Abstract
Transgenic (Tg) mice expressing ∼95% of the D166V (aspartic acid to valine) mutation in the ventricular myosin regulatory light chain (RLC) shown to cause a malignant familial hypertrophic cardiomyopathy (FHC) phenotype were generated, and the skinned and intact papillary muscle fibers from the Tg-D166V mice were examined using a Guth muscle research system. A large increase in the Ca2+ sensitivity of force and ATPase (ΔpCa50>0.25) and a significant decrease in maximal force and ATPase were observed in skinned muscle fibers from Tg-D166V mice compared with control mice. The cross-bridge dissociation rate g was dramatically decreased, whereas the energy cost (ATPase/force) was slightly increased in Tg-D166V fibers compared with controls. The calculated average force per D166V cross-bridge was also reduced. Intact papillary muscle data demonstrated prolonged force transients with no change in calcium transients in Tg-D166V fibers compared with control fibers. Histopathological examination revealed fibrotic lesions in the hearts of the older D166V mice. Our results suggest that a charge effect of the D166V mutation and/or a mutation-dependent decrease in RLC phosphorylation could initiate the slower kinetics of the D166V cross-bridges and ultimately affect the regulation of cardiac muscle contraction. Profound cellular changes observed in Tg-D166V myocardium when placed in vivo could trigger a series of pathological responses and result in poor prognosis for D166V-positive patients.—Kerrick, W. G. L., Kazmierczak, K., Xu, Y., Wang, Y., Szczesna-Cordary, D. Malignant familial hypertrophic cardiomyopathy D166V mutation in the ventricular myosin regulatory light chain causes profound effects in skinned and intact papillary muscle fibers from transgenic mice.
Keywords: phosphorylation, calcium and force transients, cross-bridge dissociation rate, energy cost, ATPase-pCa dependence
The human ventricular myosin regulatory light chain (RLC), encoded by the MYL2 gene, is one of the sarcomeric proteins associated with familial hypertrophic cardiomyopathy (FHC) (1,2,3,4,5,6,7). FHC is a relatively common autosomal dominant genetic disease characterized by ventricular hypertrophy, myofibrillar disarray, and interstitial fibrosis, often clinically manifesting as heart failure and sudden cardiac death (SCD) (8,9,10,11). The RLC FHC mutations constitute ∼2% of total FHC sarcomeric mutations (12, 13). Despite efforts by many, there is no clear understanding of the mechanisms underlying the hypertrophic heart disease and the role of myosin RLC in the pathogenesis of FHC (12, 14, 15).
The D166V mutation in myosin RLC was identified by Richard et al. (5) in 2003; similar to the previously identified R58Q mutation of RLC, it is associated with poor prognosis and SCD at young age. The D166V mutation occurs at the last amino acid residue of the human cardiac RLC and substitutes valine for the normally occurring aspartic acid (Fig. 1). It was mistakenly labeled as D166L in the original paper of Richard et al. (5) and later corrected to be D166V (ref. 16 and personal communication with Drs. P. Charron and P. Richard). In this report we characterize the transgenic (Tg) animal model for this malignant FHC mutation and present functional studies using freshly skinned and intact papillary muscle fibers from mouse hearts expressing ∼95% D166V transgene (Tg-D166V). The results are compared with those for fibers from hearts of transgenic wild-type (Tg-WT) mice expressing ∼100% of the human ventricular RLC (17) and from nontransgenic (NTg) littermates.
Figure 1.
Schematic representation of the D166V mutation (labeled in red) in the myosin RLC (National Center for Biotechnology Information Accession Number 2MYS). The heavy chain of myosin is labeled in yellow, the ELC in dark blue, and the RLC in green.
We demonstrate a large increase in the Ca2+ sensitivity of contractile force, decreased maximal ATPase and force, profoundly decreased kinetics of force-generating myosin cross-bridges g, and a lower average force per cross-bridge Fav in skinned Tg-D166V fibers compared with Tg-WT and NTg fibers. In addition, slower rates of force relaxation are observed in intact Tg-D166V papillary muscle fibers. These profound physiological alterations monitored in D166V mouse heart preparations are discussed in the context of a malignant FHC phenotype observed in the D166V-positive patients. The molecular mechanism of the D166V-induced pathological FHC response is also discussed.
MATERIALS AND METHODS
Generation and characterization of transgenic mice
All animal studies were conducted in accordance with institutional guidelines. Transgenic mouse models expressing WT or D166V FHC mutation of human ventricular RLC were generated as described previously for other RLC FHC mutations (17,18,19). In brief, the D166V-mutated RLC cDNA was cloned into the SalI site of the plasmid, α-myosin heavy chain (α-MHC) clone 26 (generously provided by Dr. J. Robbins, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA). The resulting construct contained ∼5.5 kb of the mouse α-MHC promoter, including the first two exons and part of the third, followed by the D166V (498 bp) and a 630-bp 3′ untranslated region from the human growth hormone transcript. The founder mice have been bred to NTg B6SJL mice.
Analysis of protein expression
The α-MHC-driven expression of RLC-WT and RLC-D166V in mouse hearts was quantified as outlined in Wang et al. (17). In brief, ∼10 mg of atrial tissue from Tg-WT and Tg-D166V mice was minced in a solution of 1% SDS, 1% β-mercaptoethanol, 1 mM EDTA, 1 mM PMSF, and 1 μl/ml protease inhibitor cocktail (Sigma-Aldrich Corp., St. Louis, MO, USA), homogenized, and loaded on 15% SDS-PAGE. The transgenic protein was quantified using Coomassie-stained gels and Western blots (Fig. 2A, B). The total RLC protein was detected with rabbit polyclonal RLC CT-1 antibodies produced in this laboratory (raised against 15 residues from the C terminus of human ventricular RLC) followed by a secondary goat anti-rabbit antibody conjugated with the fluorescent dye, Cy 5.5. The same RLC antibody recognized atrial (endogenous) and ventricular (transgenic) RLC proteins; however, the affinity was higher to the ventricular RLC. Myosin essential light chain (ELC) was used as a loading control and was detected with an ELC-specific monoclonal anti-human antibody (Accurate Chemical and Scientific Corporation, Westbury, NY, USA) followed by a goat anti-mouse secondary antibody labeled with the fluorescent dye, IR red 800 (shown in green in Fig. 2A). Western blots were quantitated with the Odyssey Infrared Imaging System (Li-COR, Lincoln, NE, USA).
Figure 2.
Transgenic protein expression in Tg-D166V and Tg-WT mouse hearts. A) Representative Coomassie-stained gel (top panel) and Western blot (bottom panel) of atrial muscle samples from WT and mutant mice. Lanes 1 and 2, NTg; lanes 3 and 8, Tg-WT; lanes 4–7, Tg-D166V; lane 9, D166V standard protein. B) Percent protein expression in Tg-WT and Tg-D166V mice. ELCendog, endogenous atrial essential light chain; RLCendog, endogenous atrial regulatory light chain; RLCtransg, transgenic ventricular regulatory light chain.
Analysis of protein phosphorylation
After euthanasia, the hearts from ∼6-month-old Tg-D166V, Tg-WT, and NTg mice were excised and weighted, and the ventricles were immediately isolated and frozen in liquid nitrogen. Before the experiment, the tissue was thawed in the buffer consisting of 20 mM phosphate buffer (pH 8.0), 12.5 mM MgCl2, 0.1 M CaCl2, 5 mM ATP, 0.6 mM NaN3, 0.2 mM PMSF, and 1 μl/ml protease inhibitor cocktail (Sigma-Aldrich Corp.) and homogenized. The homogenates were subsequently dissolved in SDS-PAGE buffer containing 500 mM Tris (pH 6.8), 6 M urea, 1% SDS, 10% β-mercaptoethanol, and 0.05% bromphenol blue and loaded on 15% SDS-PAGE. The phosphorylated transgenic RLC was detected with +P-human RLC antibodies, specific for the phosphorylated form of the human ventricular RLC [generously provided by Dr. Neal Epstein, National Institutes of Health (NIH) (20)] followed by a secondary goat anti-rabbit antibody conjugated with the fluorescent dye, IR red 800. Phosphorylated troponin I (TnI) was detected with Mab14 antibody (MMS-418R, Covance, Berkeley, CA, USA), sensitive to the phosphorylated serine 24 in the sequence of cardiac TnI (21), followed by a secondary goat anti-mouse antibody conjugated with the fluorescent dye, Cy 5.5 (Fig. 3). The total RLC protein was detected with rabbit polyclonal RLC CT-1 antibodies as described above, while the total TnI was detected with 6F9 antibody (Research Diagnostics Inc., Flanders, NJ, USA) (21).
Figure 3.
The effect of the D166V mutation on the phosphorylation status of RLC and TnI in transgenic mouse ventricular extracts blotted with CT-1 antibody recognizing total RLC protein and 6F9 antibody recognizing total TnI protein (A) and Mab14 MMS-418R antibody recognizing +P-TnI (top panel) and +P-human RLC antibody recognizing +P-RLC human (Tg) (bottom panel) (B). hWTst, human WT standard protein; +P-hWTst, phosphorylated human WT standard protein; +P-hTnIst, phosphorylated human TnI standard protein.
Histopathology
After euthanasia, the hearts from ∼7-, ∼11-, and ∼17-month-old Tg-D166V, Tg-WT, and NTg mice were excised, weighted, and immersed in 10% buffered formalin. Pictures of whole hearts were taken using a SteREO Discovery.V12 stereoscope and ×0.63/×5 PlanApo S objective and AxioCam HRc (Zeiss, Oberkochen, Germany). Slides of whole mouse hearts were prepared by American HistoLabs, Inc. (Gaithersburg, MD, USA). The paraffin-embedded longitudinal and cross sections of whole mouse hearts stained with hematoxylin and eosin (H&E) and Masson’s trichrome (Fig. 4A, B) were examined for overall morphology and fibrosis using a Dialux 20 microscope, ×40/0.65 Leitz Wetzlar objective, and AxioCam HRc (Zeiss).
Figure 4.
Representative ventricular heart sections from Tg-D166V, Tg-WT, and NTg mice. A) Microscopic views of ventricular cross-sections stained with H&E and Masson’s trichrome of representative ∼11-month-old mice. B) Cross-sections of ventricles stained with Masson’s trichrome from ∼7- and ∼17-month-old mutant and control mice. Note severe fibrotic lesions found in the tissue of ∼17-month-old Tg-D166V mice.
Freshly skinned papillary muscle fibers
After euthanasia, the hearts of 7 ± 1-month-old Tg-D166V, Tg-WT, and NTg control littermates were excised, and strips of mouse papillary muscle fibers (diameter: 60–70 μm) were dissected in ice-cold relaxing solution [85 mM K+, 2 mM MgATP2−, 1 mM Mg2+, 7 mM EGTA (pH 7.0), and propionate as the major anion]. The fibers were treated with 1% Triton X-100 for 30 min and processed immediately without glycerinating (19). The skinned fiber was then mounted in the Guth muscle research system, and the sarcomere length was adjusted to 2.2 μm by a laser diffraction pattern. The cross-sectional area was calculated on the basis of measurement of the fiber width by microscope and the assumption that the fiber is circular in diameter. The ATPase rate was measured using the NADH fluorescence method (22) as described earlier (17, 19). The fiber was subjected to an increasing Ca2+ gradient assessed by mixing the relaxing (pCa 9) and contracting (pCa 3.4) solutions. Both solutions contained 85 mM K+, 2 mM MgATP2−, 1 mM Mg2+, 7 mM EGTA, 5 mM phosphoenolpyruvate, 100 U/ml pyruvate kinase (PK), 0.4 mM NADH, 140 U/ml lactate dehydrogenase, ionic strength 150 mM (pH 7.0), and propionate as the major anion. Fresh, unoxidized NADH solution was introduced into the cuvette every 20 s. The decrease in NADH concentration was determined by a decrease in the fluorescence signal detected at 450 nm. The slope of the linear decrease in NADH concentration was used to calculate the ATPase rate. The Ca2+ concentration gradient was calibrated by use of the fluorescent Ca2+ indicator calcium green-2 (Molecular Probes Inc., Eugene, OR) (23). Force development was monitored simultaneously with ATPase measurements using the force transducer of the Guth apparatus.
Intact muscle fiber studies
Intact papillary muscles were quickly dissected from right ventricles in an oxygenated Krebs-Henseleit solution (119 mM NaCl, 4.6 mM KCl, 11 mM glucose, 25 mM NaHCO3, 1.2 mM KH2PO4, 1.2 MgSO4 · 7 H2O, and 1.8 mM CaCl2 · 2 H2O) containing 30 mM 2,3-butanedione monoxime and mounted in the Guth muscle research system (24). The fibers were loaded with 5 μM Fura-2 acetoxymethyl ester form (AM) for 1 h in an oxygenated Krebs-Henseleit solution containing 0.5% noncytoxic detergent Cremophor. After loading, the muscle was rinsed and then continuously perfused with Krebs-Henseleit solution. The muscle was stimulated at 1.0 Hz through the tweezers attached to the ends of the preparation. Once the preparation was mounted in the apparatus, muscle length was adjusted until maximum active twitch force was obtained. In all records force and fluorescence corresponding to either 340 or 380 nm excitation were recorded by the computer. Background fluorescence from 340 and 380 nm excitation of the unloaded preparation was subtracted from the corresponding 340 and 380 nm fluorescence signals from the Fura-2 AM loaded preparation. The fluorescence 340/380 ratio was calculated and plotted along with force. Although the off-rate of Ca2+ from Fura-2 AM is fast enough to measure the time course of [Ca2+] transients in intact papillary muscle fibers, the multiple in vivo binding sites for this hydrophobic Ca2+ indicator makes the determination of the absolute cytosolic [Ca2+] inaccurate (25). Therefore, the 340/380 fluorescence ratios were used to calculate relative changes in the intracellular [Ca2+] transients as described in Szczesna-Cordary et al. (19).
Statistical analysis
Data are expressed as the average ± se of n experiments. Statistically significant differences were determined using an unpaired Student’s t test (Sigma Plot 10.0; Systat Software, Inc., San Jose, CA, USA), with significance defined as P < 0.05.
RESULTS
In this report we present evidence that the replacement of valine (V) for the normally occurring aspartic acid (D) at the position of 166 (last amino acid) in the sequence of the ventricular myosin RLC leads to FHC through the mutant-induced alterations of the myosin cross-bridge kinetics, changes in the Ca2+ sensitivity of force development, decreased maximal contractile force, and slower rates of force relaxation in the D166V-mutated myocardium. These dramatic functional changes most likely are responsible for a severe FHC phenotype and SCD observed in the individuals carrying the D166V mutation (5). The results presented below characterize the effects of the D166V mutation at the molecular and the cellular levels.
Characterization of the D166V transgenic animal model
To examine the functional consequences of the D166V mutation in the human ventricular myosin RLC that cause the malignant FHC phenotype (5), we generated transgenic mice expressing the D166V mutation in RLC. Protein expression, phosphorylation, and mutation-induced histopathological changes were evaluated in the ventricular tissue of Tg-D166V mice compared with age-matched Tg-WT and NTg littermates.
Transgenic protein expression
Figure 2 demonstrates protein expression levels in Tg-D166V and Tg-WT mice. Determination of protein incorporation into the muscle fiber was assessed on the basis of Western blots and Coomassie-stained 15% SDS-PAGE gels of the mutant and WT cardiac muscle tissues (Fig. 2A). As shown, 97 ± 3.3% (n=12) and 93 ± 2.9% (n=6) protein expression levels were determined in Tg-WT and Tg-D166V mice, respectively (Fig. 2B). To avoid functional artifacts originating from the myc-tag sequence that is usually attached to the RLC sequence for direct ventricular protein determination, we quantified WT and D166V protein expression using mouse atrial heart samples (Fig. 2A). Because of the faster gel mobility of the transgenic human ventricular RLC (molecular mass 18.7 kDa) vs. endogenous mouse atrial RLC (molecular mass 19.3 kDa), protein (mutant and WT) expression could be easily executed. This method of protein quantitation was successfully used in our earlier studies (17) and also used by other investigators (26). Determination of the level of WT and/or mutant incorporation into the atrial muscle correlated well with their expression in the ventricles as assessed using our previously examined myc-E22K-RLC transgenic mice (18). The assessment of protein incorporation with the Coomassie-stained gels and Western blots was necessary because of the preferential binding of the CT-1 antibody to the ventricular vs. atrial RLC sequence (Fig. 2A).
Analysis of protein phosphorylation
Figure 3 demonstrates the effect of the D166V mutation on the phosphorylation status of the RLC in transgenic mouse ventricular extracts blotted with +P-human RLC antibodies, specific for the phosphorylated form of the human ventricular RLC (20). Because both Tg-WT and Tg-D166V mice express similar amounts of the human RLC protein (Fig. 2), the RLC phosphorylation level in Tg-WT and Tg-D166V ventricular extracts could be directly compared. As shown in Fig. 3A, B, the D166V mutation reduced phosphorylation of RLC as shown by the lower +P-RLC band intensity in the Tg-D166V ventricular extracts compared with that in Tg-WT extracts (Fig. 3B, lane 6 vs. lane 3). The total RLC content detected with CT-1 antibodies served as a loading control (Fig. 3A). Interestingly, the down-regulation of the phosphorylated form of RLC by the FHC D166V mutation was paralleled by a slight increase in the phosphorylated form of TnI (Fig. 3B, lane 6 vs. lane 3).
Histopathology
The histopathological examination of the ventricular heart tissue of Tg-D166V, Tg-WT, and NTg mice of different ages (∼7, 11, and 17 months old) is presented in Fig. 4. The microscopic views of the H&E-stained ventricular sections from ∼11-month-old Tg-D166V, Tg-WT, and NTg mice show no abnormal tissue morphology, and the Masson’s trichrome-stained images demonstrate no fibrosis present in any of the heart samples from all groups of mice (Fig. 4A). However, examination of the older mice (∼17 months of age) revealed severe fibrotic lesions present in the heart tissue of Tg-D166V mice compared with the age-matched Tg-WT and NTg littermates (Fig. 4B). Morphological changes found in the older Tg-D166V mice suggest that the ventricles of the FHC Tg-D166V mice undergo temporal phenotypic changes that are not present in the younger mice (≤11 months of age). Interestingly, no hypertrophy was found in any of the young or old Tg-D166V animals as determined by the heart weight to body weight ratio (data not shown). Perhaps, the histopathological changes occurring in the hearts of the older Tg-D166V mice are a more sensitive indicator of a malignant FHC phenotype than ventricular or septal hypertrophy. As we show below, however, the functional changes induced by this malignant D166V mutation largely precede any of the morphological changes and can be easily detected in the papillary muscle fibers from Tg-D166V mice as young as 6 months of age compared with the age-matched Tg-WT or NTg mice.
Functional studies performed in skinned and intact papillary muscle fibers from transgenic mice
Ca2+ dependence of ATPase and force in Tg-D166V skinned muscle fibers
As demonstrated in Fig. 5, an increase in the Ca2+ sensitivity of force and ATPase measured simultaneously under isometric conditions was determined in skinned muscle fibers from Tg-D166V mice compared with control NTg and Tg-WT mice. The pCa50 value of ATPase for Tg-D166V fibers was 5.50 ± 0.02 (n=9 fibers), whereas values for Tg-WT and NTg fibers were 5.24 ± 0.02 (n=6 fibers) and 5.23 ± 0.02 (n=7 fibers), respectively. The ΔpCa50 values of ATPase between the mutant and control fibers (>0.25) were statistically significant, with P < 0.05 (Fig. 5). Likewise, large statistically significant differences between Tg-D166V and Tg-WT/NTg fibers were observed in the force-pCa relationship. The pCa50 value of force for Tg-D166V fibers was 5.30 ± 0.02 (n=9 fibers), whereas pCa50 of force in Tg-WT and NTg fibers was 5.02 ± 0.03 (n=6 fibers) and 5.03 ± 0.01 (n=7 fibers), respectively. As in ATPase measurements, the ΔpCa50 values of force (>0.27) were statistically significant, with P < 0.05 (Fig. 5).
Figure 5.
ATPase-pCa (left panel) and force-pCa (right panel) relationships in Tg-D166V skinned muscle fibers compared with Tg-WT and NTg littermates. A statistically significant difference in the Ca2+ sensitivity of ATPase activity and force was observed between Tg-D166V muscle fibers and all other groups of fibers. No difference in either the Ca2+ sensitivity of ATPase or force between Tg-WT and NTg fibers was observed. Data are expressed as means ± se of n experiments (n individual fibers).
Maximal ATPase and force in Tg-D166V fibers
The measurements shown in Fig. 6 indicate that maximal ATPase and force per cross-section of muscle fiber were largely decreased in the mutant Tg-D166V fibers, compared with NTg and Tg-WT fibers. The ATPase rates observed in Tg-D166V fibers were 3.384 ± 0.136 s−1 per myosin head (n=9 fibers), compared with 4.331 ± 0.167 (n=6 fibers) monitored for Tg-WT fibers, and the difference (a 22% decrease in Tg-D166V) was statistically significant (P<0.05). Similarly, a 32% decrease in maximal force compared with Tg-WT fibers was measured in Tg-D166V fibers, with F = 42.2 ± 2.8 kN/m2 (Fig. 6). The differences between Tg-D166V fibers and control Tg-WT and NTg fibers were statistically significant (P<0.05).
Figure 6.
Effect of the D166V mutation in myosin RLC on maximal ATPase activity (s−1 per myosin head) and maximal force (in kN/m2) determined in Tg skinned papillary muscle fibers. Data are expressed as means ± se of n experiments (n fibers isolated from n mouse hearts).
Fraction of myosin cross-bridges attached at maximal calcium activation
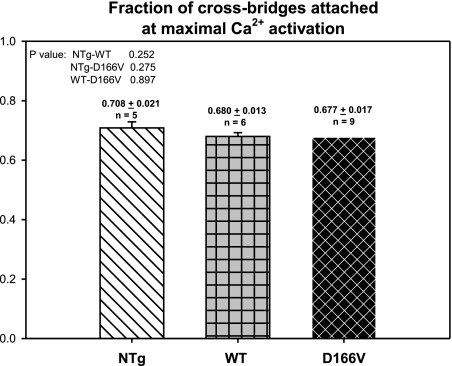
According to the hypothesis of the mechanism for muscle contraction proposed by Huxley (27), myosin cross-bridges can assume at least two distinct conformations during muscle contraction, strongly attached to actin in thin filaments and weakly attached or dissociated from thin filaments. The transition from the nonforce-generating states to the force-generating states in muscle can be characterized by the cross-bridge attachment rate f and the rate of the cross-bridge return to the nonforce-generating states g (27,28,29,30). To measure the fraction of myosin cross-bridges attached at maximal calcium activation, expressed as f/(f+g), at the end of the force/ATPase-pCa measurements, the fibers were perfused with the Ca2+-activating solution (pCa 3.4), in which 2 mM ATP was replaced with 10 mM MgADP. Under these conditions, all cycling myosin cross-bridges are forced into the force-generating state as g approaches 0, and the measured force is the maximal force that the fiber can develop. The ratio of the maximal Ca2+-activated force divided by the maximum force the muscle fiber can develop in the presence of 10 mM MgADP represents the fraction of cross-bridges attached at maximal Ca2+ activation, f/(f+g) (31, 32). Figure 7 demonstrates that no difference was observed in the fraction of cross-bridges attached at maximal Ca2+ activation among all tested groups of fibers (P>0.05).
Figure 7.
Fraction of myosin cross-bridges attached at maximal calcium activation in Tg-D166V fibers compared with Tg-WT and NTg fibers. Data are expressed as means ± se of n experiments (n fibers isolated from n mouse hearts).
Rate of dissociation of myosin cross-bridges in Tg-D166V myocardium
The formation of strongly attached myosin cross-bridges, i.e., the ones that produce contractile force (27, 28), can be characterized by the apparent rate of cross-bridge formation f. On the other hand, the dissociation of bound cross-bridges can be characterized by the rate of cross-bridge dissociation g. We have calculated g in our skinned papillary muscle fibers from the following equation: g = ATPase/concentration of cross-bridges attached at all levels of force activation. The concentration of cross-bridges attached at all levels of force activation can be calculated as normalized force × fraction of cross-bridges attached at maximal Ca2+ activation × total concentration of myosin cross-bridges (S1). The total intracellular myosin S1 concentration in muscle was assumed to be 154 μM (33). Figure 8 shows the rate of cross-bridge dissociation plotted as a function of normalized force. As demonstrated, the D166V mutation produced a large decrease in g compared with Tg-WT and NTg fibers, and the difference between Tg-D166V and Tg-WT/NTg fibers is statistically significant (P<0.05). This result suggests that the D166V mutation in RLC significantly slows down the cross-bridge turnover rate at all levels of fiber activation. As will be discussed, a large decrease in g in Tg-D166V fibers (Fig. 8) is most likely responsible for a mutation-induced dramatic increase in the Ca2+ sensitivity of force/ATPase, as demonstrated in Fig. 5. The question of the mechanism by which the D166V mutation in RLC decreases myosin cross-bridge kinetics remains. The possible mechanisms are presented in the Discussion.
Figure 8.
Effect of the D166V mutation in myosin RLC on the rate of cross-bridge dissociation g (s−1) in skinned papillary muscle fibers. Data are expressed as means ± se of n = 9 (Tg-D166V), n = 6 (Tg-WT), and n = 5 (NTg) experiments.
Energy cost of contraction in Tg-D166V papillary muscle fibers
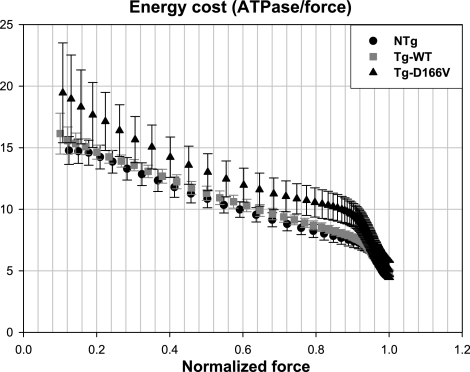
The energy cost of isometric contraction in Tg-D166V compared with control Tg-WT and NTg skinned muscle fibers was calculated by taking the ratio of fiber ATPase/fiber force and plotted as a function of the degree of force activation. As shown in Fig. 9, the energy cost in Tg-D166V skinned papillary muscle fibers was slightly higher than that in Tg-WT and/or NTg muscle fibers. The difference between the mutant and control fibers, however, was not statistically significant (Fig. 9).
Figure 9.
Energy cost of contraction expressed as the ratio of fiber ATPase/fiber force (×10−2 s−1 kN−1 m2) plotted as a function of muscle activation. Data are expressed as means ± se of n experiments (as in Fig. 8).
Force and calcium transients measured simultaneously in intact papillary muscle fibers
To investigate whether the slower kinetics of force-generating myosin cross-bridges, as determined in Tg-D166V skinned papillary muscle fibers, resulted in a mutation-dependent slower relaxation of Tg-D166V muscle, intact papillary muscles from all experimental groups were electrically stimulated at 1 Hz, and the force transients and calcium transients were simultaneously recorded. As demonstrated in Fig. 10, the rates of force relaxation in Tg-D166V muscles were significantly slower than the rates measured in Tg-WT or NTg papillary muscle fibers. However, the prolonged force transients in Tg-D166V muscle fibers were not paralleled by delayed calcium transients. No differences were observed between [Ca2+] transients of Tg-D166V vs. Tg-WT or NTg intact muscle fibers (Fig. 10).
Figure 10.
Normalized force (top panel) and [Ca2+] (bottom panel) transients in electrically stimulated intact papillary muscle fibers from Tg-D166V compared with Tg-WT and NTg mice. Note significant differences in force transients between Tg-D166V and control papillary muscle fibers (P<0.05). Data are expressed as means ± se of n = 4–6 experiments.
DISCUSSION
This study was designed to examine the molecular and functional consequences of the D166V mutation in the human ventricular myosin RLC, which was shown to cause malignant FHC phenotype (5). This D166V RLC mutation was the last identified RLC mutation associated with FHC, and it has never been studied in vitro or in transgenic animal models. The mutation occurs at the last amino acid residue of the human cardiac RLC, and the negatively charged and polar aspartic acid residue is being replaced by the hydrophobic valine (Fig. 1). The RLC mutation lies in the “elbow” of the myosin lever arm, the region of the myosin heavy chain that in the crystal structure makes a sharp bend and interacts with the N and C termini of the RLC (34) (Fig. 1). It is well accepted that the role of the myosin lever arm is to amplify the small conformational changes that occur at the nucleotide- and/or actin-binding sites of the myosin head into the large changes that ultimately result in directed movement, sarcomeric shortening, and force generation in muscle (35). The functional consequences of this D166V mutation are manifested in the skinned papillary muscle fibers by largely affecting the kinetics of myosin cross-bridges and slowing the rate of dissociation g of the myosin heads from actin, as calculated by taking the ratio of fiber ATPase/concentration of cross-bridges attached at all levels of force activation in Tg-D166V fibers (Fig. 8). One can hypothesize that the lever arm swing is inhibited by steric interference originating from the valine residue that replaces the negatively charged aspartate in the mutant heart muscle. Being hydrophobic and C-β branched, valine introduces more bulkiness near the protein backbone and puts restrictions in the conformations the main chain can adopt (36, 37). Perhaps the negative charge of the aspartic acid is necessary for the elbow of the myosin lever arm to efficiently execute its swinging motion, enabling the power stroke, and replacement of this residue with a bulky valine prevents the efficient interaction of myosin with actin. Consistent with this notion, the measurements of maximal ATPase show a large decrease in the mutant fibers compared with controls (Fig. 6).
Interestingly, the aspartate to valine substitution resulted in a decreased level of RLC phosphorylation, as measured in rapidly frozen ventricular samples from Tg-D166V compared with Tg-WT hearts (Fig. 3). This could be due to an 8-fold decrease in Ca2+ binding to the D166V RLC mutant compared with the WT (data not shown). As we showed earlier, a decrease in Ca2+ binding to the E22K mutation in RLC prevented phosphorylation of the recombinant E22K-RLC protein (12, 14). Perhaps the Ca2+-free conformation of the RLC decreases the accessibility of serine 15 for the myosin light chain kinase (MLCK) and results in a lower phosphorylation of RLC (14). As was proposed by Davis et al. (20, 38), a proper balance of RLC phosphorylation is required for proper cardiac function and is a major determinant of the stretch activation response in the heart. Data from the Moss group (39) showed that RLC phosphorylation affects cardiac muscle contractility by significantly accelerating the stretch activation response. Perhaps the aspartate to valine substitution alters the stretch activation response by decreasing the phosphorylation of RLC. Our data in skinned Tg-D166V fibers also suggested that a mutant-induced decrease in RLC phosphorylation could contribute to a lower maximal force (Fig. 6) and to largely decreased kinetics of force generation of myosin cross-bridges g (Fig. 8), as both properties were shown to increase with RLC phosphorylation (40,41,42,43,44). Reduced levels of RLC phosphorylation in Tg-D166V mice were paralleled by a slight increase in the phosphorylated TnI. No obvious explanation for this interesting observation can be offered at this time to understand the link between the PKA-mediated TnI phosphorylation and the Ca2+-calmodulin activated MLCK phosphorylation of RLC (45).
Consistent with another malignant RLC mutation, R58Q, studied in skinned papillary muscles of Tg-R58Q mice, an increase in the Ca2+ sensitivity of force and ATPase measured simultaneously under isometric conditions was determined in skinned muscle fibers from Tg-D166V mice. However, the difference in pCa50 of force/ATPase-pCa relationships between Tg-D166V and control fibers was ΔpCa50 = 0.25–0.28, which is 2-fold larger (Fig. 5) than the ΔpCa50 produced by the R58Q mutation (17). This large increase in the Ca2+ sensitivity of force and ATPase produced by the D166V mutation was most likely due to a large decrease in the cross-bridge turnover rate g (Fig. 8). Ultimately, this decrease in cross-bridge turnover rate would have been expected to cause an increase in maximal force and a decrease in ATPase if the rate of cross-bridge formation f was unchanged (27). The measurements, however, showed that maximal ATPase and force per cross-section of muscle were largely decreased in the mutant fibers (Fig. 6). One possible explanation for these unexpected results is that f was presumably also decreased in Tg-D166V fibers. This hypothesis is supported by measurements showing that the fraction of force-generating myosin cross-bridges attached at maximal Ca2+ activation expressed as f/(f+g) was the same for Tg-D166V and for Tg-WT and NTg skinned fibers (Fig. 7). Therefore, for f/(f+g) to remain constant with a largely decreased g (Fig. 8), the rate of cross-bridge attachment f would have to decrease. One can hypothesize that because g decreased ∼30%, f would also have to decrease ∼30%. According to the work of Huxley (27), the time constant for the rate of rise of force in isometrically contracting muscle is f+g. It would then be expected that this time constant in the Tg-D166V fibers was slower by ∼30%. Thus, the predicted isometric force transient would be ∼30% less during a twitch in Tg-D166V fibers compared with control intact papillary muscle fibers. These changes are anticipated to result in potential systolic dysfunction of the D166V hearts. In addition, the energy cost per cross-bridge (fiber ATPase/force) was slightly higher in Tg-D166V fibers than in controls, although the difference between Tg-D166V and control fibers was not statistically significant (Fig. 9). Because the energy cost is proportional to g/Fav (28, 31), where Fav is the average force per cross-bridge, the measured decrease in g in Tg-D166V fibers must have been accompanied by a decrease in Fav for the ratio of g/Fav to slightly increase or remain constant. In support of the slower kinetics observed in Tg-D166V muscle fibers, the intact papillary muscle fiber data demonstrate mutation-dependent prolonged force transients with no change in calcium transients in Tg-D166V intact papillary muscles compared with controls (Fig. 10). Therefore, D166V-mediated slower turnover rates of force-generating myosin cross-bridges result in slower relaxation rates of Tg-D166V papillary muscles.
In this report we demonstrated a correlation between the kinetics of force-generating myosin cross-bridges (that is largely affected by the RLC aspartate to valine substitution) and the tropomyosin-troponin (Tm-Tn)-mediated regulation of cardiac muscle contraction. We also demonstrated that the mechanism of the regulation of muscle contraction proposed by Geeves and coworkers (35, 46, 47) applies in the complex muscle fiber system. As we measured, the D166V mutation dramatically decreased the kinetics of myosin cross-bridges g, and this change was subsequently communicated to the thin filament proteins, leading to a large increase in the myofilament calcium sensitivity. One can speculate that a slower relaxation rate of the myosin cross-bridges would promote the strongly bound state and facilitate the movement of Tm-Tn on actin, promoting the “close” to “open” transition and the interaction of myosin with actin. A decrease in g would, therefore, directly result in increased calcium sensitivity (48). In addition, as was shown earlier (49), the strongly bound cross-bridges increase the affinity of troponin C for calcium, and this too can be manifested by increased myofilament calcium sensitivity. Therefore, in agreement with the model of Geeves and coworkers (35, 46, 47), we show that the myosin cross-bridges can directly affect the regulation of force development in the heart muscle. Consequently, any mutation that would affect the cross-bridge kinetics by increasing or decreasing the rate of cross-bridge dissociation from actin would result in changes in the regulation of force development and cardiac muscle contraction. One can also anticipate that these mutation-induced alterations in cross-bridge kinetics most likely trigger a series of pathological responses resulting in abnormal regulation of cardiac muscle contraction (changes in myofilament calcium sensitivity and/or force). Depending on the location of the FHC mutation in the myosin head and the type of mutation (change in charge, hydrophobicity, and others), the end effect on cross-bridge kinetics could be different for different amino acid replacements and in some cases contrary effects could be observed. As explained above, there are a few potential molecular mechanisms by which the D166V mutation in RLC decreases myosin cross-bridge kinetics, and these include a charge effect of the FHC mutation (replacement of aspartic acid with a bulky valine) and/or a mutation-dependent decrease in RLC phosphorylation.
Our cellular findings suggest several potential D166V-mediated factors that could contribute to FHC when placed in vivo. First, a large increase in Ca2+ sensitivity could contribute to decreased ventricular filling at high heart rates when the tail end of the first Ca2+ transient begins to fuse with the second. Second, the slow force relaxation rate of the fibers could also start to fuse with the next contraction when heart rates are high, also contributing to diastolic dysfunction. If severe enough, these two factors could affect diastolic filling of the heart sufficiently to result in systolic dysfunction, i.e., a decrease in stroke volume. This ultimately would cause the heart to compensate by increasing wall thickness (hypertrophy). The third factor that could result in hypertrophy is a decrease in force per cross-section of muscle caused by a decrease in force per cross-bridge Fav (systolic dysfunction). This would also cause the heart to compensate by increasing wall thickness (hypertrophy). Finally, the prediction of decreased twitch force caused by a decrease in the time constant for the rate of rise of force would result in systolic dysfunction that could only be compensated for by increases in heart rates. Unfortunately, there is no clinical information available on the individuals affected by the D166V mutation to confirm or disprove our cellular findings. However, poor prognosis of patients with the disease and multiple cases of sudden cardiac death reported for the D166V-positive patients (5) suggest that the profound functional and histopathological changes found in transgenic D166V myocardium could account for a malignant FHC phenotype associated with this mutation. The physiological alterations found in Tg-D166V myocardium will have to be further confirmed in vivo to fully understand the D166V-mediated detrimental outcome of FHC.
Acknowledgments
The authors gratefully acknowledge Dr. Neal Epstein at the National Heart, Lung, and Blood Institute, NIH, for the gift of +P-human RLC antibodies. We thank Georgianna Guzman and Zoraida Diaz-Perez for their excellent technical assistance. This work was supported by NIH grant HL071778 (D.S.-C.) and American Heart Association grant 0555320B (D.S.-C.).
References
- Poetter K, Jiang H, Hassanzadeh S, Master S R, Chang A, Dalakas M C, Rayment I, Sellers J R, Fananapazir L, Epstein N D. Mutations in either the essential or regulatory light chains of myosin are associated with a rare myopathy in human heart and skeletal muscle. Nat Genet. 1996;13:63–69. doi: 10.1038/ng0596-63. [DOI] [PubMed] [Google Scholar]
- Andersen P S, Havndrup O, Bundgaard H, Moolman-Smook J C, Larsen L A, Mogensen J, Brink P A, Borglum A D, Corfield V A, Kjeldsen K, Vuust J, Christiansen M. Myosin light chain mutations in familial hypertrophic cardiomyopathy: phenotypic presentation and frequency in Danish and South African populations. J Med Genet. 2001;38:E43. doi: 10.1136/jmg.38.12.e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabaeva Z T. The Humboldt University of Berlin: Genetic analysis in hypertrophic cardiomyopathy: missense mutations in the ventricular myosin regulatory light chain gene. Ph.D. thesis. 2002 [Google Scholar]
- Kabaeva Z T, Perrot A, Wolter B, Dietz R, Cardim N, Correia J M, Schulte H D, Aldashev A A, Mirrakhimov M M, Osterziel K J. Systematic analysis of the regulatory and essential myosin light chain genes: genetic variants and mutations in hypertrophic cardiomyopathy. Eur J Hum Genet. 2002;10:741–748. doi: 10.1038/sj.ejhg.5200872. [DOI] [PubMed] [Google Scholar]
- Richard P, Charron P, Carrier L, Ledeuil C, Cheav T, Pichereau C, Benaiche A, Isnard R, Dubourg O, Burban M, Gueffet J-P, Millaire A, Desnos M, Schwartz K, Hainque B, Komajda M, for the EUROGENE Heart Failure Project Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation. 2003;107:2227–2232. doi: 10.1161/01.CIR.0000066323.15244.54. [DOI] [PubMed] [Google Scholar]
- Morner S, Richard P, Kazzam E, Hellman U, Hainque B, Schwartz K, Waldenstrom A. Identification of the genotypes causing hypertrophic cardiomyopathy in northern Sweden. J Mol Cell Cardiol. 2003;35:841–849. doi: 10.1016/s0022-2828(03)00146-9. [DOI] [PubMed] [Google Scholar]
- Hougs L, Havndrup O, Bundgaard H, Kober L, Vuust J, Larsen L A, Christiansen M, Andersen P S. One third of Danish hypertrophic cardiomyopathy patients have mutations in MYH7 rod region. Eur J Hum Genet. 2005;13:161–165. doi: 10.1038/sj.ejhg.5201310. [DOI] [PubMed] [Google Scholar]
- Seidman C E, Seidman J G. Molecular genetic studies of familial hypertrophic cardiomyopathy. Basic Res Cardiol. 1998;93:13–16. doi: 10.1007/s003950050196. [DOI] [PubMed] [Google Scholar]
- Seidman J G, Seidman C. The genetic basis for cardiomyopathy: from mutation identification to mechanistic paradigms. Cell. 2001;104:557–567. doi: 10.1016/s0092-8674(01)00242-2. [DOI] [PubMed] [Google Scholar]
- Maron B J, Olivotto I, Spirito P, Casey S A, Bellone P, Gohman T E, Graham K J, Burton D A, Cecchi F. Epidemiology of hypertrophic cardiomyopathy-related death: revisited in a large non-referral-based patient population. Circulation. 2000;102:858–864. doi: 10.1161/01.cir.102.8.858. [DOI] [PubMed] [Google Scholar]
- Maron B J. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002;287:1308–1320. doi: 10.1001/jama.287.10.1308. [DOI] [PubMed] [Google Scholar]
- Szczesna D. Regulatory light chains of striated muscle myosin: structure, function and malfunction. Curr Drug Targets Cardiovasc Haematol Disord. 2003;3:187–197. doi: 10.2174/1568006033481474. [DOI] [PubMed] [Google Scholar]
- Alcalai R, Seidman J G, Seidman C E. Genetic basis of hypertrophic cardiomyopathy: from bench to the clinics. J Cardiovasc Electrophysiol. 2008;19:104–110. doi: 10.1111/j.1540-8167.2007.00965.x. [DOI] [PubMed] [Google Scholar]
- Szczesna D, Ghosh D, Li Q, Gomes A V, Guzman G, Arana C, Zhi G, Stull J T, Potter J D. Familial hypertrophic cardiomyopathy mutations in the regulatory light chains of myosin affect their structure, Ca2+ binding, and phosphorylation. J Biol Chem. 2001;276:7086–7092. doi: 10.1074/jbc.M009823200. [DOI] [PubMed] [Google Scholar]
- Szczesna-Cordary D, Guzman G, Ng S S, Zhao J. Familial hypertrophic cardiomyopathy-linked alterations in Ca2+ binding of human cardiac myosin regulatory light chain affect cardiac muscle contraction. J Biol Chem. 2004;279:3535–3542. doi: 10.1074/jbc.M307092200. [DOI] [PubMed] [Google Scholar]
- Richard P, Charron P, Carrier L, Ledeuil C, Cheav T, Pichereau C, Benaiche A, Isnard R, Dubourg O, Burban M, Gueffet J-P, Millaire A, Desnos M, Schwartz K, Hainque B, Komajda M. Correction (Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy) Circulation. 2004;109:3258. doi: 10.1161/01.CIR.0000066323.15244.54. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xu Y, Kerrick W G L, Wang Y, Guzman G, Diaz-Perez Z, Szczesna-Cordary D. Prolonged Ca2+ and force transients in myosin RLC transgenic mouse fibers expressing malignant and benign FHC mutations. J Mol Biol. 2006;361:286–299. doi: 10.1016/j.jmb.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Szczesna-Cordary D, Guzman G, Zhao J, Hernandez O, Wei J, Diaz-Perez Z. The E22K mutation of myosin RLC that causes familial hypertrophic cardiomyopathy increases calcium sensitivity of force and ATPase in transgenic mice. J Cell Sci. 2005;118:3675–3683. doi: 10.1242/jcs.02492. [DOI] [PubMed] [Google Scholar]
- Szczesna-Cordary D, Jones M, Moore J R, Watt J, Kerrick W G L, Xu Y, Wang Y, Wagg C, Lopaschuk G D. Myosin regulatory light chain E22K mutation results in decreased cardiac intracellular calcium and force transients. FASEB J. 2007;21:3974–3985. doi: 10.1096/fj.07-8630com. [DOI] [PubMed] [Google Scholar]
- Davis J S, Hassanzadeh S, Winitsky S, Lin H, Satorius C, Vemuri R, Aletras A H, Wen H, Epstein N D. The overall pattern of cardiac contraction depends on a spatial gradient of myosin regulatory light chain phosphorylation. Cell. 2001;107:631–641. doi: 10.1016/s0092-8674(01)00586-4. [DOI] [PubMed] [Google Scholar]
- Gomes A V, Harada K, Potter J D. A mutation in the N-terminus of troponin I that is associated with hypertrophic cardiomyopathy affects the Ca2+-sensitivity, phosphorylation kinetics and proteolytic susceptibility of troponin. J Mol Cell Cardiol. 2005;39:754–765. doi: 10.1016/j.yjmcc.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Guth K, Wojciechowski R. Perfusion cuvette for the simultaneous measurement of mechanical, optical and energetic parameters of skinned muscle fibres. Pflügers Arch. 1986;407:552–557. doi: 10.1007/BF00657515. [DOI] [PubMed] [Google Scholar]
- Allen K, Xu Y Y, Kerrick W G. Ca2+ measurements in skinned cardiac fibers: effects of Mg2+ on Ca2+ activation of force and fiber ATPase. J Appl Physiol. 2000;88:180–185. doi: 10.1152/jappl.2000.88.1.180. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xu Y, Guth K, Kerrick W G. Troponin C regulates the rate constant for the dissociation of force- generating myosin cross-bridges in cardiac muscle. J Muscle Res Cell Motil. 1999;20:645–653. doi: 10.1023/a:1005559613516. [DOI] [PubMed] [Google Scholar]
- Baylor S M, Hollingworth S. Measurement and interpretation of cytoplasmic [Ca2+] signals from calcium-indicator dyes. News Physiol Sci. 2000;15:19–26. [PubMed] [Google Scholar]
- Sanbe A, Nelson D, Gulick J, Setser E, Osinska H, Wang X, Hewett T E, Klevitsky R, Hayes E, Warshaw D M, Robbins J. In vivo analysis of an essential myosin light chain mutation linked to familial hypertrophic cardiomyopathy. Circ Res. 2000;87:296–302. doi: 10.1161/01.res.87.4.296. [DOI] [PubMed] [Google Scholar]
- Huxley A F. A hypothesis for the mechanism of contraction of muscle. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Brenner B. Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proc Natl Acad Sci U S A. 1988;85:3265–3269. doi: 10.1073/pnas.85.9.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley A F. Cross-bridge action: present views, prospects, and unknowns. J Biomech. 2000;33:1189–1195. doi: 10.1016/s0021-9290(00)00060-9. [DOI] [PubMed] [Google Scholar]
- Prakash Y S, Cody M J, Housmans P R, Hannon J D, Sieck G C. Comparison of cross-bridge cycling kinetics in neonatal vs. adult rat ventricular muscle. J Muscle Res Cell Motil. 1999;20:717–723. doi: 10.1023/a:1005585807179. [DOI] [PubMed] [Google Scholar]
- Kerrick W G, Xu Y. Inorganic phosphate affects the pCa-force relationship more than the pCa-ATPase by increasing the rate of dissociation of force generating cross-bridges in skinned fibers from both EDL and soleus muscles of the rat. J Muscle Res Cell Motil. 2004;25:107–117. doi: 10.1023/b:jure.0000035841.04314.16. [DOI] [PubMed] [Google Scholar]
- Wen Y, Pinto J R, Gomes A V, Xu Y, Wang Y, Wang Y, Potter J D, Kerrick W G. Functional consequences of the human cardiac troponin I hypertrophic cardiomyopathy mutation R145G in transgenic mice. J Biol Chem. 2008;283:20484–20494. doi: 10.1074/jbc.M801661200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi M A, Homsher E, Trentham D R. The kinetics of magnesium adenosine triphosphate cleavage in skinned muscle fibres of the rabbit. J Physiol. 1984;352:575–599. doi: 10.1113/jphysiol.1984.sp015311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayment I, Rypniewski W R, Schmidt-Base K, Smith R, Tomchick D R, Benning M M, Winkelmann D A, Wesenberg G, Holden H M. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993;261:50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- Geeves M A, Holmes K C. The molecular mechanism of muscle contraction. Adv Protein Chem. 2005;71:161–193. doi: 10.1016/S0065-3233(04)71005-0. [DOI] [PubMed] [Google Scholar]
- Omote H, Figler R A, Polar M K, Al-Shawi M K. Improved energy coupling of human P-glycoprotein by the glycine 185 to valine mutation. Biochemistry. 2004;43:3917–3928. doi: 10.1021/bi035365l. [DOI] [PubMed] [Google Scholar]
- Doolittle R F, Pandi L. Probing the β-chain hole of fibrinogen with synthetic peptides that differ at their amino termini. Biochemistry. 2007;46:10033–10038. doi: 10.1021/bi7010916. [DOI] [PubMed] [Google Scholar]
- Davis J S, Hassanzadeh S, Winitsky S, Wen H, Aletras A, Epstein N D. A gradient of myosin regulatory light-chain phosphorylation across the ventricular wall supports cardiac torsion. Cold Spring Harb Symp Quant Biol. 2002;67:345–352. doi: 10.1101/sqb.2002.67.345. [DOI] [PubMed] [Google Scholar]
- Stelzer J E, Patel J R, Moss R L. Acceleration of stretch activation in murine myocardium due to phosphorylation of myosin regulatory light chain. J Gen Physiol. 2006;128:261–272. doi: 10.1085/jgp.200609547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney H L, Bowman B F, Stull J T. Myosin light chain phosphorylation in vertebrate striated muscle: regulation and function. Am J Physiol. 1993;264:C1085–C1095. doi: 10.1152/ajpcell.1993.264.5.C1085. [DOI] [PubMed] [Google Scholar]
- Szczesna D, Zhao J, Jones M, Zhi G, Stull J, Potter J D. Phosphorylation of the regulatory light chains of myosin affects Ca2+ sensitivity of skeletal muscle contraction. J Appl Physiol. 2002;92:1661–1670. doi: 10.1152/japplphysiol.00858.2001. [DOI] [PubMed] [Google Scholar]
- Metzger J M, Greaser M L, Moss R L. Variations in cross-bridge attachment rate and tension with phosphorylation of myosin in mammalian skinned skeletal muscle fibers: implications for twitch potentiation in intact muscle. J Gen Physiol. 1989;93:855–883. doi: 10.1085/jgp.93.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney H L, Stull J T. Alteration of cross-bridge kinetics by myosin light chain phosphorylation in rabbit skeletal muscle: implications for regulation of actin-myosin interaction. Proc Natl Acad Sci U S A. 1990;87:414–418. doi: 10.1073/pnas.87.1.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann P A, Metzger J M, Greaser M L, Moss R L. Effects of partial extraction of light chain 2 on the Ca2+ sensitivities of isometric tension, stiffness, and velocity of shortening in skinned skeletal muscle fibers. J Gen Physiol. 1990;95:477–498. doi: 10.1085/jgp.95.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias F A L, Walker L A, Arteaga G M, Walker J S, Vijayan K, Pena J R, Ke Y, Fogaca R T H, Sanbe A, Robbins J, Wolska B M. The effect of myosin regulatory light chain phosphorylation on the frequency-dependent regulation of cardiac function. J Mol Cell Cardiol. 2006;41:330–339. doi: 10.1016/j.yjmcc.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Geeves M A, Lehrer S S. Cooperativity in the Ca2+ regulation of muscle contraction. Results Probl Cell Differ. 2002;36:111–132. doi: 10.1007/978-3-540-46558-4_10. [DOI] [PubMed] [Google Scholar]
- McKillop D F, Geeves M A. Regulation of the interaction between actin and myosin subfragment 1: evidence for three states of the thin filament. Biophys J. 1993;65:693–701. doi: 10.1016/S0006-3495(93)81110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J M, Wang Y, Kerrick W G, Kawai R, Cheung H C. Activation of striated muscle: nearest-neighbor regulatory-unit and cross-bridge influence on myofilament kinetics. J Mol Biol. 2002;322:1065–1088. doi: 10.1016/s0022-2836(02)00855-0. [DOI] [PubMed] [Google Scholar]
- Guth K, Potter J D. Effect of rigor and cycling cross-bridges on the structure of troponin C and on the Ca2+ affinity of the Ca2+-specific regulatory sites in skinned rabbit psoas fibers. J Biol Chem. 1987;262:13627–13635. [PubMed] [Google Scholar]