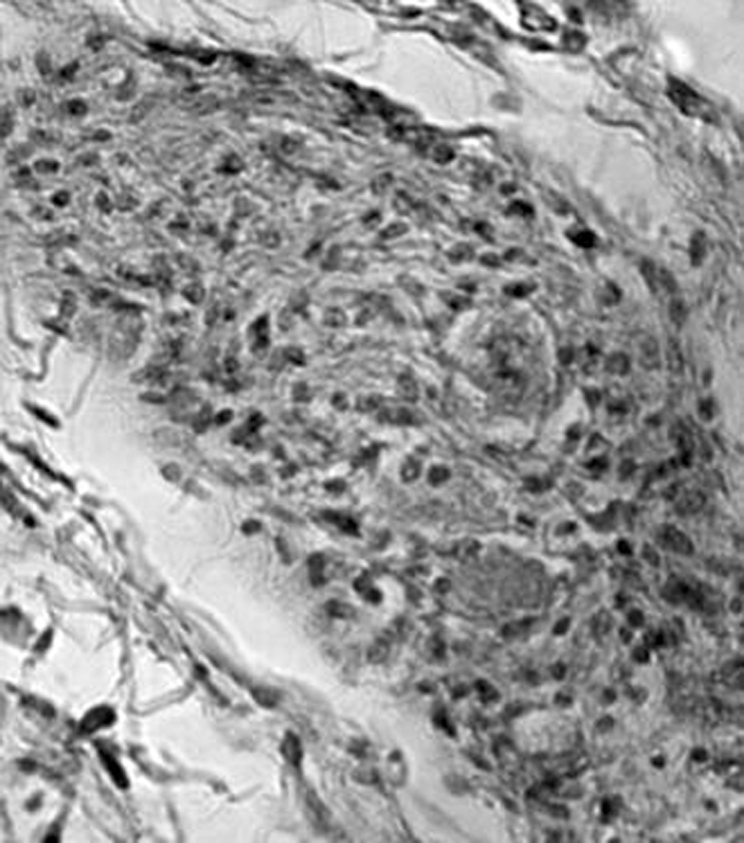
Figure 12 Ganglionitis and neurone drop‐out of the parasympathetic ganglion of a kDNA‐positive patient with Chagas disease and megacolon.
Pathological study
Acute‐phase pathology shows T cruzi forms in tissues and inflammatory infiltrates.
Chronic pathology shows striking inflammation in the absence of the parasite in situ.
Lesions affect various organs and tissues in the human body.
Muscle tissues and parasympathetic ganglia are often destroyed.
Lesions are characterised by mononuclear cell infiltrates and target cell lysis.
Parasite‐free neurone lyses are produced by inflammatory infiltrates.
The typical lesion in a patient with Chagas disease is the destruction of parasite‐free target cells.
The minimal rejection unit is the typical pathological lesion in Chagas disease.
Treatment
Nifurtimox (4‐(5‐nitro‐furylidenoamine)‐tetrahydro‐4‐4‐1,4‐thiazine‐1‐1‐dioxode) and o‐benznidazole (N‐benzyl‐2‐nitro‐imidazolacetamide) are anti‐trypanosome drugs used to treat the T cruzi infections.84,85 The cytotoxic and genotoxic effects associated with the drugs are related to their chemical structures, resulting in the release of electrophilic radicals on enzymatic nitro‐reduction. The free radicals have been identified as the nitro‐anion, H−, ½ O2 and hydrogen peroxide.86,87,88,89,90 Drug toxicity affects the parasite and also any other innocent bystander cells in the human body.85 The nitro‐group reduction alters the number of electrons at the external orbital, thus augmenting the mutagenic potential because free radicals bind to DNA double strands, forming adducts.85,86,87,88,89,90
The use of nitroderivatives in the treatment of acute Chagas disease has had limited success.91,92,93,94,95,96 Physicians have used these drugs sparingly because they do not eradicate T cruzi infections in 83.5% of acute cases. Further, an indirect indication of parasite persistence is provided by positive after‐treatment immunological tests. These observations explain why caution is required before using these drugs, in addition to reports showing that the use of anti‐trypanosome nitroderivatives does not halt progression of the disease in patients treated for Chagas disease.27 For example, electrocardiographic changes in patients treated for Chagas disease did not differ statistically from those recorded in a placebo‐treated cohort of patients with acute Chagas disease.27 Furthermore, chagasic changes on electrocardiogram have been recorded in patients 24 years after treatment with a nitroderivative compound.94,95,96 Moreover, in patients acutely infected with T cruzi, heart biopsies showed myocarditis,97 even though the patients had been treated with a nitroderivative drug. These findings strongly suggest that nitroderivative drug treatment does not prevent the onset of severe heart lesions.
In the chronic indeterminate phase, the only acceptable criterion for cure resulting from specific drug therapy for T cruzi infections is the lack of detectable specific antibodies and the results of nucleic acid tests to determine evidence of the parasite's phenotype and genotype. Results of these studies showed that 94.7% of nitroderivative drug‐treated patients with chronic Chagas disease tested positive for specific antibodies to T cruzi antigens.93 In one series that had reported 76% success in the treatment of patients with chronic Chagas disease with a nitroderivative compound, a mere 8% drop was observed in the prevalence of positive nucleic acid tests 6–18 years after chemotherapy. Importantly, it was shown that mortality was similar in treated and untreated groups of patients with chronic Chagas disease 10 years after chemotherapy.27
Treatment
Nitroderivative drugs curtail parasitaemias and clinical signs of acute infection.
Treatment does not eradicate T cruzi infection.
Progressive chronic Chagas heart disease persists in treated patients.
Amelioration of symptoms can be achieved with modern clinical and surgical techniques.
Conclusions
Drug treatment for T cruzi infections is considered unsatisfactory because it does not halt the progressive lesions of the heart and digestive tract of patients with chronic Chagas disease. It appears that effective treatment of Chagas disease would require a drug that does not produce undesirable effects and eradicates the infection or improves the prognosis of a sick patient. Specific chemotherapy with the drugs that are now available may not be cost effective. Nevertheless, treatment with a nitroderivative compound is clearly indicated in acutely infected patients, during the course of immune suppression and during organ transplantation of a patient with Chagas disease.
The controversy over the efficacy of treatment underlines the importance of sustained therapeutic research to control Chagas disease.2 The results of studies reported here suggest that the pathogenesis of the disease is related to kDNA mutations of the vertebrate host's genome. There is a vast field of research on which to base the development of drugs to prevent T cruzi‐induced kDNA mutations.3 The persistence of lifelong infections providing a source of kDNA that may result in cumulative mutations over time requries newly developed, targeted drugs. This is an area of scientific research that has the potential to generate health benefits for millions of people already infected with T cruzi, one third of whom will develop clinical manifestations of Chagas disease.
Self assessment Questions (TRUE (T) FALSE (F); answers at the end of the references)
-
Acute Chagas disease
Is usually seen in children below 15 years of age
Is often not perceived by the individual or by the doctor
Is recognised by the finding of T cruzi in blood smears
May be asymptomatic and subsides spontaneously
In <5% of the cases is associated with heart failure or meningoencephalitis
-
Chronic Chagas disease
Is usually seen 2–3 decades after the onset of T cruzi infection
Is eradicated by anti‐trypanosome drugs
Is cured by treatment as indicated by seroconversion of immunological tests and negative nucleic acid assays
Effects of treatment are beneficial because they prevent progressive Chagas heart disease
Produces megaoesophagus and megacolon in the early stages of the infection only
-
Pathogenesis
Results from a parasite‐released toxin that kills neurones
Is often associated with a neurone infection and cell death
Appears to associate minimal rejection units with autoimmunity
Is always related to the parasite's mechanic destruction of target cells
Suggests that immune system cells are not involved
-
Pathological study of chronic Chagas disease
Is related to the parasite's pseudocyst which is easily seen in histopathological sections
Leads to small lesions without clinic repercussion
Does not cause significant rates of morbidity
Is usually fatal within 2 years after the onset of symptoms of heart failure
Can occur in the absence of T cruzi infections and of specific phenotype and genotype markers of the parasite.
-
Prophylaxis
Is partly obtained by spraying pyrethroids in the domicile and peridomicile
Requires control of mosquitoes
Is controlled by vaccination in endemic areas
Is not affected by economic development
Cannot be attained by improving housing conditions
Acknowledgements
We thank Meire Maria de Lima for critical reading of the manuscript. NN is in receipt of a Post‐Doctoral Fellowship from the Ministry of Education‐CAPES, Brazil. MCG and CG are holders of scholarships from the Brazilian National Council‐CNPq.
Abbreviations
kDNA - kinetoplast DNA
Answers
1. (A) T, (B) T, (C) T, (D) T, (E) T. 2. (A) T, (B) F, (C) F, (D) F, (E) F. 3. (A) F, (B) F, (C) T, (D) F, (E) F. 4. (A) F, (B) F, (C) F, (D) T, (E) F. 5. (A) T, (B) F, (C) F, (D) F, (E) F.
Footnotes
Funding: This work has been partially supported by Financiadora de Estudos e Projetos–FINEP, Brazil, and by NIH grant I‐R03‐AI067334‐01.
Competing interests: None declared.
References
- 1.Teixeira A R L, Soulsby E S L. The Stercorarian trypanosomes. Immunopathology, immunoprophylaxis. In, ed. Immune responses in parasitic infections: immunology Boca Raton, FL, CRC Press 1987125–145.
- 2.Aufderheide A C, Salo W, Madden M.et al A 9,000‐year record of Chagas' disease. Proc Natl Acd Sci USA 20041012034–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chagas C. New human trypanosomiasis. Morphology and life cycle of Schyzotrypanum cruzi, the cause of a new human disease. Mem Inst Oswaldo Cruz 19091159–218. [Google Scholar]
- 4.Chagas C. A new human disease. Summary of etiological and clinical studies. Mem Inst Oswaldo Cruz 19113219–275. [Google Scholar]
- 5.Carcavallo R U, Jurberg J, Galindez Giron I.et alAtlas of Chagas' disease vectors in the Americas. Rio de Janeiro: Editora FIOCRUZ, 1997
- 6.Silveira A C. Current situation with the control of vector‐borne Chagas disease transmission in the Americas. Atlas of Chagas disease vector in the Americas. Rio de Janeiro: Editora FIOCRUZ, 1999
- 7.von Kreuter B F. Santos‐Buch CA. Modulation of Trypanosoma cruzi adhesion to host muscle cell membranes by ligands of muscarinic cholinergic and beta adrenergic receptors. Mol Biochem Parasitol 19893641–50. [DOI] [PubMed] [Google Scholar]
- 8.Lima M F. Villalta F. Host‐cell attachment by Trypanosoma cruzi: identification of an adhesion molecule, Biochem Biophys Res Commun 1988155256–262. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization Control of Chagas' disease: second report of a WHO expert committee. World Health Organ Tech Rep Ser 20029051–109. [PubMed] [Google Scholar]
- 10.Schmunis G A. Risk of Chagas disease through transfusions in the Americans. Medicina (Buenos Aires) 199959125–134. [PubMed] [Google Scholar]
- 11.Dias J C. Epidemiological surveillance of Chagas disease. Cad Saúde Pública 20001643–59. [PubMed] [Google Scholar]
- 12.Kirchhoff L V, Neva F A. Chagas' disease in Latin American immigrants. JAMA 19852543058–3060. [PubMed] [Google Scholar]
- 13.Schmunis G A, Cruz J R. Safety of the blood supply in Latin America. Clin Microbiol Rev 20051812–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reesink H W. European strategies against the parasite transfusion risk. Transf Clin Biol 2005121–4. [DOI] [PubMed] [Google Scholar]
- 15.Dodd R Y. Current safety of the blood supply in the United States. Int J Hematol 200480301–305. [DOI] [PubMed] [Google Scholar]
- 16.Bittencourt A L. Possible risk factors for vertical transmission of Chagas' disease. Rev Inst Med Trop São Paulo 199234403–408. [DOI] [PubMed] [Google Scholar]
- 17.Azogue E. Women and congenital Chagas' disease in Santa Cruz, Bolivia: epidemiological and sociocultural aspects. Soc Sci Med 199337503–511. [DOI] [PubMed] [Google Scholar]
- 18.Braga M S, Lauria‐Pires L, Arganaraz E R.et al Persistent infections in chronic Chagas' disease patients treated with anti‐Trypanosoma cruzi nitroderivatives. Rev Inst Med Trop São Paulo 200042157–161. [DOI] [PubMed] [Google Scholar]
- 19.Gutierrez R, Angulo V M, Tarazona Z.et al Comparison of four serological tests for the diagnosis of Chagas disease in a Colombian endemic area. Parasitology 2004129439–444. [DOI] [PubMed] [Google Scholar]
- 20.Pirard M, Iihoshi N, Boelaert M. The validity of serologic tests for Trypanosoma cruzi and the effectiveness of transfusional screening strategies in a hyperendemic region. Transfusion 200545554–561. [DOI] [PubMed] [Google Scholar]
- 21.Vexenat A C, Santana J M, Teixeira A R. Cross‐reactivity of antibodies in human infections by the kinetoplastid protozoa Trypanosoma cruzi, Leishmania chagasi and Leishmania (viannia) braziliensis. Rev Soc Bras Med Trop 199638177–185. [DOI] [PubMed] [Google Scholar]
- 22.Teixeira A R, Vexenat A C. The true significance of serological exams in the diagnosis of endemic diseases. Rev Soc Bras Med Trop 199629379–382. [DOI] [PubMed] [Google Scholar]
- 23.Tinoco D L, Garcia M P, Lauria‐Pires et al The use of 4 immunological exams for the determination of Chagas disease prevalence in streetsweepers of the City Sanitation Service in the Federal District. Rev Soc Bras Med Trop 19962933–40. [DOI] [PubMed] [Google Scholar]
- 24.Avila H A, Simpson L. Organization and complexity of minicircle‐encoded guide RNAs in Trypanosoma cruzi. RNA 19951939–947. [PMC free article] [PubMed] [Google Scholar]
- 25.Moser D R, Kirchhoff L V, Donelson J E. Detection of Trypanosoma cruzi by DNA amplification using the polymerase chain reaction. J Clin Microbiol 1989271477–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Requena J M, Jimenez‐Ruiz A, Soto R M.et al Characterization of a highly repeated interspersed DNA sequence of Trypanosoma cruzi: its potential use in diagnosis and strain classification. Mol Biochem Parasitol 199251271–280. [DOI] [PubMed] [Google Scholar]
- 27.Lauria‐Pires L, Braga M S, Vexenat A C.et al Progressive chronic Chagas heart disease ten years after treatment with anti‐Trypanosoma cruzi nitroderivatives. Am J Trop Med Hyg 200063111–118. [DOI] [PubMed] [Google Scholar]
- 28.Silveira A C, Arias A R, Segura E.et al El control de la enfermedad de Chagas en los países del cono sur de América. História de una iniciativa internacional 1991/2001. Uberaba, Brazil: Faculdade de Medicina do Triangulo Mineiro, 2002
- 29.Ebrahim G J. Eradication of American trypanosomiasis (Chagas' disease): an achievable goal? J Trop Pediatr 200450320–321. [DOI] [PubMed] [Google Scholar]
- 30.Hewitt P E, Barbara J A. Contreras M. Donor selection and microbial screening. Vox sanguinis 19946714–19. [DOI] [PubMed] [Google Scholar]
- 31.Ault S K. Environmental management: a re‐emerging vector control strategy. Am J Trop Med Hyg 19945035–49. [DOI] [PubMed] [Google Scholar]
- 32.Cohen J E, Gurtler R E. Modeling household transmission of American trypanosomiasis. Science 2001293694–698. [DOI] [PubMed] [Google Scholar]
- 33.Teixeira A R, Monteiro P S, Rebelo J M.et al Emerging Chagas disease: trophic network and cycle of transmission of Trypanosoma cruzi from palm trees in the Amazon. Emerg Infect Dis 20017100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaudhuri N. Interventions to improve children's health by improving the housing environment. Rev Environ Health 200419197–222. [PubMed] [Google Scholar]
- 35.Shikanai‐Yasuda M A, Lopes M H, Tolezano J E. Acute Chagas' disease: transmission routes, clinical aspects and response to specific therapy in diagnosed cases in an urban center. Rev Inst Med Trop São Paulo 19903216–27. [DOI] [PubMed] [Google Scholar]
- 36.Cedillos R A. Chagas' disease in El Salvador. Bull Pan Am Health Org 19759135–141. [PubMed] [Google Scholar]
- 37.Cortes‐Jimenez M, Nogueda‐Torres B, Alejandre‐Aguilar R.et al Frequency of triatomines infected with Trypanosoma cruzi collected in Cuernavaca city, Morelos, Mexico. Rev Lat Microbiol 199638115–119. [PubMed] [Google Scholar]
- 38.Vallve S L, Rojo H, Wisnivesky‐Colli C. Urban ecology of Triatoma infestans in San Juan, Argentina. Mem Inst Oswaldo Cruz 199691405–408. [DOI] [PubMed] [Google Scholar]
- 39.Aguilar V H M, Abad‐Franch F, Racines V J.et al Epidemiology of Chagas disease in Ecuador. A brief review. Mem Inst Oswaldo Cruz 199994387–393. [DOI] [PubMed] [Google Scholar]
- 40.Rangel‐Flores H, Sanchez B, Mendoza‐Duarte J.et al Serologic and parasitologic demonstration of Trypanosoma cruzi infections in an urban area of central Mexico: correlation with electrocardiographic alterations. Am J Trop Med Hyg 200165887–895. [DOI] [PubMed] [Google Scholar]
- 41.Becerril‐Flores M A, Valle‐De La Cruz A. Description of chagas disease in the Valle de Iguala, Guerrero state, Mexico‐Marco. Gac Méd Mexico 2003139539–544. [PubMed] [Google Scholar]
- 42.Fraiha Neto H, Valente S A S, Valente V C.et al Is Chagas disease endemic in the Amazon? An Acd Med Pará 1995653–57. [Google Scholar]
- 43.Valente A S, Valente V C, Fraiha Neto A. Considerations on the epidemiology and transmission of Chagas disease in the Brazilian Amazon. Mem Inst Oswaldo Cruz 199994395–398. [DOI] [PubMed] [Google Scholar]
- 44.Coura J R. Mecanismo de transmissão da infecção chagásica ao homem por via oral. Rev Inst Med Trop São Paulo 199744159–165. [Google Scholar]
- 45.Pinto A Y, Valente S A, Valente V C. Emerging acute Chagas disease in Amazonian Brazil: case reports with serious cardiac involvement. Braz J Infect Dis 20048454–460. [DOI] [PubMed] [Google Scholar]
- 46.Pereira M G. Characteristics of urban mortality from Chagas' disease in Brazil's Federal District. Bull Pan Am Health Org 1984181–9. [PubMed] [Google Scholar]
- 47.Docampo R, Moreno S N J. Free radicals metabolites in the mode of action of chemotherapeutic agents and phagocytic cells on Trypanosoma cruzi. Rev Infect Dis 19846233–238. [DOI] [PubMed] [Google Scholar]
- 48.Teixeira A R, Calixto M A, Teixeira M L. Chagas' disease: carcinogenic activity of the antitrypanosomal nitroarenes in mice. Mutat Res 1994305189–196. [DOI] [PubMed] [Google Scholar]
- 49.Teixeira A R, Cordoba J C, Souto Maior I.et al Chagas' disease: lymphoma growth in rabbits treated with Benznidazole. Am J Trop Med Hyg 199043146–158. [DOI] [PubMed] [Google Scholar]
- 50.Teixeira A R, Cunha‐Neto E, Rizzo et al Trypanocidal nitroarene treatment of experimental Trypanosoma cruzi infection does not prevent progression of chronic‐phase heart lesions in rabbits. J Infect Dis 19901621420. [DOI] [PubMed] [Google Scholar]
- 51.Flores‐Vieira C L, Antunes Barreira A. Experimental benznidazole encephalopathy. I – Clinical‐neurological alterations. J Neurol Sci 19971503–11. [DOI] [PubMed] [Google Scholar]
- 52.Flores‐Vieira C L, Chimelli L, Franca Fernandes R M.et al Experimental benznidazole encephalopathy. II‐electroencephalographic and morphological alterations. J Neurol Sci 199715013–25. [DOI] [PubMed] [Google Scholar]
- 53.Urbina J A, Docampo R. Specific chemotherapy of Chagas disease: controversies and advances. Trends Parasitol 200319495–501. [DOI] [PubMed] [Google Scholar]
- 54.Teixeira A R, Teixeira G, Macedo V.et al Acquired cell‐mediated immunodepression in acute Chagas' disease. J Clin Invest 1978621132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teixeira A R, Ripoll C M, Santos‐Buch C A. Autoimmunity in Chagas disease. In: Friedman H, Rose NR, Bendinelli M, eds. Microorganisms and autoimmune diseases. New York: Plenum Press, 1996233–250.
- 56.Prata A. Chagas' disease. Infect Dis Clin North Am 1994861–76. [PubMed] [Google Scholar]
- 57.Meneghelli U G, de Godoy R A, Macedo J F.et al Basal motility of dilated and non‐dilated sigmoid colon and rectum in Chagas' disease. Arq Gastroenterol 198219127–132. [PubMed] [Google Scholar]
- 58.Castro C, Macedo V, Rezende et al Longitudinal radiologic study of the esophagus, in an endemic area of Chagas disease, in a period of 13 years. Rev Soc Bras Med Trop 199427227–233. [DOI] [PubMed] [Google Scholar]
- 59.Galligan J J, LePard K J, Schneider D A.et al Multiple mechanisms of fast excitatory synaptic transmission in the enteric nervous system. J Auton Nerv Syst 20008197–103. [DOI] [PubMed] [Google Scholar]
- 60.Harms G, Feldmeier H. The impact of HIV infection on tropical diseases. Infect Dis Clin North Am 200519121–135. [DOI] [PubMed] [Google Scholar]
- 61.Da‐Cruz A M, Igreja R P, Dantas W.et al Long‐term follow‐up of co‐infected HIV and Trypanosoma cruzi Brazilian patients. Trans R Soc Trop Med Hyg 200498728–733. [DOI] [PubMed] [Google Scholar]
- 62.Vaidian A K, Weiss L M, Tanowitz H B. Chagas' disease and AIDS. Kinetoplastid Biol Dis 200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Castro C, Prata A, Macedo V. The influence of the parasitemia on the evolution of the chronic Chagas' disease. Rev Soc Bras Med Trop 2005381–6. [DOI] [PubMed] [Google Scholar]
- 64.Santos‐Buch C A, Teixeira A R L. The immunology of experimental Chagas disease. III. Rejection of allogeneic rabbit heart cells in vitro. J Exp Med 197414038–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Teixeira A R L. Autoimmune mechanism in Chagas' disease. In: American trypanosomiasis research. Pan Am Health Org Sci Publ 197531898–108. [Google Scholar]
- 66.Kalil J, Cunha‐Neto E. Autoimmunity in Chagas disease cardiomyopathy: fulfilling the criteria at last? Parasitol Today 199612396–399. [DOI] [PubMed] [Google Scholar]
- 67.Engman D M, Leon J S. Pathogenesis of Chagas heart disease: role of autoimmunity, Acta Trop 200281123–132. [DOI] [PubMed] [Google Scholar]
- 68.Girones N, Fresno M. Etiology of Chagas disease myocarditis: autoimmunity, parasite persistence, or both? Trends Parasitol 20031919–22. [DOI] [PubMed] [Google Scholar]
- 69.Teixeira A R, Lacava Z, Santana J M.et al Insertion of Trypanosoma cruzi DNA in the genome of mammal host cell through infection. Rev Soc Bras Med Trop 19912455–58. [DOI] [PubMed] [Google Scholar]
- 70.Teixeira A R L, Argañaraz E R, Freitas L H.et al Possible integration of Trypanosoma cruzi kNA minicircles into the host cell genome by infection. Mutat Res 1994305187–209. [DOI] [PubMed] [Google Scholar]
- 71.Simoes‐Barbosa A, Barros A M, Nitz N.et al Integration of Trypanosoma cruzi kDNA minicircle sequence in the host genome may be associated with autoimmune serum factors in Chagas disease patients. Mem Inst Oswaldo Cruz 199994(Suppl 1)249–252. [DOI] [PubMed] [Google Scholar]
- 72.Nitz N, Gomes C, Rosa A C.et al Heritable integration of kDNA minicircle sequences from Trypanosoma cruzi into the Avian genome: insights into human Chagas disease. Cell 2004118175–186. [DOI] [PubMed] [Google Scholar]
- 73.Sturm N R, Degrave W, Morel C, Simpson L. Sensitive detection and schizodeme classification of Trypanosoma cruzi cells by amplification of kinetoplast minicircle DNA sequences: use in diagnosis of Chagas' disease. Mol Biochem Parasitol 198933205–214. [DOI] [PubMed] [Google Scholar]
- 74.Price D K, Ayres J A, Pasqualone D.et al The 5′ends of LINE‐1 repeats in rabbit DNA define subfamilies and reveal a short sequence conserved between rabbits and humans. Genomics 199274320–331. [DOI] [PubMed] [Google Scholar]
- 75.Macedo V. Indeterminate form of Chagas disease. Mem Inst Oswaldo Cruz 199994(Suppl 1)311–316. [DOI] [PubMed] [Google Scholar]
- 76.Mady C, de Moraes A V, Galiano N.et al Hemodynamic study of the indeterminate form of Chagas' disease. Arq Bras Cardiol 198238271–275. [PubMed] [Google Scholar]
- 77.Oliveira J S. A natural human model of intrinsic heart nervous system denervation: Chagas' cardiopathy. Am Heart J 19851101092–1098. [DOI] [PubMed] [Google Scholar]
- 78.Koeberle F. The causation and importance of nervous lesions in American trypanosomiasis. Bull World Health Organ 197042739–743. [PMC free article] [PubMed] [Google Scholar]
- 79.Adad S J, Cançado C G, Etchebehere R M.et al Neuron count reevaluation in the myenteric plexus of chagasic megacolon after morphometric neuron analysis. Virchows Arch 2001438254–258. [DOI] [PubMed] [Google Scholar]
- 80.Tafuri W L, Maria T A, Lopes E R. Lesões do plexo mientérico do esôfago, do jejuno e do clo de chagásicos crônicos. Estudo ao microscópio eletrônico. Rev Inst Med Trop São Paulo 19711376–91. [PubMed] [Google Scholar]
- 81.Adad S J, Andrade D C, Lopes E R.et al Pathological anatomy of chagasic megaesophagus. Rev Inst Med Trop São Paulo 199133443–450. [PubMed] [Google Scholar]
- 82.Machado C R, Camargos R, Guerra L B.et al Cardiac autonomic denervation in congestive heart failure: comparison of Chagas' heart disease with other dilated cardiomyopathy. Hum Pathol 2000313–10. [DOI] [PubMed] [Google Scholar]
- 83.Vago A R, Silva D M, Adad S J.et al Chronic Chagas disease: presence of parasite DNA in the oesophagus of patients without megaoesophagus. Trans Roy Soc Trop Med Hyg 200397308–309. [DOI] [PubMed] [Google Scholar]
- 84.Onishi T, Ohashi Y, Nozu K.et al Mutagenicity of antitrypanosomal drug RO7‐1051 in Escherichia coli. Jpn J Genet 198358505–509. [Google Scholar]
- 85.Diaz de Toranzo E G, Castro J A, Franke de Cazzulo B M.et al Interaction of benznidazle reactive metabolites with nuclear and kinetoplastic DNA, proteins and lipids from Trypanosoma cruzi. Experientia 1988144880–881. [DOI] [PubMed] [Google Scholar]
- 86.Gorla N B, Ledesma O S, Barbieri G.et al Assessment of cytogenetic damage in chagasic children treated with benznidazole. Mutat Res 1988206212–220. [DOI] [PubMed] [Google Scholar]
- 87.Gorla N B, Ledesma O, Barbieri G.et al Thirteenfold increase of chromosomal aberrations non‐randomly distributed in chagasic children treated with nifurtimox. Mutat Res 1989224263–267. [DOI] [PubMed] [Google Scholar]
- 88.Docampo R, Moreno S N J. Free radicals metabolites in the mode of action of chemotherapeutic agents and phagocytic cells on Trypanosoma cruzi. Rev Infect Dis 19846233–238. [DOI] [PubMed] [Google Scholar]
- 89.Knox R J, Knight R C, Edwards D I. Interaction of nitroimidazole drugs with DNA in vitro: structure‐activity relationship. Br J Cancer 198144741–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sera N K, Fukuhara K, Myiata N.et al Mutagenicity of nitro‐azobenzeno [α]pyren and its related compounds. Mutat Res 198228081–85. [DOI] [PubMed] [Google Scholar]
- 91.Cerisola J A, Barclay E L, Lugones H.et al Results of anti Tripanosoma cruzi activity of Ro‐7‐1051 in man. Chemotherapy 1975679–85. [Google Scholar]
- 92.Soza‐Estani S, Segura E L, Ruiz A M.et al Efficacy of chemotherapy with enznidazole in children in the indeterminate phase of Chagas disease. Am J Trop Med Hyg 199859526–529. [DOI] [PubMed] [Google Scholar]
- 93.Silveira C A N, Macedo V, Prata A. Avaliação a longo prazo do tratamento específico na evolução clínica da forma indeterminada da doença de Chagas. Rev Soc Bras Med Trop 200033(Suppl II)6–8. [Google Scholar]
- 94.Andrade A L S S, Zicker F, Oliveira R M.et al Randomised trial of efficacy of benznidazole in treatment of early Tripanosoma cruzi infection. Lancet 19963481407–1413. [DOI] [PubMed] [Google Scholar]
- 95.Ianni B M, Arteaga E, Mady C.et al Uso do benzonidazol em chagásicos na forma indeterminada: resultados a longo prazo. Arq Bras Cardiol 199361(Suppl II)130–132. [Google Scholar]
- 96.Inglessis I, Carrasco H A, Anez N.et al Clinical, parasitological and histopathological follow‐up studies of acute Chagas patients treated with benznidazole. Arch Int Cardiol Mex 199868405–410. [PubMed] [Google Scholar]
- 97.Parada H, Carrasco H A, Añez N.et al Cardiac involvement is a constant findingin acute Chagas disease: a clinical, parasitological and histopathological study. Int J Cardiol 19976049–54. [DOI] [PubMed] [Google Scholar]