Abstract
Classic tissue engineering paradigms are limited by the incorporation of a functional vasculature and a reliable means for reimplantation into the host circulation. We have developed a novel approach to overcome these obstacles using autologous explanted microcirculatory beds (EMBs) as bioscaffolds for engineering complex three-dimensional constructs. In this study, EMBs consisting of an afferent artery, capillary beds, efferent vein, and surrounding parenchymal tissue are explanted and maintained for 24 h ex vivo in a bioreactor that preserves EMB viability and function. Given the rapidly advancing field of stem cell biology, EMBs were subsequently seeded with three distinct stem cell populations, multipotent adult progenitor cells (MAPCs), and bone marrow and adipose tissue-derived mesenchymal stem cells (MSCs). We demonstrate MAPCs, as well as MSCs, are able to egress from the microcirculation into the parenchymal space, forming proliferative clusters. Likewise, human adipose tissue-derived MSCs were also found to egress from the vasculature and seed into the EMBs, suggesting feasibility of this technology for clinical applications. We further demonstrate that MSCs can be transfected to express a luciferase protein and continue to remain viable and maintain luciferase expression in vivo. By using the vascular network of EMBs, EMBs can be perfused ex vivo and seeded with stem cells, which can potentially be directed to differentiate into neo-organs or transfected to replace failing organs and deficient proteins.—Chang, E. I., Bonillas, R. G., El-ftesi, S., Chang, E. I., Ceradini, D. J., Vial, I. N., Chan, D. A., Michaels, J., V, Gurtner, G. C. Tissue engineering using autologous microcirculatory beds as vascularized bioscaffolds.
Keywords: stem cells, bioreactor, gene therapy
To date, there are over 100,000 Americans awaiting organ transplantation; however, the actual number of available organs will provide for only a fraction of those with end-stage organ failure. Tissue engineering holds the promise of creating replacement tissues and organs outside the human body. Approaches based on the implantation of cells onto resorbable synthetic matrices have had success in replicating simple, relatively avascular structures such as cartilage or bone but have been unable to create more complex parenchymal organs, such as livers or kidneys (1,2,3,4). However, the classic tissue engineering paradigm utilizes cell-based approaches for organ-level problems, and consequently, seeding stem cells onto biodegradable matrices to be vascularized by angiogenesis has been unsuccessful as cellular metabolic needs cannot be met without an intact vascular network (Fig. 1a). Furthermore, the matrix itself introduces a host of additional obstacles related to immunogenic foreign material (5). Until these problems are resolved, even the most idealized results are unlikely to have true clinical applicability.
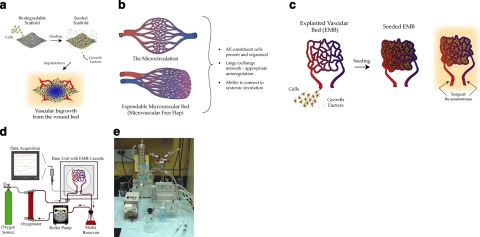
Figure 1.
Bioengineering paradigms. a) The classic tissue engineering paradigm. b) Microvascular beds are a microcosm of the circulatory system, already patterned for nutrient exchange. c) The EMB approach to tissue engineering. d) Schematic illustration of perfusion bioreactor. e) Laboratory setup.
Consequently, extensive research has been focused on designing vascularized constructs using a variety of stem cells, growth factors, sophisticated scaffolds, hydrogels, and arteriovenous loops (6,7,8,9,10,11,12). While some have demonstrated promising results (13,14,15), the ability to recreate the structural and physiological complexity of the native vascular circulation, where a tightly regulated microenvironment of constituent cells, growth factors, and matrix forming a dynamic functional unit remains a difficult challenge.
As such, the de novo creation of a functioning vasculature in vitro to support complex engineered constructs remains speculative. To circumvent the need to engineer a functional microcirculation de novo, we have devised a novel technique using the body’s own preexisting microcirculatory beds as an autologous bioscaffold that already provides a patterned vascular network that can serve as a vascularized construct for tissue engineering (16).
It has been well known that humans possess self-contained expendable microcirculatory beds that can be harvested with minimal residual disability and dysfunction (17, 18). Known as microvascular free flaps consisting of skin, muscle, adipose tissue and even bone, they are routinely employed to reconstruct congenital, traumatic, or ablative defects in our patients. Regarding tissue engineering, these vascular beds are a microcosm of the circulatory system and can serve as a bioscaffold to create autologous, vascularized constructs in vitro (19) (Fig. 1b). Since they contain a single afferent artery and efferent vein, they can be easily reintegrated into the systemic circulation by standard surgical techniques.
Our approach to organ-level tissue engineering uses explanted microcirculatory beds (EMBs), and to our knowledge, the use of free tissue transfer has not been previously described in the tissue engineering literature. We hypothesize that EMBs can be sustained in our bioreactor (ex vivo), seeded with stem/progenitor cells, and reimplanted into a recipient as a vascularized engineered construct (Fig. 1c). In this study, we not only provide proof of concept, but given the rapid advancements in stem cell research, we successfully seeded three distinct stem cell populations onto EMBs, demonstrating the broad potential applicability of our concept to the field of tissue engineering (20). We believe our approach would provide a readily available autologous bioscaffold containing a physiologically patterned vascular network capable of overcoming many hurdles limiting current tissue engineering techniques.
MATERIALS AND METHODS
Animals
A donor/recipient model was used for this study. Adult male and female Fisher rats weighing ≥300 g were used. Nude rats were employed for human mesenchymal stem cell (MSC) seeding. All animals were housed in an approved animal care center with 12:12-h light-dark cycles and provided standard rodent chow and water ad libitum. All animal studies were approved by the New York University (previous studies performed by D.J.C. and J.M.) and Stanford University Institutional Animal Care and Use Committee and were in compliance with the guidelines specified in the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
EMB harvest
The superficial inferior epigastric (SIE) fasciocutaneous EMBs were harvested as described previously (21,22,23). Briefly, rats were anesthetized using inhalational isoflurane gas. An ∼9-cm2 area was marked over the groin, and the SIE EMB was dissected based on the femoral vessels. Prior to detachment from the systemic circulation, 1 ml of heparinized saline (10 U/ml) (24) was flushed through the SIE EMB via the femoral artery. The EMB, comprising skin, adipose tissue, and its supporting vasculature, was detached with maximal femoral vessel pedicle length; transferred to our bioreactor cassette, where the vessels were cannulated onto our custom bioreactor system (Harvard Apparatus, Holliston, MA, USA); and secured using 9-0 nylon sutures.
Bioreactor perfusion system
We used the HSE-HA Perfusion System PS-1 for liver or kidney (Harvard Apparatus) as our bioreactor perfusion system (Fig. 1d, e). This system is composed of a roller pump, perfusate oxygenator, tissue chamber, and data acquisition system. The perfusate is pumped in a unidirectional manner from a reservoir through a heat exchanger to the oxygenator. From there, it passes through the arterial cannula to the EMB and exits passively through the venous cannula back to the reservoir. A popoff valve is integrated into the circuit to prevent damaging high perfusion pressures. The arterial cannula is equipped with sampling ports that allow for the measurement of perfusion pressures, perfusion sampling, and infusion of compounds. The entire system is monitored using customized data acquisition software that measures up to 10 parameters simultaneously in real time.
Bioreactor perfusion procedure
Dulbecco’s modified Eagle medium (DMEM; Invitrogen, Carslbad, CA, USA) supplemented with 5% rat serum, 2% penicillin/streptomycin (Invitrogen), and 20 U/ml heparin (American Pharmaceutical Partners, Schaumberg, IL, USA) was used as our base perfusate. Perfusion was started at 0.2 ml/min with the popoff valve set at the desired pressure setting (60–120 mmHg). The EMB was cast into a 1% PBS-agarose matrix to provide structural support and maintain hydrostatic pressure within the EMB vasculature. For perfusion under constant flow, the flow rate was adjusted to keep the perfusion pressures physiological (≤120 mmHg). For perfusion under constant pressure, the popoff valve was set at a desired physiological pressure and the flow rate gradually increased from 0.2 ml/min to the desired setting (≤5 ml/min).
Bioreactor perfusion experimental protocol
EMBs were perfused with DMEM for 6, 12, 18, and 24 h (n=12/time point). After the perfusion period, randomly selected EMBs (n=4) in each time period were replanted onto recipient rats, and the remaining (n=8) were subjected to tissue viability testing (see below). In our control group, EMBs were perfused for 10 min, detached from the bioreactor circuit, and cultivated in a tissue incubator at 37°C for the same time periods above (n=12 for each time period). Again, EMBs (n=4) were randomly selected and reimplanted while the remaining EMBs were subjected to testing. In our third group, EMBs were perfused with DMEM perfusate supplemented with a cell-free hemoglobin-based oxygen carrier [HBOC, hemoglobin glutamer-200 (bovine), HBOC-301; Oxyglobin, BioPure, Cambridge, MA, USA] (25) at a concentration of 1.3 g/dl for 24 h (n=8). Randomly selected EMBs were replanted (n=4) or subjected to viability testing (n=4).
EMB reimplantation
A recipient rat was used for EMB reimplantation. An identical SIE EMB was dissected on the ipsilateral side, ligated, and discarded, and the perfused EMB was reimplanted using standard microsurgical techniques. Reimplanted EMBs were harvested on postreimplantation day (PRD) 7 for histological analysis.
Viability analysis
We determined EMB viability based on a constellation of independent variables used in the organ perfusion literature (26).
Hemodynamics
Real-time perfusion pressures were measured throughout the perfusion period using specialized sensors that interface with our data acquisition software.
Oxygen tension
Real-time EMB tissue oxygen tensions were measured using the OxyLite oxygen tension probe (Oxford Optronix, Oxford, UK). This sensor utilizes the fluorescent quenching technique, providing a continuous quantitative oxygen tension measurement over a 0.02-mm3 area. A second sensor compensates these data for regional temperature variations, providing an accurate assessment of oxygen tension over time.
Functional vasculature analysis
Endothelial cell function was assessed in vitro by the ability to uptake acetylated LDL from the perfusate (27, 28). Dioctadecyl-tetramethylindo-carbocyanine perchlorate-labeled acetylated LDL (DiI-acLDL, Biomedical Technologies, Stoughton, MA, USA) was added to the perfusate and circulated for 2 h. Unbound DiI-acLDL was flushed from the EMB. Prior to removal from the bioreactor, FITC-labeled Lycopersicon esculentum lectin (Vector Laboratories, Burlingame, CA, USA) was added to the perfusate to stain all perfused vasculature. Functional endothelial beds were identified by colocalization of lectin stain (perfused) and uptake of DiI-acLDL (functional). Selected samples were mounted in DAPI medium (Vector Laboratories) for immunofluorescence microscopy.
Nitro-blue tetrazolium (NBT) staining
Overall tissue viability was assessed with NBT (Sigma-Aldrich, St. Louis, MO, USA). Briefly, perfused EMBs were sectioned transversely into 1-mm-thick segments and incubated in 0.2% NBT for 1 h. Sections were digitally photographed, and the percentage of NBT staining was calculated using digital analysis software (SigmaScan; Jandel Corp., San Rafael, CA, USA). Freshly harvested EMBs served as a positive control. Tissue sections in each experimental group were also processed using routine histochemical techniques [hematoxylyn-and-eosin (H&E) staining].
Reimplantation
Ultimately, reimplantation of perfused EMBs served as the gold standard of viability. Failure manifested as frank tissue necrosis within 3–4 days.
Multipotent adult progenitor cell (MAPC) experiments
MAPCs are a population of primitive progenitor cells harvested from adult bone marrow that can be sustained in an undifferentiated state over an extended period and maintain the capacity to differentiate across embryonic lineages, making them an ideal candidate for use in tissue engineering (29). MAPCs from male Fisher rats were harvested and culture-expanded as described previously (29). Briefly, bone marrow was seeded onto fibronectin-coated plates (Becton Dickinson, Bedford, MA, USA) in medium consisting of 60% DMEM-LG (Invitrogen), 40% MCDB-201 (Sigma-Aldrich), with 1× insulin-transferrin-selenium, 1× lenolenic acid-bovine serum albumin, 10−9 M dexamethasone, 10−4 M ascorbic acid 2-phosphate (Sigma-Aldrich), 2% fetal calf serum (FCS; Hyclone Laboratories, Logan, UT, USA), 10 ng/ml epidermal growth factor, 10 ng/ml platelet-derived growth factor (R&D Systems, Minneapolis, MN, USA), 100 U penicillin, and 1000 U streptomycin (Invitrogen). After 4 wk of expansion, CD45+ and Ter119+ cells were depleted using a MACS separation CS column (Miltenyi Biotec, Auburn, CA, USA).
For MAPC seeding experiments, MAPCs harvested from male donors were labeled with DiI (Invitrogen) according to manufacturer’s instructions and seeded onto female EMBs through the bioreactor for 16 h. Following perfusion, randomly selected MAPC-seeded EMBs (n=5) were reimplanted or perfused with FITC-lectin and subjected to immunohistochemical analysis (n=3). EMBs (n=5) were harvested on PRD 7 after intraperitoneal administration of bromodeoxyuridine (BrdU; 100 mg/kg; Sigma-Aldrich) for immunohistochemical analysis. Identification of seeded MAPCs was performed by immunohistochemistry and fluorescence in situ hybridization (FISH) for the Y-chromosome (CamBio, Cambridge, UK).
MSC experiments
MSCs are another self-renewing population of multipotent cells that can be harvested from either bone marrow or adipose tissue (30,31,32). Bone marrow-derived MSCs were isolated from the femurs and tibiae of newborn Fisher rats. The extracted bone marrow was passed through a 100-μm Falcon nylon cell strainer (Becton Dickinson), centrifuged at 1200 g, and plated in DMEM (Invitrogen) supplemented with 10% fetal bovine serum (FBS, Invitrogen) and 1% antibiotic-antimycotic (Invitrogen).
Adipose-derived MSCs were harvested from human lipoaspirates performed on healthy adults in accordance with Stanford University Hospital Institutional Review Board guidelines. The adipose tissue was digested with 0.075% type II collagenase (Sigma) and incubated in a 37°C shaking water bath for 30 min. Samples were then centrifuged at 1200 g, passed through a 100-μm Falcon nylon cell strainer (Becton Dickinson), and plated in DMEM supplemented with 10% FBS and 1% antibiotic-antimycotic.
Rat bone marrow-derived MSCs were labeled with PKH26 red fluorescent cell linker (Sigma), according to manufacturer’s instructions, seeded onto EMBs (n=3), and reimplanted, as described previously. Similarly, human adipose tissue-derived MSCs were also labeled with PKH26 and seeded onto nude rat EMBs (n=3) before reimplantation into nude rat recipients. EMBs were harvested on PRD7 for analysis.
MSC transfection
A Renilla luciferase construct under the CMV promoter (Promega, Madison, WI, USA) was transfected into passage 2–3 primary rat bone marrow-derived MSCs using Lipofectamine Plus (Invitrogen), according to manufacturer’s instructions. Coelenterazine (Biotium Inc., Hayward, CA, USA) substrate (1 μg/ml) was added to MSC cultures prior to imaging on IVIS Spectrum to determine efficiency of transfection (Stanford University Small Animal Imaging Facility). 2.5 × 106 transfected MSCs were seeded onto the EMB using direct subcutaneous injection (n=3) or through the bioreactor (n=3). Coelenterazine was injected through a standard tail vein injection prior to IVIS imaging. Animals with EMBs seeded with transfected MSCs that were not given coelenterazine served as controls (n=3).
Statistical analysis
Data were analyzed with SigmaStat software (Jandel Corp.). Results are presented as means ± sd. Statistical differences were analyzed by unpaired two-tailed Student’s t test, one-way ANOVA, and the Tukey test. Probability values of P < 0.05 were considered statistically significant.
RESULTS
EMBs can be successfully perfused ex vivo in a custom-designed bioreactor
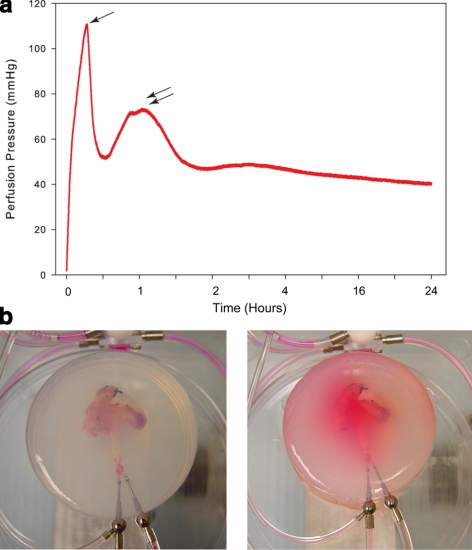
Initial studies were aimed at adjusting the flow rate to mimic physiological perfusion pressures (40–120 mmHg; measured while EMB in situ), while perfusing under constant flow conditions. A flow rate of <0.2 ml/min resulted in physiological perfusion pressures and maintenance of passive venous return throughout the extended perfusion period (≤24 h). Encasing the EMB in agarose during the initial perfusion period resulted in a reduction of early high perfusion pressures as a result of a vasodilatory effect secondary to the warm agarose. Perfusion pressures were also noted to decrease with extended perfusion as the EMB core temperature was warmed to physiological temperature (37°C) (Fig. 2a). Minimal edema and perfusate leakage were noted at the end of the perfusion period (Fig. 2b), which was further minimized by maintaining perfusion pressures within the lower physiological range (40–80 mmHg).
Figure 2.
Perfusion pressures maintained at physiological levels with use of agarose gel. a) Ex vivo perfusion pressure curve demonstrating initial perfusion period with high pressures. After addition of warm PBS-agarose matrix, perfusion pressures decrease (single arrow). Solidifying agarose caused a transient increase in pressures (double arrows), followed by a gradual decrease with the extended perfusion period. b) EMBs are cast into a PBS-agarose matrix at the start of the perfusion period. c) Following extended perfusion, there is little leakage or edema, and flow is preserved through the vascular bed.
EMBs can be maintained ex vivo and successfully reimplanted
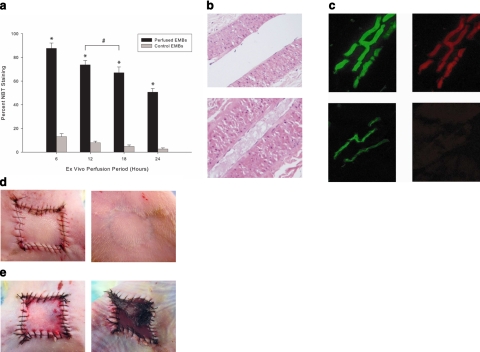
After optimizing the ex vivo perfusion of EMBs, we determined the feasibility of extending their ex vivo viability. Using the DMEM perfusate, we observed a significant survival advantage (P<0.001) in perfused EMBs compared to nonperfused EMBs for up to 24 h based on NBT staining, with greater than 60% of EMB tissue staining NBT positive after 18 h, and at least 50% at 24 h (Fig. 3a). There was no statistical difference in NBT staining between perfusion for 12 or 18 h (data not shown). NBT staining is a well-documented modality for assessing variability of multiple tissue types (33,34,35,36,37,38). Histological analysis revealed preservation of cellular and vascular architecture and endothelial function (colocalization of DiI-acLDL and lectin), as compared to nonperfused EMB controls (Fig. 3b, c). Replantation served as the gold standard for EMB viability. All EMBs perfused for 6 and 12 h survived 7 days following reimplantation, compared to 75 and 50% of EMBs perfused for 18 and 24 h, respectively. Control EMBs in all ex vivo time periods exhibited full-thickness necrosis within 2 days of reimplantation into host circulation (Fig. 3d, e). These data demonstrate proof of concept that not only can EMBs be successfully harvested and sustained ex vivo, but a time window of up to 12 h, and potentially 24 h, is available for EMB manipulation or stem cell engraftment without compromising EMB viability.
Figure 3.
Indicators of EMB viability. a) EMBs cultivated at 6, 12, 18, and 24 h (black plots) were stained with NBT and compared to control EMBs (gray plots). Statistically significant differences in percentage of viable tissue was determined for all perfused EMBs compared to controls; *P < 0.001. EMBs perfused for 12 and 18 h did not show a statistically significant difference in tissue viability; #P > 0.05. b) H&E demonstrates that perfused EMBs (top) maintained cell viability and architecture, permitting extended flow compared to nonperfused EMBs (bottom), which demonstrate loss of architecture, intravascular debris, and apoptotic cells. c) Colocalization of lectin (green, left) and DiI-acLDL uptake (red, right) indicates perfused and functional endothelium, respectively, in the perfused EMBs (top row), compared to nonperfused control EMBs (bottom row). d) Gross appearance of replanted EMB after 18 h of ex vivo perfusion at PRD 0 (immediately after replantation, left) and at PRD 28 (right). e) Nonperfused control EMBs (PRD 0, left) exhibited full thickness necrosis by PRD 2.
Supplementation with HBOC restores EMB oxygenation and extends ex vivo viability
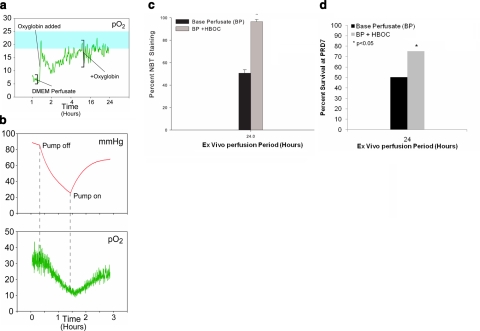
The physiological EMB tissue oxygen tension was determined to be 18–25 mmHg in vivo. Perfusion with DMEM perfusate alone resulted in ex vivo tissue oxygen tensions of 3–8 mmHg. The addition of oxyglobin to the DMEM perfusate increased perfusate oxygen tension to greater than 100 mmHg before delivery to the EMB, and increased EMB tissue oxygenation to near-physiological oxygen tension levels during ex vivo perfusion (14–35 mmHg, Fig. 4a). EMB tissue oxygenation during ex vivo perfusion was found to be flow dependent (Fig. 4b). At 24 h of ex vivo perfusion, a greater survival advantage was observed in the EMBs perfused with the HBOC-supplemented perfusate when compared to EMBs perfused without HBOC supplementation (96.5±1.94 vs. 50.6±3.11%, P<0.001; Fig. 4c). 75% of EMBs subjected to 24-h perfusion with HBOC supplementation survived at PRD 7 compared to 50% of those perfused with DMEM alone (Fig. 4d).
Figure 4.
Artificial oxygen carrier augments EMB viability. a) Ex vivo tissue oxygenation with DMEM perfusate only and supplementation with oxyglobin (blue shading indicates physiological oxygenation in vivo). b) Oxygenation (bottom) was directly related to flow and perfusion pressures (top). c) NBT viability staining of EMBs perfused for 24 h with DMEM perfusate alone compared to HBOC supplementation. There was greater EMB viability in the HBOC-supplemented perfused EMBs compared to those perfused with DMEM alone; *P < 0.001. d) Percentage EMB survival at PRD7 after 24-h perfusion with DMEM perfusate alone compared to HBOC supplementation. Seventy-five percent of EMBs subjected to 24-h perfusion with HBOC survived as compared to 50% of those perfused with DMEM alone.
MSCs and progenitor cells are efficiently seeded onto EMBs
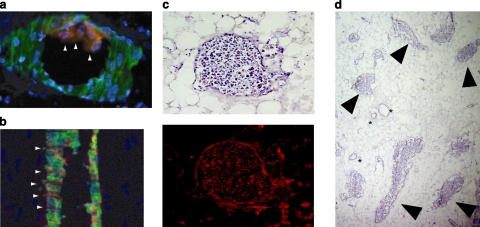
During the ex vivo perfusion of EMBs with MAPCs, an intermittent rise in perfusion pressures (90–130 mmHg) was noted with eventual return to physiological pressures and maintenance of passive venous return throughout the perfusion period. EMBs harvested immediately after ex vivo perfusion with MAPCs demonstrated adherence of cells to the luminal endothelium (Fig. 5a) and egression from the microcirculation (Fig. 5b). Following reimplantation, EMBs harvested on PRD 7 demonstrated clusters of MAPCs engrafted in close proximity to the native vasculature. On the basis of H&E, these clusters did not appear to represent an inflammatory response or phagocytosed MAPCs. Furthermore, these clusters were not emboli within the vessels, suggesting MAPCs effectively eggressed from the vasculature into the EMB (Fig. 5c, d). Whether these clusters represent bulk engraftment of MAPCs or a single engrafted MAPC that has proliferated remains to be determined.
Figure 5.
Immunohistochemistry of MAPCs mobilizing onto vascularized bioscaffold. a, b) MAPCs (red, arrowheads) adhere to (a) and egress from (b) the EMB microcirculation. c) Following reimplantation, MAPC clusters can be identified by serial sections of hematoxylin (top) and DiI fluorescence (bottom, red). d) Bulk egress of MAPCs in EMB parenchymal tissue (arrowheads). Asterisks indicate vessels.
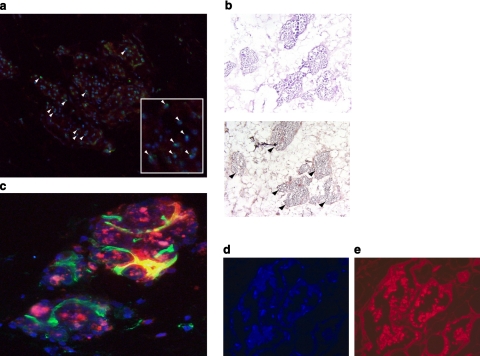
We further verified MAPC engraftment using a gender mismatch model (male donor MAPCs and female recipient EMBs). Using FISH to identify the Y-chromosome, we definitively confirmed the origin of these cell clusters were from the infused MAPCs (Fig. 6a). Furthermore, these cell clusters were BrdU+, suggesting that the engrafted MAPCs were actively proliferating following reimplantation and could therefore actively participate in the process of tissue regeneration (Fig. 6b). In addition, we found the MAPCs stimulated neovascularization or potentially differentiated into endothelial cells as new blood vessels formed around the MAPC clusters (Fig. 6c).
Figure 6.
Engraftment and proliferation of MAPCs and MSCs onto EMBs. a) Following infusion, gender-mismatched DiI-labeled MAPCs (red) can be readily identified by FISH (green, Y chromosome, white arrowheads; inset ×400). b) BrdU staining of MAPC clusters demonstrates proliferation of stem cells following engraftment (black arrowheads). c) MAPC clusters marked with DiI (red) and dapI (blue) staining stimulate angiogenesis or differentiate into functional neovessels (vasculogenesis) seen with lectin (green) perfusion within the EMB. d) Human MSCs engrafted onto EMBs and formed viable clusters, as seen on dapI staining. e) PKH26-fluorescent labeling.
A multitude of distinct stem cell populations are under intense investigation in the field of tissue engineering; however, the exact population or combination of populations that possess the greatest regenerative capacity remains to be determined (39,40,41). Thus, to broaden the potential applicability of our technology, we explored the ability of two additional stem cell populations to engraft onto EMBs. PKH26 labeled rat bone marrow-derived MSCs (data not shown), and human adipose tissue-derived MSCs were also found to engraft onto EMBs following perfusion on the bioreactor (Fig. 6d, e).
Stem cell expression of transfected luciferase protein is localized to the EMB
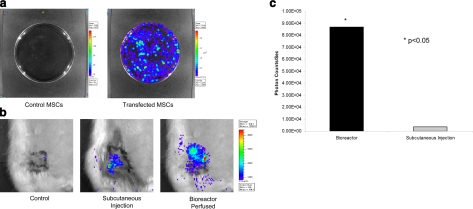
Rat bone marrow-derived MSCs were transfected with a luciferase plasmid and subsequently seeded onto the EMB using the bioreactor. In vitro, we demonstrated viable expression of luciferase in transfected MSCs (Fig. 7a). The EMBs were then seeded with transfected MSCs and successfully reimplanted following 6 h of perfusion. IVIS imaging demonstrated viable transfected MSCs in vivo. Expression of luciferase activity was localized entirely to the EMB, not only confirming successful MSC engraftment, but also demonstrating effective and targeted expression of the transfected protein (Fig. 7b). While transfected MSCs injected directly into the flap also demonstrated some luciferase activity, fluorescence was significantly diminished compared to flaps that were seeded via the bioreactor (8.68×104±8.77×101 vs. 3.61×103±3.27×101 photons/s; Fig. 7c). This suggests bioreactor-perfused flaps represent a more efficient means of delivering transfected MSCs than direct inoculation. These data further demonstrate the possibility that stem cells can be manipulated and then seeded onto an EMB to replace deficient proteins in a systemic fashion or to deliver gene therapy in a targeted fashion.
Figure 7.
MSC transfection and luciferase expression in vivo. a) Rat bone marrow-derived MSCs were transfected with a luciferase plasmid and demonstrate luciferase expression in vitro. b) Luciferase expression can be detected in EMBs receiving direct injection of transfected MSCs; however, EMBs seeded with transfected MSCs through the bioreactor demonstrated markedly greater expression of luciferase activity. c) Graphical representation of relative luminescence (photons/s) between MSC injection and perfusion.
DISCUSSION
The ability to regenerate a functional, vascularized, three-dimensional organ remains an elusive goal for tissue engineering. Current approaches are either limited to generating relatively avascular tissues or require an additional level of complexity to engineer a vascular network. Our novel approach sacrifices expendable tissue which contains not only a suitable biological matrix that can support cell adhesion but, more important, an intact microcirculatory system capable of sustaining the construct, as well as the engrafted cells. Furthermore, our approach offers the flexibility of manipulating the engineered construct ex vivo, as well as a simple means of reintegration into the host systemic circulation. To our knowledge, we are the first to describe this paradigm of using entirely native, autologous tissue as a bioscaffold for tissue engineering.
In this study, we provide proof of concept that an EMB can be sustained in our bioreactor for up to 24 h. During this time period, we successfully seeded three distinct stem cell populations onto the EMB, demonstrating the versatility of our approach. In addition, should the need arise, our system affords 24 h to seed combinations of stem cells or further manipulate the construct with growth factors without jeopardizing the ultimate viability of the EMB. We have also demonstrated active proliferation of engrafted stem cells through BrdU incorporation, as well as increased expression of transfected proteins in bioreactor-perfused EMBs. This suggests our approach not only improves stem cell engraftment compared to direct injection, but can also potentially be extrapolated to the treatment of protein deficiencies like hemophilia or diabetes.
While the bioreactor does indeed add another degree of complexity, it is necessary to sustain the EMB ex vivo and maximize stem cell engraftment. Direct perfusion of MSCs into the arterial pedicle resulted in poor EMB engraftment if the venous outflow remained patent during this process. Alternatively, EMB edema and microvessel rupture developed if the outflow was compressed during stem cell perfusion. For the current study, we adjusted perfusion parameters to maximize perfusion time in the bioreactor under the hypothesis that stem cell engraftment would increase in direct correlation with perfusion time; however, the precise parameters of cell number and length of perfusion are areas of active investigation in our laboratory. Despite the nuances in bioreactor perfusion, this technology does offer several advantages over traditional scaffold-driven approaches. As the functioning vascular network is already present, it obviates the need to recapitulate vascular patterning in vitro. Since a single afferent artery and efferent vein supply the entire explanted network, reintegration into recipient circulation is easily attainable with current microsurgical techniques. This feature also permits surgical targeting of engineered constructs, the means to transport tissue from one locale to another, and the ability to remove the engineered construct easily, should the need arise. Finally, the vast number and dimensions of EMBs makes for an abundant supply of autologous bioscaffolds, minimizing the immunological sequelae of synthetic biomaterials used in scaffold design (5).
Success in seeding EMBs with MAPCs and MSCs suggests that this system is capable of supporting a host of different cell populations (29, 32, 42). Others have also recently demonstrated the feasibility of using a bioreactor to seed a decellularized cardiac construct with stem cells able to generate coordinated action potentials and contractility (43). While this obviates the need for immunosuppresion similar to our approach, the modality still requires a donor scaffold and would therefore continue to be limited by donor scarcity. Since our approach relies on expendable scaffolds, ultimately, we hope this technology can be harnessed to fabricate organs or transfect genes to correct various protein deficiencies and inborn errors of metabolism. We demonstrate proof of principle for a novel technology to deliver stem cells to a vascularized, three-dimensionally organized autologous bioscaffold. The abundance of these bioscaffolds, the sustainability and preservation of their physiological function ex vivo, and the ability to seed them with large numbers of stem/progenitor cells can potentially overcome the current limitations and usher in new advancements in the field of tissue engineering.
Acknowledgments
Human adipo-derived MSCs were a generous gift from Dr. Michael T. Longaker (Department of Surgery, Stanford University, Stanford, CA, USA). This research was supported by National Institutes of Health/National Institute of Biomedical Imaging and Bioengineering grant EB-02265 (G.C.G.) and the National Institutes of Health Loan Repayment Program (Edward I. Chang).
References
- Stock U A, Vacanti J P. Tissue engineering: current state and prospects. Annu Rev Med. 2001;52:443–451. doi: 10.1146/annurev.med.52.1.443. [DOI] [PubMed] [Google Scholar]
- Kaihara S, Vacanti J P. Tissue engineering: toward new solutions for transplantation and reconstructive surgery. Arch Surg. 1999;134:1184–1188. doi: 10.1001/archsurg.134.11.1184. [DOI] [PubMed] [Google Scholar]
- Fiegel H C, Kaufmann P M, Bruns H, Kluth D, Horch R E, Vacanti J P, Kneser U. Hepatic tissue engineering: from transplantation to customized cell-based liver directed therapies from the laboratory. J Cell Mol Med. 2008;12:56–66. doi: 10.1111/j.1582-4934.2007.00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoganson D M, Pryor H I, 2nd, Vacanti J P. Tissue engineering and organ structure: a vascularized approach to liver and lung. Pediatr Res. 2008;63:520–526. doi: 10.1203/01.pdr.0000305879.38476.0c. [DOI] [PubMed] [Google Scholar]
- Mikos A G, McIntire L V, Anderson J M, Babensee J E. Host response to tissue engineered devices. Adv Drug Deliv Rev. 1998;33:111–139. doi: 10.1016/s0169-409x(98)00023-4. [DOI] [PubMed] [Google Scholar]
- Lokmic Z, Stillaert F, Morrison W A, Thompson E W, Mitchell G M. An arteriovenous loop in a protected space generates a permanent, highly vascular, tissue-engineered construct. FASEB J. 2007;21:511–522. doi: 10.1096/fj.06-6614com. [DOI] [PubMed] [Google Scholar]
- Messina A, Bortolotto S K, Cassell O C, Kelly J, Abberton K M, Morrison W A. Generation of a vascularized organoid using skeletal muscle as the inductive source. FASEB J. 2005;11:1570–1572. doi: 10.1096/fj.04-3241fje. [DOI] [PubMed] [Google Scholar]
- McGuigan A P, Sefton M V. Vascularized organoid engineered by modular assembly enables blood perfusion. Proc Natl Acad Sci U S A. 2006;31:11461–11466. doi: 10.1073/pnas.0602740103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa E R, Vacanti J P. An overview of the pathology and approaches to tissue engineering. Ann N Y Acad Sci. 2002;979:10–26. doi: 10.1111/j.1749-6632.2002.tb04863.x. discussion 35–18. [DOI] [PubMed] [Google Scholar]
- Shea L D, Smiley E, Bonadio J, Mooney D J. DNA delivery from polymer matrices for tissue engineering. Nat Biotechnol. 1999;17:551–554. doi: 10.1038/9853. [DOI] [PubMed] [Google Scholar]
- Iwaguro H, Yamaguchi J, Kalka C, Murasawa S, Masuda H, Hayashi S, Silver M, Li T, Isner J M, Asahara T. Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration. Circulation. 2002;105:732–738. doi: 10.1161/hc0602.103673. [DOI] [PubMed] [Google Scholar]
- Black A F, Berthod F, L'Heureux N, Germain L, Auger F A. In vitro reconstruction of a human capillary-like network in a tissue-engineered skin equivalent. FASEB J. 1998;12:1331–1340. doi: 10.1096/fasebj.12.13.1331. [DOI] [PubMed] [Google Scholar]
- Dolderer J H, Abberton K M, Thompson E W, Slavin J L, Stevens G W, Penington A J, Morrison W A. Spontaneous large-volume adipose tissue generation from a vascularized pedicled fat flap inside a chamber space. Tissue Eng. 2007;13:673–681. doi: 10.1089/ten.2006.0212. [DOI] [PubMed] [Google Scholar]
- Morritt A N, Bortolotto S K, Dilley R J, Han X, Kompa A R, McCombe D, Wright C E, Itescu S, Angus J A, Morrison W A. Cardiac tissue engineering in an in vivo vascularized chamber. Circulation. 2007;115:353–360. doi: 10.1161/CIRCULATIONAHA.106.657379. [DOI] [PubMed] [Google Scholar]
- Sekine H, Shimizu T, Yang J, Kobayashi E, Okano T. Pulsatile myocardial tubes fabricated with cell sheet engineering. Circulation. 2006;114:87–93. doi: 10.1161/CIRCULATIONAHA.105.000273. [DOI] [PubMed] [Google Scholar]
- Michaels J V, Levine J P, Hazen A, Ceradini D J, Galiano R D, Soltanian H, Gurtner G C. Biologic brachytherapy: ex vivo transduction of microvascular beds for efficient, targeted gene therapy. Plast Reconstr Surg. 2006;118:54–65. doi: 10.1097/01.prs.0000220466.27521.22. [DOI] [PubMed] [Google Scholar]
- Armstrong M B, Masri N, Venugopal R. Reconstructive microsurgery: reviewing the past, anticipating the future. Clin Plast Surg. 2001;28:671–686. [PubMed] [Google Scholar]
- Buncke H J, Buncke G M, Kind G M. The early history of microsurgery. Plast Reconstr Surg. 1996;98:1122–1123. doi: 10.1097/00006534-199611000-00052. [DOI] [PubMed] [Google Scholar]
- Taylor G I, Palmer J H. The vascular territories (angiosomes) of the body: experimental study and clinical applications. Br J Plast Surg. 1987;40:113–141. doi: 10.1016/0007-1226(87)90185-8. [DOI] [PubMed] [Google Scholar]
- Tae S K, Lee S H, Park J S, Im G I. Mesenchymal stem cells for tissue engineering and regenerative medicine. Biomed Mater. 2006;2:63–71. doi: 10.1088/1748-6041/1/2/003. [DOI] [PubMed] [Google Scholar]
- Dempsey M P, Hamou C, V J M, Ghali S, Jazayeri L, Grogan R H, Gurtner G C. Using genetically modified microvascular free flaps to deliver local cancer immunotherapy with minimal systemic toxicity. Plast Reconstr Surg. 2008;12:1541–1453. doi: 10.1097/PRS.0b013e31816ff6aa. [DOI] [PubMed] [Google Scholar]
- Zhang F, Sones W D, Lineaweaver W C. Microsurgical flap models in the rat. J Reconstr Microsurg. 2001;17:211–221. doi: 10.1055/s-2001-14353. [DOI] [PubMed] [Google Scholar]
- Petry J J, Wortham K A. The anatomy of the epigastric flap in the experimental rat. Plast Reconstr Surg. 1984;74:410–413. doi: 10.1097/00006534-198409000-00014. [DOI] [PubMed] [Google Scholar]
- Pantazi G, Knight K R, Romeo R, Hurley J V, Hennessy O, Willemart G, Penington A J, Morrison W A. The beneficial effect of heparin in preischemic perfusion solutions for cold-stored skin flaps. Ann Plast Surg. 2000;44:304–310. doi: 10.1097/00000637-200044030-00009. [DOI] [PubMed] [Google Scholar]
- Callan M B, Rentko V T. Clinical application of a hemoglobin-based oxygen-carrying solution. Vet Clin North Am Small Anim Pract. 2003;33:1277–1293. doi: 10.1016/s0195-5616(03)00119-0. [DOI] [PubMed] [Google Scholar]
- Butler A J, Rees M A, Wight D G, Casey N D, Alexander G, White D J, Friend P J. Successful extracorporeal porcine liver perfusion for 72 hr. Transplantation. 2002;73:1212–1218. doi: 10.1097/00007890-200204270-00005. [DOI] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner J M. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher A M, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar B N, Reinhardt R L, Schwartz R E, Keene C D, Ortiz-Gonzalez X R, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low W C, Largaespada D A, Verfaillie C M. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Schäffler A, Büchler C. Concise review: adipose tissue-derived stromal cells–basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818–827. doi: 10.1634/stemcells.2006-0589. [DOI] [PubMed] [Google Scholar]
- Jori F P, Napolitano M A, Melone M A, Cipollaro M, Cascino A, Altucci L, Peluso G, Giordano A, Galderisi U. Molecular pathways involved in neural in vitro differentiation of marrow stromal stem cells. J Cell Biochem. 2005;94:645–655. doi: 10.1002/jcb.20315. [DOI] [PubMed] [Google Scholar]
- Pittenger M F, Mackay A M, Beck S C, Jaiswal R K, Douglas R, Mosca J D, Moorman M A, Simonetti D W, Craig S, Marshak D R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Hickey M J, Hurley J V, Morrison W A. Temporal and spatial relationship between no-reflow phenomenon and postischemic necrosis in skeletal muscle. Am J Physiol Heart Circ Physiol. 1996;271:H1277–H1286. doi: 10.1152/ajpheart.1996.271.4.H1277. [DOI] [PubMed] [Google Scholar]
- Zacharowski K, Otto M, Hafner G, Chatterjee P K, Thiemermann C. Endotoxin induces a second window of protection in the rat heart as determined by using p-nitro-blue tetrazolium staining, cardiac troponin T release, and histology. Arterioscler Thromb Vasc Biol. 1999;19:2276–2280. doi: 10.1161/01.atv.19.9.2276. [DOI] [PubMed] [Google Scholar]
- Ridenour T R, Warner D S, Todd M M, McAllister A C. Mild hypothermia reduces infarct size resulting from temporary but not permanent focal ischemia in rats. Stroke. 1992;23:733–738. doi: 10.1161/01.str.23.5.733. [DOI] [PubMed] [Google Scholar]
- Wang H D, Pagano P J, Du Y, Cayatte A J, Quinn M T, Brecher P, Cohen R A. Superoxide anion from the adventitia of the rat thoracic aorta inactivates nitric oxide. Circ Res. 1998;82:810–818. doi: 10.1161/01.res.82.7.810. [DOI] [PubMed] [Google Scholar]
- Sipowicz M A, Chomarat P, Diwan B A, Anver M A, Awasthi Y C, Ward J M, Rice J M, Kasprzak K S, Wild C P, Anderson L M. Increased oxidative DNA damage and hepatocyte overexpression of specific cytochrome P450 isoforms in hepatitis of mice infected with Helicobacter hepaticus. Am J Pathol. 1997;151:933–941. [PMC free article] [PubMed] [Google Scholar]
- Tomino Y, Saitoh A, Kuramoto T, Ohmuro H, Wang H, Shirato I, Koide H, Iijima T, Rinno H, Taneda A. Detection of glycosylated protein in renal tissues and dermal vascular vessels in the microalbuminuric stage of diabetic nephropathy using the nitro blue tetrazolium reaction. Nephron. 1994;68:207–211. doi: 10.1159/000188258. [DOI] [PubMed] [Google Scholar]
- Laflamme M A, Murry C E. Regenerating the heart. Nat Biotechnol. 2005;23:845–856. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- Melero-Martin J M, De Obaldia M E, Kang S Y, Khan Z A, Yuan L, Oettgen P, Bischoff J. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res. 2008;103:194–202. doi: 10.1161/CIRCRESAHA.108.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Kuroda R, Mifune Y, Kawamoto A, Shoji T, Miwa M, Asahara T, Kurosaka M. Circulating endothelial/skeletal progenitor cells for bone regeneration and healing. Bone. 2008;43:434–439. doi: 10.1016/j.bone.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Reyes M, Verfaillie C M. Characterization of multipotent adult progenitor cells, a subpopulation of mesenchymal stem cells. Ann N Y Acad Sci. 2001;938:231–233; discussion 233–235. doi: 10.1111/j.1749-6632.2001.tb03593.x. [DOI] [PubMed] [Google Scholar]
- Ott H C, Matthiesen T S, Brechtken J, Grindle S, Goh S K, Nelson W, Taylor D A. The adult human heart as a source for stem cells: repair strategies with embryonic-like progenitor cells. Nat Clin Pract Cardiovasc Med. 2007;4:S27–S39. doi: 10.1038/ncpcardio0771. [DOI] [PubMed] [Google Scholar]