Abstract
Engagement of the costimulatory protein ICOS activates effector/memory T cells in tissue by enhancing TCR-mediated proliferation and cytokine production. We now report that in an antigen-independent manner, ICOS also induces adhesion and spreading in human effector/memory T cells, consequently inhibiting cell migration. T cell spreading and elongation after ICOS ligation are accompanied by the formation of two types of actin-rich membrane protrusions: thin, finger-like structures similar to filopodia and short, discrete microspikes. Although filopodia/microspike formation occurs independently of the PI-3K signaling cascade, ICOS-mediated T cell elongation depends on PI-3K activity, which inhibits the accumulation of GTP-bound RhoA. Further inhibition of RhoA activation exacerbates the ICOS-mediated, elongated phenotype. We propose that in inflamed tissue, ICOS engagement by ICOS ligand on a professional or nonprofessional APC prevents the forward motility of the T cell by inhibiting RhoA-dependent uropod retraction. The resulting ICOS-induced T cell spreading and filopodia/microspike formation may promote antigen recognition by enhancing a T cell’s scanning potential of an adherent APC surface.
Keywords: signal transduction, human, Rho-GTPases, filopodia, microspikes
INTRODUCTION
Antigen-specific T lymphocyte activation, expansion, and acquisition of effector functions are critical components of an adaptive immune response. Furthermore, regulation of immunity is dependent on the ability of antigen-specific T lymphocytes to respond to invading pathogens and still maintain tolerance against self-proteins. One mechanism by which the immune system adjusts the balance between T cell activation and tolerance is the requirement for costimulatory signals to optimize T cell responses [1]. According to the currently accepted model of T cell activation, optimal T cell effector function requires not only engagement of the TCR but also a series of additional receptor–ligand interactions that contribute to T cell responsiveness by augmenting those biochemical signals that emanate from the TCR [1, 2]. Failure to receive these additional signals results in clonal anergy or apoptosis [1, 2].
The CD28 family of costimulatory receptors plays a prominent role in regulating T cell activation and peripheral tolerance by providing positive and negative modulatory signals, respectively. Although CD28 has been demonstrated to be essential for naïve T cell activation, the majority of effector and memory T cell responses is CD28-independent [3, 4]. As a result, investigators have suspected that costimulatory receptors other than CD28 may regulate immune responses in infected and inflamed tissue. One such costimulatory molecule is ICOS, a disulfide-linked homodimeric glycoprotein of 55–60 kDa that shares ∼20% sequence homology with CD28 [5]. As has been described for CD28, ICOS costimulation enhances anti-CD3-induced T cell proliferation and secretion of effector cytokines [6]. In contrast to CD28, which is constitutively expressed on naïve T lymphocytes, ICOS is not expressed until after T cell activation [6]. Similarly, unlike B7.1 and B7.2, ligands for CD28 expressed only in lymphoid tissues, the ICOS ligand (ICOSL; B7h, B7RP, B7-H2, and LICOS) is detected in lymphoid and nonlymphoid tissues [7]. The expression of ICOSL in nonlymphoid tissues, such as the brain, heart, kidney, liver, and intestine, suggests that unlike CD28, ICOS regulates the activation of antigen-experienced effector/memory T cells, which are recruited to or reside within these peripheral tissues [8,9,10]. Several in vivo studies support this hypothesis. Inhibition of B7–CD28 interactions during mucosal T cell priming in the inflamed lung prevents leukocyte activation, cytokine production, and chemokine expression, and blocking CD28 during the effector phase of an immune response has no effect [11]. Conversely, only ICOS blockade during the effector phase ameliorates lung inflammation [11], can prolong murine cardiac allograft survival [12], and inhibits experimental autoimmune encephalitis, a murine model of human multiple sclerosis [13], all supporting the role of ICOS in costimulating effector/memory T cell function.
Recently, ICOSL expression, induced on the surface of endothelial cells following exposure to inflammatory cytokines, has been proposed to mediate effector/memory but not naïve T cell transendothelial migration [14, 15]. However, evidence for this conclusion is limited to microscopic images of T cell/endothelial cell cocultures or the adherence of T cells to ICOSL-coated plates, in which distinct morphological modifications were induced. Although these results indicate that ICOS is capable of mediating antigen-independent activities that regulate effector/memory T cell adhesion and cytoskeletal reorganization, in addition to its costimulatory role in T cell activation, the biological functions associated with adhesion remain to be investigated directly. It is possible in these earlier reports that the endothelial cells used may have acted as nonprofessional APCs and that ICOSL expression on them actually enhanced T cell activation and not migration [14].
Once embedded within inflamed sites in peripheral tissues, a T cell must search for its cognate peptide:MHC ligand by binding activated professional and nonprofessional APCs. Initial contact between a T cell and APC occurs in the absence of antigen or at low antigen concentrations [16]. Analysis of T cell–APC interactions at the single-cell level using time-lapse video microscopy has revealed that highly dynamic cytoskeletal and morphological modifications occur in T cells as they scan an APC’s surface [16]. Once antigen recognition occurs, additional cytoskeletal rearrangement results in the formation of the immunological synapse and is believed to promote sustained T cell signaling and the subsequent T cell commitment for differentiation and proliferation [16]. In naïve T cells, these early cytoskeletal changes appear to be regulated by intracellular signals initiated by the costimulatory receptor CD28 and accessory proteins such as CD2 and LFA-1 [16,17,18]. However, little is known about what receptors regulate the retention of effector/memory T cells at sites of inflammation and promote their activation.
ICOS contains two tyrosine residues within its cytoplasmic domain that may potentially mediate intracellular protein–protein interactions [5]. However, PI-3K is the only signaling protein known to interact with ICOS [19]. This interaction is regulated by the binding of the p85 regulatory subunit of PI-3K to the YMFM sequence located between residues 180 and 183 of the cytoplasmic tail of ICOS [6]. The activation of PI-3K following ICOS ligation is believed to be essential for the ability of ICOS to costimulate T cell activation [20]. PI-3K activation results in phosphorylation of phosphatidylinositol 4,5 bis-phosphate (PIP2) membrane lipids to form phosphatidylinositol 3,4,5 tris-phosphate (PIP3) and the subsequent recruitment of intracellular signaling proteins containing pleckstrin homology (PH) domains to the plasma membrane [21]. Among the proteins regulated by PI-3K lipid phosphorylation is Akt [21], a downstream effector molecule known to participate in stimulating protein synthesis, cell growth, cell cycle, and survival, as well as guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs) that regulate the activation of the Rho family GTPases [16, 22]. These Rho GTPases form a subgroup of the Ras superfamily of 20–30 kDa GTP-binding proteins and are associated with remodeling the actin cytoskeleton in many cell types, including T lymphocytes [16, 23]. Rac1, Cdc42, and RhoA are the most well-characterized Rho family GTPases and play distinct roles in cytoskeletal reorganization that regulates cell motility. Rac1 activation is believed to be important for lamellipodia formation at the leading edge of the cells [23], and Cdc42 triggers the formation of filopodia, slender actin-rich cytoplasmic projections extending from the leading edge of migrating cells [23], which function as sensory structures and as effectors of motility. RhoA activation controls F-actin contractility and uropod retraction [23].
We demonstrated recently that the ability of ICOS to costimulate anti-CD3-mediated effector/memory T cell proliferation and cytokine production depends on the expression of ICOS and TCR ligands on the same surface (Jennifer L. Franko, Alan D. Levine, manuscript submitted). This requirement for colocalization of TCR and ICOS signals suggests that ICOS-mediated adhesion and cytoskeletal reorientation may in fact be antimigratory and promote antigen recognition, thus enabling ICOS to carry out its costimulatory function. This is consistent with a recent report demonstrating the recruitment of PI-3K regulatory subunits to the immunological synapse following ICOS ligation [24]. To test this hypothesis, we investigated the biochemical and structural processes that contribute to the activity of ICOS, which may in turn lead to sustained TCR signaling. Our results demonstrate that ICOS-induced adhesion does not support T cell migration, as reported previously [15, 25], but instead, prevents T cell detachment through actin cytoskeletal reorganization that increases T cell elongation. In response to ICOS ligation, T cell spreading is visualized by the induction of an elongated morphology accompanied by the formation of two types of actin-rich membrane protrusions: thin, finger-like structures similar to filopodia and short, discrete microspikes. In addition, we show that ICOS-dependent T cell elongation, but not filopodia/microspike formation, is mediated by the induction of PI-3K activity that leads to a subsequent decrease in GTP-bound RhoA. Therefore, we propose that in the ICOSL-adherent T cell, RhoA functions as a molecular switch in cell phenotype. If the ICOS-mediated decline in RhoA activity is inhibited, adherent T cells become highly motile. In contrast, if the decrease in GTP-bound RhoA levels is maintained or enhanced, ICOS-adherent T cells are unable to retract their uropods and instead undergo cytosolic extension, causing them to oscillate between their poles while extending and retracting their filopodia, as if scanning an adherent surface.
MATERIALS AND METHODS
Reagents
For functional studies, anti-CD3 was purchased from Ortho Diagnostic Systems (Raritan, NJ, USA). ICOSL.Ig, B7.1.Ig, and programmed death ligand 1 (PD-L1).Ig were all purchased from R&D Systems (Minneapolis, MN, USA). HRP-conjugated goat anti-rabbit secondary antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Total and phospho-specific antibodies toward Akt, ERK1/2, p38, and JNK were all purchased from Cell Signaling (Danvers, MA, USA). The antivimentin primary antibody used for confocal microscopy was from Chemicon International (Temecula, CA, USA), and cytoskeletal components were visualized with Alexafluor-488 phalloidin and Alexafluor-568 donkey anti-human IgG from Molecular Probes (Eugene, OR, USA). The pharmacological agents used were PI-3K inhibitors LY294002 (Cell Signaling) and wortmannin (Calbiochem, San Diego, CA, USA), MEK1 inhibitor U0126 (Cell Signaling), Akt inhibitor II (SH-5), and Rock inhibitor Y27632 (Calbiochem). Toxin B and exoenzyme C3 transferase were purchased from Cytoskeleton (Denver, CO, USA).
Cells
PBMC were purified from healthy donors by Ficoll-Hypaque density gradient centrifugation (Histopaque, Sigma-Aldrich, St. Louis, MO, USA). Peripheral blood T lymphoblasts (PBT) were prepared by resuspending PBMC at a concentration of 1 × 106 cells/ml in RPMI 1640, 25 mM Hepes, and 10% FCS (all from BioWhittaker, Walkersville, MD, USA) and stimulating with 5 ng/ml IL-2 (R&D Systems) and 0.5% PHA (Invitrogen Life Technologies, Carlsbad, CA, USA) for the first 48 h of culture. Thereafter, media were changed every 48 h and supplemented with IL-2 (5 ng/ml) for >8 days in culture to obtain a population of >96% highly enriched CD3+CD45RO+ PBT lymphoblasts. The institutional review board of University Hospital Case Medical Center (Cleveland, OH, USA) approved this study.
Cell adhesion
Twenty-four-well tissue-culture plates (Corning Life Sciences, Lowell, MA, USA) were precoated overnight at 4°C with 300 μl 5 μg/ml ICOSL.Ig, PD-L1.Ig, or B7.1.Ig in the presence or absence of 2.5 μg/ml anti-CD3, diluted in borate buffer (25 mM NaBorate, 150 mM NaCl, 100 mM boric acid, pH 8.5). BSA (3%) and 0.01% poly-L-lysine (PLL; Sigma-Aldrich)-coated plates, prepared in the same manner, were used as a negative and positive control, respectively. Plates were washed three times with PBS immediately before use. PBT lymphoblasts were fluorescently labeled with 4 μM calcein-acetoxymethylester (AM; Molecular Probes) for 20 min at 37°C in a 5% CO2 humidified incubator at a concentration of 2 × 106 cells/ml in Dulbecco’s PBS (DPBS; BioWhittaker) containing 5% FCS. Calcein-labeled T cells were washed three times with DPBS and resuspended in RPMI 1640 without phenol red (BioWhittaker), supplemented with 10% FCS. Cells were then seeded onto the 24-well culture plates at 1 × 106 cells per well and incubated for 2 h. Nonadherent cells were removed by washing plates twice with DPBS to minimize background binding before adhesion was assessed by epifluorescence microscopy on an Olympus IMT-2 inverted microscope (Olympus, Lake Success, NY, USA) using Magnafire 2.0 software (Obtronics, Goleta, CA, USA).
Agonist-coated microspheres
ICOSL.Ig-coated microspheres are made by incubating 24 × 106 polystyrene, sulfate-coated beads (5 μm diameter, Interfacial Dynamics, Portland, OR, USA) overnight at room temperature, gently rocking in 1 ml 0.2 M carbonate-bicarbonate buffer, pH 9.5, and 5 μg/ml ICOSL.Ig, anti-CD3, or both. Beads are then washed three times with DPBS and resuspended in RPMI 1640, 2.5 mM Hepes, and used immediately.
Cytokine production, growth, and proliferation analysis
PBT lymphoblasts were cultured in 96-well flat-bottom plates (Costar, Cambridge, MA, USA) at a density of 200,000 cells per well and stimulated with antibody-coated beads at a 1:1 bead:cell ratio for 72 h, unless otherwise indicated. Culture supernatants were removed after 66 h for analysis of IFN-γ production using commercially available paired antibodies (BD Bioscience, San Jose, CA, USA), according to the manufacturer’s instructions. Cultures were replenished with new media and pulsed with 0.5 μCi/well [3H] thymidine (New England Nuclear, Boston, MA, USA). Following an additional 6 h incubation, T cells were harvested, and incorporated radioactivity was measured using a cell harvester and 1205 Betaplate™ counter (Wallac, Gaithersburg, MD, USA). To follow T cell growth, PBT lymphoblasts were cultured in triplicate in 12-well plates at a density of 500,000 cells per well, coated with 1 μg/ml anti-CD3 and 5 μg/ml ICOSL.Ig, alone or in combination. Every 24 h for 5 days, the cells are harvested by a brief treatment with 0.05% trypsin in 0.53 mM EDTA (Invitrogen Life Technologies), stained with trypan blue, and counted with a hemacytometer.
TCR activation and immunoblotting
Following stimulation of effector/memory T lymphoblasts with ICOSL-Ig-coated microspheres at a 3:1 bead:cell ratio for the indicated times, cells were lysed in an equal volume of 2× Laemmli buffer plus 2-ME and boiled for 10 min. Proteins were resolved by SDS-PAGE on a 10% gel under reducing conditions and then transferred to nitrocellulose membranes (Invitrogen Life Technologies) in a transfer buffer containing 20 mM Tris-HCl, 150 mM glycine, and 20% methanol. Membranes were incubated for 1 h at room temperature in blocking buffer [5% nonfat milk, 0.1% Tween 20 (Sigma-Aldrich) in PBS], followed by an additional hour incubation with primary antibodies diluted in blocking buffer, according to the manufacturer’s instructions. After washing, the bound, primary antibodies were detected by incubating the membrane for 1 h at room temperature with a secondary goat anti-rabbit HRP-conjugated antibody and visualized using Supersignal (Pierce, Rockford, IL, USA).
Confocal microscopy
Coverslips (Corning Life Sciences) were placed into six-well tissue-culture plates (Falcon, Franklin Lakes, NJ, USA) and coated with ICOSL.Ig, B7.1.Ig, or PD-L1.Ig by incubating with 300 μl protein at a concentration of 5 μg/ml overnight at 4°C. After washing the coverslips with DPBS, 750,000 T lymphoblasts in RPMI 1640 with 5% FCS were adhered to the coverslips for 2 h, then fixed with 4% formaldehyde, permeabilized in 0.1% Triton X-100, and blocked with 3% BSA in DPBS for 45 min. Primary antibodies against vimentin were diluted 1:2000 into 3% BSA in DPBS and incubated with the coverslips for 1 h. The wells were washed three times in DPBS and then incubated for an additional hour with Alexafluor-488 phalloidin or Alexafluor-568 donkey anti-goat IgG (Molecular Probes). Cells were observed using confocal-scanning microscopy (LSM510 META, Carl Zeiss, Jena, Germany) using a 100× oil immersion lens. Images were recorded on disk and analyzed using LSM Image Browser 4.2 (Carl Zeiss).
Video microscopy
For video microscopy, 35 mm glass-bottom microwell dishes (MatTek Corp., Ashland, MA, USA) were coated at 4°C overnight with 300 μl ICOSL.Ig (5 μg/ml), diluted in borate buffer. T cells/dish (7.5×105) were incubated for 2 h at 37°C, and nonadherent cells were removed by washing twice with DPBS. Images (350) were recorded at three locations in the well at 15 s intervals using a Leica DMI 3000 B widefield microscope (Wetzar, Germany) with a humidified, environmental chamber supported with 5% CO2 and 37°C. The digitized video files were saved in Quicktime format (Apple, Cupertino, CA, USA), and cell mobility and oscillations were calculated frame-by-frame using Metamorph software (Molecular Devices, Sunnyvale, CA, USA).
Rho GTPase activity assay
RhoA GTPase activity was measured using appropriate colorimetric activation gold-labeled immunosorbent assay (G-LISA; Cytoskeleton), performed according to the manufacturer’s instructions. Levels of luminescence were detected using a VERSAmax microplate reader (Molecular Devices).
Statistical analysis
Where indicated, statistical analysis was performed using a two-tailed t-test with type I error of 0.05. A P value ≤0.05 is considered to be statistically significant.
RESULTS
ICOS engagement enhances T cell adhesion
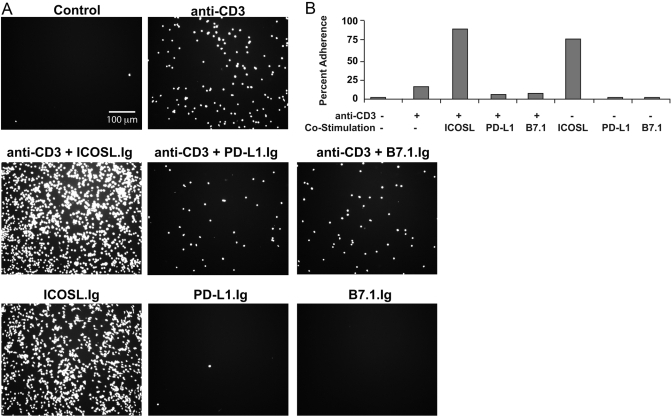
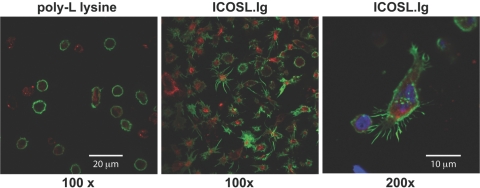
To investigate if the dual functions of ICOS, as a costimulator and an adhesion molecule, may cooperate to regulate T cell activation, we investigated the antigen-independent ability of ICOS to mediate T cell adhesion, an important first step in T cell retention in inflamed tissue as well as antigenic peptide:MHC ligand recognition. As a result of the proposed role of ICOS in regulating effector/memory T cell responses, we focused specifically on this T cell subset. Calcein-AM-labeled effector/memory PBT lymphoblasts were adhered to uncoated plates and plates precoated with ICOSL.Ig, PD-L1.Ig, or B7.1.Ig in the presence or absence of anti-CD3. Following a 2-h incubation, wells were washed to remove nonadherent cells, and adherence was visualized using epifluorescence microscopy. T cell adhesion to anti-CD3 alone was minimal, increasing slightly above levels observed for nonstimulated, control cells (Fig. 1A). However, T cells incubated on plates coated with ICOSL-Ig showed a dramatic, sixfold increase in adhesion (Fig. 1B). Cells incubated in PD-L1.Ig- or B7.1.Ig-coated wells adhered at negligible levels comparable with those of anti-CD3 alone, demonstrating that the adhesion was specific for ICOS ligation and not a result of nonspecific binding of the Fc portion of the chimeric protein to FcRs on the surface of T cells. Interestingly, no increase in adhesion was observed when anti-CD3 antibody was included in combination with any of these costimulatory ligands, including B7.1, the ligand for CD28, thus demonstrating that ICOS is unique among the CD28 family of costimulatory receptors in functioning as an adhesion molecule. These latter findings are consistent with an earlier report that also demonstrated that CD28–B7 interactions do not support T cell adhesion [26]. Furthermore, upon close examination, ICOS-adherent T cells spread on this substratum, assuming an elongated morphology (Fig. 1, and see Figs. 3 and 5 below).
Fig. 1.
Effector/memory ICOS+ T cells adhere to plate-bound ICOSL and assume an elongated morphology. Calcein-AM-labeled PBT lymphoblasts were incubated for 2 h on ICOSL.Ig-, PD-L1.Ig-, B7.1.Ig-, or BSA-precoated (control) plates in the presence or absence of anti-CD3. Plates were washed to remove nonadherent cells and visualized by epifluorescent microscopy. (B) The percentage of adherent cells was calculated by counting the number of cells present in five random fields (surface area visible with a 10× objective=0.5934 mm2), normalizing that cell number to the 180-mm2 surface area for an entire well of a 24-well plate, and dividing that total by the 300,000 cells that saturate all binding sites in the well.
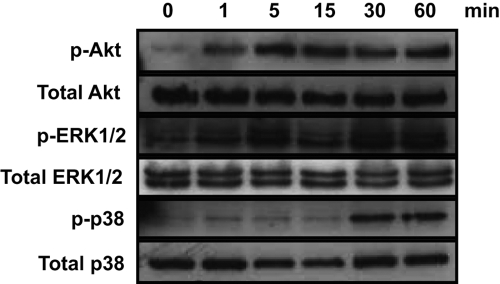
Fig. 2.
ICOS engagement alone activates Akt, ERK1/2, and p38 kinases. PBT lymphoblasts were rested overnight in the absence of IL-2 and then stimulated with microspheres coated with 5 μg/ml ICOSL.Ig for 1, 5, 15, 30, and 60 min. Cell lysates were analyzed for Akt, ERK1/2, and p38 phosphorylation (p-Akt, p-ERK1/2, p-p38, respectively) by immunoblot. Protein levels for total Akt, ERK1/2, and p38 expression were accessed as a loading control.
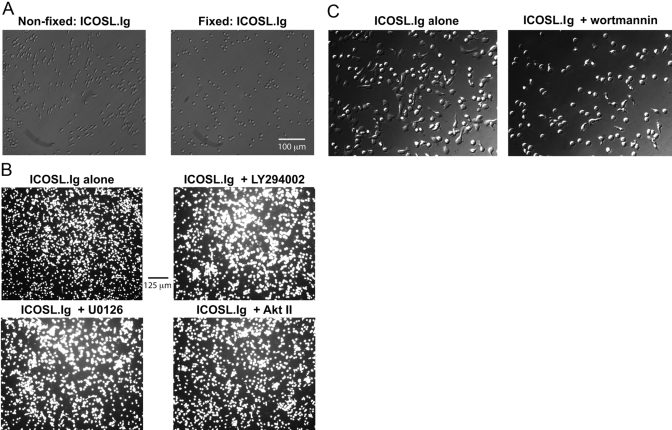
Fig. 3.
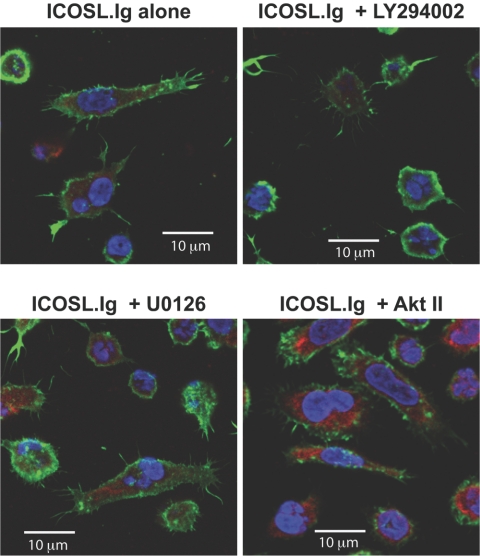
ICOS-mediated T cell elongation, but not adhesion, is dependent on induction of intracellular signaling pathways. Rested effector/memory T lymphoblasts were left unfixed, fixed with 4% formaldehyde, (C) pretreated with 20 nM wortmannin for 20 min, or (B) labeled with calcein-AM and pretreated with 50 μM LY294002, 20 nM U0126, or 20 μM Akt inhibitor II (Akt II; SH-5) for 20 min before their incubation on ICOSL.Ig (5 μg/ml)-coated plates for 2 h. Cellular adhesion, spreading, and elongation were observed by phase-contrast (A and C) or epifluorescent microscopy (B).
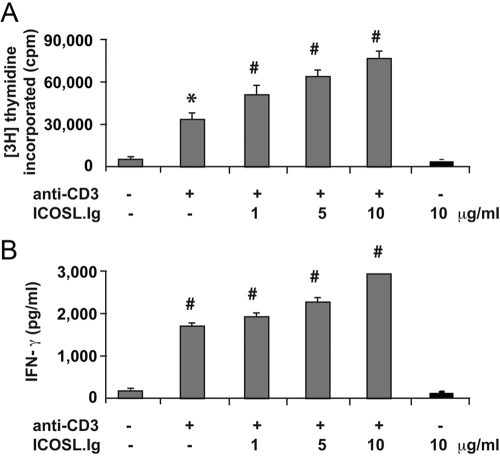
Fig. 4.
ICOS-induced signal transduction does not enhance T cell proliferation or cytokine production in the absence of TCR engagement. Rested PBT lymphoblasts were stimulated on plates precoated with 1 μg/ml anti-CD3 and increasing concentrations of ICOSL.Ig (gray bars) or with 10 μg/ml ICOSL.Ig alone (black bars). After 3 days in culture, proliferation was measured by [3H]-thymidine incorporation and (B) IFN-γ production by ELISA. Error bars were determined using the sd from replicate wells. *, P < 0.01, versus anti-CD3 stimulation alone; #, P < 0.001, versus anti-CD3 stimulation alone. No significant difference was observed between no stimulation and ICOSL.Ig alone.
Fig. 5.
ICOS engagement induces cytoplasmic extensions and the formation of F-actin-enriched filopodia and microspikes in human effector/memory T cells. T lymphoblasts were incubated for 2 h on coverslips precoated with PLL (0.01%) or ICOSL.Ig (5 μg/ml). After fixation and permeabilization of the cells, F-actin was visualized by staining with Alexofluor-488-labeled phalloidin. Vimentin was immunostained with goat anti-human vimentin antisera in combination with an Alexofluor-568-conjugated donkey anti-goat, affinity-purified polyclonal antibody. Images were recorded using a LSM510 META laser-scanning confocal microscope under a 100× oil immersion objective.
TCR-independent, ICOS-induced morphological changes, but not the initial adhesion event itself, are dependent on cellular metabolism and signal transduction
The biological function of most costimulatory receptors is believed to be dependent on antigen-specific signals initiated by engagement of the TCR [27]. In contrast, the adhesion and elongation of T cells are mediated by an ICOSL–ICOS interaction in the absence of TCR ligation, suggesting that ICOS engagement can activate an intracellular signal in an antigen-independent manner. ICOS is known to bind the p85 subunit of the lipid kinase PI-3K via a conserved Tyr-Met-X-Met motif within its cytoplasmic tail [6]. Not surprisingly, ICOS costimulation enhances TCR-induced PI-3K lipid kinase activity as well as the phosphorylation of the downstream serine/threonine protein kinase Akt [28]. As it is likely that ICOS-induced PI-3K activation modulates additional second messenger signaling pathways important to T cell activation, we investigated the ability of ICOS ligation to induce Akt phosphorylation as well as the activation of critical T cell-associated signaling proteins demonstrated previously to be regulated by PI-3K, including the three MAPK pathways [29, 30]. T lymphoblasts were rested overnight in the absence of IL-2 and then stimulated with latex beads coated with ICOSL.Ig alone for times that varied between 1 and 60 min. The level of Akt, ERK1/2, p38, and JNK phosphorylation was analyzed by fractionating the cell lysates via SDS-PAGE and immunoblotting with phosphospecific antibodies (Fig. 2). Basal levels of Akt and ERK1/2 phosphorylation were low in rested effector/memory T lymphoblasts, and ICOS stimulation alone resulted in a marked increase in the phosphorylation of both within 5 min that was maintained for up to 60 min. p38 activation was also induced following ICOS engagement, although with delayed kinetics in comparison with Akt and ERK, reaching its maximum at 30 min. ICOS ligation did not induce JNK activation (data not shown).
To examine the contribution of the signals induced by ICOS engagement to T cell adhesion and elongation, effector/memory T lymphoblasts were rendered metabolically inactive by fixation with 4% formaldehyde. Fixed and nonfixed T cells were incubated on ICOSL.Ig-coated plates for 2 h, washed, and examined by phase-contrast microscopy (Fig. 3A). Little difference in the adhesive capability was observed between the two groups. However, as noted earlier, ICOS-mediated adhesion induced morphological changes in these effector/memory T cells, characterized by an elongated, spread phenotype. These changes were not observed in cells fixed before ICOS stimulation and adhesion. This finding suggests that although ICOS-mediated adhesion is independent of signal transduction, ICOS-induced morphological changes require the activation of signaling cascades emanating from the ICOS receptor. To confirm this result and to investigate the biochemical pathways required for this elongated phenotype, T cells were pretreated for 20 min with inhibitors of PI-3K (LY294002), Akt (SH-5, an Akt inhibitor II), and the ERK1/2 cascade (U0126, an inhibitor of MEK1 and -2, kinases directly upstream of ERK1/2), all of which are induced by ICOS engagement (as shown in Fig. 2). Following 2 h incubation on ICOSL.Ig-coated plates, levels of adhesion were recorded by epifluorescent microscopy (Fig. 3B). In agreement with the metabolically inactivated cells, inhibition of all known signaling pathways initiated by ICOS ligation did not reduce T cell adhesion to ICOSL.Ig. However, it appears that only signaling through the PI-3K pathway (wortmannin is a PI-3K inhibitor) is required for the induction of T cell spreading and the elongated morphology exhibited in response to ICOS ligation (Fig. 3, B and C).
Other T cell effector functions, such as proliferation and cytokine production, are not stimulated by ICOS engagement alone
In light of the ability of ICOS to induce a signaling cascade, cell adhesion, and changes in cell morphology in the absence of TCR ligation, we re-examined whether ICOS engagement alone could also stimulate effector/memory T cell proliferation, growth, and cytokine production (Fig. 4). Rested CD4+CD45RO+ T lymphoblasts were left unstimulated or stimulated with 1 μg/ml plate-bound anti-CD3 in the presence or absence of increasing concentrations of immobilized ICOSL.Ig chimeric protein or with ICOSL.Ig alone. Following 66 h of stimulation, conditioned media were collected and analyzed for IFN-γ and IL-2 production by ELISA. T cell proliferation was measured by pulsing the cells with [3H] thymidine for the final 6 h of culture, and the expansion of the T cells was followed by daily counting of the viable cells. As expected, engagement of ICOS during suboptimal anti-CD3-mediated T cell activation leads to a significant dose-dependent enhancement of T cell proliferation (P<0.01 for anti-CD3 alone and 0.001 for ICOSL costimulation) and IFN-γ secretion (P<0.001), and stimulation with ICOSL.Ig alone had no effect on any parameter, as the responses with no stimulation or ICOSL.Ig alone were not significantly different. As reported previously [5, 31,32,33,34,35,36], costimulation with anti-CD3 and ICOSL.Ig did not enhance IL-2 production above CD3 cross-linking alone, and stimulation with ICOSL.Ig alone did not induce the secretion of IL-2 (data not shown). CD28 costimulation, however, did enhance anti-CD3-mediated IL-2 secretion in effector/memory T cells (data not shown). Consistent with the thymidine incorporation results, T cell growth was induced by CD3 cross-linking, further enhanced by ICOSL-mediated costimulation, and unaffected by ICOSL.Ig treatment alone. During the first 3 days of culture, T cell density remained unchanged. On Day 4, CD3 cross-linking induced a 20% increase in T cell concentration, and the costimulated culture showed an 80% expansion in T cell number. In contrast, there was a precipitous drop (55%) in viable T cells in those wells with no stimulation or coated with ICOSL.Ig alone.
ICOS engagement induces cytoplasmic elongation and the formation F-actin-enriched filopodia and microspikes in human effector/memory T cells
To further characterize the effect of ICOS engagement on T cell spreading and morphological changes possibly linked to cytoskeletal reorganization, effector/memory T lymphoblasts were exposed to coverslips precoated with ICOSL.Ig or as a control, PLL, a positively charged amino acid polymer known to be highly adhesive through interactions with the anionic glycocalyx on the T cell surface but not to induce intracellular signals. Following 2 h of incubation, cells were fixed, permeabilized, stained for vimentin and F-actin, two cytoskeletal components of the intermediate and microfilaments, respectively, and studied by confocal microscopy (Fig. 5). As expected, cells that adhered to PLL maintained the spherical shape characteristic of blood-derived T cells and exhibited a prominent cortical ring of F-actin just beneath the cell’s plasma membrane and a more diffuse, cage-like distribution of vimentin throughout the cytosol. In contrast, ICOSL-adherent cells were induced to undergo distinct morphological changes involving elongation of their cell body and reorganization of actin microfilaments leading to the development of multiple, thin, finger-like, actin-rich projections, described here as filopodia, which were accompanied by numerous smaller plasma membrane protrusions or microspikes, which appeared quite similar to developing, actin-rich pseudopodia (Fig. 5, right panel). In these static micrographs, ∼30% of cells acquired the highly elongated phenotype, and >98% of the cells demonstrated actin-rich membrane projections when all focal plains were scanned. It is important to note that these percentages underestimate the number of T cells that have elongated during the 2 h of ICOS ligation. At the time of imaging, many cells will not have initiated the process or may have released from the surface. This concept will be investigated further in the time-lapse video microscopic images described (see Fig. 8 and the Supplemental Videos).
Fig. 6.
PI-3K blockade inhibits T cell cytoplasmic extension, but not filopodia or microspike formation. Rested effector/memory T cells were pretreated with 50 μM LY294002, 20 nM U0126, or 20 μM Akt inhibitor II (SH-5) for 20 min before their incubation on ICOSL.Ig (5 μg/ml)-coated plates for 2 h. After fixation and permeabilization of the cells, F-actin and vimentin were visualized by scanning confocal microscopy, as described in the legend to Figure 5.
Fig. 7.
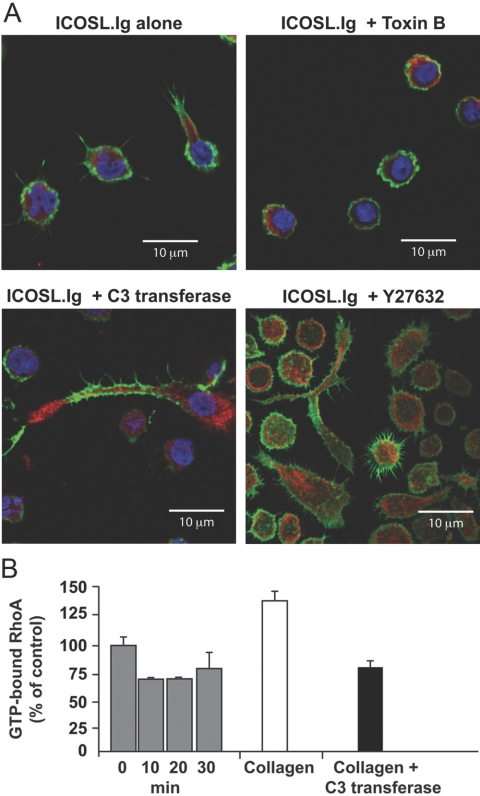
Distinct functions for individual members of the Rho GTPase family in ICOS-mediated T cell cytosolic extension and filopodia formation. Rested T lymphoblasts were left untreated or pretreated with Toxin B for 6 h, exoenzyme C3 transferase for 4 h, or Y27632 for 30 min before their incubation on ICOSL.Ig (5 μg/ml)-coated plates for 2 h. After fixation and permeabilization of the cells, F-actin and vimentin were visualized by scanning confocal microscopy, as described in the legend to Figure 5. (B) Effector/memory T cells were adhered to and stimulated by plate-bound ICOSL.Ig for 10, 20, or 30 min (gray bars), and cell lysates were prepared. GTP-bound, active RhoA concentrations were determined by G-LISA. As a positive (white bar) and negative (black bar) control on the same population of T cells, integrins were activated with Mn2+ pretreatment for 30 min, and the cells were adhered to collagen in the absence and presence, respectively, of the RhoA inhibitor, exoenzyme C3 transferase. Error bars were determined using the sd from replicate wells.
Fig. 8.
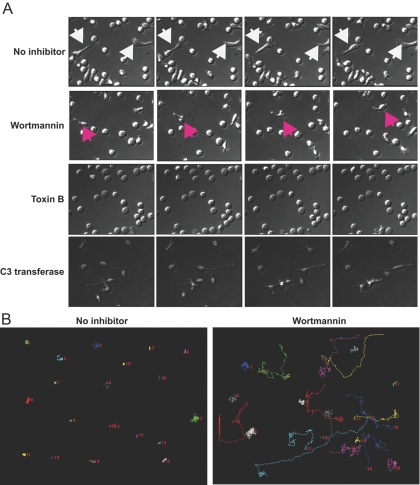
Activation of PI-3K by ICOS engagement induces T cell oscillation, not migration. Rested effector/memory T cells were incubated for 2 h on ICOSL.Ig-coated, glass-bottom microwells before being visualized for an additional 1.5 h by time-lapse microscopy. Images were acquired every 15 s. Still photographs taken 1.25 min apart for cells treated with ICOSL.Ig alone and 3.75 min apart for those treated with inhibitors are shown to illustrate the oscillations of the cytosol in untreated cells and cells pretreated with exoenzyme C3 transferase, cell migration after wortmannin pretreatment for 30 min, and a rounded, stagnant cell after a 6-h Toxin B pretreatment. White arrowheads highlight an untreated cell undergoing rapid oscillations, and pink arrowheads identify a cell migrating in the presence of wortmannin. (B) Migration paths of individual T cells incubated on ICOSL.Ig-coated plates in the absence or presence of wortmannin (20 nM) pretreatment were tracked for 250 frames and graphed using Metamorph software.
Induction of cell elongation and cytoskeletal rearrangement after ICOS engagement depends on PI-3K activity
Although ICOSL adhesion was unaffected by metabolic inactivation, elongation of the T cell body induced by ICOS engagement was not observed in PI-3K-inhibited cells or those fixed prior to ICOS ligation, suggesting the involvement of ICOS-dependent signaling pathways in the regulation of cytoskeletal reorganization and filopodia/microspike formation. To investigate a role for ICOS-mediated signal transduction in the development of the cytoplasmic extension and the actin-rich membrane protrusions, effector/memory T cells were pretreated with inhibitors to the various signaling pathways enhanced by ICOS stimulation, including PI-3K, ERK1/2 (MEK1/2), and Akt. Cells were then incubated on ICOSL.Ig-coated plates for 2 h and visualized by phase-contrast microscopy (Fig. 3, and see Fig. 8) or fixed, permeabilized, and stained for F-actin and vimentin confocal localization (Fig. 6). ICOS-induced T cell elongation, as witnessed by cytoplasmic extension, and cytoskeletal rearrangement of actin-rich membrane protrusions (filopodia and microspikes) were unaffected by inhibition of the ERK1/2 or Akt signaling pathways (Fig. 6). In contrast, inhibition of PI-3K activity by wortmannin (Fig. 3C, and see Fig. 8) and LY294002 (Figs. 3B and 6) led to the inability of T lymphoblasts to undergo ICOS-mediated cytoplasmic extension, decreasing the percentage of elongated cells following ICOS stimulation from 29.9% to 13.4% following wortmannin pretreatment and with a similar decline after LY294002 exposure. Interestingly, PI-3K inhibition had a minimal effect on filopodia and microspike formation, suggesting that these structures are regulated by different biochemical mechanisms than elongation.
ICOS-induced filopodia and microspike protrusions require small Rho-GTPase activity
Among many activities, the PI-3K pathway is known to regulate the activation of the small molecular weight GTPases of the Rho family, Cdc42, Rac, and RhoA, which play a critical role in remodeling the actin cytoskeleton in many cell types, including T lymphocytes. To investigate a role for the Rho family of GTPases in ICOS-induced T cell elongation, and filopodia/microspike formation, effector/memory T cells were pretreated for 6 h with Clostridium difficile Toxin B, an enzyme that uses UDP-glucose as a substrate to collectively inhibit all members of the Rho family [37], followed by incubation on ICOSL.Ig-coated plates for 2 h. When visualized by phase-contrast microscopy, Toxin B pretreatment inhibited ICOS-mediated T cell morphological changes dramatically, such as spreading and elongation (see Fig. 8, and see Supplemental Video 3 online). As shown earlier for cells that underwent metabolic inactivation and those adherent to PLL, Toxin B-pretreated cells retained their spherical shape. Upon further high-resolution examination by confocal microscopy and staining for vimentin and actin, Toxin B-treated cells were unable to undergo actin polymerization after ICOS stimulation and thus, did not form filopodia, microspikes, or cytoplasmic extension (Fig. 7A).
Although Toxin B inhibition confirmed the participation of the Rho family GTPases in ICOS-mediated cytoskeletal rearrangement and membrane protrusion, Rac, Cdc42, and RhoA have been described to be responsible for distinct patterns of actin reorganization, such as the formation of lamellipodia, filopodia, and actin contractility, respectively. To elucidate the function of the individual Rho GTPases in ICOS-mediated events, we compared the often-opposing contributions of RhoA versus Rac/Cdc42 in the formation of ICOS-induced filopodia/microspikes and cell elongation through pharmacological inhibition of the RhoA pathway. T cells pretreated with exoenzyme C3 transferase, a specific inhibitor of RhoA activity [38], demonstrated a morphological phenotype distinct from cells stimulated with ICOSL.Ig alone or those pretreated with Toxin B (Figs. 7A and 8, and see Supplemental Videos 1, 3, and 4 online). Although the ability to induce actin-rich membrane protrusions was unaffected by C3 transferase pretreatment, RhoA inhibition in some cells resulted in substantially increased elongation of what appeared to be the trailing edge of ICOS-stimulated T cells. To confirm the unique function of RhoA in ICOS-stimulated cells, we also demonstrated that inhibition of the RhoA-dependent kinase Rock with Y27632 yielded the same cell morphology (Fig. 7A). As a result of the altered phenotype in C3 transferase- and Y27632-treated cells, we directly investigated the effect of ICOS stimulation on RhoA GTPase activity. GTP-bound, active RhoA concentrations were determined by G-LISA following stimulation of effector/memory T cells with plate-bound ICOSL.Ig for times varying from 10 to 30 min (Fig. 7B). Plate-bound ICOS stimulation reduced RhoA activity to levels below that of unstimulated cells. As a positive control against premature GTP hydrolysis, the same preparation of T lymphoblasts was incubated with Mn2+ before adherence to collagen-coated plates, a procedure demonstrated previously in our laboratory to increase RhoA activity. As expected, RhoA activity was elevated in Mn2+-treated effector/memory T cells adherent to collagen. This activation was reversed by pretreatment with C3 transferase.
ICOS adhesion and activation initiate rapid T cell oscillations but not cell migration
Although ICOS-induced filopodia, microspikes, and cytosolic extension in effector/memory T cells are suggestive of a motile phenotype, ICOS-mediated inhibition of RhoA activity is not. To investigate this apparent discrepancy and to ascribe a biological function to ICOS-mediated events, we captured the kinetic response of T lymphoblasts to plate-bound ICOS activation with time-lapse video microscopy. Rested effector/memory T cells were incubated for 2 h on ICOSL.Ig-coated, glass-bottom microwells before being visualized for an additional 1.5 h by time-lapse microscopy (Fig. 8; Supplemental Video 1 online). Images were acquired every 15 s. Despite their spread, seemingly highly polarized morphology, typically characteristic of migrating cells, T lymphocytes stimulated with ICOSL.Ig do not migrate. However, these cells did not remain stagnate. The impact to ICOSL.Ig adherence yielded two distinct phenotypic responses in T cells. The first involves the shifting of the cell cytoplasm back and forth between the two opposing poles of highly elongated cells, referred to as oscillations. The second effect is the random projection and retraction of numerous filopodia and microspikes, accompanied by a pivoting-like movement in the less highly elongated cells. Neither phenotype resulted in displacement of the cells along the ICOSL.Ig-coated plate. As underscored by these videos, the events associated with the projection and retraction of filopodia and cytoplasmic extensions are dynamic. Thus, the static micrographs in previous figures underestimate the percentage of T cells undergoing elongation, as at the time of imaging, many cells will not have initiated the process or may have retracted to their starting position.
To examine the participation of PI-3K and Rho GTPases in ICOS-induced oscillations, effector/memory T cells were exposed to wortmannin, Toxin B, and exoenzyme C3 transferase pretreatment, stimulated on ICOSL.Ig-coated microwells for 2 h and then visualized by time-lapse microscopy every 15 s for 1.5 h (Fig. 8A; Supplemental Videos 2–4 online). Consistent with our findings by phase-contrast and confocal microscopy and with a recent report [15], wortmannin reduced ICOS-induced T cell cytoplasmic extension but had little effect on the ability of effector/memory T cells to produce filopodia, although these membrane protrusions seemed to be reduced in length. In contrast, wortmannin pretreatment stimulated effector/memory T cell migration on ICOSL.Ig-coated plates, raising the percentage of cells undergoing a net displacement greater than one cell length in the 1.5 h of filming from 3.84% ± 3.0% in cells stimulated with ICOSL.Ig alone to 26.7% ± 5.0% for the wortmannin-pretreated culture (Table 1). The individual migration pattern of a single cell pretreated with wortmannin is illustrated (Fig. 8B). Tracking of individual cells demonstrated that they followed a random course of migration with an average speed of 0.124 ± 0.026 μm/s. Although the illustration shows the migration track for a single, highly motile cell, in general, the majority of T cells pretreated with wortmannin did not migrate continuously across the surface of the ICOSL.Ig-coated well but instead, often stopped and started, reversing directions frequently while extending and retracting their membrane protrusions (Supplemental Video 2 online).
TABLE 1.
Inhibition of ICOS-Induced PI-3K Activity Promotes T Cell Migration
| Total distance (μm) | Distance from origin (μm) | Velocity (μm/s) | |
|---|---|---|---|
| No inhibitor | 35.5 ± 49.60a | 3.84 ± 3.00b | 0c |
| Wortmannin | 378.8 ± 80.04 | 66.2 ± 49.84 | 0.124 ± 0.026 |
Average total distance traveled by individual cells over 250 frames taken at 15 s intervals. All calculations were an average of 20 individual cell migration patterns for each treatment group.
Average distance between where a cell was located at the start of filming (Frame 1) to where it was located after 250 frames.
Average velocity of individual cells during their 250 frames of filming.
In contrast, Toxin B inhibition of cytoskeletal rearrangement, cytoplasmic extension, and filopodia and microspike protrusions yielded stagnant, spherical T cells, bound to the ICOSL.Ig-coated wells (Fig. 8A; Supplemental Video 3 online). Pretreatment with the RhoA inhibitor, exoenzyme C3 transferase, again resulted in an oscillatory phenotype distinct from those induced by the other two pharmacological inhibitors or by stimulation with ICOSL.Ig alone (Fig. 8A; Supplemental Video 4 online). In contrast to cells pretreated with wortmannin, C3 transferase did not enhance T cell migration on ICOSL.Ig-coated plates but induced an exaggerated, elongated phenotype (Fig. 8A). These elongated, RhoA-inhibited cells, when bound to ICOSL, displayed the cytosolic oscillations seen in cells stimulated with ICOSL.Ig alone (Supplemental Video 4 online). We propose that the combination of a decrease in GTP-bound RhoA as a result of ICOS ligation with the additional decline in active RhoA as a result of C3 transferase-mediated inhibition yields even less-efficient uropod retraction and thus, even longer cytoplasmic extension.
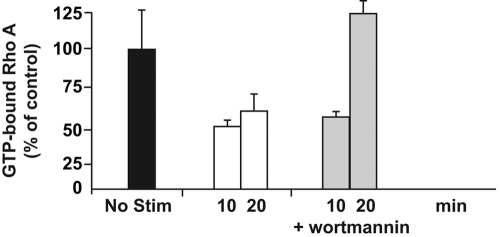
Decline in ICOS-induced, GTP-bound RhoA is PI-3K-dependent
On the one hand, it appears that disrupting the PI-3K signaling pathway after ICOS engagement results in deceased cytoplasmic extension and increased cell mobility. On the other hand, inhibition of RhoA activation generates a more extreme, ICOS-mediated phenotype that includes a highly elongated cytoplasmic extension undergoing cell-body oscillation but is not motile. We therefore speculated that the ICOS-mediated decline in GTP-bound RhoA and the subsequent cytoskeletal reorganization, cytoplasmic extension, and cellular oscillations were all initiated by a PI-3K-dependent mechanism. To test this hypothesis, effector/memory T cells were pretreated with wortmannin and then adhered to ICOSL.Ig-coated plates for 10 and 20 min before lysates were prepared and RhoA activity measured by G-LISA (Fig. 9). PI-3K inhibition not only prevented the ICOS-mediated decrease in activated RhoA in a cyclic manner as expected but increased the concentration of GTP-bound RhoA to levels above that of resting T cells.
Fig. 9.
Inhibiting PI-3K-dependent signal transduction from ICOS reverses the loss in GTP-bound RhoA. Effector/memory T cells were left unstimulated (black bar) or incubated on ICOSL.Ig (5 μg/ml)-coated plates for 10 or 20 min in the absence (white bars) or presence (gray bars) of a pretreatment with wortmannin (20 nM) for 30 min before adhesion. Lysates were prepared and GTP-bound RhoA quantified by G-LISA, as described in the legend to Figure 7. Error bars were determined using the sd from replicate wells.
DISCUSSION
The adaptive immune response is initiated when T lymphocytes recognize and respond to their cognate antigenic peptides bound to MHC molecules on the surface of APC. However, a TCR-transmitted signal alone is not sufficient to induce full T cell activation [28, 39]. Optimal T cell responses are dependent on additional modulation by a constantly growing number of coreceptors, accessory proteins, and costimulatory pathways with diverse regulatory functions. Although costimulatory receptors, such as CD28, transduce intracellular signaling cascades that modify the TCR-mediated cascades [28, 39], adhesion molecules such as ICAM-1 are believed to enhance T cell activation by facilitating contact between T cells and APCs [39]. In the present report, we demonstrate that in addition to its costimulatory role in effector/memory T cell activation, the CD28 family member ICOS acts in an antigen-independent manner to promote T cell adhesion, induce the extension of exploratory, actin-rich membrane projections, increase cytoplasmic spreading, and inhibit cell migration. Although adhesion via ICOS is independent of its ability to transduce intracellular signals, initiation of cytoplasmic elongation and filopodia and microspike formation depend on ICOS-induced intracellular signal transduction. Although the elongated phenotype exhibited by ICOS-stimulated T cells requires PI-3K activation, the induction of actin-rich membrane protrusions does not. Filopodia and microspike formation are, however, dependent on the Rho family of small GTPases. Interestingly, inhibition of PI-3K activation not only reduces cell elongation and reverses ICOS-mediated inhibition of RhoA, it also induces effector/memory T cell migration, as determined by time-lapse microscopy. These results combined with the finding that exoenzyme C3 transferase pretreatment of ICOSL.Ig-stimulated cells produces an exacerbated, elongated phenotype compared with ICOSL.Ig-stimulated controls led us to propose that RhoA functions as a molecular switch in T cell morphology in response to ICOS stimulation. Induction of PI-3K activity by ICOS inhibits RhoA activity, thereby eliminating the contractile force necessary for uropod retraction, which leads to decreased effector/memory T cell migration and thus, retention of the T cell at sites of inflammation.
The best-characterized adhesion pathways involved in T cell activation include the interaction between LFA-1 and its ligands ICAM-1, -2, and -3 and CD2 with LFA-3 (CD58) [40]. These adhesion molecules are generally expressed on the surface of resting T cells in an inactive conformation that requires activation through the TCR/CD3 complex, chemokine receptors, or stimulation with PMA to induce their binding to their respective ligands, a process known as “inside out signaling” [41]. ICOS-mediated adhesion differs from these receptor systems characterized previously in two important ways: Adhesion between ICOSL and ICOS is independent of TCR or chemokine receptor ligation, and TCR engagement does not further enhance T cell adhesion via ICOS. In fact, ICOS-mediated adhesion does not require intracellular signaling at all, as metabolic inactivation of cells prior to ICOS stimulation had no effect on adhesion. These findings indicate that events involved in initial ICOS–ICOSL interactions are distinct from those involved in traditional T cell adhesion receptor–ligand interactions. Further studies investigating the binding affinity of ICOS–ICOSL interactions will help elicit how ICOS can function as such a strong adhesion molecule in the absence of traditional inside out signaling.
Although initial adhesive interactions between ICOS and ICOSL are independent of ICOS-mediated signal transduction, morphological changes induced by ICOS engagement are not seen in cells fixed before ICOS stimulation, suggesting that ICOS possesses the ability to induce intracellular signals that are responsible for cytoskeletal rearrangement. The p85 regulatory subunit of the lipid kinase PI-3K is recruited to the cytoplasmic tail of ICOS during costimulation via a conserved Tyr-Met-X-Met motif, resulting in enhanced PI-3K activity [6, 28, 35, 42,43,44,45,46]. It was assumed previously that phosphorylation of the cytoplasmic tail of ICOS, by components of the TCR signaling cascade, was a prerequisite for the costimulatory function of ICOS [28]. Although these events may be necessary for ICOS enhancement of TCR-mediated proliferation and cytokine production, our results indicate that they are not necessary for ICOS-induced intracellular signal transduction that results in PI-3K, Akt, p38, and ERK1/2 activation or cytoskeletal changes. Although the mechanism responsible for their activation is not the focus of this particular study, the ability of ICOS engagement to induce ERK1/2 and p38 MAPK activity is intriguing, as the p85 regulatory subunit is the only signaling molecule currently identified to interact with ICOS [19]. Whether the activation of ERK1/2 and p38 does occur downstream of PI-3K or is a result of an alternate, yet unidentified signaling pathway associated with ICOS ligation has not been determined. The potential for an alternate, PI-3K-independent, ICOS-mediated signaling pathway is intriguing, in light of the PI-3K-independent nature of ICOS-induced filopodia and microspike formation reported herein. This possibility is consistent with a recent suggestion that HS1-associated protein X-1 is a novel binding partner for CD28 and ICOS [47].
Upon activation, PI-3K catalyzes the phosphorylation of phosphatidylinositols at the D3 position of their myoinositol ring and the subsequent production of PIP2 and PIP3 [48], which recruit intracellular signaling molecules to the plasma membrane via binding to their PH domains. GEFs and GAPs, which regulate the activity of Rho family members, contain PH-binding domains and are regulated by PI-3K activity [16, 22]. Rho GTPases are known to control a variety of cellular events including lymphocyte survival, proliferation, and differentiation, in addition to their important role in regulating cellular adhesion, cell shape, and motility though actin cytoskeletal reorganization [23]. It is therefore not surprising that the activity of Rho family members is regulated by signals transduced from a receptor possessing costimulatory and adhesion properties. Consistent with their role in cytoskeletal rearrangement, the elongated phenotype and formation of filopodia/microspikes induced by ICOS stimulation are abrogated by the collective inhibition of all members of the Rho family. However, despite being responsible for the induction of cytoskeletal changes, ICOS stimulation results in a decrease in RhoA GTPase activity. In contrast with fibroblasts, where microinjection of the C3 exoenzyme results in retraction of pseudopodia, cell rounding, and the eventual loss of adhesion [49], RhoA inactivation in cells of hematopoietic lineage is associated with actin polymerization [50,51,52]. For example, in chemoattractant-stimulated neutrophils, monocytic cell lines, and fresh blood monocytes, RhoA inactivation leads to accelerated filopodial formation [51, 52]. In T cells specifically, aberrant extension of finger-like dendritic processes on substrates that include VCAM-1, fibronectin, ICAM-1, and mAb, specific to β1 integrins, has been described following RhoA inactivation [53, 54]. As an extension of these previous reports, we show that further inhibition of RhoA activity by pretreatment with exoenzyme C3 transferase results in an exacerbation of the elongated phenotype induced by ICOS stimulation alone. Furthermore, in agreement with recent studies, inhibition of PI-3K activity, which prevents a loss in RhoA activity following ICOS stimulation, decreases ICOS-mediated T cell elongation [15].
RhoA activity can be modulated by regulating the activity of a RhoA GAP or GEF protein. We hypothesize that the down-regulation of RhoA GTPase activity in ICOS-stimulated cells is a consequence of PI-3K enhancing the activation of a RhoA GAP. As an example, PI-3K activity in other cell types has previously been linked to the regulation of the Rho GAP Arf GAP, Rho GAP, Ankyrin repeat and PH domain 3 (ARAP3) [22]. As a result, the cyclic nature of Rho family GTP loading and hydrolysis, decreasing the rate of GTP hydrolysis by inhibiting a PI-3K-dependent RhoA GAP protein, would allow for the accumulation of GTP-bound RhoA and explain not only the reversal but also the enhancement of RhoA activity at 20 min following ICOS ligation. Although currently under intense investigation, the identity and specificities of GAP proteins expressed in T lymphocytes are not well characterized.
Cell motility is a dynamic process involving F-actin polymerization at the cell’s leading edge, attachment to the substratum with filopodia, generation of contractile forces and traction, and ultimate release of the cell’s trailing anchor or uropod [23]. Consistent with our findings, previous studies have demonstrated that ICOS signaling promotes morphological changes in activated T cells. These changes include the development of a seemingly polarized, elongated cell phenotype, similar to that which is often associated with migrating cells. Based solely on these static images, it was proposed previously that ICOS engagement is capable of promoting T cell migration [15, 25]. However, static images acquired by traditional light microscopic techniques capture only a single instance of an often complex biological event. In this respect, time-lapse microscopy provides a more complete analysis of the responses associated with ICOS ligation. Thus, we demonstrate here that the morphological changes associated with ICOS stimulation do not equate to T cell migration but rather, an oscillatory movement of the cytoplasm between the opposing poles in cells fixed in position. In addition, PI-3K inhibition prior to ICOS engagement increased the number of cells migrating on ICOSL.Ig-coated plates significantly. These results, combined with the observation that PI-3K inhibition reverses the decrease in RhoA activity seen following ICOS stimulation, led us to speculate that ICOS-mediated enhancement of PI-3K activity acts to inhibit effector/memory T lymphocyte migration by reducing RhoA activity. Although the elongated phenotype exhibited by ICOS-stimulated cells is clearly the product of a complex interaction among all three Rho GTPases, it appears that the decrease in RhoA activity, in particular, prevents the contractile force necessary for uropod retraction, thereby inhibiting forward migration. The resulting generation of an artificially elongated T cell is therefore the consequence of two opposing forces: the leading edge continuing to process forward and the trailing edge remaining firmly attached to ICOSL.Ig. A similar role for RhoA was described previously in the detachment and contractility of the trailing edge of migrating eosinophils [55], monocytes [56], and LFA-1 [54] and CD2-stimulated T lymphocytes [57]. Our observations that exoenzyme C3 transferase pretreatment results in further elongation of effector/memory T cells following ICOS engagement support this mechanism of action.
Toxin B inhibition of all Rho family GTPases results in the inability of the cell to induce any cytoskeletal changes. This indicates that another member of the Rho family must regulate the elaboration of ICOS-induced filopodia and microspike membrane protrusions. Cdc42 activity has been demonstrated previously to promote formation of filopodial protrusions in human CD4+ T cells and the Jurkat lymphoma cell line following CD28 engagement [58]. It is thus likely that increased Cdc42 activation following ICOS stimulation may be responsible for the driving force necessary for actin polymerization. Although the Cdc42 GEF Vav-1 is associated with PI-3K activity [16, 58], inhibition of PI-3K prior to ICOS stimulation does not inhibit filopodia/microspike formation. This suggests that there is an additional, unidentified signaling pathway not involving PI-3K associated with ICOS intracellular responses. This is particularly interesting, as PI-3K is the only signaling molecule currently known to interact with ICOS. Despite the seemingly important link between Cdc42 activation and the PI-3K-independent, ICOS-mediated filopodia/microspike formation, our analysis of Cdc42 activation following ICOS stimulation was encumbered by low Cdc42 expression in T lymphocytes that caused traditional GTPase pull-down assays to lack sensitivity and the current unavailability of a Cdc42 G-LISA assay.
Identification of the pathways initiated by ICOS engagement that direct the activity of the Rho family GTPases and thus, modulate their role in regulating T cell motility, cytoskeletal rearrangement, and immune recognition is underway. Overall, the studies presented here introduce new insights into antigen-independent mechanisms used by effector/memory T cells to enhance immune responses in the periphery and support an alternate interpretation of the antigen-independent function for ICOS proposed previously [15, 25]. Our findings suggest that the costimulatory ligand–receptor (ICOSL–ICOS) system optimizes T cell activation in inflamed tissue by reducing T cell migration and promoting the exploratory interactions necessary for antigen recognition by effector/memory T cells bound to activated APCs.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (DK-54213 and AI-53188) to A. D. L. and to the Confocal Microscopy and Cytometry Core Facilities of the Case Comprehensive Cancer Center (P30 CA43703). We thank all of the members of the A. D. L. laboratory in taking turns to carry the primary T cell culture, especially Feng Xue and Drs. Lopamudra Das, Tamara Fink, and Brenda Rivera Reyes, and Dr. Pingfu Fu for help with statistical evaluation. Dr. Mary Laughlin allowed us to use her equipment in the proliferation assays, and Dr. Minh Lam was invaluable in training us in the use of the confocal and video imaging equipment. We appreciate the imaging support and use of software provided by Drs. Josephine Adams, Pin Xu, and Alison Hall.
References
- Coyle A J, Gutierrez-Ramos J C. The expanding B7 superfamily: increasing complexity in costimulatory signals regulating T cell function. Nat Immunol. 2001;2:203–209. doi: 10.1038/85251. [DOI] [PubMed] [Google Scholar]
- Schwartz R H. A cell culture model for T lymphocyte clonal anergy. Science. 1990;248:1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- London C A, Lodge M P, Abbas A K. Functional responses and costimulator dependence of memory CD4+ T cells. J Immunol. 2000;164:265–272. doi: 10.4049/jimmunol.164.1.265. [DOI] [PubMed] [Google Scholar]
- Liang L, Sha W C. The right place at the right time: novel B7 family members regulate effector T cell responses. Curr Opin Immunol. 2002;14:384–390. doi: 10.1016/s0952-7915(02)00342-4. [DOI] [PubMed] [Google Scholar]
- Hutloff A, Dittrich A M, Beier K C, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek R A. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- Coyle A J, Lehar S, Lloyd C, Tian J, Delaney T, Manning S, Nguyen T, Burwell T, Schneider H, Gonzalo J A, Gosselin M, Owen L R, Rudd C E, Gutierrez-Ramos J C. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity. 2000;13:95–105. doi: 10.1016/s1074-7613(00)00011-x. [DOI] [PubMed] [Google Scholar]
- Swallow M M, Wallin J J, Sha W C. B7h, a novel costimulatory homolog of B7.1 and B7.2, is induced by TNFα. Immunity. 1999;11:423–432. doi: 10.1016/s1074-7613(00)80117-x. [DOI] [PubMed] [Google Scholar]
- Sato T, Kanai T, Watanabe M, Sakuraba A, Okamoto S, Nakai T, Okazawa A, Inoue N, Totsuka T, Yamazaki M, Kroczek R A, Fukushima T, Ishii H, Hibi T. Hyperexpression of inducible costimulator and its contribution on lamina propria T cells in inflammatory bowel disease. Gastroenterology. 2004;126:829–839. doi: 10.1053/j.gastro.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Totsuka T, Kanai T, Iiyama R, Uraushihara K, Yamazaki M, Okamoto R, Hibi T, Tezuka K, Azuma M, Akiba H, Yagita H, Okumura K, Watanabe M. Ameliorating effect of anti-inducible costimulator monoclonal antibody in a murine model of chronic colitis. Gastroenterology. 2003;124:410–421. doi: 10.1053/gast.2003.50050. [DOI] [PubMed] [Google Scholar]
- Nakazawa A, Dotan I, Brimnes J, Allez M, Shao L, Tsushima F, Azuma M, Mayer L. The expression and function of costimulatory molecules B7H and B7–H1 on colonic epithelial cells. Gastroenterology. 2004;126:1347–1357. doi: 10.1053/j.gastro.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Gonzalo J A, Tian J, Delaney T, Corcoran J, Rottman J B, Lora J, Al-garawi A, Kroczek R, Gutierrez-Ramos J C, Coyle A J. ICOS is critical for T helper cell-mediated lung mucosal inflammatory responses. Nat Immunol. 2001;2:597–604. doi: 10.1038/89739. [DOI] [PubMed] [Google Scholar]
- Ozkaynak E, Gao W, Shemmeri N, Wang C, Gutierrez-Ramos J C, Amaral J, Qin S, Rottman J B, Coyle A J, Hancock W W. Importance of ICOS-B7RP-1 costimulation in acute and chronic allograft rejection. Nat Immunol. 2001;2:591–596. doi: 10.1038/89731. [DOI] [PubMed] [Google Scholar]
- Rottman J B, Smith T, Tonra J R, Ganley K, Bloom T, Silva R, Pierce B, Gutierrez-Ramos J C, Ozkaynak E, Coyle A J. The costimulatory molecule ICOS plays an important role in the immunopathogenesis of EAE. Nat Immunol. 2001;2:605–611. doi: 10.1038/89750. [DOI] [PubMed] [Google Scholar]
- Khayyamian S, Hutloff A, Buchner K, Grafe M, Henn V, Kroczek R A, Mages H W. ICOS-ligand, expressed on human endothelial cells, costimulates Th1 and Th2 cytokine secretion by memory CD4+ T cells. Proc Natl Acad Sci USA. 2002;99:6198–6203. doi: 10.1073/pnas.092576699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nukada Y, Okamoto N, Konakahara S, Tezuka K, Ohashi K, Mizuno K, Tsuji T. AILIM/ICOS-mediated elongation of activated T cells is regulated by both the PI3-kinase/Akt and Rho family cascade. Int Immunol. 2006;18:1815–1824. doi: 10.1093/intimm/dxl115. [DOI] [PubMed] [Google Scholar]
- Acuto O, Cantrell D. T cell activation and the cytoskeleton. Annu Rev Immunol. 2000;18:165–184. doi: 10.1146/annurev.immunol.18.1.165. [DOI] [PubMed] [Google Scholar]
- Hauss P, Selz F, Cavazzana-Calvo M, Fischer A. Characteristics of antigen-independent and antigen-dependent interaction of dendritic cells with CD4+ T cells. Eur J Immunol. 1995;25:2285–2294. doi: 10.1002/eji.1830250826. [DOI] [PubMed] [Google Scholar]
- Miller M J, Hejazi A S, Wei S H, Cahalan M D, Parker I. T cell repertoire scanning is promoted by dynamic dendritic cell behavior and random T cell motility in the lymph node. Proc Natl Acad Sci USA. 2004;101:998–1003. doi: 10.1073/pnas.0306407101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang X, Loke P, Kim J, Wojnoonski K, Kusdra L, Allison J P. A genetic library screen for signaling proteins that interact with phosphorylated T cell costimulatory receptors. Genomics. 2006;88:841–845. doi: 10.1016/j.ygeno.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Okamoto N, Tezuka K, Kato M, Abe R, Tsuji T. PI3-kinase and MAP-kinase signaling cascades in AILIM/ICOS- and CD28-costimulated T-cells have distinct functions between cell proliferation and IL-10 production. Biochem Biophys Res Commun. 2003;310:691–702. doi: 10.1016/j.bbrc.2003.09.065. [DOI] [PubMed] [Google Scholar]
- Parry R V, Riley J L, Ward S G. Signaling to suit function: tailoring phosphoinositide 3-kinase during T-cell activation. Trends Immunol. 2007;28:161–168. doi: 10.1016/j.it.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Krugmann S, Williams R, Stephens L, Hawkins P T. ARAP3 is a PI3K- and rap-regulated GAP for RhoA. Curr Biol. 2004;14:1380–1384. doi: 10.1016/j.cub.2004.07.058. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid F, del Pozo M A. Leukocyte polarization in cell migration and immune interactions. EMBO J. 1999;18:501–511. doi: 10.1093/emboj/18.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fos C, Salles A, Lang V, Carrette F, Audebert S, Pastor S, Ghiotto M, Olive D, Bismuth G, Nunes J A. ICOS ligation recruits the p50α PI3K regulatory subunit to the immunological synapse. J Immunol. 2008;181:1969–1977. doi: 10.4049/jimmunol.181.3.1969. [DOI] [PubMed] [Google Scholar]
- Okamoto N, Nukada Y, Tezuka K, Ohashi K, Mizuno K, Tsuji T. AILIM/ICOS signaling induces T-cell migration/polarization of memory/effector T-cells. Int Immunol. 2004;16:1515–1522. doi: 10.1093/intimm/dxh153. [DOI] [PubMed] [Google Scholar]
- Bromley S K, Iaboni A, Davis S J, Whitty A, Green J M, Shaw A S, Weiss A, Dustin M L. The immunological synapse and CD28-CD80 interactions. Nat Immunol. 2001;2:1159–1166. doi: 10.1038/ni737. [DOI] [PubMed] [Google Scholar]
- Beier K C, Kallinich T, Hamelmann E. Master switches of T-cell activation and differentiation. Eur Respir J. 2007;29:804–812. doi: 10.1183/09031936.00094506. [DOI] [PubMed] [Google Scholar]
- Rudd C E, Schneider H. Unifying concepts in CD28, ICOS and CTLA4 co-receptor signaling. Nat Rev Immunol. 2003;3:544–556. doi: 10.1038/nri1131. [DOI] [PubMed] [Google Scholar]
- Ward S G, June C H, Olive D. PI 3-kinase: a pivotal pathway in T-cell activation? Immunol Today. 1996;17:187–197. doi: 10.1016/0167-5699(96)80618-9. [DOI] [PubMed] [Google Scholar]
- Seminario M C, Wange R L. Lipid phosphatases in the regulation of T cell activation: living up to their PTEN-tial. Immunol Rev. 2003;192:80–97. doi: 10.1034/j.1600-065x.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- Yoshinaga S K, Whoriskey J S, Khare S D, Sarmiento U, Guo J, Horan T, Shih G, Zhang M, Coccia M A, Kohno T, Tafuri-Bladt A, Brankow D, Campbell P, Chang D, Chiu L, Dai T, Duncan G, Elliott G S, Hui A, McCabe S M, Scully S, Shahinian A, Shaklee C L, Van G, Mak T W, Senaldi G. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402:827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- McAdam A J, Chang T T, Lumelsky A E, Greenfield E A, Boussiotis V A, Duke-Cohan J S, Chernova T, Malenkovich N, Jabs C, Kuchroo V K, Ling V, Collins M, Sharpe A H, Freeman G J. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells. J Immunol. 2000;165:5035–5040. doi: 10.4049/jimmunol.165.9.5035. [DOI] [PubMed] [Google Scholar]
- McAdam A J, Greenwald R J, Levin M A, Chernova T, Malenkovich N, Ling V, Freeman G J, Sharpe A H. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409:102–105. doi: 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- Witsch E J, Peiser M, Hutloff A, Buchner K, Dorner B G, Jonuleit H, Mages H W, Kroczek R A. ICOS and CD28 reversely regulate IL-10 on re-activation of human effector T cells with mature dendritic cells. Eur J Immunol. 2002;32:2680–2686. doi: 10.1002/1521-4141(200209)32:9<2680::AID-IMMU2680>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Parry R V, Rumbley C A, Vandenberghe L H, June C H, Riley J L. CD28 and inducible costimulatory protein Src homology 2 binding domains show distinct regulation of phosphatidylinositol 3-kinase, Bcl-xL, and IL-2 expression in primary human CD4 T lymphocytes. J Immunol. 2003;171:166–174. doi: 10.4049/jimmunol.171.1.166. [DOI] [PubMed] [Google Scholar]
- Harada Y, Ohgai D, Watanabe R, Okano K, Koiwai O, Tanabe K, Toma H, Altman A, Abe R. A single amino acid alteration in cytoplasmic domain determines IL-2 promoter activation by ligation of CD28 but not inducible costimulator (ICOS) J Exp Med. 2003;197:257–262. doi: 10.1084/jem.20021305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aktories K. Bacterial toxins that target Rho proteins. J Clin Invest. 1997;99:827–829. doi: 10.1172/JCI119245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aktories K, Braun U, Rosener S, Just I, Hall A. The Rho gene product expressed in E. coli is a substrate of botulinum ADP-ribosyltransferase C3. Biochem Biophys Res Commun. 1989;158:209–213. doi: 10.1016/s0006-291x(89)80199-8. [DOI] [PubMed] [Google Scholar]
- Dustin M L. Role of adhesion molecules in activation signaling in T lymphocytes. J Clin Immunol. 2001;21:258–263. doi: 10.1023/a:1010927208180. [DOI] [PubMed] [Google Scholar]
- Bierer B E, Burakoff S J. T cell adhesion molecules. FASEB J. 1988;2:2584–2590. doi: 10.1096/fasebj.2.10.2838364. [DOI] [PubMed] [Google Scholar]
- Lollo B A, Chan K W, Hanson E M, Moy V T, Brian A A. Direct evidence for two affinity states for lymphocyte function-associated antigen 1 on activated T cells. J Biol Chem. 1993;268:21693–21700. [PubMed] [Google Scholar]
- Feito M J, Vaschetto R, Criado G, Sanchez A, Chiocchetti A, Jimenez-Perianez A, Dianzani U, Portoles P, Rojo J M. Mechanisms of H4/ICOS costimulation: effects on proximal TCR signals and MAP kinase pathways. Eur J Immunol. 2003;33:204–214. doi: 10.1002/immu.200390023. [DOI] [PubMed] [Google Scholar]
- Gonzalo J A, Delaney T, Corcoran J, Goodearl A, Gutierrez-Ramos J C, Coyle A J. Cutting edge: the related molecules CD28 and inducible costimulator deliver both unique and complementary signals required for optimal T cell activation. J Immunol. 2001;166:1–5. doi: 10.4049/jimmunol.166.1.1. [DOI] [PubMed] [Google Scholar]
- Truitt K E, Hicks C M, Imboden J B. Stimulation of CD28 triggers an association between CD28 and phosphatidylinositol 3-kinase in Jurkat T cells. J Exp Med. 1994;179:1071–1076. doi: 10.1084/jem.179.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K V, Cai Y C, Raab M, Duckworth B, Cantley L, Shoelson S E, Rudd C E. T-cell antigen CD28 interacts with the lipid kinase phosphatidylinositol 3-kinase by a cytoplasmic Tyr(P)-Met-Xaa-Met motif. Proc Natl Acad Sci USA. 1994;91:2834–2838. doi: 10.1073/pnas.91.7.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secrist J P, Burns L A, Karnitz L, Koretzky G A, Abraham R T. Stimulatory effects of the protein tyrosine phosphatase inhibitor, pervanadate, on T-cell activation events. J Biol Chem. 1993;268:5886–5893. [PubMed] [Google Scholar]
- Van Berkel M E, Oosterwegel M A. CD28 and ICOS: similar or separate costimulators of T cells? Immunol Lett. 2006;105:115–122. doi: 10.1016/j.imlet.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Ward S G, Cantrell D A. Phosphoinositide 3-kinases in T lymphocyte activation. Curr Opin Immunol. 2001;13:332–338. doi: 10.1016/s0952-7915(00)00223-5. [DOI] [PubMed] [Google Scholar]
- Paterson H F, Self A J, Garrett M D, Just I, Aktories K, Hall A. Microinjection of recombinant p21rho induces rapid changes in cell morphology. J Cell Biol. 1990;111:1001–1007. doi: 10.1083/jcb.111.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrengruber M U, Boquet P, Coates T D, Deranleau D A. ADP-ribosylation of Rho enhances actin polymerization-coupled shape oscillations in human neutrophils. FEBS Lett. 1995;372:161–164. doi: 10.1016/0014-5793(95)00880-i. [DOI] [PubMed] [Google Scholar]
- Aepfelbacher M, Essler M, Huber E, Czech A, Weber P C. Rho is a negative regulator of human monocyte spreading. J Immunol. 1996;157:5070–5075. [PubMed] [Google Scholar]
- Allen W E, Jones G E, Pollard J W, Ridley A J. Rho, Rac and Cdc42 regulate actin organization and cell adhesion in macrophages. J Cell Sci. 1997;110:707–720. doi: 10.1242/jcs.110.6.707. [DOI] [PubMed] [Google Scholar]
- Woodside D G, Wooten D K, Teague T K, Miyamoto Y J, Caudell E G, Udagawa T, Andruss B F, McIntyre B W. Control of T lymphocyte morphology by the GTPase Rho. BMC Cell Biol. 2003;4:2–13. doi: 10.1186/1471-2121-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Bracke M, Leitinger B, Porter J C, Hogg N. LFA-1-induced T cell migration on ICAM-1 involves regulation of MLCK-mediated attachment and ROCK-dependent detachment. J Cell Sci. 2003;116:3123–3133. doi: 10.1242/jcs.00606. [DOI] [PubMed] [Google Scholar]
- Alblas J, Ulfman L, Hordijk P, Koenderman L. Activation of RhoA and ROCK are essential for detachment of migrating leukocytes. Mol Biol Cell. 2001;12:2137–2145. doi: 10.1091/mbc.12.7.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthylake R A, Lemoine S, Watson J M, Burridge K. RhoA is required for monocyte tail retraction during transendothelial migration. J Cell Biol. 2001;154:147–160. doi: 10.1083/jcb.200103048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibaldi E V, Salgia R, Reinherz E L. CD2 molecules redistribute to the uropod during T cell scanning: implications for cellular activation and immune surveillance. Proc Natl Acad Sci USA. 2002;99:7582–7587. doi: 10.1073/pnas.112212699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Fontana L I, Barr V, Samelson L E, Bierer B E. CD28 engagement promotes actin polymerization through the activation of the small Rho GTPase Cdc42 in human T cells. J Immunol. 2003;171:2225–2232. doi: 10.4049/jimmunol.171.5.2225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.