Abstract
Sepsis is a severe, life-threatening infection and a leading cause of death in hospitals. A hallmark of sepsis is the profound apoptosis-induced depletion of lymphocytes generating a lymphopenic environment. As lymphopenia can induce nonantigen-driven homeostatic proliferation (HP), we examined this process during sepsis. CD4+ and CD8+ T cells, which were depleted within 24 h of sepsis induction, remained at significantly reduced levels until Day 21 when normal numbers were detected. When HP was examined, naïve CD8+ T cells proliferated between Day 7 and Day 21 post-cecal ligation and puncture, developing into memory cells with relatively few cells expressing an activation phenotype. Conversely, naïve CD4+ T cells did not undergo HP, but proportionally higher numbers expressed activation markers. Adoptive transfer studies revealed that T cells from mice that had recovered from sepsis were not protective when transferred to naïve mice undergoing sepsis. In addition, the TCR repertoire was not skewed toward any specific Vβ type but resembled the repertoire found in normal mice, suggesting that T cells were not primed to antigens resulting from the infection. Interestingly, depletion of endogenous CD8+ but not CD4+ T cells restored the ability of naive CD4+ T cells to undergo HP, increasing the number of CD4+ T cells with memory but not activation markers. We conclude that homeostatic control in the postseptic environment permits recovery of the T cell repertoire to normal levels without generating antigen-specific memory or aberrant T cell specificities. Restoration of homeostatic control mechanisms might be a rational therapy for this disorder.
Keywords: T cells, apoptosis, T cell repertoire, sepsis
INTRODUCTION
Sepsis represents the leading cause of death in many intensive care units and now ranks as the 12th leading cause of death in the United States [1, 2]. Death results from multi-organ failure as a result of the inability of the host to control infections. Although the innate immune system reacts to septic challenge by engulfing bacteria and secreting cytokines, the capacity of the innate immune response to remove bacteria is one factor that can determine life or death [3]. Although the loss of monocytes/macrophages through apoptosis likely contributes to the host’s inability to control the initial infection, depletion of dendritic cells (DC) and T cells affects acquired immunity by depleting APC and potential effector cells. This defect in acquired immunity is attributed to the massive apoptosis of these cells, a severe depression in important cytokines in the immune response [4, 5], and an increase in immune-suppressive cytokines such as IL-10 [6]. Cell loss during sepsis creates a lymphopenic environment that must be filled to replenish the immune response and restore the system to homeostasis. Although many studies have reported the loss of function early in the septic response [6,7,8,9,10], few studies have explored the mechanism by which immune cells are returned to normal function in recovering septic individuals. This is significant, as the amount of T cell loss from peripheral lymphoid organs by apoptosis can play a significant role in patient survival [3].
During recovery from lymphopenia, the thymic pathway is commonly compromised, and T cells rely on peripheral expansion to restore their numbers. This process is called lymphopenia-induced homeostatic proliferation (HP) and can be driven by cytokines or specific antigen. HP can favor certain T cell clones, resulting in a skewed repertoire and the loss of repertoire diversity [11, 12], predisposing the individual to autoimmunity [11] or alloimmunity [13]. HP has been studied in naïve and memory CD4+ and CD8+ T cells, where it has been observed that transfer of naïve T cells into a lymphocyte-deficient environment initiates spontaneous proliferation. Although the process is not understood fully, a number of factors have been shown to be important. First, HP in naïve T cells is driven by recognition of self-peptide/MHC complexes [14, 15] presented by DC [16]. There does not seem to be this requirement for HP in memory cells [17]. Second, naïve and memory CD8+ T cells do not compete with each other for survival, and CD4+ T cells will compete at the clonal level if they are of similar specificity [18, 19]. Third, the cytokines IL-7 and IL-15 are important driving forces for HP, with CD8+ T cells responding to IL-7 or IL-15 [20] and CD4+ T cells dependent on IL-7 [21]. In fact, IL-15 appears to be important for the expansion of memory CD8+ T cells [22]. Fourth, presentation of cognate antigen during lymphopenic conditions increases the response of individual clones [13, 23]. This has been observed in burn-induced lymphopenia with an increased production of alloantigen-specific CD8+ T cells that lead to increased rejection of allografts [13]. Finally, T cells that arise from HP without participation of cognate antigen express memory markers but not activation markers [11, 24, 25].
We showed recently that sepsis does not significantly activate or induce proliferation of naïve T cells for the first 3 days following cecal ligation and puncture (CLP). This is not unexpected, given that naïve T cells must engage their antigen receptor via antigen/MHC presentation to proliferate and become activated. However, a significant loss of T cell numbers and the creation of space as a result of apoptosis might create the necessity for the T cell compartment to be subject to nonantigen-induced HP mechanisms. This would ensure repopulation of T cell numbers, thereby restoring the repertoire to normal levels. As HP can result in the generation of memory and effector T cells, the possibility exists that this process could lead to the generation of self-reactive clones that mediate autoimmunity or to primed memory cells that would be protective upon rechallenge with the same organisms. However, a connection between sepsis and autoimmunity has not been reported, and immunological memory in septic individuals has not been examined. The purpose of the present study was to determine if T cell HP occurs during or following sepsis and to evaluate potential mechanisms. Our data show that CD4+ and CD8+ T cells use different mechanisms to repopulate their respective compartments. Naïve CD8+ T cells undergo HP becoming memory cells, and naïve CD4+ T cells fail to undergo HP. There are, however, high numbers of activated CD4+ T cells when the T cell compartment has recovered, indicating that other non-HP mechanisms are responsible for CD4+ T cell recovery. Interestingly, T cells from recovered septic mice are not protective in sepsis and display a normal TCR repertoire. In addition, CD8+ T cells control naive CD4+ T cell HP, and CD4+ T cells perform no such function. We conclude that HP following sepsis is under strict control to restore the normal T cell repertoire.
MATERIALS AND METHODS
Mice
C57BL/6 (CD45.2+, Stock #000664), C57BL/6 (CD45.1+, Stock #002014), OT-I, and OT-II mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Mice weighing 18–28 g were housed for at least 1 week before manipulations. Male or female mice were age- and sex-matched for all experiments. All animal procedures were performed according to National Institutes of Health guidelines and approved by the Washington University Institutional Animal Care and Use Committee (St. Louis, MO, USA). Experimental groups consisted of at least five mice, and each experiment was repeated a minimum of two times.
Antibodies and reagents
Unless otherwise indicated, all antibodies were purchased from BD PharMingen (San Diego, CA, USA) or eBioscience (San Diego, CA, USA). CFSE was purchased from Molecular Probes/Invitrogen (Carlsbad, CA, USA). The OVA323–339 peptide was synthesized by Sigma Genosys (Dallas, TX, USA). Magnetic bead-based T cell isolation kits were purchased from Miltenyi Co. (Auburn, CA, USA). The negative-selection mouse T cell enrichment kits were purchased from Stem Cell Technologies (Canada; Cat. #19752A for CD4+ T cells and Cat. #19753A for CD8+ T cells). Recombinant mouse (rm)IL-7 was purchased from R&D Systems (Minneapolis, MN, USA; Cat. #407-ML/CF). Dr. Thomas S. Griffith (University of Iowa, Iowa City, IA, USA) provided the CD4 T cell-depleting antibody GK1.5 and the CD8 T cell-depleting antibody (mAb 2.43). The mouse TCR Vβ screening panel kit was purchased from BD PharMingen (San Diego, CA, USA). Collagenase Type II was purchased from Worthington (Lakewood, NJ, USA; Cat. #LS004176). DNase was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
Sepsis model—CLP
The CLP model was used to induce intra-abdominal peritonitis as described previously [26, 27]. Mice were anesthetized with isoflurane, and an abdominal incision was performed. The cecum was identified, ligated, and punctured twice with a #27-gauge needle. The abdomen was closed in two layers, and 1 ml 0.9% saline was administered s.c. Sham-operated mice were treated identically, except the cecum was not ligated or punctured. Spleens were harvested at different time-points postsurgery as indicated.
Irradiation of mice
For the induction of lymphopenia, C57BL/6 mice received 600 R irradiation in a γ-cell 40 irradiator. Mice received 1 × 106 CFSE-labeled T cells 24 h later.
Quantification of spleen cell numbers
Mice were killed and spleens harvested at various time-points after CLP or sham surgery. Isolated cells were prepared by gently pressing the organ between frosted glass slides. After spinning, the pellet is resuspended in 3 ml RPMI 10% FCS + 500 ng/ml collagenase type 2 and 20 pg/ml DNase and incubated at 37°C for 45 min on an orbital shaker. Samples were vortexed every 15 min. EDTA (40 μl 0.5 M) was added, and samples were shaken for an additional 10 min. Cells were washed and red cells lysed. After washing, cells were resuspended in 1 ml FACS buffer. The total splenic cell count was determined by counting aliquots using the Vi-Cell cell counter (Beckman Coulter, Fullerton, CA, USA; done in duplicate). The percentage of each cell type in the spleen was identified using fluorescently labeled mAb directed against their respective CD surface markers: Flow cytometric analysis (50,000–150,000 events/sample) was performed on FACScan (Becton Dickinson, San Jose, CA, USA). Total cell counts were determined using the following formula: Subpopulation cell counts = total cells recovered × percentage of cells in subpopulation/100.
In vivo HP
Purified OT-I and OT-II T cells and wild-type (wt) CD4+ or CD8+ T cells were suspended at 1 × 107 cells/ml in warmed PBS + 0.1% BSA. CFSE (1.25 μl/ml of a 5-mM stock) was added, and the cells were incubated for 10 min in a 37°C water bath. The reaction was stopped by the addition of ice-cold PBS containing 10% FBS. Cells were washed 3× with PBS/FBS, counted, and resuspended in saline to the appropriate volume. One million CFSE-labeled cells were injected i.v. into recipient C57BL/6 mice that had undergone CLP 7 days earlier. T cell proliferation was assessed 14 days later by determining CFSE content (3–5 million events per sample) in the T cell population.
Adoptive transfer of splenocytes and serum into irradiated recipients
C57BL/6 mice received sham or CLP surgery. At Day 7 postsurgery, these mice were used as donors for the adoptive transfer of whole spleen cells and serum. Mice underwent anesthesia using isoflurane, and blood was drawn via cardiac puncture using a 25-gauge needle. Blood was allowed to clot for 30 min at room temperature. After spinning for 20 min at 2000 g, serum was removed carefully. Serum (600 μl) was injected i.v. into recipient mice that received irradiation 1 day before adoptive transfer. Donor splenocytes were prepared from CLP mice by gently grinding the organ through a 70-μm cell strainer. Cells were washed and red cells lysed. After washing, the cell pellet was resuspended in 0.9% saline. Cells were counted using the Vi-Cell (Beckman Coulter). Splenocytes (5×106 or 5×107) were adoptively transferred i.v into irradiated recipient animals. At the same time, irradiated recipient animals also received 1 × 106 naïve, CFSE-labeled CD4+ T cells i.v. Proliferation of CD4+ T cells was analyzed 7 days postadoptive transfer.
In vivo IL-7 treatment
C57BL/6 mice underwent sham or CLP surgery. Seven days postsurgery, mice received 1 × 106 CFSE-labeled, naïve CD4+ T cells i.v. Each mouse received 5 μg recombinant human (rh)IL-7 via i.p. injection every other day for a total of 14 days. Mice were then killed, and proliferation of CFSE-labeled CD4+ T cells was determined by flow cytometry.
Peptide stimulation of CD4+ T cells
C57BL/6 mice underwent sham or CLP surgery. At Day 7 postsurgery, animals were given 1 × 106 CFSE-labeled, naïve OT-II CD4+ T cells i.v. Twenty-four hours later, mice received 100 μg Ova peptide (OVA323–339) i.v., and the proliferation of OT-II CD4+ T cells was evaluated 3 days later.
CD4+ and CD8+ T cell depletion
Depletion of CD8+ T cells was achieved by three daily 100 μg doses of anti-CD8 (αCD8; mAb 2.43), beginning 3 days prior to infusion of CFSE-labeled CD4+ T cells. Mice then received two maintenance doses per week for the duration of the experiment. To deplete CD4+ T cells, mice received three daily i.v. doses of 100 μg GK1.5 on Days 1–3 postsurgery. Seven days postsurgery, 1 × 106 CFSE-labeled OT-2 CD4+ T cells were adoptively transferred i.v. On Day 21, postsham or CLP mice were killed, and OT-2 CD4+ T cell proliferation was determined by CFSE content.
Statistical analysis
Data are reported as the mean ± sem. Data were analyzed using the statistical software program Prism (GraphPad Software, San Diego, CA, USA). Data involving two groups only were analyzed by a Student’s t-test, and data involving more than two groups were analyzed using one-way ANOVA with Tukey’s multiple comparison test. Significance was accepted at P< 0.05.
RESULTS
Loss and gain of T cells during sepsis
The loss of CD4+ and CD8+ T cells in the spleen and thymus by apoptosis during the first week following CLP is well established [26, 27]. However, it is unclear when the lymphoid organs recover their preseptic T cell numbers in surviving mice. Consequently, mice were subjected to CLP, and absolute numbers of T cells in the spleen and thymus were monitored for 56 days. Figure 1A shows that splenic CD4+ T cells were reduced by 50% on Day 1 and 65% by Day 5 following CLP. Similarly CD8+ T cells were reduced by 40% on Day 1 and 50% by Day 5. Both populations remained reduced significantly for the first 2–3 weeks and by Day 21, had returned to near-normal numbers. When examined 56 days post-CLP, CD4+ and CD8+ T cells were at their normal, presepsis numbers. In the thymus (Fig. 1B), all major T cell populations were depleted severely by Day 5 including DN (CD4–CD8–), DP (CD4+CD8+), and SP (CD4+ and CD8+) T cells. All populations recovered by 25 days post-CLP. Thus, central and peripheral lymphoid organs show parallel depletion and recovery during sepsis.
Fig. 1.
T cell depletion in the spleen and thymus. Mice underwent a sham operation or CLP. At various times postsurgery, spleen and thymus were harvested and the absolute number of T cell subsets enumerated. (A) CD4+ and CD8+ splenic T cells; (B) Double-positive (DP; CD4+CD8+ T cells), single-positive (SP; CD4+ or CD8+ T cells), and double-negative (DN; CD4–CD8– T cells) thymic T cells. Values are expressed as total counts per spleen or thymus. Each value represents the average of three to five mice. Significance: ***, P < 0.001; **, P < 0.01; *, P < 0.05.
CD8+ T cells but not CD4+ T cells undergo HP during recovery from sepsis
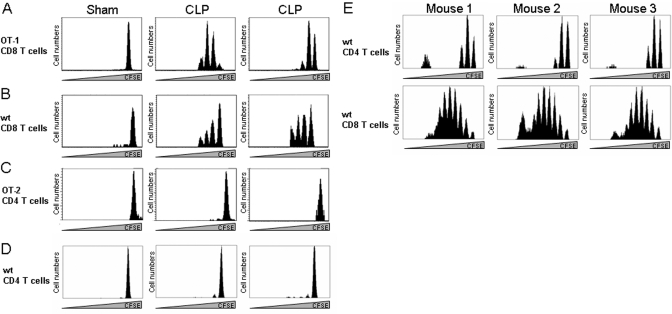
The loss of T cells in the spleen creates significant “space” in the T cell pool, suggesting conditions for HP might exist within the first 2–3 weeks following CLP. Therefore, we examined HP by the standard adoptive transfer technique using naïve T cells labeled with CFSE. Initially, we used OVA-reactive TCR transgenic T cells by transferring 106 CFSE-labeled OT-I (CD8+) or OT-II (CD4+) T cells to septic mice 7 days following surgery. Two weeks later (Day 21 post-CLP), we examined proliferation by determining CFSE content in these cells. Figure 2A shows that naïve CD8+ OT-I T cells fail to proliferate in nonlymphopenic, sham-operated mice but underwent proliferation in septic animals. In contrast, OT-II CD4+ T cells did not show proliferation in sham or CLP animals (Fig. 2C). To rule out the possibility that this might be peculiar to TCR-transgenic T cells, we examined HP of naïve CD4+ and CD8+ T cells. Figure 2D shows that naïve, nontransgenic CD4+ T cells also did not undergo HP in septic mice, and naïve CD8+ T cells underwent homeostatic division (Fig. 2B). Both populations of naïve T cells (CD4+ and CD8+) were capable of HP in irradiated mice (Fig. 2E), a standard model for measuring T cell HP [19], demonstrating that our naïve T cells were functional.
Fig. 2.
HP of CD4+ and CD8+ T cells during CLP. Recipient mice underwent a sham operation or CLP. Seven days later, mice received 1 × 106 CFSE-labeled: (A) OT-I CD8+ T cells; (B) naïve (wt) CD8+ T cells; (C) OT-II CD4+ T cells; (D) naïve CD4+ T cells. On Day 21 postsurgery, spleens were harvested, and CFSE content was assessed by flow cytometry. Data shown represent one of three sham mice and two of five CLP animals examined. (E) Mice received 600 R γ-irradiation, and 1 day later, they were infused with 1 × 106 CFSE-labeled, naïve CD4+ or CD8+ T cells. CFSE content in splenic cells was assessed 7 days later by flow cytometry. Three of five representative mice are shown for CD4+ and CD8+ T cells.
Naïve CD4+ T cells are responsive to IL-7 and cognate antigen in septic mice
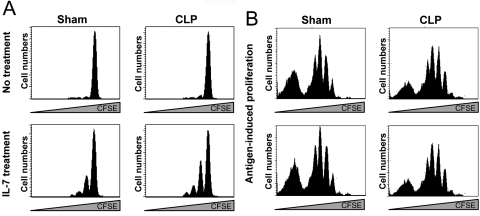
We considered the idea that the lack of HP of naïve CD4+ T cells might be because they were unable to respond to stimuli in the postseptic environment such as IL-7 or cognate antigen. We explored these possibilities with the experiments in Figure 3. First, naïve CD4+ T cells were labeled with CFSE and transferred to recipient mice that underwent CLP 7 days earlier. The mice were given rIL-7 beginning on Day 7 and every other day until Day 21 when spleens were harvested to examine HP by CFSE content. Figure 3A shows that CD4+ T cells from mice given IL-7 underwent several rounds of proliferation compared with untreated mice, which did not proliferate.
Fig. 3.
Proliferation of naïve CD4+ T cells to IL-7 and cognate antigen. (A) Mice underwent a sham operation or CLP. Seven days later, they were given 1 × 106 naïve, CFSE-labeled CD4+ T cells. Each mouse received 5 μg rIL-7 via i.p. injection every other day for a total of 14 days. Mice were then killed on Day 21 postsurgery and proliferation determined by CFSE content. (B) Mice underwent a sham operation or CLP. Seven days later, they were given 1 × 106 naïve, CFSE-labeled OT-II T cells. Twenty-four hours later, mice received 100 μg Ova peptide (OVA323–339) i.v., and the proliferation of OT-II CD4+ T cells was evaluated 3 days later. Two of five representative mice are shown for sham and CLP.
To examine antigen-induced proliferation, we adoptively transferred CFSE-labeled OT-II CD4+ T cells to mice that had been septic for 7 days. We then administered specific peptide (OVA323–339) to these animals and assessed proliferation 3 days later. We observed that OT-II CD4+ T cells underwent multiple rounds of proliferation in sham and CLP mice. We conclude from the data in Figure 3 that T cells placed into septic mice can respond to signals such as IL-7 and cognate antigen.
Differential activation and memory cell development following sepsis for CD4+ and CD8+ T cells
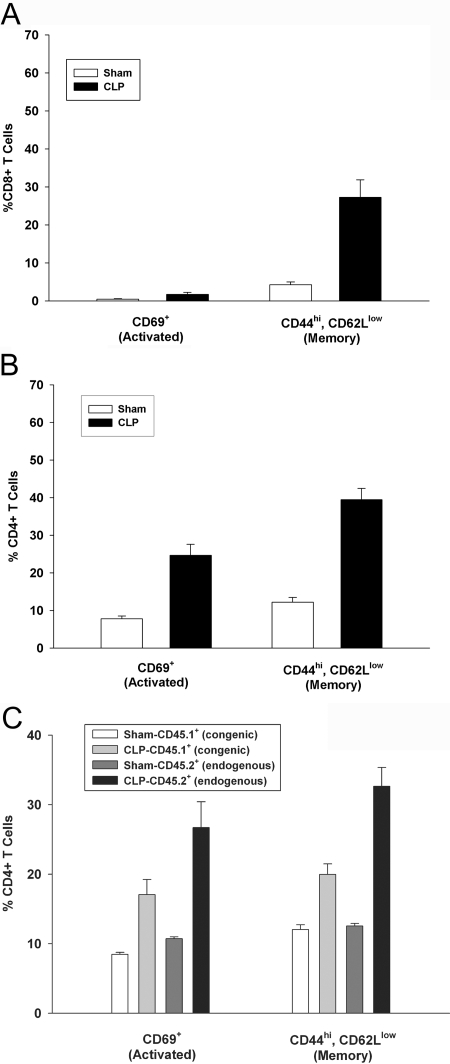
One hallmark of HP in many systems is that the proliferation is not driven by cognate antigen but results in the generation of a significant number of cells with a memory phenotype but lacking activation markers such as CD69 [11, 17, 24, 25]. This was indeed the case for CD8+ T cells as shown in Figure 4A. When analyzed on Day 21 post-CLP, 27.3% of CD8+ T cells from septic mice were CD44hi, CD62Llow compared with only 4.3% in sham-operated animals (Fig. 4A), indicating significant memory. However, only 1.75% of CD8+ T cells recovered at Day 21 expressed the activation marker CD69, compared with 0.5% in sham animals. This is consistent with what has been found in other systems, where cells undergoing HP acquire a memory phenotype (CD44hi, CD62Llow) more readily than they do activation markers [24, 25].
Fig. 4.
Activated and memory cells following CLP. Mice underwent a sham operation or CLP. Twenty-one days later, spleens were harvested and analyzed by flow cytometry for activation (CD69+) and memory (CD44hiCD62Llow) T cells in the (A) CD8+ T cell population and (B) CD4+ T cell population. (C) CD45.2-expressing C57BL/6 mice underwent a sham operation or CLP. At Day 7 post-CLP, mice received 2 × 106 naïve, congenic (CD45.1-expressing), CFSE-labeled CD4+ T cells. Twenty-one days postsurgery, spleens were harvested, and the percentage of activation and memory endogenous (CD45.2+) and transferred (CD45.1+) CD4+ T cells was evaluated by flow cytometry. Each value represents the average of eight to 10 mice over two separate experiments.
When CD4+ T cells were examined 21 days post-CLP, 39% were CD44hi, CD62Llow (memory) compared with only 12.2% in sham animals (Fig. 4B). In addition, 24.7% of the CD44hi, CD62Llow expressed the activation marker CD69 compared with 12.2% in sham animal. As naïve CD4+ T cells do not undergo HP (see Fig. 2), this suggests that CD4+ T cells may have arisen from the endogenous pool (nonadoptively transferred). We tested this possibility by performing the experiment in Figure 4C. Naïve CD4+ T cells were purified from CD45.1+ congenic C57BL/6 mice and labeled with CFSE. These cells were then transferred to CD45.2+ C57BL/6 mice 7 days post-CLP. On Day 21, memory and activation were assessed. We observed a significant increase in activated (CD69+) and memory (CD44hi, CD62Llow) markers in CD4+ T cells derived from the endogenous T cell pool (CLP CD45.2+ endogenous) compared with the transferred T cells (CLP-CD45.1+ congenic). We conclude that the majority of activated and memory CD4+ T cells arises from endogenous sources.
Lack of TCR Vβ repertoire skewing and immunologic memory in recovered mice
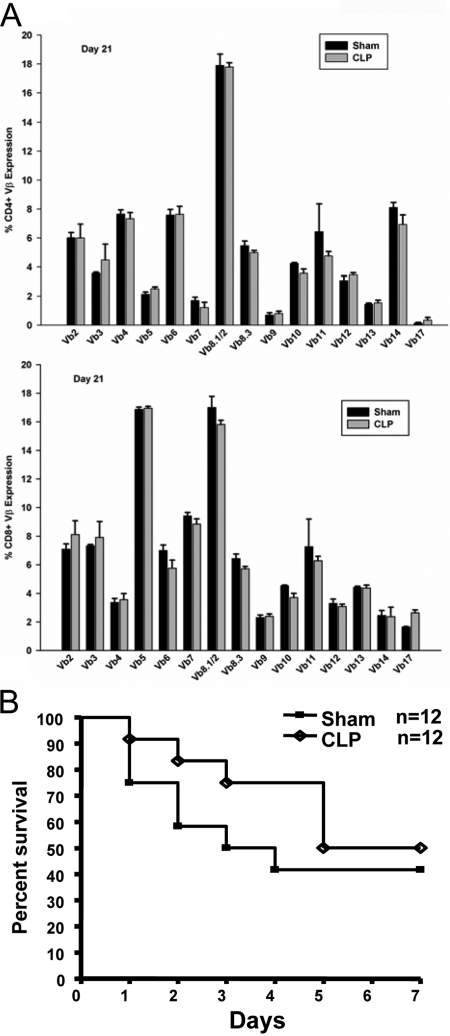
If the accumulation of activated and memory CD4+ T cells was driven by encounter with endogenous antigens, one might expect that individual T cells clones would be responding and that the T cell repertoire might skew toward specific TCR Vβ types. This would explain our inability to detect HP in naïve cells, as fewer clones would be responding. In fact, it has been observed that HP in other systems can skew the TCR repertoire toward certain Vβ TCR epitopes [11, 12, 23]. This can occur without the presence of known exogenous antigen [18, 28] and does not involve normal host bacterial flora [29]. In fact, HP can result in autoimmunity [11] and disease [30]. As CLP results in large numbers of intestinal bacteria being spilled into the blood, there would be ample opportunity for bacterial antigens and superantigens to be presented to T cells. Thus, it is possible that this interaction might lead to increases in some Vβ subtypes in the T cell repertoire. Consequently, we examined the TCR Vβ expression on Day 21 when T cells numbers were at normal levels. Surprisingly, we detected no skewing of the repertoire toward any particular Vβ type. In fact, it is difficult to tell sham from CLP animals by this analysis (Fig. 5A). We also observed a lack of TCR skewing at 7 days (data not shown), suggesting that earlier effects on CD4+ T cells may not be antigen- or superantigen-driven. This is consistent with our previous studies showing that sepsis does not stimulate T cells to proliferation or become activated early in the disease [10].
Fig. 5.
Vβ analysis and immunologic memory following recovery from sepsis. (A) On Day 21 following a sham or CLP operation, spleens were harvested, and the expression of Vβ TCR epitopes was assessed by flow cytometry. Each value represents the average of five mice. (B) Mice received sham or CLP surgery. At Day 21 postsurgery, these mice were used as donors for the adoptive transfer of 50 × 106 whole spleen cells to naïve mice. Recipient mice then underwent sham or CLP surgery 24 h later. Survival was monitored for 7 days. Each group consisted of 12 mice.
Although the TCR repertoire was identical in sham and CLP animals, we did detect the presence of memory CD8+ T cells and memory/activated CD4+ T cells in mice recovering from septic insult. These cells could have arisen from rapid, antigen-induced proliferation of a small number of clones occurring over a few days rather than the 14 days used to examine HP. Therefore, it is possible that CFSE detection and Vβ analysis might miss these rare cells. Therefore, we attempted to detect primed clones by determining if they could provide protection to naïve mice undergoing sepsis. T cells were recovered from mice 21 days post-CLP (when activated and memory cells were detected) and adoptively transferred them to naïve C57BL/6 mice 24 h prior to CLP surgery. We then monitored survival for 1 week. As shown in Figure 5B, T cells from 21-day CLP offered no survival advantage over cells from sham-operated animals. These survival curves mirror those obtained from C57BL/6 mice that do not receive a T cell transfer (data not shown). Thus, in spite of the presence of memory and activated CD4+ and CD8+ T cells, there is no T cell-mediated protection afforded to mice undergoing sepsis. We conclude that restoration of the T cell compartment involves replenishing lost T cell clones but does not result in protective, immunological memory.
Spleen cells from septic mice do not suppress HP in irradiated mice
Although we observed that naïve T cells could respond to antigen and IL-7 in the septic environment (see Fig. 3), we could not rule out other endogenous, suppressive mechanisms. Recent reports suggest that during CLP, regulatory cells can be generated that prohibit CD4+ T cell responses [9]. Although functional CD4+CD25+ T regulatory cells have been shown to influence the outcome of sepsis in some cases [31] but not others [32, 33], myeloid suppressor cells have been demonstrated [9]. In addition, inhibitory cytokines might be present in the serum of septic mice that could regulate CD4+ T cell HP [6]. Consequently, we tested for the ability of spleen cells or serum from septic mice to inhibit naïve CD4+ T cell HP in a model of radiation-induced lymphopenia. C57BL/6 mice were irradiated (600 R) and infused with serum (600 μl) or spleen cells (5×106 or 50×106) from 7-day septic mice. At the same time, mice were given CFSE-labeled, naïve CD4+ T cells. HP was assessed 7 days later by CFSE content. Figure 6 shows that neither cells nor serum from septic mice could prevent CD4+ T cell HP in irradiated mice.
Fig. 6.
Adoptive transfer of cells or serum from septic mice, which received sham or CLP surgery. At Day 7 postsurgery, these mice were used as donors for the adoptive transfer of whole spleen cells and serum, which were transferred to mice that had received 600 R irradiation and 2 × 106 naïve, CFSE-labeled CD4+ T cells 24 h earlier. Proliferation was assessed by CFSE content 7 days later. One of two sham and one of three CLP mice are shown for each group.
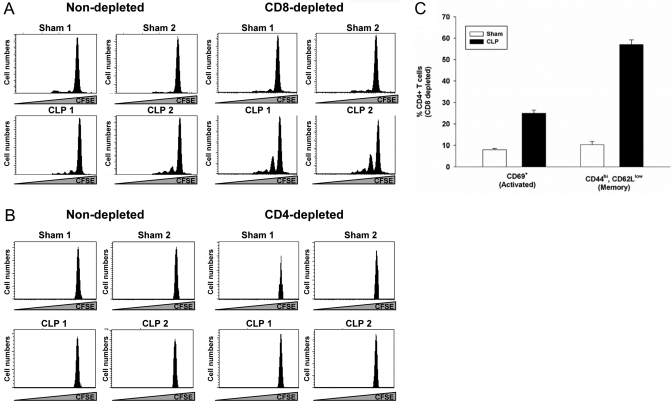
Depletion of CD8+ but not CD4+ T cells restores CD4+ T cell HP
Spleens from septic mice recovered at Days 7–10 contain large numbers of myeloid cells identified by the CD11b and Gr-1 surface markers (ref. [9], and our unpublished data). Thus, our transfers may have missed other cell populations (such as T cells), which are found in greatly reduced numbers at that time (see Fig. 1; ref. [27]). Consequently, we examined the role of T cells in regulating CD4+ HP by in vivo antibody-mediated depletion in septic mice. Mice were subjected to a sham operation or CLP and depleted of CD8+ T cells by injecting a depleting antibody. Mice were then infused with CFSE-labeled, naïve CD4+ T cells on Day 7, and CD4+ HP was examined on Day 21. Data in Figure 7A show that depletion of CD8+ T cells improved naïve CD4+ T cell HP. This effect was not seen in sham-operated animals. Depletion of CD4+ T cells prior to injection of CFSE-labeled, naïve CD4+ T cells had no effect (Fig. 7B). Interestingly, CD8 depletion (Fig. 7C) did not increase the number of activated CD69+ CD4+ T cells in septic animals (25±1.5% vs. 24.7%±2.9% in Fig. 4B), but it was effective at increasing the number of CD4+ T cells expressing a CD44high, CD62Llow memory phenotype (57+2.2% vs. 39.5+3% in Fig. 4B). Thus, the development of CD4+ T cell memory during HP is under the control of the CD8+ T cell population, and the development of activated CD4+ T cells is not.
Fig. 7.
In vivo depletion of CD4+ and CD8+ T cells. Mice underwent a sham or CLP operation. (A) Depletion of CD8+ T cells was achieved by three daily, 100 μg doses of αCD8 (mAb 2.43) beginning 3 days prior to infusion of CFSE-labeled, naïve CD4+ T cells on Day 7. (B) CD4+ T cells were depleted by three daily i.v. doses of 100 μg GK1.5 on Days 1–3 postsurgery. Seven days postsurgery, 1 × 106 CFSE-labeled, naïve CD4+ T cells were adoptively transferred i.v. HP was assessed on Day 21 by flow cytometry. (C) On Day 21 post-CLP, the numbers of activated (CD69+) and memory (CD44hiCD62low) CD4+ T cells in CD8-depleted mice were assessed by flow cytometry.
DISCUSSION
Sepsis is the host response that occurs as a result of a severe, life-threatening infection and a common cause of death in intensive care units [1, 2]. Sepsis results in the generation of an immunosuppressive environment mediated by several factors, including the loss of lymphoid and myeloid cells as a result of apoptosis [4, 8]. Apoptotic cell death presents serious problems for the immune system due to the loss of cells that mediate the innate and acquired immune responses. In addition, apoptotic cells themselves are immunosuppressive [34, 35], creating additional problems for an immune response attempting to recover after such a severe insult.
Autopsy studies of patients dying of sepsis have shown that there is often a substantial loss of splenic T and B lymphocytes [4, 36, 37]. The fact that many patients dying with sepsis have such profound lymphocyte depletion is consistent with ongoing apoptosis and/or a failure of the system to recover its lost cell numbers. It is essential that patients with sepsis reconstitute their immune effector cells if they are to eradicate their primary infection and avoid contracting secondary hospital-acquired infections. Thus, it is important to understand the mechanisms responsible for lymphocyte recovery with the idea that early intervention might prevent some of the more serious sequelae of this disease.
Our studies were undertaken to examine the reconstitution of the T cell compartment during postseptic lymphopenia. Our data show that CD4+ and CD8+ T cells return to normal numbers by Day 21 postsepsis induction; however, they use different mechanisms. Naïve CD8+ T cell recovery is under homeostatic control, as shown by the ability of these cells to undergo lymphopenia-induced HP and become memory cells without activation markers; a hallmark of HP that suggests repopulation was driven by low-affinity TCR/MHC interaction and not cognate antigen [17]. Naïve CD4+ T cells, on the other hand, do not undergo HP but appear to arise from an endogenous pool of memory T cells. Interestingly, T cells from septic mice, which had recovered their cell numbers, were not protective when transferred to other mice. In addition, these populations did not contain a disproportionate number of any TCR Vβ clonotypes, suggesting that repopulation was not driven by encounter with cognate antigen. Based on these results, we conclude that the expansion of T cells following sepsis must be under tight control to restore the T cell compartment without the development of aberrant T cell specificities. It may be that the system is geared to restore the T cell repertoire at the expense of developing acquired immunity to endogenous microorganisms. However, it is also possible that we do not observe protective immunity following CLP, as T cells are not required for the types of infections released by CLP. Thus, the role of memory T cells in fighting infections following a CLP injury will require further study.
HP studies in other systems have also revealed that CD4+ and CD8+ T cells have different requirements for reconstitution of their respective niches. CD4+ T cells compete at the clonal level such that only missing clones undergo HP to replenish the repertoire [18, 19]. CD8+ T cells have been shown to fill space regardless of the specific hole in the repertoire [17]. During sepsis, however, there is remarkable stability as the repertoire recovers. CD4+ and CD8+ T cells expand with the primary goal of replacing lost specificities, suggesting that septic insult leads to a unique set of circumstances that focus on restoring the T cell repertoire.
Recently, it was shown that the spleen of septic mice contained a high number of myeloid suppressor cells expressing the CD11b and Gr-1 markers on Days 7–10 following CLP. Although these cells can suppress certain CD4-mediated T cell functions [9], apparently, they do not regulate CD4+ T cell HP as shown by our studies. When we adoptively transferred spleens, which contain large numbers of these myeloid suppressors, they were unable to regulate HP in irradiated hosts. However, in vivo depletions of T cells in the lymphopenic environment revealed that naïve CD4+ T cell HP is under the control of CD8+ T cells. Removal of CD8+ T cells permitted CD4+ T cells to proliferate, leading to an increase in the number of CD4+ T cells with memory markers. The mechanism by which CD8+ T cells regulate the CD4+ T cell compartment is currently unknown, but understanding these issues may have important implications for understanding methods to restore T cell responses during recovery from sepsis.
Our data demonstrate that during sepsis, T cell numbers in the thymus are reduced severely but recover to normal levels in parallel with the spleen. It is established that cells exported from the thymus during lymphopenia maintain their naïve status [38], and as we observed large numbers of memory cells in mice recovering from sepsis, it is unlikely that the thymus can participate in the restoration of the T cell pool. Thymic cells are likely restored by emigrants from the bone marrow, as this organ recovers from sepsis, and peripheral T cells return to normal levels from the residual peripheral T cell pool of naïve CD8+ T cells and memory CD4+ T cells.
Studies about CD4+ T cell HP during sepsis also revealed two additional insights. First, naïve T cells are capable of responding to IL-7, the major cytokine driving T cell expansion during lymphopenia. Thus, one mechanism for the lack of CD4+ T cell HP during sepsis may be the lack of available IL-7. We also found that naïve T cells are capable of responding to cognate antigen during lymphopenic conditions created following sepsis, suggesting that the addition of naive cells at this time did not reveal any additional, suppressive mechanisms directed toward these cells. Understanding the mechanisms of T cell recovery following septic insult will help in the design of rational interventions that could accelerate reconstitution of the immune system and reduce the number of deaths in intensive care units.
Acknowledgments
This work was supported by National Institutes of Health grants EY06765 (T. A. F.), EY015570 (T. A. F.), EY02687 (Department of Ophthalmology and Visual Science Core grant), GM44118 (R. S. H.), and GM55194 (R. S. H.) and a Department of Ophthalmology and Visual Science grant from Research to Prevent Blindness (New York, NY, USA).
References
- Angus D C, Linde-Zwirble W T, Lidicker J, Clermont G, Carcillo J, Pinsky M R. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Murphy S L. Deaths: final data for 1998. Natl Vital Stat Rep. 2000;48:1–105. [PubMed] [Google Scholar]
- Hotchkiss R S, Swanson P E, Freeman B D, Tinsley K W, Cobb J P, Matuschak G M, Buchman T G, Karl I E. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- Hotchkiss R S, Karl I E. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- Hotchkiss R S, Tinsley K W, Swanson P E, Grayson M H, Osborne D F, Wagner T H, Cobb J P, Coopersmith C, Karl I E. Depletion of dendritic cells, but not macrophages, in patients with sepsis. J Immunol. 2002;168:2493–2500. doi: 10.4049/jimmunol.168.5.2493. [DOI] [PubMed] [Google Scholar]
- Ayala A, Chung C S, Song G Y, Chaudry I H. IL-10 mediation of activation-induced TH1 cell apoptosis and lymphoid dysfunction in polymicrobial sepsis. Cytokine. 2001;14:37–48. doi: 10.1006/cyto.2001.0848. [DOI] [PubMed] [Google Scholar]
- Kell M R, Kavanaugh E G, Goebel A, Soberg C C, Lederer J A. Injury primes the immune system for an enhanced and lethal T-cell response against bacterial superantigen. Shock. 1999;12:139–144. doi: 10.1097/00024382-199908000-00008. [DOI] [PubMed] [Google Scholar]
- Wesche D E, Lomas-Neira J L, Perl M, Chung C S, Ayala A. Leukocyte apoptosis and its significance in sepsis and shock. J Leukoc Biol. 2005;78:325–337. doi: 10.1189/jlb.0105017. [DOI] [PubMed] [Google Scholar]
- Delano M J, Scumpia P O, Weinstein J S, Coco D, Nagaraj S, Kelly-Scumpia K M, O'Malley K A, Wynn J L, Antonenko S, Al-Quran S Z, Swan R, Chung C S, Atkinson M A, Ramphal R, Gabrilovich D I, Reeves W H, Ayala A, Phillips J, Laface D, Heyworth P G, Clare-Salzler M, Moldawer L L. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204:1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsinger J, Herndon J M, Davis C G, Muenzer J T, Hotchkiss R S, Ferguson T A. The role of TCR engagement and activation-induced cell death in sepsis-induced T cell apoptosis. J Immunol. 2006;177:7968–7973. doi: 10.4049/jimmunol.177.11.7968. [DOI] [PubMed] [Google Scholar]
- Marleau A M, Sarvetnick N. T cell homeostasis in tolerance and immunity. J Leukoc Biol. 2005;78:575–584. doi: 10.1189/jlb.0105050. [DOI] [PubMed] [Google Scholar]
- La Gruta N L, Driel I R, Gleeson P A. Peripheral T cell expansion in lymphopenic mice results in a restricted T cell repertoire. Eur J Immunol. 2000;30:3380–3386. doi: 10.1002/1521-4141(2000012)30:12<3380::AID-IMMU3380>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Maile R, Barnes C M, Nielsen A I, Meyer A A, Frelinger J A, Cairns B A. Lymphopenia-induced homeostatic proliferation of CD8+ T cells is a mechanism for effective allogeneic skin graft rejection following burn injury. J Immunol. 2006;176:6717–6726. doi: 10.4049/jimmunol.176.11.6717. [DOI] [PubMed] [Google Scholar]
- Martin B, Bourgeois C, Dautigny N, Lucas B. On the role of MHC class II molecules in the survival and lymphopenia-induced proliferation of peripheral CD4+ T cells. Proc Natl Acad Sci USA. 2003;100:6021–6026. doi: 10.1073/pnas.1037754100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Becourt C, Bienvenu B, Lucas B. Self-recognition is crucial for maintaining the peripheral CD4+ T-cell pool in a nonlymphopenic environment. Blood. 2006;108:270–277. doi: 10.1182/blood-2006-01-0017. [DOI] [PubMed] [Google Scholar]
- Zaft T, Sapoznikov A, Krauthgamer R, Littman D R, Jung S. CD11chigh dendritic cell ablation impairs lymphopenia-driven proliferation of naive and memory CD8+ T cells. J Immunol. 2005;175:6428–6435. doi: 10.4049/jimmunol.175.10.6428. [DOI] [PubMed] [Google Scholar]
- Jameson S C. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. 2002;2:547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- Moses C T, Thorstenson K M, Jameson S C, Khoruts A. Competition for self ligands restrains homeostatic proliferation of naive CD4 T cells. Proc Natl Acad Sci USA. 2003;100:1185–1190. doi: 10.1073/pnas.0334572100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy A E, Shen H. Cutting edge: homeostatic proliferation of peripheral T lymphocytes is regulated by clonal competition. J Immunol. 2003;170:672–676. doi: 10.4049/jimmunol.170.2.672. [DOI] [PubMed] [Google Scholar]
- Schluns K S, Kieper W C, Jameson S C, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol. 2003;4:680–686. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- Sandau M M, Winstead C J, Jameson S C. IL-15 is required for sustained lymphopenia-driven proliferation and accumulation of CD8 T cells. J Immunol. 2007;179:120–125. doi: 10.4049/jimmunol.179.1.120. [DOI] [PubMed] [Google Scholar]
- Stockinger B, Kassiotis G, Bourgeois C. Homeostasis and T cell regulation. Curr Opin Immunol. 2004;16:775–779. doi: 10.1016/j.coi.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Goldrath A W, Bevan M J. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 1999;11:183–190. doi: 10.1016/s1074-7613(00)80093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehen S, Brduscha-Riem K. Naive cytotoxic T lymphocytes spontaneously acquire effector function in lymphocytopenic recipients: a pitfall for T cell memory studies? Eur J Immunol. 1999;29:608–614. doi: 10.1002/(SICI)1521-4141(199902)29:02<608::AID-IMMU608>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Hotchkiss R S, Swanson P E, Knudson C M, Chang K C, Cobb J P, Osborne D F, Zollner K M, Buchman T G, Korsmeyer S J, Karl I E. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J Immunol. 1999;162:4148–4156. [PubMed] [Google Scholar]
- Hotchkiss R S, Tinsley K W, Swanson P E, Chang K C, Cobb J P, Buchman T G, Korsmeyer S J, Karl I E. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc Natl Acad Sci USA. 1999;96:14541–14546. doi: 10.1073/pnas.96.25.14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B, Yamane H, Hu-Li J, Paul W E. Spontaneous and homeostatic proliferation of CD4 T cells are regulated by different mechanisms. J Immunol. 2005;174:6039–6044. doi: 10.4049/jimmunol.174.10.6039. [DOI] [PubMed] [Google Scholar]
- Min B, Thornton A, Caucheteux S M, Younes S A, Oh K, Hu-Li J, Paul W E. Gut flora antigens are not important in the maintenance of regulatory T cell heterogeneity and homeostasis. Eur J Immunol. 2007;37:1916–1923. doi: 10.1002/eji.200737236. [DOI] [PubMed] [Google Scholar]
- Milner J D, Ward J M, Keane-Myers A, Paul W E. Lymphopenic mice reconstituted with limited repertoire T cells develop severe, multiorgan, Th2-associated inflammatory disease. Proc Natl Acad Sci USA. 2007;104:576–581. doi: 10.1073/pnas.0610289104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer J G, Zhang T, Zhao J, Ding C, Cramer M, Justen K L, Vonderfecht S L, Na S. Adoptive transfer of in vitro-stimulated CD4+CD25+ regulatory T cells increases bacterial clearance and improves survival in polymicrobial sepsis. J Immunol. 2005;174:7141–7146. doi: 10.4049/jimmunol.174.11.7141. [DOI] [PubMed] [Google Scholar]
- Scumpia P O, Delano M J, Kelly K M, O'Malley K A, Efron P A, McAuliffe P F, Brusko T, Ungaro R, Barker T, Wynn J L, Atkinson M A, Reeves W H, Salzler M J, Moldawer L L. Increased natural CD4+CD25+ regulatory T cells and their suppressor activity do not contribute to mortality in murine polymicrobial sepsis. J Immunol. 2006;177:7943–7949. doi: 10.4049/jimmunol.177.11.7943. [DOI] [PubMed] [Google Scholar]
- Wisnoski N, Chung C S, Chen Y, Huang X, Ayala A. The contribution of CD4+ CD25+ T-regulatory-cells to immune suppression in sepsis. Shock. 2007;27:251–257. doi: 10.1097/01.shk.0000239780.33398.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson T A, Griffith T S. Cell death and the immune response: a lesson from the privileged. J Clin Immunol. 1997;17:1–10. doi: 10.1023/a:1027343210080. [DOI] [PubMed] [Google Scholar]
- Ferguson T A, Herndon J, Elzey B, Griffith T S, Schoenberger S, Green D R. Uptake of apoptotic antigen-coupled cells by lymphoid dendritic cells and cross-priming of CD8(+) T cells produce active immune unresponsiveness. J Immunol. 2002;168:5589–5595. doi: 10.4049/jimmunol.168.11.5589. [DOI] [PubMed] [Google Scholar]
- Felmet K A, Hall M W, Clark R S, Jaffe R, Carcillo J A. Prolonged lymphopenia, lymphoid depletion, and hypoprolactinemia in children with nosocomial sepsis and multiple organ failure. J Immunol. 2005;174:3765–3772. doi: 10.4049/jimmunol.174.6.3765. [DOI] [PubMed] [Google Scholar]
- Toti P, De Felice C, Occhini R, Schuerfeld K, Stumpo M, Epistolato M C, Vatti R, Buonocore G. Spleen depletion in neonatal sepsis and chorioamnionitis. Am J Clin Pathol. 2004;122:765–771. doi: 10.1309/RV6E-9BMC-9954-A2WU. [DOI] [PubMed] [Google Scholar]
- Bourgeois C, Kassiotis G, Stockinger B. A major role for memory CD4 T cells in the control of lymphopenia-induced proliferation of naive CD4 T cells. J Immunol. 2005;174:5316–5323. doi: 10.4049/jimmunol.174.9.5316. [DOI] [PubMed] [Google Scholar]