Abstract
Changes in synaptic strength are important for synaptic development and synaptic plasticity. Most directly responsible for these synaptic changes are alterations in synaptic receptor number and density. Although alterations in receptor density mediated by the insertion, lateral mobility, removal, and recycling of receptors have been extensively studied, the dynamics and regulators of intracellular scaffolding proteins have only recently begun to be illuminated. In particular, a closer look at the receptor-associated proteins, which bind to receptors and are necessary for their synaptic localization and clustering, has revealed broader functions than previously thought and some rather unexpected thematic similarities. More than just “placeholders” or members of a passive protein “scaffold,” receptor-associated proteins in every synapse studied have been shown to provide a number of signaling roles. In addition, the most recent state-of-the-art imaging has revealed that receptor-associated proteins are highly dynamic and are involved in regulating synaptic receptor density. Together, these results challenge the view that receptor-associated proteins are members of a static and stable scaffold and argue that their dynamic mobility may be essential for regulating activity-dependent changes in synaptic strength.—Bruneau, E. G., Esteban, J. A., Akaaboune, M. Receptor-associated proteins and synaptic plasticity.
Keywords: rapsyn, gephyrin, PSD-95, scaffold, dynamics
The recruitment and maintenance of a high density of ionotropic receptors at postsynaptic sites is necessary for proper synaptic function. Associated with receptors is a group of proteins that make up the postsynaptic density and provide an intracellular scaffold for receptor anchoring. Although traditionally thought to serve primarily as receptor anchors or placeholders, receptor-associated proteins have been shown recently to play a central role in the regulation of intracellular signaling and the maintenance of the scaffolding complex. Through their many protein-interacting domains, receptor-associated proteins are able to regulate directly and/or indirectly the dynamics of postsynaptic receptors and other scaffold proteins, implicating them as central organizers of the postsynaptic apparatus.
Mounting evidence has also challenged the view of the receptor-associated protein as a member of a relatively stable postsynaptic scaffold. Interestingly, the picture emerging from recent work is a postsynaptic structure in which receptor-associated proteins are highly dynamic, selectively regulated, and crucially involved in modulating changes in receptor density, both during development and at the mature, dynamic postsynaptic apparatus.
At the cholinergic neuromuscular junction (NMJ), the receptor-associated protein of the synapse (rapsyn) is responsible for receptor anchoring and is crucial for the initiation of acetylcholine receptor (AChR) clustering (1, 2). Analogously, at inhibitory synapses in the central nervous system, the receptor-associated protein gephyrin is necessary for the aggregation of glycine (3, 4) and many subtypes of γ-aminobutyric acid (GABAA) receptors (5, 6). In excitatory central synapses, the glutamatergic AMPA and NMDA receptors are similarly tethered in place by protein linkers. Anchoring at glutamatergic synapses, however, is not mediated by a single receptor-associated protein, but instead by a family of PDZ-domain-containing proteins, including the most prevalent member, PSD-95, which binds directly to NMDA receptors and indirectly to AMPA receptors (7).
Rapsyn, gephyrin, and PSD-95 are not the only receptor-associated proteins that have been identified at cholinergic, GABAergic, glycinergic, and glutamatergic synapses. A handful of other important proteins have been shown to directly bind to and potentially regulate receptor trafficking, activity, or surface expression. However, rapsyn, gehpyrin, and PSD-95 have been most fully described in terms of structure, binding partners, and dynamics, and they have all been shown to be critically involved in receptor clustering at postsynaptic sites. Although the roles that a number of other scaffolding proteins play in the trafficking and clustering of receptors will be discussed, we will focus this review on rapsyn, gephyrin, and PSD-95: components of the intracellular scaffold that illustrate a number of the ways in which the character of the receptor-associated protein is being re-envisioned.
RAPSYN AND CHOLINERGIC SYNAPSES
At the peripheral NMJ, pentameric AChRs mediate the activation of muscle action potentials. As with all synaptic sites, the clustering of receptors in direct apposition to the presynaptic nerve terminal is important for strong and effective synaptic transmission. Proteins that regulate the accumulation of AChRs at the synapse are therefore essential for establishing synaptic transmission and regulating synaptic strength. One protein shown to bind to the AChR through yeast two-hybrid studies is the wnt signaling component, adenomatous polyposis coli (APC) (8). Interference of this binding with dominant negative constructs decreased the number of AChR clusters on cultured muscle cells, indicating that wnt signaling may have some regulatory effect on AChR clustering. The other identified binding partner of the AChR is rapsyn. Rapsyn binds both to β-catenin (9) and to β–dystroglycan (2, 10, 11), which both have the potential to anchor receptors to the intracellular protein scaffold (Fig. 1A).
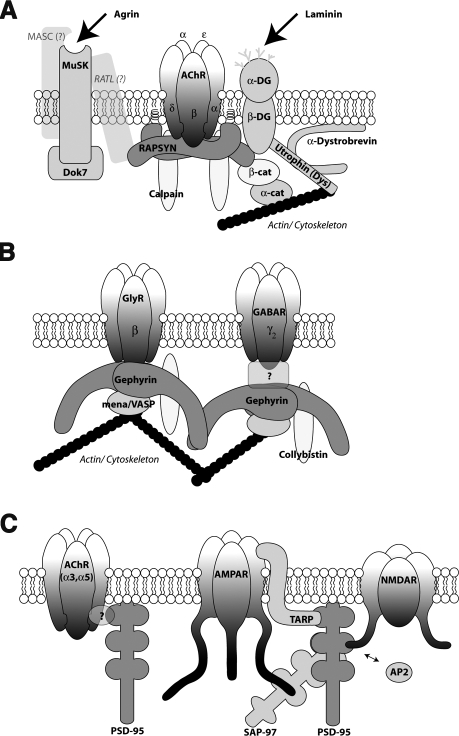
Figure 1.
Rapsyn, gephyrin, and PSD-95 as scaffolding proteins and signaling molecules. An illustration of rapsyn, gephyrin, and PSD-95 (red) and some of the scaffolding proteins (blue) and signaling molecules (yellow) with which they associate. A) At acetylcholine receptor clusters, rapsyn binds the intracellular portion of the receptor subunits. The activation of rapsyn and the clustering of receptors are initiated by agrin or laminin, and rapsyn stabilizes receptors through a direct association with β-dystroglycan and β-catenin. Cytoskeletal reorganization is controlled by the association of rapsyn to calpain. B) At inhibitory synapses, glycine and/or GABA receptors mediate neurotransmission. The activation of gephyrin clustering requires glycine receptor activity, and gephyrin binds directly to the intracellular loop of the glycine receptor γ-subunit. Direct binding of gephyrin to the GABA receptor has not been observed, but gephyrin is required for γ2-GABA receptor subunit clustering. Like rapsyn, gephyrin links receptors to the intracellular cytoskeleton (through mena/VASP) and regulates signaling through interactions with collybistin. C) The clustering of AMPA, NMDA, and nACh receptors at excitatory central synapses is mediated by PDZ proteins, including the most prevalent member of that family, PSD-95. PSD-95 binds directly to the NMDA receptor and indirectly to AMPA receptors through intermediate TARP and/or SAP-97 proteins. Although PSD-95 associates with nAChRs in central synapses, it is not known whether this interaction is direct.
The essential role of rapsyn in the anchoring of AChRs to the scaffold and the clustering of receptors at postsynaptic sites in vivo is illustrated by the complete lack of clustered receptors at nerve endplates in rapsyn−/− mice and the lack of receptor clusters on myotubes generated from these mice, even as overall surface AChR expression does not decrease (12, 13). Interestingly, it has recently been shown that depletion of rapsyn expression with RNAi in adult rat muscle fibers in vivo also results in the rapid disappearance of AChR clusters, showing that rapsyn is necessary for both the formation and the maintenance of AChR clustering (14). In contrast to muscle cells, the size and density of nicotinic AChR clusters in neurons of the superior cervical ganglia are not altered in rapsyn−/− mice, indicating that rapsyn may play a different role in muscle cells and neurons (4).
Rapsyn and AChR are expressed on the muscle surface in a 1:1 ratio (15). Changes in this ratio have been shown to alter surface AChR density, suggesting that modulation of rapsyn expression could regulate receptor density (16,17,18). One scaffolding component that has been shown to alter rapsyn protein expression is β-catenin. Aside from potentially linking the rapsyn-AChR complex to cytoskeletal components through α-catenin, β-catenin has been shown recently to regulate rapsyn protein levels by altering mRNA expression (19). In this study, the introduction of a metabolically stabilized β-catenin construct decreased both the production of a rapsyn reporter and the number of AChR clusters on muscle cells. Electroporation of this construct in vivo also caused a decrease in the size of NMJs. Conversely, decreases in β-catenin have been shown to increase AChR expression both in vitro and in vivo (9, 20). The generation of β-catenin conditional-knockout mice has provided evidence that muscle β-catenin is not only involved in the postsynaptic maturation but also in presynaptic differentiation by controlling retrograde signals that are required for presynaptic differentiation through a canonical signaling pathway. Further work will be needed to determine the identity of these retrograde signals generated by β-catenin action. Another recent study has shown that receptor clustering at postsynaptic sites is mediated by the increased association of rapsyn to calpain, a protease that initiates AChR dispersal when unbound (21). When calpain activity was blocked, either by pharmacological agents or RNAi, receptor stability was compromised. Since rapsyn is expressed almost exclusively at the synapse in the mature muscle cell, rapsyn’s association to calpain prevents declustering only at synaptic sites and allows extrasynaptic dispersal. It is possible that rapsyn/calpain association/dissociation may be one of mechanisms that underlies the dramatic difference in receptor density at synaptic and extrasynaptic sites.
In addition to its critical role in AChR clustering, rapsyn also may play a similar role in clustering other scaffolding proteins. Studies investigating the agrin-signaling cascade, which initiates receptor clustering at nerve endplates in vivo, support this conclusion, implicating rapsyn not only as an integral component of this clustering cascade, but a central component that actively recruits other scaffolding components (22). This conclusion is supported by evidence that postsynaptic sites on muscles from rapsyn−/− mice show disruptions of the dystrophin-glycoprotein complex, with near-complete absence of utrophin and syntrophin (13). It is not clear whether the lack of intracellular scaffolding proteins in rapsyn−/− mice is directly linked to the absence of rapsyn itself, or whether the resulting lack of AChR clustering prevents the accumulation of scaffolding proteins. Evidence for the latter possibility is supported by recent findings from our laboratory. In this work, we demonstrated that exposure of AChRs labeled with Alexa 594 conjugates on cultured myotubes to low-powered laser light caused the dissipation of the illuminated AChRs from clusters, which, in turn, induced the removal of scaffolding proteins and prevented the accumulation of new AChRs and associated scaffolding proteins at the illuminated site. Genetic approaches from other labs have similarly found that AChRs are required for the proper subsynaptic localization of rapsyn in zebrafish (23, 24). Regardless of the particular mechanism, the lack of scaffolding proteins at postsynaptic sites in rapsyn−/− mice indicates that rapsyn is involved, at least indirectly, in the clustering of the intracellular scaffolding complex. These emerging data implicate rapsyn not only as an AChR anchor, but also as a regulator of signaling cascades, which themselves are capable of altering AChR clustering.
The regulation of receptor density at synaptic sites has been extensively studied at the NMJ, and the insertion, removal, recycling, and lateral migration of receptors into and out of the postsynaptic membrane have all been shown to be involved in maintaining a dynamic equilibrium of synaptic AChRs (25,26,27,28). However, little is known about the dwell-time of the rapsyn molecule at the postsynaptic scaffold. Recently, our lab has begun to examine the dynamics of rapsyn, yielding interesting observations about the synaptic stability and regulation of this protein. Using fluorescent recovery after photobleaching (FRAP), we have found that rapsyn turns over at aneural cluster sites on cultured muscle cells more rapidly than AChRs and that rapsyn is unaffected by protein phosphatase inhibition that causes significant changes in receptor removal and insertion (29). This result supports previous biochemical analysis indicating that the metabolic turnover of rapsyn in muscle cells is very rapid (30). Recent in vivo studies in our lab have confirmed that rapsyn turns over rapidly at the innervated NMJ (unpublished data).
While AChR turnover and trafficking are dramatically altered by activity (25, 28, 31), rapsyn turnover in vivo appears to be unaffected by postsynaptic activity blockade (unpublished data), indicating that both in vitro and in vivo rapsyn and AChRs are regulated by different mechanisms. It is possible that changing the turnover of receptor-associated proteins at cluster sites may be a factor regulating receptor density, and this intriguing possibility is currently under investigation in our lab. At the very least, the fast turnover of rapsyn could allow for rapid changes in rapsyn density within synaptic domains, which could drive alterations in the intracellular scaffold morphology and local AChR density during development and aging.
In humans, rapsyn has been implicated in muscular dystrophies, including myasthenic syndrome. One hallmark of this illness is a dramatic decrease in postsynaptic AChR density, and mutations in the rapsyn gene or promoter region have been shown to be the primary cause of synaptic pathogenesis in a number of reported cases (32,33,34,35). In addition to the disruption of junctional folding caused by rapsyn mutations or deficiencies, mutations in the rapsyn protein cause a decrease in the number of postsynaptic rapsyn and receptor proteins. The use of mutant rapsyn-GFP fusion proteins should enable a very complete investigation of the molecular-level pathophysiology of these muscular dystrophies.
GEPHYRIN AND GLYCINERGIC SYNAPSES
In inhibitory synapses of the brain and spinal cord, the majority of synaptic transmission is enabled by glycine and GABA receptors. GABAA receptors are pentameric ionotropic receptors composed of a wide diversity of receptor subunits. The intracellular loops of these receptor subtypes have been shown to associate with a number of proteins, including the Golgi-specific DHHC zinc finger protein (GODZ), phospholipase C-related, catalytically inactive proteins (PRIP), Plic-1, and the GABA receptor-associated protein (GABARAP), all of which will be described below. However, one protein that has been shown to be crucial for the clustering of glycine receptors and many types of GABAA receptors is the 93-kDa protein gephyrin.
Analogous to rapsyn, gephyrin has been shown to play a crucial role in the clustering and localization of glycine and GABAA receptors at inhibitory synapses in the brain and spinal cord (3,4,5,6, 36, 37). Gephyrin is a tubulin-binding protein that associates directly with the intracellular loop of the glycine receptor β-subunit and indirectly through a putative linker protein to the intracellular portion of γ-subunit containing GABAA receptors (Fig. 1B) (38). Similar to rapsyn knockouts, glycine receptor clustering is absent in mice that are lacking gephyrin either because of genetic deletion or RNAi (3, 4, 39); at the same time, α2- and γ2-containing GABAA receptor clustering is dramatically decreased in gephyrin knockout mice (6, 40, 41), even though overall GABAAR surface expression remains unaffected (6, 42). Further, it has been shown that GABAergic inhibitory postsynaptic currents, synaptic plasticity and performance on learning tasks are all significantly compromised when gephyrin expression or clustering is disrupted (41, 43), while whole cell GABA-mediated currents are unchanged (41). This indicates that gephyrin may be necessary for both the initiation and maintenance of inhibitory receptor clustering, which is required for proper synaptic function.
In addition to functional binding domains on gephyrin, which allow self-association and the association of gephyrin with scaffolding proteins, gephyrin binds to a number of signaling molecules that may help regulate synaptic structure, including collybistin a guanine nucleotide exchange factor. Similar to the role of calpain in AChR clustering at the NMJ, collybistin has been proposed to modulate cytoskeletal remodeling and be involved in gephyrin and GABAAR clustering (43,44,45). For example, collybistin knockout mice display dramatic decreases in GABAAR clustering in amygdala and hippocampus and show deficits in synaptic plasticity and learning (43). Also, on the list of regulatory proteins that bind to and are potentially modulated by gephyrin is RAFT1 (mTOR), which is a regulator of mRNA translation that has been shown to be active only if bound to gephyrin (46). Finally, gephyrin binds to the actin-associating mena/VASP (vasodilator stimulated protein) and profilin proteins (47), which are involved in actin regulation, implying that gephyrin not only relies on the cytoskeleton for anchoring but may be directly involved in regulating cytoskeletal dynamics and thereby altering synaptic structure.
Although its association with GABAARs is likely transient and restricted to export from the Golgi, GODZ has been shown to bind directly to the palmitoylated fragments of the GABAAR intracellular loop (48). GODZ is localized to Golgi regions, so it has been suggested that it palmitoylates GABAARs before exocytosis. A protein that links to GABAARs both intracellularly and at synaptic sites is Plic-1 (49). Overexpression of the ubiquitin-like Plic-1 protein has been shown to increase the intracellular half-life of the GABAAR, potentially by disrupting polyubiquitination, and increase surface expression of GABAARs. Introduction of a GABAAR-Plic-1 interfering peptide decreases surface expression of GABAARs. This suggests that Plic-1 may be involved in mediating GABAAR interactions with the intracellular scaffold.
By far, the most studied of the GABAAR associated proteins is the ubiquitin-like, 117-aa GABARAP. GABARAP binds to the large intracellular loop of the gamma GABAA subunits and colocalizes with GABAA, mostly in intracellular compartments. Initial findings that GABARAP increases GABAA clustering when coexpressed in heterologous cells suggested that GABARAP may serve to link GABAA receptors to an intracellular scaffold, much like the role of rapsyn in AChR clustering. However, GABARAP knockout mice were shown to cluster GABARs normally and were phenotypically indistinguishable from wild-type mice (50). The intracellular colocalization of GABARAP with GABAARs in neurons suggests that GABARAP may be more involved with the regulation of GABAAR trafficking or targeting, a supposition that has been supported by recent work (51, 52).
GABARAP binds with a number of other proteins that serve to potentially modify and regulate the GABAAR. For example, GABARAP binds to the PDZ protein GRIP-1 (53) and to NSF (51, 54), which are both involved in the regulation of AMPAR trafficking in excitatory synapses (55). GABARAP has also been shown to associate, mostly in intracellular compartments, with PRIP, a protein that also binds to protein phosphatase 1-α (PP1-α) (56). Mice deficient in PRIP show significant increases in PP1-α activity, suggesting that PRIP modifies the GABAAR by indirectly bringing it into close association with PP1-α. While PRIP knockout mice show no change in GABAA surface receptor number, binding sites, or electrophysiology, they do show alterations in response to GABAAR drugs and cofactors, and the mice have behavioral impairments in coordination (56). It has therefore been suggested that PRIP and PP1-α are involved in phospho-dependent modulation of GABAA that is enabled by their association with GABARAP.
A more direct role of GABARAP in GABAAR-mediated synaptic plasticity was recently demonstrated in cerebellar Perkinje neurons (57). In cell culture preparations, it was shown that the association of GABARAP with both GABAARs and microtubules was required for the late phase of rebound potential (RP). In this study, they found that competitive inhibition of GABARAP-GABAAR association prevented late-phase RP without altering subcellular localization or surface expression patterns of GABAARs. A GABARAP truncation mutant unable to bind to tubulin similarly prevented RP while leaving surface expression and localization unaltered. Together, these studies suggest that GABARAP is a signaling platform that may aid in the trafficking, delivery, and modification of GABAARs but is likely not involved in the anchoring of GABAARs to the intracellular scaffold or the accumulation of receptors at synaptic sites.
The dynamics of the glycine receptor have recently been investigated in a series of elegant single-particle tracking experiments. Twenty-five years ago, investigation of AChR lateral diffusion led to the “diffusion-trap” model of synaptic receptor accumulation (58). Single-particle imaging studies have allowed a more direct investigation of receptor mobility at glycinergic synapses. Using coated beads, rapidly bleaching protein fluorophores and nonbleaching quantum dots, we have shown that individual glycine receptors move with near-Brownian motion outside of synaptic sites but slow considerably once they are within the synaptic region (59,60,61). Recently, in a series of elegant experiments, the Triller group has shown that glycine receptors, but not GABAARs, are highly dynamic in response to NMDAR activity and intracellular calcium release (36).
Gephyrin appears to be critical for the trapping and stabilization of glycine receptors, as cotransfection of glycine receptors and gephyrin in heterologous cells or the overexpression of gephyrin in neuronal cells dramatically decreases receptor lateral mobility (59). Similarly, when gephyrin surface expression is decreased with shRNA or with a dominant-negative gephyrin construct, glycine receptor mobility increases (61).
Although the dynamics of GABAA receptors have not been examined as extensively as ACh or glycine receptors, studies using recombinant GABAARs containing either the bungarotoxin binding site (62) or a tag allowing electrophysiological identification (63) have found that GABAARs are rapidly inserted and removed at the neuronal membrane and that they shuttle rapidly between synaptic and extrasynaptic regions by lateral diffusion. As with glycine receptors, gephyrin appears to play a critical role in the regulation of GABAAR dynamics, as disruption of gephyrin with RNAi decreases the number and density of GABAAR clusters, while increasing GABAAR cluster mobility on the surface of cultured hippocampal neurons (40).
The dynamics of gephyrin itself have also recently begun to be investigated. Using recombinant gephyrin fused to a fluorescent marker and time-lapse imaging, Hanus et al. (64) measured the overall movement of individual gephyrin clusters in cultured spinal cord neurons following pharmacological manipulation. By examining intracluster fluctuations over short time periods and the movement of the entire cluster over longer time periods, we found that gephyrin dynamics within clusters were decreased when F-actin was disrupted, while movements of the entire clusters were tubulin dependent. Interestingly, synaptic activity specifically stabilized the intracluster movement of gephyrin. Although these studies did not examine the insertion, removal, or lateral mobility of gephyrin into or out of individual synaptic sites, they nevertheless support a model in which receptor-associated protein dynamics potentially play a role in regulating changes in receptor density at postsynaptic sites.
PSD-95 AND GLUTAMATERGIC SYNAPSES
Most excitatory transmission in the mammalian brain is mediated by glutamate. At glutamatergic synapses, anchoring of the main receptor types is facilitated by a family of PDZ binding proteins. Most ubiquitous and best characterized of these glutamatergic postsynaptic density (PSD) proteins is PSD-95, which binds directly to NMDARs through the C terminus of the NR2 subunit, and indirectly to AMPARs through interactions with SAP-97 and auxiliary TARPs (Fig. 1C) (65,66,67,68,69). Unlike rapsyn or gephyrin, PSD-95 does not appear to be necessary for initial NMDA receptor clustering: although PSD-95 clusters NMDARs when the two proteins are coexpressed in heterologous cells (70), genetic knockouts still display NMDAR clusters, and loss-of-function constructs have no effect on postsynaptic NMDAR accumulation or NMDAR-mediated excitatory postsynaptic currents (EPSCs) (71, 72). Further, time-lapse imaging indicates that NMDA receptors actually begin to cluster at postsynaptic sites prior to the arrival of PSD-95 (73). However, PSD-95 does appear to contribute to some extent to NMDAR clustering in mature synapses. For example, simultaneous shRNA-mediated knockdown of PSD-95 and the closely related protein PSD-93 (chapsyn-110) produces a small decrease in NMDAR-mediated synaptic transmission, whereas each individual knockdown does not (74). Nevertheless, and in contrast to its limited role on NMDAR targeting, there is abundant evidence that PSD-95 is most critically involved in regulating AMPA receptor density at synapses. For example, acute reduction in PSD-95 levels results in decreased AMPA receptor-mediated synaptic responses (71, 72). Conversely, overexpression of PSD-95 greatly increases AMPA receptor presence at synapses, with no effect on NMDA receptors (68, 71, 72, 75,76,77,78). On the basis of these combined observations, PSD-95 is thought to be a fundamental molecular scaffold for the anchoring of AMPA receptors at the postsynaptic membrane. In agreement with this interpretation, it has been recently shown that PSD-95 retains (or stabilizes) AMPA receptors at synapses by restricting their lateral diffusion within the postsynaptic membrane (79). This mechanism can effectively modulate synaptic strength in a subsecond timescale (80).
Similar to rapsyn and gephyrin, PSD-95 has multiple interaction domains capable of mediating self-association and binding structural proteins (81,82,83). PSD-95 also serves as a central organizer of a number of signaling molecules (82, 83,84,85,86,87). For example, PSD-95 associates with Src-family kinases, reduces Src-kinase activity and inhibits the phosphorylation of NMDARs (87, 88). Also, associated with PSD-95 are Kalirin-7, a GEF that promotes spine formation, and SPAR, a Rap-specific GTPase-activating protein (RapGAP), which similarly stimulates spine growth (86, 89). This function of PSD-95 as a signaling scaffold is likely to be responsible for its role in the modulation of synaptic plasticity (90, 91).
A final receptor that has been shown to associate with PSD-95 in central synapses is the heteromeric nicotinic AChR (92). Although rapsyn has been shown to be inessential for the clustering on nonmuscle isoforms of the AChR expressed in the CNS and ciliary ganglion (4), PSD-95 has been shown to bind to α3- and α5-containing AChRs in vitro. PDZ association has been shown to be essential for the maturation of nonmuscle nicotinic synapses, and PSD-95 also assists in ACh receptor-mediated signal transduction (92). In central synapses, AChRs are thought to play a regulatory role in neurotransmission, and their activity can be modulated by nicotine (93). It is not entirely clear how nicotine regulates these receptors, but studies in postmortem human brain tissue and in mice chronically exposed to nicotine have shown an increase in the number of nAChRs in central synapses (94, 95). Decreased AChR expression in the CNS has been linked to a number of disorders, including attention deficit disorder, Alzheimer’s disease, Parkinson’s disease, and schizophrenia (96,97,98). Because most nAChRs in the CNS are localized presynaptically rather than postsynaptically (99), more work will have to be done to illuminate the significance of PSD-95-nAChR association in these diseases.
As with rapsyn and gephyrin, recent work has focused on the turnover of PSD-95 as a means of controlling the synaptic localization and density of receptors. Using a PSD-95 fusion construct in hippocampal cultures in vitro and FRAP experiments similar to the ones used to examine rapsyn turnover, investigators have shown that PSD-95 is a fairly stable component of excitatory synapses under basal conditions (100, 101). However, in vivo two-photon excitation of photoactivating GFP (paGFP) fused to PSD-95 indicated that PSD-95 residence time at synaptic sites varies with postnatal age and is regulated by sensory experience (102). This observation suggests that synaptic activity may control the removal and/or insertion of PSD-95 at synaptic sites, which would, in turn, dramatically alter AMPA receptor levels and reset receptor density. Indeed, as mentioned above, experimental manipulation of PSD-95 levels have been shown to dramatically alter AMPAR-mediated currents by altering synaptic expression of AMPA receptors (68, 71, 72, 75,76,77,78).
Although the role of PSD-95 in synaptic plasticity is still debated, a number of studies have shown that the down-regulation of AMPARs resulting from an activation of NMDARs is mediated by changes in synaptic expression of PSD-95. For example, NMDAR activation has been shown to trigger PSD-95 ubiquitination and proteasomal degradation, which, in turn, leads to AMPAR down-regulation and synaptic depression (103). The PSD-95 presence at synapses is also regulated by activity-dependent phosphorylation at Ser-295. In this case, activation of NMDARs during long-term depression has been shown to cause PSD-95 dephosphorylation and dissociation from synapses (104). Finally, PSD-95 is targeted and anchored to synaptic membranes through N-terminal palmitoylation (105, 106). Interestingly, PSD-95 palmitoylation, and therefore synaptic association, has been shown to be reversible and regulated by glutamate receptor activation (107). In conclusion, these three layers of regulation (ubiquitination, phosphorylation, and palmitoylation) strongly suggest that PSD-95 presence at synapses can be dynamically adjusted in response to neuronal activity, and therefore, constitute a mechanism to modify AMPA receptor synaptic number during plasticity.
PSD-95 belongs to a family of membrane-associated guanylate kinases, or MAGUKs (108). There is growing evidence that several MAGUKs, and not only PSD-95, are important players in the regulation of synaptic function and AMPA receptor trafficking (109). However, their high structural similarity has made it difficult to ascertain the specific roles of these synaptic scaffolding proteins, which are likely to display partially redundant functions in most experimental settings.
Only recently, acute knockdown (shRNA) and gene-replacement strategies have started to unveil a more complex, but hopefully more accurate, picture of the specific roles of these proteins in synaptic function and plasticity (74, 91). According to this new evidence, PSD-93 and PSD-95 together are responsible for most AMPA receptor presence at synapses in mature neurons. Thus, acute removal of either MAGUK leads to the loss of roughly 50% of synaptic AMPA receptors, with the double knockdown producing an almost complete depletion of AMPA receptors from synapses (74). The almost additive effect of the PSD-93 and PSD-95 knockdowns strongly suggests that these two MAGUKs control largely separate sets of synapses. Interestingly, this MAGUK specialization is also reflected at the level of postnatal development. Thus, immature (<2 wk old) rodent synapses mostly rely on a different synaptic MAGUK, SAP-102 (74), which is more prevalently expressed in early postnatal development (110). Further work will be necessary to determine whether differences in MAGUK identity endow individual synapses with distinct functional properties.
The repertoire of AMPA receptor binding proteins responsible for receptor trafficking is not limited to these synaptic scaffolding molecules. In fact, the early steps of AMPA receptor transport along the biosynthetic pathway are likely to be controlled by a different set of PDZ proteins. For example, SAP-97, a different MAGUK that binds the GluR1 subunit of AMPA receptors (111), has been shown to interact with the receptor at the level of the endoplasmic reticulum and/or cis-Golgi (112). On a similar note, a macromolecular complex containing the multi-PDZ protein GRIP1 couples GluR2-containing AMPA receptor with kinesin motor proteins for receptor transport along the microtubular cytoskeleton in dendrites (113, 114). In addition to their role in early trafficking, these PDZ proteins may also participate in local changes in synaptic AMPA receptors during plasticity. Thus, SAP-97 has been implicated in activity-dependent targeting of AMPA receptors at synapses (71, 72). Conversely, GRIP1 acts in concert with another PDZ protein, PICK1, to control the regulated removal and recycling of receptors during long-term depression (115,116,117). More extensive information on the variety of AMPA receptor binding proteins and their role in receptor trafficking has been recently presented in several excellent reviews (7, 109, 119, 120).
FUTURE DIRECTIONS AND UNANSWERED QUESTIONS
Extensive study of AChRs at the peripheral NMJ and AMPARs, GABAARs, and glycine receptors at central synapses has detailed much about receptor dynamics. At the NMJ, for example, changes in postsynaptic activity are able to alter the rates of receptor insertion, recycling, and removal (25, 28, 31). At glutamatergic synapses, activity similarly is able to alter the fate of internalized AMPARs (121). The ways in which receptor-associated proteins are regulated and how they, in turn, modulate receptor density are only now beginning to be understood. The work reviewed in this paper represents the initial steps into understanding the dynamics and behavior of receptor-associated proteins; there remain a host of unanswered questions (Fig. 2). For example, it has been shown that rapsyn appears to accompany AChRs in the exocytic pathway (122, 123), but it is not known whether rapsyn similarly traffics with AChRs on internalization or during recycling. This question is fundamental to understanding whether receptor-associated proteins serve as anchors to receptors once they are trapped at synaptic sites, or if the receptor-associated proteins play a more active role, chaperoning receptors during trafficking. Other questions about the cycling and trafficking of receptor-associated proteins remain: are receptor-associated proteins internalized individually or collectively with receptors? Can all receptor-associated proteins internalize and then recycle back to the surface at the same synapse or to neighboring synapses or folds? Do receptor-associated proteins maintain their association with receptors extrasynaptically? In fact, because they are already intracellularly localized, the definitions of such terms as “internalization” and “recycling” are potentially distinct from transmembrane receptors. As the regulated trafficking of receptors has proven to be essential for altering synaptic receptor number so, too, may the regulation of receptor-associated protein trafficking alter synaptic efficacy. A clearer understanding of receptor-associated protein dynamics will be essential to further characterize these essential proteins.
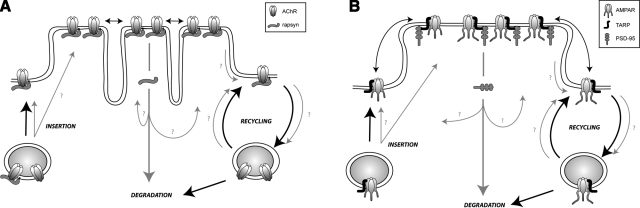
Figure 2.
Receptor and receptor-associated protein dynamics at peripheral cholinergic and central glutamatergic synapses. A) Model suggesting the way by which AChR-associated rapsyn scaffolding protein (red arrows) is removed from and inserted into the neuromuscular junction. There are two possibilities by which rapsyn can be removed: 1) rapsyn migrates with receptors into the perijunctional region (as a transitory station) and then dissociates from receptor before being internalized or translocated along the membrane, 2) rapsyn is directly internalized from the junctional region. The fact that receptors and rapsyn are differentially regulated implies that rapsyn does not follow the same path as the receptor. To maintain normal equilibrium, the insertion rate of rapsyn must equal the removal rate. The insertion could occur either into the perijunctional pool, the junctional pool, or both as denoted by question marks. The recycling of rapsyn is also not yet resolved. B) Model suggesting the way PSD-95 is removed from and inserted into the excitatory synapse. As with rapsyn in peripheral cholinergic synapses, AMPA receptor dynamics have been relatively well characterized (black arrows) while the details of PSD-95 insertion, removal and recycling at central synapses are largely unknown (red arrows).
Because of their intracellular localization, it seems likely that genetic tagging and fluorescent microscopy will continue to drive the research on the trafficking and dynamics of receptor-associated proteins. In fact, previous work has investigated some aspects of receptor-associated protein trafficking using PSD-95-GFP fusion proteins (91, 124). Although it is not known whether it takes an intracellular path or moves exclusively in the plane of the membrane, these studies showed that PSD-95 is able to insert into synapses and rapidly migrate between synaptic sites over time. This indicates that PSD-95 enters and exits a synapse a number of times before being degraded, which is supported by the disparity between PSD-95 synaptic dwell-time (∼1 h) and metabolic half-life (∼36 h) (91, 107). It will be interesting to see whether the recycling of receptor-associated proteins is as prevalent as the recycling of receptors and whether that aspect of dynamics provides another major mechanism by which protein dynamics can be rapidly modulated in order to regulate synaptic strength.
CONCLUSIONS
The rapid turnover rate of postsynaptic density proteins illustrated by rapsyn, gephyrin, and PSD-95 appears to be the rule rather the exception. Along with PSD-95, other PDZ proteins studied as GFP fusion proteins with FRAP both at the drosophila NMJ and in hippocampal neuronal cell cultures have proven to be very dynamic, turning over even more rapidly than PSD-95 (100, 101, 125). Together, these emerging data challenge a view of the postsynaptic scaffold as a static structure. Because many of these scaffolding proteins play an important role in the density, turnover, and localization of postsynaptic receptors, this also indicates that changes in scaffolding protein turnover may have dramatic effects on synaptic structure, development, and plasticity. Given the growing list of roles that postsynaptic proteins play in establishing, maintaining, and modifying the postsynaptic receptor density, it may be time to imagine a different, more all-encompassing moniker for these PSD regulators: the term “scaffolding proteins” seems insufficient to describe the multifaceted nature of these dynamic postsynaptic regulators.
Acknowledgments
This work was supported by the U.S. National Institutes of Health (M.A.) and a National Research Service Award (G.B.), the National Science Foundation (M.A.), and the Muscular Dystrophy Association (M.A.).
References
- Maimone M M, Enigk R E. The intracellular domain of the nicotinic acetylcholine receptor alpha subunit mediates its coclustering with rapsyn. Mol Cell Neurosci. 1999;14:340–354. doi: 10.1006/mcne.1999.0779. [DOI] [PubMed] [Google Scholar]
- Bartoli M, Ramarao M K, Cohen J B. Interactions of the rapsyn RING-H2 domain with dystroglycan. J Biol Chem. 2001;276:24911–24917. doi: 10.1074/jbc.M103258200. [DOI] [PubMed] [Google Scholar]
- Kirsch J, Wolters I, Triller A, Betz H. Gephyrin antisense oligonucleotides prevent glycine receptor clustering in spinal neurons. Nature. 1993;366:745–748. doi: 10.1038/366745a0. [DOI] [PubMed] [Google Scholar]
- Feng G, Tintrup H, Kirsch J, Nichol M C, Kuhse J, Betz H, Sanes J R. Dual requirement for gephyrin in glycine receptor clustering and molybdoenzyme activity. Science. 1998;282:1321–1324. doi: 10.1126/science.282.5392.1321. [DOI] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson J A, Fritschy J M, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Brandstatter J H, Laube B, Stahl S, Muller U, Betz H. Loss of postsynaptic GABA(A) receptor clustering in gephyrin-deficient mice. J Neurosci. 1999;19:9289–9297. doi: 10.1523/JNEUROSCI.19-21-09289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- Wang J, Jing Z, Zhang L, Zhou G, Braun J, Yao Y, Wang Z Z. Regulation of acetylcholine receptor clustering by the tumor suppressor APC. Nat Neurosci. 2003;6:1017–1018. doi: 10.1038/nn1128. [DOI] [PubMed] [Google Scholar]
- Zhang B, Luo S, Dong X P, Zhang X, Liu C, Luo Z, Xiong W C, Mei L. Beta-catenin regulates acetylcholine receptor clustering in muscle cells through interaction with rapsyn. J Neurosci. 2007;22:3968–3973. doi: 10.1523/JNEUROSCI.4691-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel E D, Roberds S L, Campbell K P, Merlie J P. Rapsyn may function as a link between the acetylcholine receptor and the agrin-binding dystrophin-associated glycoprotein complex. Neuron. 1995;15:115–126. doi: 10.1016/0896-6273(95)90069-1. [DOI] [PubMed] [Google Scholar]
- Cartaud A, Coutant S, Petrucci T C, Cartaud J. Evidence for in situ and in vitro association between beta-dystroglycan and the subsynaptic 43K rapsyn protein. Consequence for acetylcholine receptor clustering at the synapse. J Biol Chem. 1998;273:11321–11326. doi: 10.1074/jbc.273.18.11321. [DOI] [PubMed] [Google Scholar]
- Gautam M, DeChiara T M, Glass D J, Yancopoulos G D, Sanes J R. Distinct phenotypes of mutant mice lacking agrin, MuSK, or rapsyn. Brain Res Dev Brain Res. 1999;114:171–178. doi: 10.1016/s0165-3806(99)00013-9. [DOI] [PubMed] [Google Scholar]
- Gautam M, Noakes P G, Mudd J, Nichol M, Chu G C, Sanes J R, Merlie J P. Failure of postsynaptic specialization to develop at neuromuscular junctions of rapsyn-deficient mice. Nature. 1995;377:232–236. doi: 10.1038/377232a0. [DOI] [PubMed] [Google Scholar]
- Kong X C, Barzaghi P, Ruegg M A. Inhibition of synapse assembly in mammalian muscle in vivo by RNA interference. EMBO Rep. 2004;5:183–188. doi: 10.1038/sj.embor.7400065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRochelle W J, Froehner S C. J Biol Chem. 1987;262:8190–8195. [PubMed] [Google Scholar]
- Phillips W D, Vladeta D, Han H, Noakes P G. Rapsyn and agrin slow the metabolic degradation of the acetylcholine receptor. Mol Cell Neurosci. 1997;10:16–26. doi: 10.1006/mcne.1997.0634. [DOI] [PubMed] [Google Scholar]
- Wang Z Z, Mathias A, Gautam M, Hall Z W. Metabolic stabilization of muscle nicotinic acetylcholine receptor by rapsyn. J Neurosci. 1999;19:1998–2007. doi: 10.1523/JNEUROSCI.19-06-01998.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervasio O L, Phillips W D. Increased ratio of rapsyn to ACh receptor stabilizes postsynaptic receptors at the mouse neuromuscular synapse. J Physiol. 2005;562:673–685. doi: 10.1113/jphysiol.2004.077685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ruan N J, Qian L, Lei W L, Chen F, Luo Z G. Wnt/beta-catenin signaling suppresses rapsyn expression and inhibits acetylcholine receptor clustering at the neuromuscular junction. J Biol Chem. 2008;283:21668–21675. doi: 10.1074/jbc.M709939200. [DOI] [PubMed] [Google Scholar]
- Li X M, Dong X P, Luo S W, Zhang B, Lee D H, Ting A K, Neiswender H, Kim C H, Carpenter-Hyland E, Gao T M, Xiong W C, Mei L. Retrograde regulation of motoneuron differentiation by muscle beta-catenin. Nat Neurosci. 2008;11:262–268. doi: 10.1038/nn2053. [DOI] [PubMed] [Google Scholar]
- Chen F, Qian L, Yang Z H, Huang Y, Ngo S T, Ruan N J, Wang J, Schneider C, Noakes P G, Ding Y Q, Mei L, Luo Z G. Rapsyn interaction with calpain stabilizes AChR clusters at the neuromuscular junction. Neuron. 2007;55:247–260. doi: 10.1016/j.neuron.2007.06.031. [DOI] [PubMed] [Google Scholar]
- Apel E D, Glass D J, Moscoso L M, Yancopoulos G D, Sanes J R. Rapsyn is required for MuSK signaling and recruits synaptic components to a MuSK-containing scaffold. Neuron. 1997;18:623–635. doi: 10.1016/s0896-6273(00)80303-7. [DOI] [PubMed] [Google Scholar]
- Bruneau E G, Brenner D I, Kuwada J Y, Akaaboune M. Acetylcholine receptor clustering is required for the accumulation and maintenance of scaffolding proteins. Curr Biol. 2008;18:109–115. doi: 10.1016/j.cub.2007.12.029. [DOI] [PubMed] [Google Scholar]
- Ono F, Higashijima S, Shcherbatko A, Fetcho J R, Brehm P. Paralytic zebrafish lacking acetylcholine receptors fail to localize rapsyn clusters to the synapse. J Neurosci. 2001;21:5439–5448. doi: 10.1523/JNEUROSCI.21-15-05439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaaboune M, Culican S M, Turney S G, Lichtman J W. Rapid and reversible effects of activity on acetylcholine receptor density at the neuromuscular junction in vivo. Science. 1999;286:503–507. doi: 10.1126/science.286.5439.503. [DOI] [PubMed] [Google Scholar]
- Akaaboune M, Grady R M, Turney S, Sanes J R, Lichtman J W. Neurotransmitter receptor dynamics studied in vivo by reversible photo-unbinding of fluorescent ligands. Neuron. 2002;34:865–876. doi: 10.1016/s0896-6273(02)00739-0. [DOI] [PubMed] [Google Scholar]
- Bruneau E G, Macpherson P C, Goldman D, Hume R I, Akaaboune M. The effect of agrin and laminin on acetylcholine receptor dynamics in vitro. Dev Biol. 2005;288:248–258. doi: 10.1016/j.ydbio.2005.09.041. [DOI] [PubMed] [Google Scholar]
- Bruneau E G, Akaaboune M. The dynamics of recycled acetylcholine receptors at the neuromuscular junction in vivo. Development. 2006;133:4485–4493. doi: 10.1242/dev.02619. [DOI] [PubMed] [Google Scholar]
- Bruneau E G, Akaaboune M. The dynamics of the rapsyn scaffolding protein at individual acetylcholine receptor clusters. J Biol Chem. 2007;282:9932–9940. doi: 10.1074/jbc.M608714200. [DOI] [PubMed] [Google Scholar]
- Musil L S, Frail D E, Merlie J P. The mammalian 43-kD acetylcholine receptor-associated protein (RAPsyn) is expressed in some nonmuscle cells. J Cell Biol. 1989;108:1833–1840. doi: 10.1083/jcb.108.5.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneau E, Sutter D, Hume R I, Akaaboune M. Identification of nicotinic acetylcholine receptor recycling and its role in maintaining receptor density at the neuromuscular junction in vivo. J Neurosci. 2005;25:9949–9959. doi: 10.1523/JNEUROSCI.3169-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno K, Sadeh M, Blatt I, Brengman J M, Engel A G. E-box mutations in the RAPSN promoter region in eight cases with congenital myasthenic syndrome. Hum Mol Genet. 2003;12:739–748. doi: 10.1093/hmg/ddg089. [DOI] [PubMed] [Google Scholar]
- Maselli R A, Dunne V, Pascual-Pascual S I, Bowe C, Agius M, Frank R, Wollmann R L. Rapsyn mutations in myasthenic syndrome due to impaired receptor clustering. Muscle Nerve. 2003;28:293–301. doi: 10.1002/mus.10433. [DOI] [PubMed] [Google Scholar]
- Ohno K, Engel A G, Shen X M, Selcen D, Brengman J, Harper C M, Tsujino A, Milone M. Rapsyn mutations in humans cause endplate acetylcholine-receptor deficiency and myasthenic syndrome. Am J Hum Genet. 2002;70:875–885. doi: 10.1086/339465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banwell B L, Ohno K, Sieb J P, Engel A G. Novel truncating RAPSN mutations causing congenital myasthenic syndrome responsive to 3,4-diaminopyridine. Neuromuscul Disord. 2004;14:202–207. doi: 10.1016/j.nmd.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Levi S, Schweizer C, Bannai H, Pascual O, Charrier C, Triller A. Homeostatic regulation of synaptic GlyR numbers driven by lateral diffusion. Neuron. 2008;59:261–273. doi: 10.1016/j.neuron.2008.05.030. [DOI] [PubMed] [Google Scholar]
- Triller A, Choquet D. New concepts in synaptic biology derived from single-molecule imaging. Neuron. 2008;59:359–374. doi: 10.1016/j.neuron.2008.06.022. [DOI] [PubMed] [Google Scholar]
- Meyer G, Kirsch J, Betz H, Langosch D. Identification of a gephyrin binding motif on the glycine receptor beta subunit. Neuron. 1995;15:563–572. doi: 10.1016/0896-6273(95)90145-0. [DOI] [PubMed] [Google Scholar]
- Levi S, Logan S M, Tovar K R, Craig A M. Gephyrin is critical for glycine receptor clustering but not for the formation of functional GABAergic synapses in hippocampal neurons. J Neurosci. 2004;24:207–217. doi: 10.1523/JNEUROSCI.1661-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob T C, Bogdanov Y D, Magnus C, Saliba R S, Kittler J T, Haydon P G, Moss S J. Gephyrin regulates the cell surface dynamics of synaptic GABAA receptors. J Neurosci. 2005;25:10469–10478. doi: 10.1523/JNEUROSCI.2267-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Jiang M, Miralles C P, Li R W, Chen G, de Blas A L. Gephyrin clustering is required for the stability of GABAergic synapses. Mol Cell Neurosci. 2007;36:484–500. doi: 10.1016/j.mcn.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneussel M, Brandstatter J H, Gasnier B, Feng G, Sanes J R, Betz H. Gephyrin-independent clustering of postsynaptic GABA(A) receptor subtypes. Mol Cell Neurosci. 2001;17:973–982. doi: 10.1006/mcne.2001.0983. [DOI] [PubMed] [Google Scholar]
- Papadopoulos T, Korte M, Eulenburg V, Kubota H, Retiounskaia M, Harvey R J, Harvey K, O'Sullivan G A, Laube B, Hulsmann S, Geiger J R, Betz H. Impaired GABAergic transmission and altered hippocampal synaptic plasticity in collybistin-deficient mice. EMBO J. 2007;26:3888–3899. doi: 10.1038/sj.emboj.7601819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kins S, Betz H, Kirsch J. Collybistin, a newly identified brain-specific GEF, induces submembrane clustering of gephyrin. Nat Neurosci. 2000;3:22–29. doi: 10.1038/71096. [DOI] [PubMed] [Google Scholar]
- Erickson J W, Cerione R A. Multiple roles for Cdc42 in cell regulation. Curr Opin Cell Biol. 2001;13:153–157. doi: 10.1016/s0955-0674(00)00192-7. [DOI] [PubMed] [Google Scholar]
- Sabatini D M, Barrow R K, Blackshaw S, Burnett P E, Lai M M, Field M E, Bahr B A, Kirsch J, Betz H, Snyder S H. Interaction of RAFT1 with gephyrin required for rapamycin-sensitive signaling. Science. 1999;284:1161–1164. doi: 10.1126/science.284.5417.1161. [DOI] [PubMed] [Google Scholar]
- Giesemann T, Schwarz G, Nawrotzki R, Berhorster K, Rothkegel M, Schluter K, Schrader N, Schindelin H, Mendel R R, Kirsch J, Jockusch B M. Complex formation between the postsynaptic scaffolding protein gephyrin, profilin, and Mena: a possible link to the microfilament system. J Neurosci. 2003;23:8330–8339. doi: 10.1523/JNEUROSCI.23-23-08330.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller CA, Yuan X, Panzanelli P, Martin ML, Alldred M, Sassoe-Pognetto M, Luscher B. The gamma2 subunit of GABA(A) receptors is a substrate for palmitoylation by GODZ. J Neurosci. 2004;24:5881–5891. doi: 10.1523/JNEUROSCI.1037-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford F K, Kittler J T, Muller E, Thomas P, Uren J M, Merlo D, Wisden W, Triller A, Smart T G, Moss S J. GABA(A) receptor cell surface number and subunit stability are regulated by the ubiquitin-like protein Plic-1. Nat Neurosci. 2001;4:908–916. doi: 10.1038/nn0901-908. [DOI] [PubMed] [Google Scholar]
- O'Sullivan G A, Kneussel M, Elazar Z, Betz H. GABARAP is not essential for GABA receptor targeting to the synapse. Eur J Neurosci. 2005;22:2644–2648. doi: 10.1111/j.1460-9568.2005.04448.x. [DOI] [PubMed] [Google Scholar]
- Leil T A, Chen Z W, Chang C S, Olsen R W. GABAA receptor-associated protein traffics GABAA receptors to the plasma membrane in neurons. J Neurosci. 2004;24:11429–11438. doi: 10.1523/JNEUROSCI.3355-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z W, Chang C S, Leil T A, Olsen R W. C-terminal modification is required for GABARAP-mediated GABA(A) receptor trafficking. J Neurosci. 2007;27:6655–6663. doi: 10.1523/JNEUROSCI.0919-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler J T, Arancibia-Carcamo I L, Moss S J. Association of GRIP1 with a GABA (A) receptor associated protein suggests a role for GRIP1 at inhibitory synapses. Biochem Pharmacol. 2004;68:1649–1654. doi: 10.1016/j.bcp.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Kittler J T, Rostaing P, Schiavo G, Fritschy J M, Olsen R, Triller A, Moss S J. The subcellular distribution of GABARAP and its ability to interact with NSF suggest a role for this protein in the intracellular transport of GABA (A) receptors. Mol Cell Neurosci. 2001;18:13–25. doi: 10.1006/mcne.2001.1005. [DOI] [PubMed] [Google Scholar]
- Hanley J G, Khatri L, Hanson P I, Ziff E B. NSF ATPase and alpha-/beta-SNAPs disassemble the AMPA receptor-PICK1 complex. Neuron. 2002;34:53–67. doi: 10.1016/s0896-6273(02)00638-4. [DOI] [PubMed] [Google Scholar]
- Kanematsu T, Jang I S, Yamaguchi T, Nagahama H, Yoshimura K, Hidaka K, Matsuda M, Takeuchi H, Misumi Y, Nakayama K, Yamamoto T, Akaike N, Hirata M, Nakayama K. Role of the PLC-related, catalytically inactive protein p130 in GABA(A) receptor function. EMBO J. 2002;21:1004–1011. doi: 10.1093/emboj/21.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi S Y, Hirano T. Sustained structural change of GABA(A) receptor-associated protein underlies long-term potentiation at inhibitory synapses on a cerebellar Purkinje neuron. J Neurosci. 2007;27:6788–6799. doi: 10.1523/JNEUROSCI.1981-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S H, Poo M M. Rapid lateral diffusion of extrajunctional acetylcholine receptors in the developing muscle membrane of Xenopus tadpole. J Neurosci. 1983;3:225–231. doi: 10.1523/JNEUROSCI.03-01-00225.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier J, Vannier C, Serge A, Triller A, Choquet D. Fast and reversible trapping of surface glycine receptors by gephyrin. Nat Neurosci. 2001;4:253–260. doi: 10.1038/85099. [DOI] [PubMed] [Google Scholar]
- Dahan M, Levi S, Luccardini C, Rostaing P, Riveau B, Triller A. Diffusion dynamics of glycine receptors revealed by single-quantum dot tracking. Science. 2003;302:442–445. doi: 10.1126/science.1088525. [DOI] [PubMed] [Google Scholar]
- Charrier C, Ehrensperger M V, Dahan M, Levi S, Triller A. Cytoskeleton regulation of glycine receptor number at synapses and diffusion in the plasma membrane. J Neurosci. 2006;26:8502–8511. doi: 10.1523/JNEUROSCI.1758-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanov Y, Michels G, Armstrong-Gold C, Haydon P G, Lindstrom J, Pangalos M, Moss S J. Synaptic GABAA receptors are directly recruited from their extrasynaptic counterparts. EMBO J. 2006;25:4381–4389. doi: 10.1038/sj.emboj.7601309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Mortensen M, Hosie A M, Smart T G. Dynamic mobility of functional GABAA receptors at inhibitory synapses. Nat Neurosci. 2005;8:889–897. doi: 10.1038/nn1483. [DOI] [PubMed] [Google Scholar]
- Hanus C, Ehrensperger M V, Triller A. Activity-dependent movements of postsynaptic scaffolds at inhibitory synapses. J Neurosci. 2006;26:4586–4595. doi: 10.1523/JNEUROSCI.5123-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter A R, Stephenson F A. Coexpression of postsynaptic density-95 protein with NMDA receptors results in enhanced receptor expression together with a decreased sensitivity to L-glutamate. J Neurochem. 2000;75:2501–2510. doi: 10.1046/j.1471-4159.2000.0752501.x. [DOI] [PubMed] [Google Scholar]
- Chetkovich D M, Chen L, Stocker T J, Nicoll R A, Bredt D S. Phosphorylation of the postsynaptic density-95 (PSD-95)/discs large/zona occludens-1 binding site of stargazin regulates binding to PSD-95 and synaptic targeting of AMPA receptors. J Neurosci. 2002;22:5791–5796. doi: 10.1523/JNEUROSCI.22-14-05791.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Ko J, Park E, Lee J R, Yoon J, Lim S, Kim E. Phosphorylation of stargazin by protein kinase A regulates its interaction with PSD-95. J Biol Chem. 2002;277:12359–12363. doi: 10.1074/jbc.M200528200. [DOI] [PubMed] [Google Scholar]
- Schnell E, Sizemore M, Karimzadegan S, Chen L, Bredt D S, Nicoll R A. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc Natl Acad Sci U S A. 2002;99:13902–13907. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C, Li H, Rivera C, Keinänen K. Interaction between SAP97 and PSD-95, two Maguk proteins involved in synaptic trafficking of AMPA receptors. J Biol Chem. 2006;281:4267–4273. doi: 10.1074/jbc.M505886200. [DOI] [PubMed] [Google Scholar]
- Kim E, Niethammer M, Rothschild A, Jan Y N, Sheng M. Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature. 1995;378:85–88. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Futai K, Lashuel H A, Lo I, Okamoto K, Walz T, Hayashi Y, Sheng M. Quaternary structure, protein dynamics, and synaptic function of SAP97 controlled by L27 domain interactions. Neuron. 2004;44:453–467. doi: 10.1016/j.neuron.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Schlüter O M, Xu W, Malenka R C. Alternative N-terminal domains of PSD-95 and SAP97 govern activity-dependent regulation of synaptic AMPA receptor function. Neuron. 2006;51:99–111. doi: 10.1016/j.neuron.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Washbourne P, Bennett J E, McAllister A K. Rapid recruitment of NMDA receptor transport packets to nascent synapses. Nat Neurosci. 2002;5:751–759. doi: 10.1038/nn883. [DOI] [PubMed] [Google Scholar]
- Elias G M, Funke L, Stein V, Grant S G, Bredt D S, Nicoll R A. Synapse-specific and developmentally regulated targeting of AMPA receptors by a family of MAGUK scaffolding proteins. Neuron. 2006;52:307–320. doi: 10.1016/j.neuron.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Béïque J C, Andrade R. PSD-95 regulates synaptic transmission and plasticity in rat cerebral cortex. J Physiol. 2003;546:859–867. doi: 10.1113/jphysiol.2002.031369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Malinow R. Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity. J Neurosci. 2004;24:916–927. doi: 10.1523/JNEUROSCI.4733-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini A E, Schnell E, Chetkovich D M, Nicoll R A, Bredt D S. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- Stein V, House D R, Bredt D S, Nicoll R A. Postsynaptic density-95 mimics and occludes hippocampal long-term potentiation and enhances long-term depression. J Neurosci. 2003;23:5503–5506. doi: 10.1523/JNEUROSCI.23-13-05503.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bats C, Groc L, Choquet D. The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron. 2007;53:719–734. doi: 10.1016/j.neuron.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Heine M, Groc L, Frischknecht R, Béïque J C, Lounis B, Rumbaugh G, Huganir R L, Cognet L, Choquet D. Surface mobility of postsynaptic AMPARs tunes synaptic transmission. Science. 2008;320:201–205. doi: 10.1126/science.1152089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Naisbitt S, Hsueh Y P, Rao A, Rothschild A, Craig A M, Sheng M. GKAP, a novel synaptic protein that interacts with the guanylate kinase-like domain of the PSD-95/SAP90 family of channel clustering molecules. J Cell Biol. 1997;136:669–678. doi: 10.1083/jcb.136.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colledge M, Dean R A, Scott G K, Langeberg L K, Huganir R L, Scott J D. Targeting of PKA to glutamate receptors through a MAGUK-AKAP complex. Neuron. 2000;27:107–119. doi: 10.1016/s0896-6273(00)00013-1. [DOI] [PubMed] [Google Scholar]
- Romorini S, Piccoli G, Jiang M, Grossano P, Tonna N, Passafaro M, Zhang M, Sala C. A functional role of postsynaptic density-95-guanylate kinase-associated protein complex in regulating Shank assembly and stability to synapses. J Neurosci. 2004;24:9391–9404. doi: 10.1523/JNEUROSCI.3314-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J H, Liao D, Lau L F, Huganir R L. SynGAP: a synaptic RasGAP that associates with the PSD-95/SAP90 protein family. Neuron. 1998;20:683–691. doi: 10.1016/s0896-6273(00)81008-9. [DOI] [PubMed] [Google Scholar]
- Tezuka T, Umemori H, Akiyama T, Nakanishi S, Yamamoto T. PSD-95 promotes Fyn-mediated tyrosine phosphorylation of the N-methyl-D-aspartate receptor subunit NR2A. Proc Natl Acad Sci U S A. 1999;96:435–440. doi: 10.1073/pnas.96.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Johnson R C, Sattler R, Zhang X, Huganir R L, Kambampati V, Mains R E, Eipper B A. The neuronal Rho-GEF Kalirin-7 interacts with PDZ domain-containing proteins and regulates dendritic morphogenesis. Neuron. 2001;29:229–242. doi: 10.1016/s0896-6273(01)00193-3. [DOI] [PubMed] [Google Scholar]
- Kalia L V, Salter M W. Interactions between Src family protein tyrosine kinases and PSD-95. Neuropharmacology. 2003;45:720–728. doi: 10.1016/s0028-3908(03)00313-7. [DOI] [PubMed] [Google Scholar]
- Kalia L V, Pitcher G M, Pelkey K A, Salter M W. PSD-95 is a negative regulator of the tyrosine kinase Src in the NMDA receptor complex. EMBO J. 2006;25:4971–4982. doi: 10.1038/sj.emboj.7601342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak D T, Yang S, Rudolph-Correia S, Kim E, Sheng M. Regulation of dendritic spine morphology by SPAR, a PSD-95-associated RapGAP. Neuron. 2001;31:289–303. doi: 10.1016/s0896-6273(01)00355-5. [DOI] [PubMed] [Google Scholar]
- Migaud M, Charlesworth P, Dempster M, Webster L C, Watabe A M, Makhinson M, He Y, Ramsay M F, Morris R G, Morrison J H, O'Dell T J, Grant S G. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature. 1998;396:433–439. doi: 10.1038/24790. [DOI] [PubMed] [Google Scholar]
- Xu W, Schluter O M, Steiner P, Czervionke B L, Sabatini B, Malenka R C. Molecular dissociation of the role of PSD-95 in regulating synaptic strength and LTD. Neuron. 2008;57:248–262. doi: 10.1016/j.neuron.2007.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy W G, Liu Z, Nai Q, Coggan J S, Berg D K. PDZ-containing proteins provide a functional postsynaptic scaffold for nicotinic receptors in neurons. Neuron. 2003;38:759–771. doi: 10.1016/s0896-6273(03)00324-6. [DOI] [PubMed] [Google Scholar]
- Role L W, Berg D K. Nicotinic receptors in the development and modulation of CNS synapses. Neuron. 1996;16:1077–1085. doi: 10.1016/s0896-6273(00)80134-8. [DOI] [PubMed] [Google Scholar]
- Marks M J, Burchs J B, Collins A C. Effects of chronic nicotine infusion on tolerance development and cholinergic receptors. J Pharmacol Exp Ther. 1983;226:817–825. [PubMed] [Google Scholar]
- Breese C R, Marks M J, Logel J, Adams C E, Sullivan B, Collins A C, Leonard S. Effect of smoking history on [3H]nicotine binding in human postmortem brain. J Pharmacol Exp Ther. 1997;282:7–13. [PubMed] [Google Scholar]
- Perry E K, Perry R H, Smith C J, Dick D J, Candy J M, Edwardson J A, Fairbairn A, Blessed G. Nicotinic receptor abnormalities in Alzheimer’s and Parkinson’s diseases. J Neurol Neurosurg Psychiatry. 1987;50:806–809. doi: 10.1136/jnnp.50.6.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro C, Deakin J F. NMDA receptor subunit NRI and postsynaptic protein PSD-95 in hippocampus and orbitofrontal cortex in schizophrenia and mood disorder. Schizophr Res. 2005;80:323–330. doi: 10.1016/j.schres.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Kristiansen L V, Beneyto M, Haroutunian V, Meador-Woodruff J H. Changes in NMDA receptor subunits and interacting PSD proteins in dorsolateral prefrontal and anterior cingulate cortex indicate abnormal regional expression in schizophrenia. Mol Psychiatry. 2006;11:737–747, 705. doi: 10.1038/sj.mp.4001844. [DOI] [PubMed] [Google Scholar]
- Wonnacott S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- Kuriu T, Inoue A, Bito H, Sobue K, Okabe S. Differential control of postsynaptic density scaffolds via actin-dependent and -independent mechanisms. J Neurosci. 2006;26:7693–7706. doi: 10.1523/JNEUROSCI.0522-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K, Fong D K, Craig A M. Postsynaptic protein mobility in dendritic spines: long-term regulation by synaptic NMDA receptor activation. Mol Cell Neurosci. 2006;31:702–712. doi: 10.1016/j.mcn.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Gray N W, Weimer R M, Bureau I, Svoboda K. Rapid redistribution of synaptic PSD-95 in the neocortex in vivo (Online) PLoS Biol. 2006;4:e370. doi: 10.1371/journal.pbio.0040370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colledge M, Snyder E M, Crozier R A, Soderling J A, Jin Y, Langeberg L K, Lu H, Bear M F, Scott J D. Ubiquitination regulates PSD-95 degradation and AMPA receptor surface expression. Neuron. 2003;40:595–607. doi: 10.1016/s0896-6273(03)00687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M J, Futai K, Jo J, Hayashi Y, Cho K, Sheng M. Synaptic accumulation of PSD-95 and synaptic function regulated by phosphorylation of serine-295 of PSD-95. Neuron. 2007;56:488–502. doi: 10.1016/j.neuron.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Craven S E, El-Husseini A E, Bredt D S. Synaptic targeting of the postsynaptic density protein PSD-95 mediated by lipid and protein motifs. Neuron. 1999;22:497–509. doi: 10.1016/s0896-6273(00)80705-9. [DOI] [PubMed] [Google Scholar]
- El-Husseini A E, Craven S E, Chetkovich D M, Firestein B L, Schnell E, Aoki C, Bredt D S. Dual palmitoylation of PSD-95 mediates its vesiculotubular sorting, postsynaptic targeting, and ion channel clustering. J Cell Biol. 2000;148:159–172. doi: 10.1083/jcb.148.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini Ael-D, Schnell E, Dakoji S, Sweeney N, Zhou Q, Prange O, Gauthier-Campbell C, Aguilera-Moreno A, Nicoll R A, Bredt D S. Synaptic strength regulated by palmitate cycling on PSD-95. Cell. 2002;108:849–863. doi: 10.1016/s0092-8674(02)00683-9. [DOI] [PubMed] [Google Scholar]
- Funke L, Dakoji S, Bredt D S. Membrane-associated guanylate kinases regulate adhesion and plasticity at cell junctions. Annu Rev Biochem. 2005;74:219–245. doi: 10.1146/annurev.biochem.74.082803.133339. [DOI] [PubMed] [Google Scholar]
- Elias G M, Nicoll R A. Synaptic trafficking of glutamate receptors by MAGUK scaffolding proteins. Trends Cell Biol. 2007;17:343–352. doi: 10.1016/j.tcb.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Sans N, Petralia R S, Wang Y X, Blahos J, 2nd, Hell J W, Wenthold R J. A developmental change in NMDA receptor-associated proteins at hippocampal synapses. J Neurosci. 2000;20:1260–1271. doi: 10.1523/JNEUROSCI.20-03-01260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard A S, Davare M A, Horne M C, Garner C C, Hell J W. SAP97 is associated with the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit. J Biol Chem. 1998;273:19518–19524. doi: 10.1074/jbc.273.31.19518. [DOI] [PubMed] [Google Scholar]
- Sans N, Racca C, Petralia R S, Wang Y X, McCallum J, Wenthold R J. Synapse-associated protein 97 selectively associates with a subset of AMPA receptors early in their biosynthetic pathway. J Neurosci. 2001;21:7506–7516. doi: 10.1523/JNEUROSCI.21-19-07506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setou M, Seog D H, Tanaka Y, Kanai Y, Takei Y, Kawagishi M, Hirokawa N. Glutamate-receptor-interacting protein GRIP1 directly steers kinesin to dendrites. Nature. 2002;417:83–87. doi: 10.1038/nature743. [DOI] [PubMed] [Google Scholar]
- Shin H, Wyszynski M, Huh K H, Valtschanoff J G, Lee J R, Ko J, Streuli M, Weinberg R J, Sheng M, Kim E. Association of the kinesin motor KIF1A with the multimodular protein liprin-alpha. J Biol Chem. 2003;278:11393–11401. doi: 10.1074/jbc.M211874200. [DOI] [PubMed] [Google Scholar]
- Hanley J G. Molecular mechanisms for regulation of AMPAR trafficking by PICK1. Biochem Soc Trans. 2006;34:931–935. doi: 10.1042/BST0340931. [DOI] [PubMed] [Google Scholar]
- Lu W, Ziff E B. PICK1 interacts with ABP/GRIP to regulate AMPA receptor trafficking. Neuron. 2005;47:407–421. doi: 10.1016/j.neuron.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Lin D T, Huganir R L. PICK1 and phosphorylation of the glutamate receptor 2 (GluR2) AMPA receptor subunit regulates GluR2 recycling after NMDA receptor-induced internalization. J Neurosci. 2007;27:13903–13908. doi: 10.1523/JNEUROSCI.1750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd J D, Huganir R L. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- Greger I H, Esteban J A. AMPA receptor biogenesis and trafficking. Curr Opin Neurobiol. 2007;17:289–297. doi: 10.1016/j.conb.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka R C. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Ehlers M D. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- Marchand S, Bignami F, Stetzkowski-Marden F, Cartaud J. The myristoylated protein rapsyn is cotargeted with the nicotinic acetylcholine receptor to the postsynaptic membrane via the exocytic pathway. J Neurosci. 2000;20:521–528. doi: 10.1523/JNEUROSCI.20-02-00521.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand S, Devillers-Thiery A, Pons S, Changeux J P, Cartaud J. Rapsyn escorts the nicotinic acetylcholine receptor along the exocytic pathway via association with lipid rafts. J Neurosci. 2002;22:8891–8901. doi: 10.1523/JNEUROSCI.22-20-08891.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe S, Kim H-D, Miwa A, Kuriu T, Okado H. Continual remodeling of postsynaptic density and its regulation by synaptic activity. Nat Neurosci. 1999;2:804–811. doi: 10.1038/12175. [DOI] [PubMed] [Google Scholar]
- Rasse T M, Fouquet W, Schmid A, Kittel R J, Mertel S, Sigrist C B, Schmidt M, Guzman A, Merino C, Qin G, Quentin C, Madeo F F, Heckmann M, Sigrist S J. Glutamate receptor dynamics organizing synapse formation in vivo. Nat Neurosci. 2005;8:898–905. doi: 10.1038/nn1484. [DOI] [PubMed] [Google Scholar]