Abstract
P-selectin glycoprotein ligand-1 (PSGL-1) is constitutively expressed on leukocytes and was thought to be down-regulated with cell activation. However, this work shows the surprising finding of functional PSGL-1 up-regulation during acute inflammation. PSGL-1 function was studied in our autoperfusion assay, in which blood from a mouse carotid flows through a microchamber coated with a fixed density of P-selectin. Under the inflammatory conditions—uveitis induced by systemic lipopolysaccharide injection—we recorded significantly reduced leukocyte rolling velocity, which suggests PSGL-1 up-regulation; however, flow cytometry showed reduced PSGL-1. When bound leukocytes were released from the vasculature by PSGL-1 blockade, a large peripheral blood leukocyte (PBL) population showed elevated PSGL-1, which could account for the reduced PSGL-1 in the remaining unbound population. In the eye, systemic blockade of PSGL-1 with a monoclonal antibody or recombinant soluble PSGL-1 drastically reduced the severe manifestations of uveitis. Furthermore, PSGL-1 blockade was significantly more effective in reducing retinal leukostasis than was P-selectin blockade. Our results provide surprising evidence for functional PSGL-1 up-regulation in PBLs during acute inflammation. The temporal overlap between PSGL-1 and P-selectin up-regulation reveals an as yet unrecognized collaboration between this receptor-ligand pair, increasing efficiency of the first steps of the leukocyte recruitment cascade.—Almulki, L., Noda, K., Amini, R., Schering, A., Garland, R. C., Nakao, S., Nakazawa, T., Hisatomi, T., Thomas, K. L., Masli, S., Hafezi-Moghadam, A. Surprising up-regulation of P-selectin glycoprotein ligand-1 (PSGL-1) in endotoxin-induced uveitis.
Keywords: leukocyte rolling velocity, autoperfused micro-flow chamber, lipopolysaccharides, adhesion molecules, acute immune response
P-selectin glycoprotein ligand-1 (PSGL-1), a 240-kDa disulfide-bonded homodimeric mucin-like glycoprotein, is expressed on myeloid cells and a subset of lymphocytes (1). PSGL-1 binds to P-, E-, and L-selectin and mediates the interaction of leukocytes with endothelial cells, platelets, and other leukocytes (2,3,4,5). Under flow conditions, PSGL-1 interaction with P-selectin on the surface of activated endothelium (6) mediates the initial steps of the leukocyte recruitment cascade, tethering and rolling (7,8,9). This initial binding of PSGL-1 to P-selectin critically contributes to the vascular damage after ischemia reperfusion (IR) or during atherosclerosis (10, 11).
The predominant role of PSGL-1 in the initial steps of the leukocyte recruitment cascade makes it an attractive target for the treatment of vascular inflammatory diseases. For instance, in a model of IR injury to the rat liver, PSGL-1 blockade with a monoclonal antibody (mAb) significantly reduces intrahepatic leukocyte infiltration, cytokine gene expression, and hepatocellular damage (12, 13). In addition, soluble PSGL-1, which binds to all selectins in vivo (14), diminishes the localized production of proinflammatory cytokines during IR kidney injury (15, 16).
Uveitis is a common inflammatory eye disease that can cause loss of vision. In the endotoxin-induced model of uveitis (EIU) (17, 18), adhesion molecules critically contribute to the inflammatory outcome. P- and E-selectin (19, 20), β2-integrins (21, 22), ICAM-1 (23, 24), VCAM-1, and VLA-4 (25) play distinct roles in the ocular inflammatory responses to endotoxin. P-selectin blockade results in only a partial reduction of leukocyte infiltration into the vitreous of EIU animals, whereas blockade of both P- and E-selectin causes additional reduction (20). Similarly, blockade of all selectins with sialyl Lewis X, a tetrasaccharide expressed on cell surface molecules, reduces leukocyte infiltration into the aqueous humor of EIU animals more than P-selectin blockade alone (19). Because of the various functional overlaps between the molecules involved in the inflammatory outcome in EIU (26), it is of great clinical interest to identify molecular targets, the blockade of which is least compensated for by other molecules (27).
During EIU, leukocyte rolling flux and firm adhesion peak in the iris venules 4–8 h after lipopolysaccharide (LPS) injection (28) and also coincide with reduced rolling velocity in the retinal vessels (29). Thus far, these phenomena have been attributed to the increased expression of endothelial P-selectin (19, 30), whereas the regulation of P-selectin’s leukocyte ligand, PSGL-1, has remained unexplored. Indeed, the current paradigm suggests that endotoxin down-regulates PSGL-1 expression (31), further emphasizing the role of P-selectin up-regulation in the aforementioned changes.
The complexity of the leukocyte endothelial interaction in vivo, together with the fact that endothelial P-selectin expression varies during inflammation, makes elucidation of potential changes in PSGL-1 function during disease challenging. To study such molecular changes in leukocyte surface molecules during diseases, we previously developed the autoperfused micro-flow chamber assay (32). Using this technique, we recently showed functional up-regulation of the leukocyte integrin VLA-4 during EIU and the important role of this molecule for leukocyte recruitment in the eye (25).
In this study, we investigate the role of PSGL-1 during acute ocular inflammation on a functional level by using the established model of EIU in mice together with the autoperfused micro-flow chamber assay. In addition, we assess the effect of PSGL-1 inhibition on the inflammatory outcome during EIU by using a functionally blocking mAb or recombinant PSGL-1 protein.
MATERIALS AND METHODS
Animals and induction of EIU
C57BL/6 wild-type (WT) male mice at 8–12 wk of age were purchased from Jackson Laboratory (Bar Harbor, ME, USA). For autoperfused flow chamber experiments, 10- to 14-wk-old male green fluorescent protein (GFP) transgenic mice [C57BL/6-Tg (UBC-GFP) 30Scha/J; Charles River, Wilmington, MA, USA] were used. Mice were anesthetized with ketamine at 100 mg/kg and xylazine at 10 mg/kg, and EIU was induced by a single i.p. injection of 150 μg Salmonella typhimurium LPS (Sigma-Aldrich, St. Louis, MO, USA). All animal experiments were approved by the Animal Care Committee of the Massachusetts Eye & Ear Infirmary.
Treatments
Mice received a single i.p. injection of 100 μg of mAbs against either PSGL-1 (4RA10), P-selectin (RB40.34), or an isotype-matched control Ab (BD Biosciences, Franklin Lakes, NJ, USA), immediately after LPS stimulation. Soluble recombinant PSGL-1 fused to the Fc domain of human immunoglobulin IgG1 (rPSGL-Ig; Y’s Therapeutics, Burlingame, CA, USA) was systemically administered to the mice via femoral vein injection at a concentration of 7.5 mg/kg body weight, and control mice received isotype-matched human IgG1 (Invitrogen, San Francisco, CA, USA). The effects of the treatments on ocular inflammation were evaluated 24 h after the injections.
Peripheral blood counts
Blood was harvested from the heart of control and EIU mice into EDTA-coated syringes. Complete blood counts were performed by using a Coulter Counter (T-890; Beckman Coulter, Inc., Fullerton, CA, USA).
Autoperfused micro-flow chamber assay
Leukocyte rolling velocity was analyzed in control and EIU mice by using our autoperfused micro-flow chamber assay (32). Briefly, translucent microchambers (inner diameter 0.4×0.04 mm; VitroCom, Mountain Lakes, NJ, USA) were coated with recombinant murine P-selectin (5 μg/ml; R&D, Minneapolis, MN, USA) at 4°C overnight. P-selectin-coated microslides were connected to biocompatible polyester tubing (PE10; Becton Dickinson, Franklin Lakes, NJ, USA) at both ends. The tubing and microslide were incubated with 1% bovine serum albumin (Sigma-Aldrich) for 1 h to block nonspecific leukocyte interactions with the inner surfaces. Subsequently, the tubing ends were microsurgically connected to the right carotid artery and the left jugular vein of an anesthetized mouse, as previously described (32).
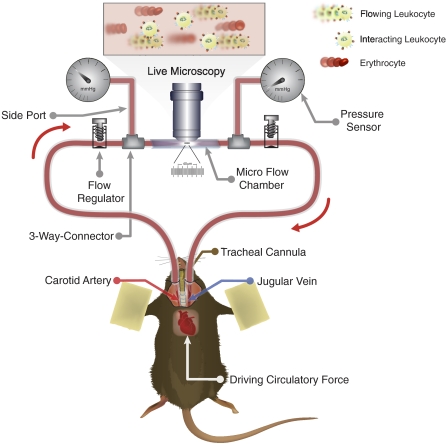
In this model, blood flows from the carotid artery into the biocompatible inlet tubing, then through the microslides, proceeds through outlet tubing, and reenters the animal’s body via the jugular vein (Fig. 1). To regulate the blood velocity, the diameter of the inlet tubing is altered by adjustable screw valves. To continuously measure the blood pressure, microtransducers (SPR-671, Millar Instruments, Houston, TX, USA) are attached to the chamber by 3-way connectors embedded before and after the microslide. The transducers are connected to a pressure control unit (TCB-600, Millar Instruments). The analog output of the pressure control unit was digitalized by an A/D converter (ML785 PowerLab/8SP, ADInstruments; Colorado Springs, CO, USA) connected to a Macintosh computer containing CHART 5™ software (ADInstruments). The measured values at the inlet and the outlet of the chamber were used to calculate the pressure drop (Δp) in the micro-flow chamber. The shear stress (τ) was derived from Δp, by using our previously described custom software, Rectflow®, allowing 3-D determination of hemodynamic conditions within the micro-flow chamber (32).
Figure 1.
Schematic of the autoperfused micro-flow chamber assay, originally introduced by Hafezi-Moghadam et al. (32). The micro-flow chamber assembly, microsurgically connected to the carotid artery and jugular vein of a live mouse under anesthesia, creates a closed circuit with the animal’s vascular system. The native blood cells flow from the carotid artery, pass through the translucent area of the chamber for microscopy, and subsequently reenter the animal’s body through the jugular vein. The pressure gradient between the artery (high pressure) and the vein (low pressure) provides a continuous flow of cells through the chamber. Circulating PBLs interact with immobilized adhesion molecules (i.e., P-selectin at 5 μg/ml coating concentration) on translucent microslides. Regulation of the entry pressure (i.e., 40 mmHg) before the microslide provides controlled flow conditions. Interacting leukocytes are observed and recorded by using transluminescence video microscopy.
Under an upright, fixed-stage intravital microscope (Leica, Wetzlar, Germany), leukocyte rolling on immobilized P-selectin was videotaped by using a color charge-coupled device camera (Dage, Michigan City, IN, USA) for subsequent analysis. Ten different fields of view were each recorded for at least 1 min/field in each chamber (32). Leukocyte rolling velocity was defined as displacement of individual cells interacting with the chamber surfaces over time (Δd/Δt). The displacement of leukocytes was determined by using Exbem software (version 3.0; Pixlock, Münster, Germany).
Flow cytometry
Flow cytometry was performed as previously described (33) with slight modifications. Briefly, blood freshly drawn from the mouse heart through use of an EDTA-treated syringe was aliquoted and incubated for 30 min on ice with 1 μg/106 cells of either phycoerythrin (PE) -conjugated anti-PSGL-1 mAb (2PH-1), anti-PSGL-1 mAb (4RA10) as a primary antibody followed by PE-conjugated goat–anti-rat Ig, or their respective isotype controls (BD Biosciences, Franklin Lakes, NJ, USA). Red blood cells were lysed by using Easy-Lyse (Leinco Technologies, St. Louis, MO, USA). Subsequently, blood cells were centrifuged and resuspended into fixative according to the manufacturer’s protocol for flowcytometric analysis. Peripheral blood lymphocytes (PBLs) were gated according to their characteristic forward and side scatter by using Coulter EPICS XL; System Work II software (Beckman Coulter) was used for data acquisition.
Aqueous humor analysis
Inflammatory cell infiltration in aqueous humor, obtained from normal and EIU animals 24 h after LPS injection with or without PSGL-1 antibody treatment, was analyzed. In brief, aqueous humor was collected by anterior chamber puncture with a 30-gauge needle. Subsequently, 1 μl of the aqueous humor samples was pipetted onto microscopy slides and air-dried. The slides were processed with Wright Giemsa staining solution (Fisher Scientific, Kalamazoo, MI, USA), and the total number of stained cells on each slide was counted under a light microscope (Leica) (34).
Ex vivo visualization of retinal vasculature and adherent leukocytes
Leukocyte adhesion to the retinal vessels was evaluated 24 h after EIU induction by using the Concanavalin A (ConA) lectin staining technique (25, 34). Briefly, under deep anesthesia, a 27-gauge cannula was introduced into the left ventricle. Mice were then perfused with 3 ml of PBS to remove erythrocytes and nonadherent leukocytes, followed by 3 ml of fluorescein isothiocyanate-conjugated ConA lectin (50 μg/ml in PBS; Vector Laboratories, Burlington, CA, USA) to label adherent leukocytes and vascular endothelial cells. Mice were then perfused with PBS for removal of residual unbound lectin. The eyes were enucleated and fixed in 1% paraformaldehyde for 10 min, and retinas were carefully flatmounted. The flatmounts were imaged by using an epifluorescence microscope (DM RXA; Leica), and the total number of ConA-stained leukocytes per retina was counted.
Reverse transcription-polymerase chain reaction (RT-PCR)
Dissected retinas from mouse eyes at 6 and 24 h after treatments were homogenized in extraction reagent (TRIzol Reagent; Invitrogen), and total RNA was extracted according to the manufacturer’s protocol. Subsequently, reverse transcription was conducted from 2 μg of total RNA at 37°C for 1 h by using a First-Strand cDNA synthesis kit (GE Healthcare, Buckinghamshire, UK). PCR was performed with a Platinum PCR SuperMix (Invitrogen) with a thermal controller (GeneAmp PCR System 9700; PE Biosystems, Foster City, CA, USA). The thermal cycle was 1 min of denaturation at 94°C; then either 1 min of annealing at 55°C for GAPDH, 2 min at 50°C for tumor necrosis factor (TNF) -α, or 1 min at 62°C for monocyte chemoattractant protein (MCP)-1; and 1 min of elongation at 72°C. This thermal sequence was conducted for 30 cycles and ended with an additional 3 min at 72°C. The nucleotide sequences of the PCR primers were as follows: 5′-ACC CAG AAG ACT GTG GAT GG-3′ (sense) and 5′-GGG TCT TAC TCC TTG GAG GC-3′ (antisense) for GAPDH, 5′-ATG AGC ACA GAA AGC ATG ATC CGC-3′ (sense), and 5′-CCA AAG TAG ACC TGC CCG GAC TC-3′ (antisense) for TNF-α, and 5′-ATC CCA ATG AGT AGG CTG GAG AG-3′ (sense) and 5′-CAG AAG TGC TTG AGG TGG TTG TG-3′ (antisense) for MCP-1 (34,35,36). PCR products were analyzed by electrophoresis in 1.5% agarose gel and stained with ethidium bromide (0.2 μg/ml). Quantification of the band density was performed by using ImageJ 1.37 software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD, USA; available at http://rsb.info.nih.gov/ij/).
Statistical analysis
Student’s t test was used for comparison of groups. All values were expressed as means ± se. A value of P < 0.05 was considered statistically significant.
RESULTS
Reduced leukocyte rolling velocity on immobilized P-selectin during EIU
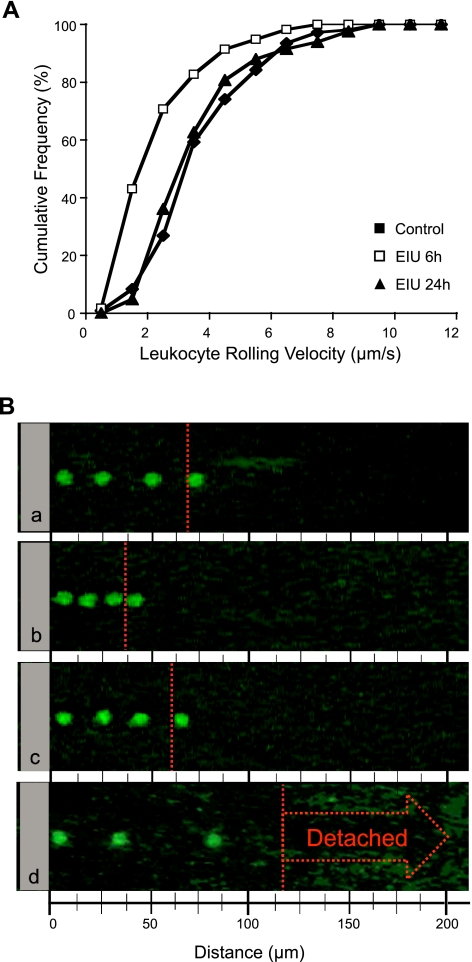
To investigate PSGL-1 function during EIU, we performed autoperfused micro-flow chamber experiments with normal and EIU animals (Fig. 1). PBLs from normal animals rolled on immobilized P-selectin (5 μg/ml) at an average velocity of 4.1 ± 0.2 μm/s (n=6; Fig. 2). At 6 h after LPS injection, however, the velocity of rolling leukocytes on immobilized P-selectin was significantly reduced by 34% to 2.7 ± 0.2 μm/s (n=4, P<0.05), which suggests functional up-regulation of PSGL-1 during EIU. However, 24 h after LPS injection, leukocytes rolled at similar velocities (4.0±0.6 μm/s, n=4) as those from normal control animals (P=0.8), which suggests a recovery from the initial functional up-regulation of PSGL-1 at this time point (Fig. 2). To examine whether the reduced leukocyte rolling velocity in EIU animals at 6 h is PSGL-1-mediated, 50 μl of the neutralizing mAb, 4RA10, was injected through the side port into the chamber. Within the first minute after the mAb injection, rolling leukocytes sped up and detached from the chamber surfaces and joined the stream of the blood flow (n=2). Thereafter, no leukocyte rolling was observed in any area of the chamber (Fig. 2B).
Figure 2.
Visualization of PSGL-1-mediated leukocyte rolling during EIU. A) Cumulative histograms of the velocities of rolling leukocytes on immobilized P-selectin in micro-flow chambers connected to control or EIU mice at various time points after LPS stimulation. The average rolling velocity was decreased in EIU animals 6 h after LPS injection (n=4) compared with control animals (n=6, P<0.05). However, 24 h after LPS injection, the average rolling velocity in EIU animals was similar to that of control animals (n=4, P=0.4). Each curve represents rolling velocities of 50 leukocytes. B) Composite micrographs of representative rolling leukocytes on immobilized P-selectin in the autoperfused micro-flow chamber: control mouse (a), EIU mouse 6 h after LPS injection (b), EIU mouse 24 h after LPS injection (c), and EIU mouse treated with anti-PSGL-1 mAb injected through the side port into the chamber, 6 h after LPS injection (d). The displacement of a representative rolling leukocyte from each group from the left border (0 μm) to the red dotted line within 15 s illustrates the differences in leukocyte rolling velocity.
To account for the hemodynamic conditions within the chamber, the blood pressure before the inlet and after the outlet of the chamber was measured, from which the drop of pressure Δp and the shear stress τ were derived (32). The average shear values did not differ significantly among the experimental groups (Table 1).
TABLE 1.
PBL counts in control or EIU animals 6 or 24 h after LPS injection
| Variable | Control | EIU
|
|
|---|---|---|---|
| 6 h | 24 h | ||
| ΔP (mmHg) | 38.7 ± 2.5 | 37.2 ± 3.8 | 37 ± 3.3 |
| τ (dyn/cm2) | 20.6 ± 1.3 | 19.8 ± 2 | 19.7 ± 1.8 |
| PBL count (μl−1) | 2795 ± 337 | 1074 ± 218 | 2160 ± 336 |
Pressure drop (ΔP) and shear stress (τ) values in the flow chambers in the various experimental groups.
We also quantified the total PBLs of normal (n=5) and EIU mice at 6 (n=6) and 24 h (n=4) after LPS injection. The PBL counts of EIU animals were significantly reduced 6 h after LPS injection (P<0.01) and returned to normal values by 24 h (P=0.1; Table 1).
Numerical PSGL-1 up-regulation on PBLs
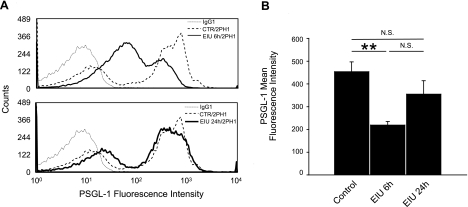
To further investigate the cause of the reduction in P-selectin-mediated rolling velocity in EIU animals, we quantified the expression of PSGL-1 on the surface of the PBLs in normal and EIU animals. PBLs from lysed blood of control and LPS-treated mice at 6 and 24 h were labeled with either anti-PSGL-1 or IgG control antibodies conjugated with PE and analyzed using flow cytometry. In line with previous reports (31, 37), at 6 h after LPS injection, PBLs showed a significantly lower PSGL-1 mean fluorescence intensity (MFI; 219.2±15.2, n=4) compared with control mice (453.9±43.1, n=4, P<0.01). PBLs from EIU mice at 24 h after LPS did not show a difference in PSGL-1 MFI compared with that of normal animals (355±59, n=4, P=0.2; Fig. 3).
Figure 3.
PSGL-1 expression on PBLs during EIU. A) Representative histograms of PSGL-1 expression on PBLs from control (CTR) or EIU mice at 6 and 24 h after LPS injection. Leukocytes were stained with anti-PSGL-1 (2PH1) or IgG1 control antibodies conjugated with PE. PBLs from EIU animals 6 h after LPS injection showed decreased PSGL-1 mean fluorescence intensity values compared with normal controls. In contrast, leukocytes from EIU animals at 24 h after LPS injection showed PSGL-1 mean fluorescence values similar to control animals. Each curve is representative of 4 independent experiments. B) Quantification of PSGL-1 mean fluorescence intensity in control and EIU animals at 6 and 24 h after LPS injection. N.S., not significant. **P < 0.01.
Because the reduction of PSGL-1 expression on PBLs 6 h after LPS injection appears in contrast to what could explain the reduced rolling velocity at this time point on immobilized P-selectin, we hypothesized that the population of the cells examined for rolling velocity and those obtained for flow cytometry might be distinct.
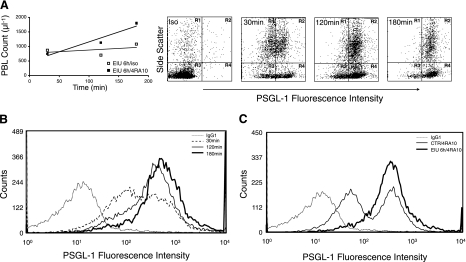
To address this, we treated EIU mice with the PSGL-1 neutralizing antibody, 4RA10, 6 h after LPS treatment and quantified PSGL-1 surface expression by using flow cytometry. At 30, 120, and 180 min after anti-PSGL-1 mAb treatment, the PBL counts of the EIU animals rapidly increased to 749 ± 27, 1361 ± 34, and 1799 ± 44, respectively, compared with 875 ± 12, 707 ± 18, and 1081 ± 12 leukocytes/μl in the isotype-treated mice (Fig. 4A). Concomitant with the increase in the PBL counts, the PSGL-1 MFI increased to 441 ± 6, 558 ± 6, and 781 ± 9 at 30, 120, and 180 min, respectively, after anti-PSGL-1 mAb treatment (Fig. 4A, B). These results suggest that the higher PSGL-1-expressing leukocytes in EIU animals were interacting with the vascular endothelium and were released into the circulation only with the anti-PSGL-1 treatment.
Figure 4.
PBL counts and PSGL-1 expression in EIU animals with anti-PSGL-1-neutralizing mAb. A) At 6 h after LPS injection, mice were treated with PSGL-1-neutralizing mAb (4RA10) or isotype control. The animal blood was harvested at 30, 120, and 180 min after antibody treatment, and the PBL counts were obtained. In parallel, leukocytes were evaluated for their PSGL-1 surface expression through indirect immunofluorescence. B) PSGL-1 surface expression on PBLs from EIU mice 6 h after LPS injection at 30, 120, and 180 min after antibody treatment. PBLs from animals 120 and 180 min after PSGL-1 blockade showed higher expression of PSGL-1 compared with the cells obtained 30 min after mAb treatment. C) Increased PSGL-1 surface expression on PBLs from EIU mice 6 h after simultaneous administration of PSGL-1-neutralizing mAb (4RA10) and LPS, compared with 4RA10-treated controls. Each curve is representative of 3 independent experiments.
Similarly, PBLs from animals that received the anti-PSGL-1 mAb (4RA10) at the same time as the LPS injection showed higher PSGL-1 MFI (484.7±52.9, n=3) compared with that on PBLs from control mice 6 h after antibody treatments (386.7±40.9, n=3). This suggests a previously unknown numerical PSGL-1 up-regulation in LPS-treated animals (Fig. 4C). The PBL counts of the animals 6 h after the simultaneous treatment with the anti-PSGL-1 mAb and LPS (2656±438, n=3) did not differ from that of control animals that received the antibody alone (2439±150, n=3, P=0.6), which suggests a prominent role for PSGL-1 in the LPS-induced leukopenia.
Role of PSGL-1 in leukocyte infiltration into the anterior chamber
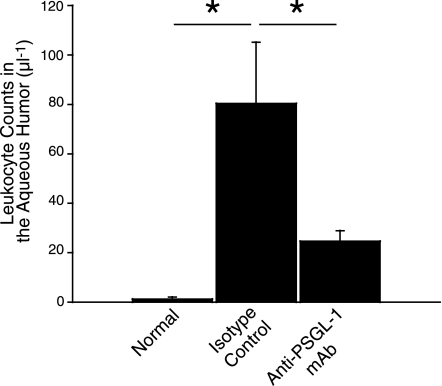
To investigate whether PSGL-1 contributes to acute ocular inflammation, we quantified the number of leukocytes in the anterior chamber of normal control and EIU mice with or without blockade of PSGL-1. Twenty-four hours after LPS injection, there was a significantly larger number of infiltrated cells in the aqueous humor of the EIU mice treated with control IgG (80±25 cells/μl, n=7) compared with normal controls (1.2±0.3 cells/μl, n=5, P<0.05). However, blockade of PSGL-1 with a functionally blocking mAb caused a significant 69.3% reduction in the number of the infiltrated cells in the aqueous humor of EIU mice (25±4 cells/μl, n=9, P<0.05; Fig. 5). There was no difference in PBL counts between the mice treated with the anti-PSGL-1 mAb (2667±341, n=6) and those treated with isotype control (2396±106, n=3, P=0.4).
Figure 5.
PSGL-1 blockade diminishes inflammatory cell infiltration into the aqueous humor during EIU. The number of infiltrated cells counted in 1 μl of aqueous humor of normal and EIU mice 24 h after LPS injection, treated with either anti-PSGL-1 mAb (n=9) or control IgG (n=7). Values are means ± se. *P < 0.05.
These data suggest an important role for PSGL-1 in the acute inflammatory response in the anterior chamber.
Role of PSGL-1 in retinal leukostasis during EIU
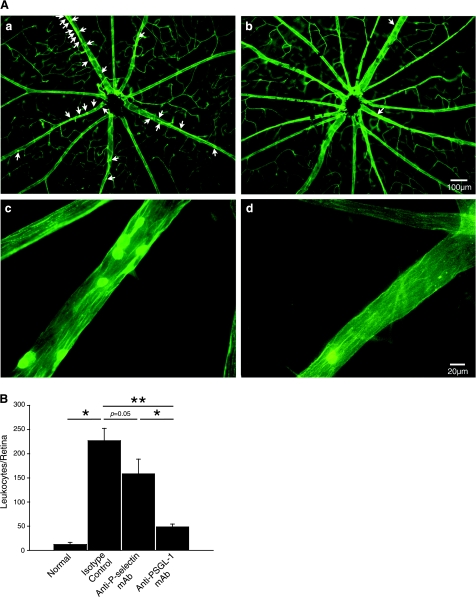
To investigate whether PSGL-1 plays a role in the inflammatory outcome of the posterior segment of the eye, we quantified firm leukocyte adhesion in the retinal vessels of normal vehicle-treated mice and EIU mice treated with anti-PSGL-1 or isotype-matched control Abs. In line with previous reports (19, 20), a large number of leukocytes firmly adhered to the retinal vessels 24 h after LPS injection (227±26 leukocytes/retina, n=8) in contrast to the small number found in saline-treated, normal control mice (12±4 leukocytes/retina, n=4). Blockade of PSGL-1 with a neutralizing mAb caused a pronounced 78.5% reduction in retinal leukostasis in EIU mice (49±6 leukocytes/retina, n=12, P=0.0001), compared with the IgG-treated EIU animals, 24 h after LPS injection (Fig. 6A, B).
Figure 6.
Effect of PSGL-1 blockade on retinal leukostasis. A) Representative micrographs of flatmounted retinas from EIU mice at 24 h treated with isotype control mAb show a large number of firmly adhering leukocytes on the retinal vasculature (a, c; arrows). In contrast, anti-PSGL-1-treated EIU animals show very few firmly adhering leukocytes (b, d). B) Quantification of firmly adhering leukocytes in the retina of normal and EIU animals treated with IgG control or mAb against P-selectin or PSGL-1. EIU mice treated with anti-PSGL-1 mAb (n=12) showed significantly fewer firmly adhering leukocytes than those treated with either anti-P-selectin mAb (n=8) or IgG control (n=8). Results represent means ± se; *P < 0.05; **P < 0.01.
To compare the contribution of PSGL-1 and its endothelial ligand, P-selectin, with the firm leukocyte adhesion in the retinal vessels, we also treated a group of EIU animals with the P-selectin neutralizing mAb, RB40.34. Blockade of P-selectin in EIU mice caused a significant 30% reduction in firm leukocyte adhesion to the retinal vessels (159±30 leukocytes/retina, n=8, P=0.05). However, the reduction in the number of firm leukocyte adhesions in the retinal vessels of EIU animals with PSGL-1 blockade was significantly larger than after P-selectin blockade (P<0.01; Fig. 6B), which indicates that PSGL-1 blockade may be a more effective target for blockade of acute inflammatory response in the posterior chamber.
Role of PSGL-1 in the expression of inflammatory mediators during EIU
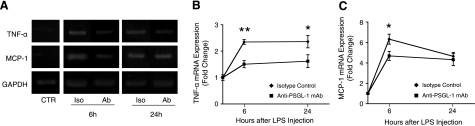
To investigate the effect of PSGL-1 blockade on local proinflammatory cytokines during EIU, the mRNA expression levels of TNF-α and MCP-1 were assessed in retinal extracts from EIU mice treated with anti-PSGL-1 or control Ab at 6 and 24 h after LPS. RT-PCR showed down-regulation of retinal TNF-α and MCP-1 in animals treated with the anti-PSGL-1-neutralizing mAb, mainly 6 h after LPS injection (Fig. 7A). Semiquantitative analysis showed significant reduction of TNF-α retinal mRNA expression by 36% at 6 h and by 35% at 24 h after LPS injection (n=10 and 8, P<0.01, and n=8 each, P<0.05, respectively; Fig. 7B). In addition, MCP-1 mRNA expression was attenuated with PSGL-1 mAb treatment by 25% at 6 h (n=10 and 6, P<0.05) but not at 24 h after LPS administration (n=8 each, P=0.3), which suggests that anti-PSGL-1-neutralizing mAb was effective in reducing proinflammatory cytokines at earlier time points during EIU (Fig. 7C).
Figure 7.
Effect of PSGL-1 blockade on retinal expression of inflammatory mediators. A) TNF-α and MCP-1 expression in retinal tissue of EIU mice. Retinal mRNA expression of TNF-α and MCP-1 in control (CTR) and EIU mice, treated with PSGL-1-neutralizing mAb (Ab) or isotype-matched control IgG (Iso), 6 and 24 h after LPS injection. B, C) Semiquantitative analysis showing the relative amount of TNF-α (B) and MCP-1 mRNA expression (C) as a ratio of the gene product relative to the expression of the GAPDH (arbitrary units). Each data point represents an average of 6 to 10 experiments. *P < 0.05; **P < 0.01.
Therapeutic potential of soluble PSGL-1 in acute ocular inflammation
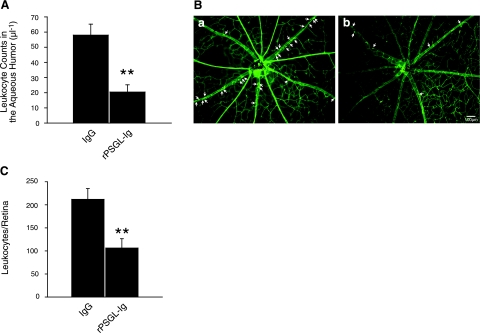
To investigate whether soluble PSGL-Ig has anti-inflammatory effects during acute ocular inflammation, EIU mice were treated systemically with rPSGL-Ig at the same time as the LPS-injection, and the inflammatory reaction was assessed 24 h later. Quantification of the infiltration of inflammatory cells into the aqueous humor of EIU animals showed a significant 65% reduction with rPSGL-Ig treatment (21±5 vs. 58±7 cells/μl from those IgG-treated EIU mice, n=10 and 12, respectively, P=0.001; Fig. 8A). Similarly, the number of leukocytes firmly adhering to the retinal vessels of rPSGL-Ig treated EIU mice (106±20 leukocytes/retina, n=9) was significantly reduced (by 50%) compared with the IgG-treated control animals (212±23 leukocytes/retina, n=6, P=0.001; Fig. 8B, C), which suggests that human rPSGL-Ig may be useful in the treatment of acute ocular inflammatory diseases.
Figure 8.
Effect of competitive blockade of PSGL-1 on inflammatory outcome during EIU. A) Leukocyte infiltration into the aqueous humor. Number of infiltrated cells was counted in 1 μl of aqueous humor of EIU mice at 24 h after LPS injection and treatment with rPSGL-Ig (n=10) or as a control with human IgG (n=12). Values are means ± se. **P < 0.01. B) Representative micrographs of flatmounted retinas from human IgG-treated (a) and rPSGL-Ig-treated EIU mice (b). rPSGL-Ig suppressed firm leukocyte adhesion to the retinal vasculature. Arrows indicate firmly adhering leukocytes. C) Quantification of adherent retinal leukocytes. EIU mice treated with rPSGL-Ig (n=9) showed significant reduction of adherent leukocytes to the retinal vessels compared with IgG-treated EIU mice (n=6). **P < 0.01.
DISCUSSION
In this study, we investigate the expression and function of the pan-selectin ligand PSGL-1 in endotoxin-induced systemic inflammation. We show the critical role of PSGL-1 in the severe ocular manifestation of EIU. Our results suggest the novel concept that during acute inflammation, functional PSGL-1 up-regulation on the leukocyte surface concomitant with endothelial P-selectin up-regulation promotes leukocyte-endothelial interaction.
To elucidate the isolated contribution of PSGL-1 to systemic disease, we performed experiments in the micro-flow chamber assay. In these experiments, the concentration of PSGL-1’s immobilized ligand, P-selectin, was kept at a constant level, which allowed us to attribute the quantitative changes in rolling velocity to changes in PSGL-1 expression and function during the disease state, a distinction that would be difficult in other experimental systems (25, 32). These experiments, surprisingly, revealed that leukocytes from uveitic animals roll at significantly lower velocities 6 h after LPS injection. Even though lower leukocyte rolling velocity during acute inflammation had previously been noted in vivo, the underlying mechanism has been mostly attributed to endothelial P-selectin up-regulation (28, 29).
Indeed, recent experiments have suggested a down-regulation of PSGL-1 with endotoxin treatment, presumably through elevated shedding (31). With lower PSGL-1 expression and increased shedding, a higher PSGL-1-mediated leukocyte rolling velocity would have been expected (33, 38). In contrast, our autoperfused micro-flow chamber experiments show an intriguing reduction in PSGL-1-mediated rolling velocity that could not be explained with the existing reports (31).
Because our autoperfused micro-flow chamber experiments distinguish between the contribution of P-selectin and PSGL-1, they reveal that the previously reported lower rolling velocity during acute inflammation is the result of not only endothelial P-selectin up-regulation (28, 29) but also of functional up-regulation of PSGL-1 on PBLs. This finding is also supported by our flow cytometric analysis of PSGL-1 expression on PBLs. Even though our initial experiments confirmed the reduced PSGL-1 expression on PBLs 6 h after LPS injection (31), blockade of PSGL-1 in endotoxin-treated animals revealed significantly higher PSGL-1 surface expression. This may result from the release of higher PSGL-1-expressing leukocytes from the vascular wall of the animal, a population that may have been unaccounted for in the previous studies (37). Such a scenario would explain the discrepancy between the previously found lower expression of PSGL-1 (31) and our flow chamber results that show lower rolling velocities on immobilized P-selectin. The up-regulation of PSGL-1 during the early phase of systemic inflammation overlaps with the time course of P-selectin up-regulation, which suggests the existence of a previously unrecognized cooperative action between these molecules.
Our studies further show the critical role of PSGL-1 in the severe ocular inflammation during EIU. PSGL-1 blockade significantly reduces leukocyte infiltration into the aqueous humor and firm adhesion to the retinal vessels, as well as retinal TNF-α and MCP-1 mRNA expression. In addition, our data indicate that blockade of PSGL-1 is more effective than P-selectin blockade in reducing posterior segment inflammation during EIU. This difference suggests that PSGL-1 interaction with its other ligands of the selectin family, L- and E-selectin, contributes to the EIU outcome. Thus, PSGL-1 appears to be a key regulatory molecule in endotoxin-induced inflammation, blockade of which has a profound effect on the pathological signs of the disease and may offer an attractive target for treatment of uveitis.
Current treatments of uveitis include corticosteroids and immunosuppressive agents, which have considerable side effects (39, 40). There is an urgent need for alternative immunomodulatory drugs that can preserve vision while avoiding the severe side effects of the conventional regimens. Direct blockade or competitive inhibition of PSGL-1 may thus provide an effective and tolerable treatment for uveitis. The translational potential of this approach to treat or prevent recurrence of uveitis is further supported by the fact that the soluble PSGL-Ig we used in our study for competitive inhibition is a fully human recombinant fusion protein of PSGL-1 and IgG-1 (41). This protein has been used in clinical trials for acute myocardial infarction (42) and for renal (43) and liver transplant patients for prevention of IR injury (Elizabeth C. Squire, Y’s Therapeutics, personal communication, June 20, 2007).
Moreover, our results suggest a critical role for PSGL-1 in the severe leukopenia during the early phase of endotoxemia (6 h after LPS injection) and show that the systemic leukocyte counts recover to normal levels with PSGL-1 blockade. These insights may be of value for the treatment of such life-threatening conditions as bacteremia or septic shock, in which patients exhibit a progressive decrease in their PBL counts.
In summary, our findings show, for the first time, a functional up-regulation of PSGL-1 during systemic inflammatory disease. Our findings are an advance over the conventional paradigm that the initial step of leukocyte-endothelial interaction is mainly facilitated by the up-regulation of endothelial P-selectin by revealing a temporal and functional cooperation between P-selectin and PSGL-1 up-regulation. Furthermore, our results show the critical role of PSGL-1 in the pathogenesis of acute ocular inflammation. Blockade of PSGL-1, either by a neutralizing mAb or, competitively, through a recombinant protein, effectively reduces various inflammatory parameters in vivo. PSGL-1 may thus become an attractive therapeutic target for treatment and prevention of such devastating ocular inflammatory diseases as uveitis.
Acknowledgments
We are grateful to Dr. P. Cheung, Director of Preclinical Research and Discovery at Y’s Therapeutics for graciously providing research-grade YSPSL (recombinant soluble P-selectin glycoprotein ligand IgG fusion protein, rPSGL-Ig). The authors thank Dr. S. E. Leeman for a critical review of the manuscript and R. Huang for his assistance with flow cytometry. This work was supported by U.S. National Institue of Health grants (HL086933, AI050775) and a National Eye Institute core grant (EY14104). We are indebted to the Massachusetts Lions Foundation for generous funds provided for laboratory equipment used in this project. We thank Research to Prevent Blindness for unrestricted funds awarded to the Department of Ophthalmology at Harvard Medical School. We are grateful to the Marion W. and Edward F. Knight Fund for support of A.H.-M.’s research.
References
- Laszik Z, Jansen P J, Cummings R D, Tedder T F, McEver R P, Moore K L. P-selectin glycoprotein ligand-1 is broadly expressed in cells of myeloid, lymphoid, and dendritic lineage and in some nonhematopoietic cells. Blood. 1996;88:3010–3021. [PubMed] [Google Scholar]
- Spertini O, Cordey A S, Monai N, Giuffre L, Schapira M. P-selectin glycoprotein ligand 1 is a ligand for L-selectin on neutrophils, monocytes, and CD34+ hematopoietic progenitor cells. J Cell Biol. 1996;135:523–531. doi: 10.1083/jcb.135.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K L. Structure and function of P-selectin glycoprotein ligand-1. Leuk Lymphoma. 1998;29:1–15. doi: 10.3109/10428199809058377. [DOI] [PubMed] [Google Scholar]
- Hirata T, Merrill-Skoloff G, Aab M, Yang J, Furie B C, Furie B. P-Selectin glycoprotein ligand 1 (PSGL-1) is a physiological ligand for E-selectin in mediating T helper 1 lymphocyte migration. J Exp Med. 2000;192:1669–1676. doi: 10.1084/jem.192.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenette P S, Denis C V, Weiss L, Jurk K, Subbarao S, Kehrel B, Hartwig J H, Vestweber D, Wagner D D. P-Selectin glycoprotein ligand 1 (PSGL-1) is expressed on platelets and can mediate platelet-endothelial interactions in vivo. J Exp Med. 2000;191:1413–1422. doi: 10.1084/jem.191.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng J G, Bevilacqua M P, Moore K L, McIntyre T M, Prescott S M, Kim J M, Bliss G A, Zimmerman G A, McEver R P. Rapid neutrophil adhesion to activated endothelium mediated by GMP-140. Nature. 1990;343:757–760. doi: 10.1038/343757a0. [DOI] [PubMed] [Google Scholar]
- Butcher E C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Norman K E, Moore K L, McEver R P, Ley K. Leukocyte rolling in vivo is mediated by P-selectin glycoprotein ligand-1. Blood. 1995;86:4417–4421. [PubMed] [Google Scholar]
- Moore K L, Patel K D, Bruehl R E, Li F, Johnson D A, Lichenstein H S, Cummings R D, Bainton D F, McEver R P. P-selectin glycoprotein ligand-1 mediates rolling of human neutrophils on P-selectin. J Cell Biol. 1995;128:661–671. doi: 10.1083/jcb.128.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Dulkanchainun T S, Goss J A, Imagawa D K, Shaw G D, Anselmo D M, Kaldas F, Wang T, Zhao D, Busuttil A A, Kato H, Murray N G, Kupiec-Weglinski J W, Busuttil R W. Reduction of hepatic ischemia/reperfusion injury by a soluble P-selectin glycoprotein ligand-1. Ann Surg. 1998;227:832–840. doi: 10.1097/00000658-199806000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchihashi S, Fondevila C, Shaw G D, Lorenz M, Marquette K, Benard S, Shen X D, Ke B, Busuttil R W, Kupiec-Weglinski J W. Molecular characterization of rat leukocyte P-selectin glycoprotein ligand-1 and effect of its blockade: protection from ischemia-reperfusion injury in liver transplantation. J Immunol. 2006;176:616–624. doi: 10.4049/jimmunol.176.1.616. [DOI] [PubMed] [Google Scholar]
- Hicks A E, Nolan S L, Ridger V C, Hellewell P G, Norman K E. Recombinant P-selectin glycoprotein ligand-1 directly inhibits leukocyte rolling by all 3 selectins in vivo: complete inhibition of rolling is not required for anti-inflammatory effect. Blood. 2003;101:3249–3256. doi: 10.1182/blood-2002-07-2329. [DOI] [PubMed] [Google Scholar]
- Takada M, Nadeau K C, Shaw G D, Marquette K A, Tilney N L. The cytokine-adhesion molecule cascade in ischemia/reperfusion injury of the rat kidney. Inhibition by a soluble P-selectin ligand. J Clin Invest. 1997;99:2682–2690. doi: 10.1172/JCI119457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller T F, Sattler B, Binder L, Vetterlein F, Ringe B, Lorf T. Reduction of severe ischemia/reperfusion injury in rat kidney grafts by a soluble P-selectin glycoprotein ligand. Transplantation. 2001;72:216–222. doi: 10.1097/00007890-200107270-00008. [DOI] [PubMed] [Google Scholar]
- Rosenbaum J T, McDevitt H O, Guss R B, Egbert P R. Endotoxin-induced uveitis in rats as a model for human disease. Nature. 1980;286:611–613. doi: 10.1038/286611a0. [DOI] [PubMed] [Google Scholar]
- Smith J R, Hart P H, Williams K A. Basic pathogenic mechanisms operating in experimental models of acute anterior uveitis. Immunol Cell Biol. 1998;76:497–512. doi: 10.1046/j.1440-1711.1998.00783.x. [DOI] [PubMed] [Google Scholar]
- Suzuma K, Mandai M, Kogishi J, Tojo S J, Honda Y, Yoshimura N. Role of P-selectin in endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 1997;38:1610–1618. [PubMed] [Google Scholar]
- Whitcup S M, Kozhich A T, Lobanoff M, Wolitzky B A, Chan C C. Blocking both E-selectin and P-selectin inhibits endotoxin-induced leukocyte infiltration into the eye. Clin Immunol Immunopathol. 1997;83:45–52. doi: 10.1006/clin.1996.4324. [DOI] [PubMed] [Google Scholar]
- Rosenbaum J T, Boney R S. Efficacy of antibodies to adhesion molecules, CD11a or CD18, in rabbit models of uveitis. Curr Eye Res. 1993;12:827–831. doi: 10.3109/02713689309020387. [DOI] [PubMed] [Google Scholar]
- Whitcup S M, DeBarge L R, Caspi R R, Harning R, Nussenblatt R B, Chan C C. Monoclonal antibodies against ICAM-1 (CD54) and LFA-1 (CD11a/CD18) inhibit experimental autoimmune uveitis. Clin Immunol Immunopathol. 1993;67:143–150. doi: 10.1006/clin.1993.1057. [DOI] [PubMed] [Google Scholar]
- Becker M D, Garman K, Whitcup S M, Planck S R, Rosenbaum J T. Inhibition of leukocyte sticking and infiltration, but not rolling, by antibodies to ICAM-1 and LFA-1 in murine endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 2001;42:2563–2566. [PubMed] [Google Scholar]
- Whitcup S M, Hikita N, Shirao M, Miyasaka M, Tamatani T, Mochizuki M, Nussenblatt R B, Chan C C. Monoclonal antibodies against CD54 (ICAM-1) and CD11a (LFA-1) prevent and inhibit endotoxin-induced uveitis. Exp Eye Res. 1995;60:597–601. doi: 10.1016/s0014-4835(05)80001-6. [DOI] [PubMed] [Google Scholar]
- Hafezi-Moghadam A, Noda K, Almulki L, Iliaki E F, Poulaki V, Thomas K L, Nakazawa T, Hisatomi T, Miller J W, Gragoudas E S. VLA-4 blockade suppresses endotoxin-induced uveitis: in vivo evidence for functional integrin up-regulation. FASEB J. 2007;21:464–474. doi: 10.1096/fj.06-6390com. [DOI] [PubMed] [Google Scholar]
- Planck S R, Han Y B, Park J M, O'Rourke L, Gutierrez-Ramos J C, Rosenbaum J T. The effect of genetic deficiency of adhesion molecules on the course of endotoxin-induced uveitis. Curr Eye Res. 1998;17:941–946. doi: 10.1076/ceyr.17.9.941.5139. [DOI] [PubMed] [Google Scholar]
- Strauss E C, Larson K A, Brenneise I, Foster C S, Larsen G R, Lee N A, Lee J J. Soluble P-selectin glycoprotein ligand 1 inhibits ocular inflammation in a murine model of allergy. Invest Ophthalmol Vis Sci. 1999;40:1336–1342. [PubMed] [Google Scholar]
- Baatz H, Pleyer U, Thiel H J, Hammer C. In vivo study of leukocyte-endothelium interaction in endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 1995;36:1960–1967. [PubMed] [Google Scholar]
- Miyamoto K, Ogura Y, Hamada M, Nishiwaki H, Hiroshiba N, Honda Y. In vivo quantification of leukocyte behavior in the retina during endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 1996;37:2708–2715. [PubMed] [Google Scholar]
- Miyamoto K, Ogura Y, Hamada M, Nishiwaki H, Hiroshiba N, Tsujikawa A, Mandai M, Suzuma K, Tojo S J, Honda Y. In vivo neutralization of P-selectin inhibits leukocyte-endothelial interactions in retinal microcirculation during ocular inflammation. Microvasc Res. 1998;55:230–240. doi: 10.1006/mvre.1998.2084. [DOI] [PubMed] [Google Scholar]
- Marsik C, Mayr F, Cardona F, Schaller G, Wagner O F, Jilma B. Endotoxin down-modulates P-selectin glycoprotein ligand-1 (PSGL-1, CD162) on neutrophils in humans. J Clin Immunol. 2004;24:62–65. doi: 10.1023/B:JOCI.0000018064.13793.83. [DOI] [PubMed] [Google Scholar]
- Hafezi-Moghadam A, Thomas K L, Cornelssen C. A novel mouse-driven ex vivo flow chamber for the study of leukocyte and platelet function. Am J Physiol. 2004;286:C876–C892. doi: 10.1152/ajpcell.00500.2003. [DOI] [PubMed] [Google Scholar]
- Hafezi-Moghadam A, Thomas K, Prorock A, Huo Y, Ley K. L-selectin shedding regulates leukocyte recruitment. J Exp Med. 2001;193:863–872. doi: 10.1084/jem.193.7.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai N, Oike Y, Noda K, Urano T, Kubota Y, Ozawa Y, Shinoda H, Koto T, Shinoda K, Inoue M, Tsubota K, Yamashiro K, Suda T, Ishida S. Suppression of ocular inflammation in endotoxin-induced uveitis by blocking the angiotensin II type 1 receptor. Invest Ophthalmol Vis Sci. 2005;46:2925–2931. doi: 10.1167/iovs.04-1476. [DOI] [PubMed] [Google Scholar]
- Yang Y, Zhe X, Phan S H, Ullenbruch M, Schuger L. Involvement of serum response factor isoforms in myofibroblast differentiation during bleomycin-induced lung injury. Am J Respir Cell Mol Biol. 2003;29:583–590. doi: 10.1165/rcmb.2002-0315OC. [DOI] [PubMed] [Google Scholar]
- Marie O, Thillaye-Goldenberg B, Naud M C, de Kozak Y. Inhibition of endotoxin-induced uveitis and potentiation of local TNF-alpha and interleukin-6 mRNA expression by interleukin-13. Invest Ophthalmol Vis Sci. 1999;40:2275–2282. [PubMed] [Google Scholar]
- Davenpeck K L, Brummet M E, Hudson S A, Mayer R J, Bochner B S. Activation of human leukocytes reduces surface P-selectin glycoprotein ligand-1 (PSGL-1, CD162) and adhesion to P-selectin in vitro. J Immunol. 2000;165:2764–2772. doi: 10.4049/jimmunol.165.5.2764. [DOI] [PubMed] [Google Scholar]
- Hafezi-Moghadam A, Ley K. Relevance of L-selectin shedding for leukocyte rolling in vivo. J Exp Med. 1999;189:939–948. doi: 10.1084/jem.189.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamesis R R, Rodriguez A, Christen W G, Akova Y A, Messmer E, Foster C S. Systemic drug toxicity trends in immunosuppressive therapy of immune and inflammatory ocular disease. Ophthalmology. 1996;103:768–775. doi: 10.1016/s0161-6420(96)30618-0. [DOI] [PubMed] [Google Scholar]
- Thadani S M, Foster C S. Treatment of ocular inflammation in children. Paediatr Drugs. 2004;6:289–301. doi: 10.2165/00148581-200406050-00003. [DOI] [PubMed] [Google Scholar]
- Khor S P, McCarthy K, DuPont M, Murray K, Timony G. Pharmacokinetics, pharmacodynamics, allometry, and dose selection of rPSGL-Ig for phase I trial. J Pharmacol Exp Ther. 2000;293:618–624. [PubMed] [Google Scholar]
- Mertens P, Maes A, Nuyts J, Belmans A, Desmet W, Esplugas E, Charlier F, Figueras J, Sambuceti G, Schwaiger M, Mortelmans L, Van de Werf F. Recombinant P-selectin glycoprotein ligand-immunoglobulin, a P-selectin antagonist, as an adjunct to thrombolysis in acute myocardial infarction. The P-Selectin Antagonist Limiting Myonecrosis (PSALM) trial. Am Heart J. 2006;152:e121–e128. doi: 10.1016/j.ahj.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Gaber A O, Mulgaonkar S, Kahan B, Bowers V, Woodle S, Bajjoka I, Butt K, Jensik S, Nezakatgoo N, Klintmalm G, Patton P. YSPSL (rPSGL-Ig) for the prevention of delayed graft function (DGF)preliminary results of ongoing dose escalation and efficacy study. 2007 [Poster presentation] American Transplant Congress, http://www.atcmeeting.org/2007/index.php. [Google Scholar]