Abstract
In this study, the inhibitor 2 of protein phosphatase 2A (I2PP2A) was identified in vitro and in situ as a ceramide-binding protein, which exhibits stereoisomer specificity and fatty acid chain length preference. Site- directed mutagenesis coupled with structural details of I2PP2A suggested that VIK 207-209 residues localized on helix 7 are important for ceramide binding and single mutation of K209D altered this interaction. Notably, I2PP2A-ceramide binding decreased the association between PP2A and the inhibitor, preventing the inhibition of PP2A activity in vitro. In addition, studies in A549 human lung cancer cells revealed that ceramide mediates c-Myc degradation via its PP2A-dependent dephosphorylation at S62, and treatment with okadaic acid and expression of c-Myc mutants with S62A or S62D conversions resulted in resistance to ceramide-mediated degradation. Importantly, whereas down-regulation of I2PP2A enhanced PP2A-mediated c-Myc degradation in response to ceramide, ectopic expression of wild-type I2PP2A but not of its K209D mutant protected this degradation in A549 cells. Moreover, expression of wild-type I2PP2A prevented the growth-inhibitory effects of ceramide both against A549 cells and xenograft-driven tumors in situ and in vivo compared with that in controls. Thus, these results suggest that direct interaction of I2PP2A with ceramide plays important biological roles via the regulation of PP2A activity and signaling, which in turn control ceramide-mediated degradation of c-Myc and antiproliferation.—Mukhopadhyay, A., Saddoughi, S. A., Song, P., Sultan, I., Ponnusamy, S., Senkal, C. E., Snook, C. F., Arnold, H. K., Sears, R. C., Hannun, Y. A., Ogretmen, B. Direct interaction between the inhibitor 2 and ceramide via sphingolipid-protein binding is involved in the regulation of protein phosphatase 2A activity and signaling.
Keywords: bioactive lipids, c-Myc degradation
Ceramide mediates antiproliferative signaling events in response to various stress stimuli, predominantly by controlling specific downstream protein targets, such as protein kinases and phosphatases, that in turn modulate multiple signaling pathways (1, 2). One of the immediate downstream targets of ceramide is protein phosphatase 2A (PP2A), whose activity is induced by ceramide (3, 4). Activation of PP2A then leads to growth inhibition and/or apoptotic cell death via regulation of a myriad of cellular targets, including c-Myc (5, 6). It has been well documented that there are orchestrated phosphorylation and dephosphorylation events at S62/T58 residues of c-Myc that regulate its proteasomal degradation (5, 6). Phosphorylation at S62 stabilizes c-Myc, whereas its subsequent phosphorylation at T58 by glycogen synthase kinase-3-β (GSK3-β) is required for dephosphorylation at S62 by PP2A, which then leads to the ubiquitination and degradation of c-Myc (5, 6).
PP2A is a heterotrimer complex, which contains the catalytic (C), structural (A), and regulatory (B) subunits. In addition to pharmacological inhibitors, there exist noncompetitive biological inhibitors of PP2A, including proteins such as I1PP2A and I2PP2A, that associate with PP2A and inhibit its activity (7, 8). I2PP2A belongs to the SET domain proteins, which are abnormally translocated and fused to nucleoporin (Nup214) in some nonlymphocytic acute myeloid leukemias (9). It has also been reported that I2PP2A modulates PP2A, leading to blastic transformation in chronic myeloid leukemia models (10).
In this study, because it is well established that sphingolipid-protein interactions play significant roles in the regulation of various signaling events (11,12,13,14), we explored ceramide-binding proteins in A549 cells. Interestingly and unexpectedly, I2PP2A, also known as putative histocompatibility leukocyte antigen class II protein (PHAP-II) (8, 15), template activating factor 1β (TAF1β) (16), or inhibitor of histone acetyltransferase (INHAT) (17), was identified as one of the major ceramide-binding proteins, which exhibits stereoisomer and fatty acid chain length preference. Notably, these studies demonstrated that the I2PP2A-ceramide binding is involved in the regulation of PP2A activity, preventing its inhibition by I2PP2A in vitro. To determine whether the I2PP2A-ceramide association plays any biological roles, we focused our efforts on the regulation of c-Myc in A549 cells. Because ceramide has been shown to induce the ubiquitination and proteasome-dependent degradation of c-Myc in A549 cells (18), albeit by an unknown mechanism, it is hypothesized that the I2PP2A-ceramide interaction might play a role in the degradation of c-Myc at least in part by regulating its dephosphorylation at S62 by PP2A. Indeed, although downregulation of I2PP2A enhanced PP2A-mediated c-Myc degradation in response to ceramide, ectopic expression of wild-type (wt)-I2PP2A, but not of its K209D mutant, protected this degradation. This action was concomitant with the reduction of growth inhibitory functions of ceramide by overexpression of I2PP2A in A549 cells. In summary, these data suggest a novel mechanism by which I2PP2A-ceramide binding is involved in the regulation of PP2A activity and signaling, which in turn modulates c-Myc degradation and antiproliferation.
MATERIALS AND METHODS
Association of I2PP2A with ceramide in vitro and in situ
The interaction between purified recombinant I2PP2A or endogenously expressed I2PP2A (wt or mutants) and biotin-labeled d-erythro-C6-ceramide (B-C6-Cer), obtained from the Lipidomics Core Facility (Department of Biochemistry and Molecular Biology, Medical University of South Carolina, Charleston, SC, USA), was detected using the Immobilized Monomeric Avidin kit (Pierce Biotechnology, Rockford, IL, USA) as described by the manufacturer. In brief, A549 cell pellets were lysed in the cell lysate buffer containing 10 mM Tris HCl (pH 8.0), 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 1% sodium deoxycholate, 1% Triton-X-100, and protease inhibitor cocktail (Sigma-Aldrich Corp., St. Louis, MO, USA). Cell lysate containing 1 mg of total protein was incubated with 10 μM biotin or B-C6-Cer (solubilized in ethanol) for 30 min at room temperature. After incubation, 2 ml of PBS was added to the reaction mixture, which was then applied to the avidin column (2-ml bed volume) and passed through the column twice. Then the column was washed with 20 ml of PBS (10× bed volume). Bound proteins were eluted by 2 ml (2 mM) of biotin solution, and concentrated with a Centricon (Millipore, Billerica, MA, USA) filter device to 100 μl for further analysis by SDS-PAGE.
To examine the direct association between I2PP2A and ceramide in vitro, the following procedure was used: the recombinant protein, expressed in Escherichia coli and purified by metal ion column chromatography, was dialyzed in a buffer containing 50 mM Tris HCl (pH 7.5), 150 mM NaCl, 1 mM dithiothreitol, and 1 mM EDTA and was concentrated by a Centricon (Sigma-Aldrich Corp.) filtering device. Recombinant I2PP2A (10 nM), was incubated with 10 μM biotin or B-C6-Cer in 2 ml of PBS containing 0.05% Briij-35 for 30 min, and then the reaction mixture was applied to the avidin column. Bound proteins were eluted after washes with PBS (20 ml) as described above.
For in situ analysis, A549 cells were pretreated with either 50 μM fumonisin B1 (FB1) or 50 nM myriocin (MYR) and labeled with 10 μM biotin-labeled sphingosine (B-Sph) (Avanti Polar, Alabaster, AL, USA) in the presence or absence of 10 μM stearate or palmitate for 5 h to generate the biotin-labeled endogenous ceramides. Then an equal amount of cell lysate (1–2 mg of total protein) for each sample was applied to the avidin column, and ceramide-bound proteins were eluted as described above. The generation of ceramides was confirmed by lipid extraction followed by thin-layer chromatography or liquid chromatography (LC)/mass spectrometry (MS), as we described previously (19). Ceramide-binding proteins were then determined by SDS-PAGE and Western blotting using anti-I2PP2A antibodies (GloboZymes, Carlsbad, CA, USA) as described (19). Exogenous ceramides used in these studies were obtained from the Lipidomics Core Facility at the Medical University of South Carolina.
Plasmids, site-directed mutagenesis, and protein purification
The mutant forms of I2PP2A were generated using site-directed mutagenesis, as described previously (19). After expression of wt I2PP2A-green fluorescent protein (GFP) or its mutant forms, containing VIK/SSS, FFT/YYA, R/A, or K/D conversions, cloned into pEGFP-C3 vector (BD Biosciences, San Jose, CA, USA) with the GFP tag in A549 cells, the proteins were pulled down from cell extracts using the μMACS GFP Tag Protein Isolation Kit (Miltenyi Biotec, Auburn, CA, USA) as described by the manufacturer.
Recombinant I2PP2A cloned into pTrcHisA with the 6xHis tag (Invitrogen, Carlsbad, CA, USA) was expressed in E. coli BL21 Codon Plus-competent cells and purified using columns (Chelating Sepharose Fast Flow; Amersham Biosciences Corp., Piscataway, NJ, USA) that had been charged with 0.2 M NiSO4. The bound proteins were eluted using increasing concentrations of imidazole (50–500 mM) in the lysis buffer (50 mM Tris HCl, pH 7.5; 150 mM NaCl; and 5% glycerol). Purified fractions were then run on SDS-PAGE and analyzed by Western blotting using rabbit polyclonal anti-I2PP2A (GloboZymes) or mouse monoclonal anti-6xHis (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) antibodies.
Cell lines and transfections
A549 human lung adenocarcinoma cells were grown in Dulbecco’s modified Eagle’s medium containing 10% fetal calf serum and 1% penicillin/streptomycin (PerkinElmer Life and Analytical Sciences, Boston, MA, USA). Expression vectors containing wt and mutant forms of human c-Myc with the V5 tag on their C termini (S62A, S62D, T58A, or T58E) were used for their expression in A549 cells (18, 19). For the knockdown experiments, 50 nM double-stranded small interfering (siRNA) oligonucleotides (Dharmacon, Inc., Chicago, IL, USA) were transfected into A549 cells using Oligofectamine reagent (Invitrogen) for 48 h as described by the manufacturer. The sequences of I2PP2A-siRNA molecules were as follows: I2PP2A siRNA-1, 5′-(CAAGCCAGUGAGGAGAUUU)-3′; and I2PP2A siRNA-2, 5′-(AAAUCUCCUC-ACUGGCUUG)-3′. The siRNA molecules containing nontargeting sequences (Dharmacon, Inc.) were used as controls. The expression levels of proteins were analyzed by Western blotting using the following antibodies: rabbit polyclonal anti-I2PP2A (GloboZymes), anti-β-actin (Sigma-Aldrich Corp.), and anti-phospho-T58-c-Myc (GenScript Corp., Piscataway, NJ, USA) or the mouse monoclonal anti-c-Myc (Santa Cruz Biotechnology, Inc.), anti-PP2Ac (Upstate Biotechnology, Temecula, CA, USA), anti-GFP (Abcam Inc., Cambridge, MA, USA), anti-V5 (Invitrogen), and anti-HA (Cell Signaling Technology, Danvers, MA, USA), as described previously (18, 19). The nuclear localization of wt and mutant I2PP2A-containing GFP tags in A549 cells was determined using confocal microscopy, as described previously (18).
Protein phosphatase assay
Catalytic activity of PP2Ac and PP2A (trimer) (purified forms obtained from GloboZymes) was measured as described by the manufacturer using 32P-labeled myelin basic protein (MBP) as a substrate. The association between PP2A and I2PP2A was examined by immunoprecipitation of I2PP2A-GFP, performed using A549 cell extracts treated with C16- or C18-pyridinium-ceramide (Pyr-Cer) using the anti-GFP antibody, followed by Western blotting using the anti-PP2A antibody that recognizes the catalytic subunit of PP2A, as described above.
Analysis of anchorage-independent growth and tumor progression
Possible roles of I2PP2A in ceramide-mediated regulation of anchorage-independent cell growth were examined using soft agar growth assay, as described previously (20). In addition, effects of I2PP2A overexpression in the regulation of tumor progression and/or growth in vivo were determined by implantation of 0.5 × 106 A549 cells that transiently overexpress I2PP2A into the flanks of SCID mice (n=6 tumors/group). After tumors reached to 50–100 mm3 volume, animals were treated with d-e-C6-ceramide (20 mg/kg) every 3 days for 28 days. Tumor formation was evaluated every 3 days for 28 days using digital calipers, as described previously (21). A549 cells transfected with the vector only were used as controls in these studies. Chromophore was used as a control for C6-ceramide. Statistical analysis was performed as we described previously (21), and P < 0.05 was regarded as significant.
RESULTS
I2PP2A binds exogenous C6-ceramide directly and specifically in vitro
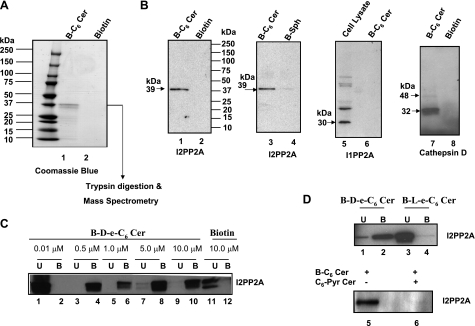
To identify ceramide-binding proteins, total A549 cellular extracts (1 mg of total protein) were incubated with B-C6-Cer in vitro (Supplemental Fig. S1A), and proteins that bound ceramide (10 μM) were isolated using avidin-conjugated Sepharose columns. Eluted ceramide-binding proteins were then separated by SDS-PAGE and visualized by silver or Coomassie blue staining. Although there were various minor bands visible after silver staining (data not shown), one major band was detected after Coomassie blue staining in extracts incubated with B-C6-Cer and not with the biotin control (Fig. 1A). After extraction from the stained gel, this band was digested with trypsin, and peptides were analyzed using LC/MS. Ten tryptic peptides (Supplemental Fig. S1B) of the ceramide-associated protein band were identified as fragments of I2PP2A (7, 8).
Figure 1.
Identification of I2PP2A as a ceramide-binding protein. A) Cell lysates (1 mg) were incubated with B-C6-Cer (10 μM) for 30 min, and then B-C6-Cer-bound proteins were eluted from the avidin columns and separated by SDS-PAGE. Eluted proteins that associated with B-C6-Cer or biotin only (used as controls) were then visualized by Coomassie blue staining (lanes 1 and 2, respectively). Then, after in-gel digestion of the band with trypsin, LC-tandem mass spectrometry analysis identified 10 unique peptides that matched the sequence of I2PP2A (shown in Supplemental Fig. S1B). B) Association of I2PP2A with ceramide was also confirmed by Western blot analysis using anti-I2PP2A, I1PP2A, or cathepsin-D antibodies in fractions eluted after incubations with 10 μM of biotin, B-C6-Cer, or B-Sph (lanes 1, 2; 3, 4; 5, 6; and 7, 8, respectively). The total cellular extracts (30 μg) were used as positive controls for the detection of I1PP2A (30 kDa) protein (lane 5). C) Purified I2PP2A-His (10 nM) was used for the binding assay with 0.01–10 μM d-e-B-C6-Cer or biotin (10 μM). After elution from the columns, an equal volume of unbound (U) proteins (30 μl) in flow-through fractions (lanes 1, 3, 5, 7, and 9), or B-C6-Cer-bound (B) proteins obtained from the eluted fractions (lanes 2, 4, 6, 8, and 10) were used. Lanes 11 and 12 contain U and B fractions obtained from the biotin-incubated samples. D) Purified I2PP2A (10 nM) was also used for binding assays with 10 μM d-e-B-C6-Cer or l-e-B-C6-Cer to determine the stereospecificity of the interaction (top panel: lanes 1, 2 and 3, 4, respectively). The competition of B-C6-Cer for the I2PP2A interaction in the absence or presence of highly water soluble C6-Pyr-Cer (bottom panel: lanes 5 and 6, respectively) was also examined.
The association of I2PP2A with B-C6-Cer and not with biotin or B-Sph in cell extracts was further confirmed by Western blot analysis using the rabbit polyclonal anti-I2PP2A antibody (Fig. 1B, lanes 1–4). It is also curious that I2PP2A seemed to interact slightly with B-Sph (Fig. 1B, lane 4); however, the binding of I2PP2A to B-C6-Cer was much (10-fold) greater than its binding to B-Sph (Fig. 1B, lanes 3 vs. 4). Notably, B-C6-Cer-bound fractions were probed with an antibody that recognizes I1PP2A, and no signal was detected (Fig. 1B, lane 6). Total A549 cell extracts (30 μg) were used as a positive control for I1PP2A detection (Fig. 1B, lane 5). Thus, these results indicate the specificity of I2PP2A over I1PP2A for ceramide binding. In addition, other known ceramide-associated proteins (12, 13), such as cathepsin D and protein kinase C-ζ (data not shown) were detected by Western blot analysis in B-C6-Cer-eluted and not in biotin-eluted fractions (Fig. 1B, lanes 7 and 8, respectively). These results further validated the successful use of the B-C6-Cer to identify ceramide-interacting proteins (directly or indirectly) in vitro.
Next, to examine whether ceramide directly binds I2PP2A in vitro, purified recombinant I2PP2A (10 nM) was incubated with various concentrations of B-C6-Cer (0.01–10 μM), and after the unbound proteins were cleared by washes in flow-through fractions, the ceramide-bound protein was eluted from the columns as described in Materials and Methods. The data showed that ceramide at 0.5–10 μM and not at 0.01 μM directly associated with purified I2PP2A in vitro (Fig. 1C, lanes 3–10, 1, and 2, respectively). As a control, biotin was also incubated with purified I2PP2A, and no significant binding was detectable (Fig. 1C, lanes 11 and 12).
Then we determined the requirement of stereoisomer specificity of I2PP2A for ceramide binding. Purified I2PP2A was incubated with d-erythro-B-C6-Cer or its enantiomeric l-erythro stereoisomer (10 μM), and the association with I2PP2A was examined as described above. Interestingly, the data revealed that I2PP2A interacted only with d-e-B-C6-Cer and not with l-e-B-C6-Cer in vitro (Fig. 1D, lanes 2 and 4, respectively), suggesting a d-erythro stereoisomer preference of I2PP2A for ceramide binding.
It should be noted here that, theoretically, the levels of total unbound I2PP2A, shown in lanes 1 and 2 in Fig. 1C and in lanes 3 and 4 in Fig. 1D, should have been equal to the levels of the total ceramide-bound protein, shown in lanes 3 and 4 in Fig. 1C and lanes 1 and 2 in Fig. 1D. These differences between bound and unbound levels of I2PP2A can be due to a variety of experimental conditions, which are still unknown. However, it can be speculated that this discrepancy may be due to extensive washes of the avidin columns to remove any nonspecifically interacting proteins after incubation of B-C6-Cer with cell extracts in these experiments, which may result in the removal of some of the bound I2PP2A also. This, in turn, may have resulted in the loss of I2PP2A signal in these samples. The possible loss of ceramide-bound I2PP2A from these columns makes it very difficult to determine exactly what percentage of the cellular I2PP2A binds ceramide.
Moreover, binding of I2PP2A to ceramide was further confirmed with the use of highly soluble d-e-C6-Pyr-Cer (21, 22) as a non-biotin-labeled competitor. After cell extracts were preincubated with 10 μM C6-Pyr-Cer, equal amounts of B-C6-Cer were added, and interaction of I2PP2A with ceramide was examined as described above. The presence of C6-Pyr-Cer almost completely prevented the association of B-C6-Cer with I2PP2A compared with controls in vitro (Fig. 1D, bottom panel, lanes 6 and 5, respectively), confirming that the biotin label is not required for I2PP2A-ceramide interaction. Taken together, these data suggest for the first time that I2PP2A but not I1PP2A directly and specifically binds ceramide and that the I2PP2A-ceramide interaction prefers the naturally occurring ceramide in its d-erythro configuration in vitro.
I2PP2A interacts with endogenously generated C18-ceramide in A549 cells
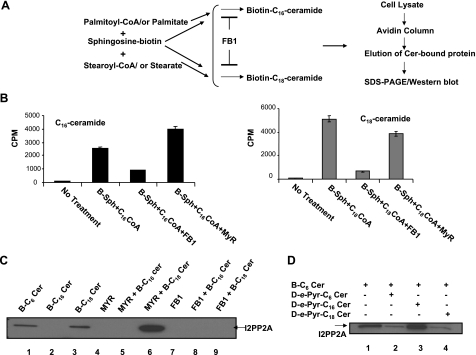
To determine whether endogenously generated ceramides with longer fatty acid chain lengths, such as C16- and C18-ceramides, also interact with I2PP2A, we took advantage of the sphingosine-recycling pathway (18) for the generation of endogenous ceramides. Cells were coincubated with B-Sph in the presence of palmitate or stearate, which, on acylation of B-Sph, led to the generation of biotin-labeled endogenous C16- or C18-ceramides, respectively (Fig. 2A). The generation of C18- and C16-ceramides via the recycling pathway using B-Sph and radiolabeled fatty acyl-CoAS ([3H]stearoyl- or -palmitoyl-CoA) was confirmed by thin-layer chromatography and scintillation counting after lipid extractions (Fig. 2B). Notably, as reported previously (18), treatment with FB1, a potent inhibitor of ceramide synthase (CerS), inhibited generation of C18- and C16-ceramide ∼90 and 70%, respectively, whereas MYR, an inhibitor of serine palmitoyl transferase, had no significant inhibitory effect (Fig. 2B).
Figure 2.
I2PP2A preferentially binds endogenous C18-ceramide in cells. A) Generation of biotin-labeled C16- or C18-ceramides in A549 cells was induced via the sphingosine recycling pathway. B) Generation of C16- or C18-ceramides in A549 cells was confirmed using B-Sph and 3H-labeled palmitoyl- or stearoyl-CoA (left and right panels) in the absence or presence of FB1or MYR as described in Materials and Methods. C) After forced generation of B-C16- or B-C18-Cer in cells (lanes 2 and 3), the I2PP2A-ceramide interaction in the absence or presence of MYR or FB1 (lanes 5, 8 and 6, 9, respectively) was detected by Western blotting. D) Cells were pretreated with exogenous C6-Pyr-Cer, C16-Pyr-Cer, or C18-Pyr-Cer before treatment with B-C6-Cer (each 10 μM). The competition of the exogenous ceramides with B-C6-Cer in I2PP2A binding (lanes 2–4, respectively) compared with that in controls (lane 1) was then determined as described above.
Interestingly, isolation of ceramide-binding proteins after pulsing of the cells with B-Sph and fatty acids (palmitate or stearate) showed that I2PP2A was detected in extracts after forced generation of B-C18- but not B-C16-ceramide in A549 cells (Fig. 2C, lanes 3 and 2, respectively). Treatment with FB1 prevented the association of C18-ceramide with I2PP2A, whereas MYR did not have any inhibitory effects, and it seemed to induce the interaction between C18-ceramide and I2PP2A (Fig. 2C, lanes 9 and 6, respectively). These data suggest that I2PP2A might prefer C18-ceramide over C16-ceramide for binding in these cells.
Consistent with these results, treatment of cells with d-e-B-C6-Cer in the absence or presence of exogenous C6- or C18-Pyr-Cer at 10 μM competed with the association of I2PP2A and B-C6-Cer (Fig. 2D, lanes 4, 2, and 1, respectively). On the other hand, the incubation of A549 cell extracts with exogenous C16-Pyr-Cer at 10 μM did not have any effect on I2PP2A/B-C6-Cer binding (Fig. 2D, lanes 3 and 1, respectively), supporting the preference of C6- and C18-ceramide in the I2PP2A interaction over C16-ceramide in A549 cells.
Determination of amino acid residues of I2PP2A involved in ceramide binding
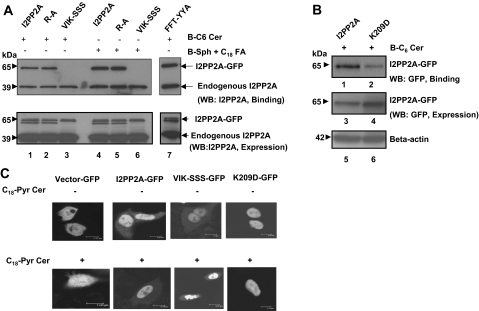
To determine the amino acid residues of I2PP2A that are involved in ceramide binding, we first examined the presence of hydrophobic regions of the protein, using its recently solved crystal structure (23) (Supplemental Fig. S1C). Then site-directed mutagenesis of VIK-SSS and FFT-YYA residues of I2PP2A (highlighted as red and blue, respectively, in Supplemental Fig. S1C), which are localized in the opposing surfaces of the highly hydrophobic pocket, was performed. After expression in A549 cells with the GFP tag on their N termini, binding of these mutant proteins to ceramide was analyzed and compared with that of wt I2PP2A-GFP using the antibody that detects I2PP2A (Fig. 3A). This antibody detected both the ectopically and endogenously expressed I2PP2A proteins with 65- and 39-kDa mass on Western blots (Fig. 3A). The results show that although the FFT-YYA mutation did not affect binding to B-C6-Cer compared with that of wt I2PP2A-GFP (Fig. 3A, top panel, lanes 7 and 1, respectively), the VIK-SSS mutant of I2PP2A almost completely lost its ability to bind B-C6-Cer (Fig. 3A, top panel, lane 3). In addition, mutation of VIK to SSS also caused alterations for binding to endogenous C18-ceramide (Fig. 3A, top panel, lane 6), generated by pulsing the cells in the presence of B-Sph and stearate, as shown in Fig. 2. However, the binding of endogenous I2PP2A (bottom bands) to ceramide was still detectable in these experiments (Fig. 3A, top panel). Thus, these data suggest that VIK (207-209) residues are involved in ceramide binding. The equal expression of wt and the mutants of I2PP2A-GFP was confirmed by Western blotting (Fig. 3A, bottom panel).
Figure 3.
Identification of the amino acid residues of I2PP2A that are involved in ceramide interaction. A) Site-directed mutagenesis to generate V207S/I208S/K209S, F189Y/F190Y/T191A, R169A, or K209D conversions were performed, and the wt and mutant forms of I2PP2A containing the GFP tag were expressed in A549 cells. Equal expression of these proteins was confirmed by Western blotting using anti-I2PP2A antibody (bottom panel). The binding of wt, R/A, VIK/SSS, or FFT/YYA mutants of I2PP2A with the exogenous B-C6-Cer or endogenously generated B-C18-Cer via the recycling pathway (top panel) were then examined. B) The possible role of the K209 residue of I2PP2A in ceramide binding was determined after expression of the K209D mutant in A549 cells compared with that of wt I2PP2A containing GFP (lanes 2 and 1, respectively). The expression levels of wt and the K209D mutant of I2PP2A-GFP were confirmed by Western blotting using anti-GFP or anti-β-actin antibodies (lanes 3, 4 and 5, 6, respectively). C) Subcellular localization of wt and mutant forms of I2PP2A-GFP in the absence (top panel) or presence (bottom panel) of ceramide in A549 cells were examined using confocal microscopy.
Notably, VIK residues are localized on helix 7 of I2PP2A with K209 interacting with the β-sheet below. This helix has long loops on either side, which suggested a potential flap at the head of the hydrophobic pocket (Supplemental Fig. S1C). To examine the role of K209, localized at the tip of helix 7, which might help optimize ceramide positioning and/or binding, we generated a single K209D mutation of I2PP2A and analyzed its ceramide-binding ability in A549 cells. We also generated an R169A mutation, which is not localized within helix 7, as a control. The results showed that the K209D mutation decreased ceramide binding ∼75% compared with that of wt I2PP2A (Fig. 3B, upper panel, lanes 2 and 1, respectively). However, mutation of R169A did not have any inhibitory role in ceramide binding compared with controls (Fig. 3A, top panel, lanes 2 and 5 vs. 1 and 4, respectively). To eliminate the possibility that the lack of ceramide binding of the VIK/SSS and K209D mutants of I2PP2A with GFP is due to the alterations in their nuclear localization, we examined their subcellular localization in A549 cells using confocal microscopy. The data showed that wt I2PP2A-GFP localized mainly to the nucleus, and mutations of VIK/SSS or K209D did not alter the nuclear localization of I2PP2A-GFP in the absence or presence of ceramide (Fig. 3C). Taken together, these data suggest that VIK 207-209 residues, and in particular K209, which are localized in helix 7 of I2PP2A, are probably involved in ceramide binding in A549 cells.
Ceramide prevents the inhibition of PP2A by I2PP2A in vitro
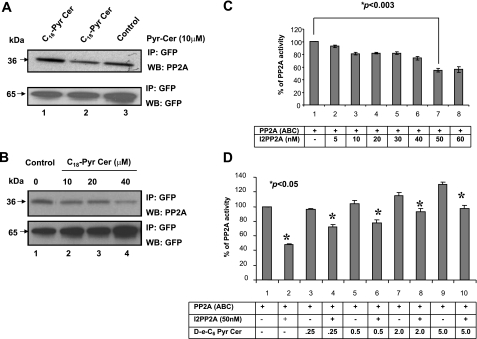
The direct association between ceramide and I2PP2A in vitro and in A549 cells also suggested that this sphingolipid-protein binding might play a role in the regulation of PP2A activity. To evaluate this hypothesis, first we examined whether ceramide affects the interaction between I2PP2A and PP2A, which leads to the inhibition of PP2A activity, using coimmunoprecipitation assays in the absence or presence of ceramide. C18-Pyr-Cer but not C16-Pyr-Cer at 10 μM decreased the interaction between I2PP2A and PP2Ac (∼45%) compared with controls in cell extracts (Fig. 4A, lanes 2, 1, and 3, respectively). These results were also consistent when A549 cells were treated with increasing concentrations of C18-Pyr-Cer, which decreased the association between I2PP2A and PP2A compared with that in untreated controls in a dose-dependent manner (Fig. 4B, lanes 4, 3, 2, and 1, respectively). These data suggest that increasing concentrations of ceramide might prevent the inhibitory effects of the inhibitor 2 against PP2A activity in vitro.
Figure 4.
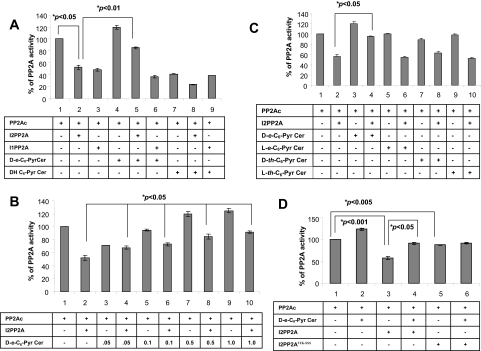
Ceramide relieves I2PP2A from PP2A and prevents the inhibitory effects of I2PP2A on PP2A activity. A) Possible roles of ceramide in the regulation of I2PP2A/PP2A association, which leads to the inhibition of PP2A activity, were examined in cells that express wt I2PP2A-GFP in the absence or presence of C16- or C18-Pyr-Cer (each 10 μM) compared with untreated controls (top panel). The association of I2PP2A-GFP with PP2A was detected by immunoprecipitation (IP) using the anti-GFP antibody, followed by Western blotting (WB) using the anti-PP2Ac antibody. Equal expression of I2PP2A-GFP in these samples was confirmed by Western blotting using the antibody that recognizes GFP (bottom panel). B) Effects of increasing concentrations of ceramide (0–40 μM) on the interaction between PP2Ac and I2PP2A was determined by immunoprecipitation followed by Western blotting as described above. C) PP2A trimer was incubated with increasing concentrations of I2PP2A (5–60 nM), and its effects on PP2A activity was determined in vitro using the phosphatase assay, as described in Materials and Methods. D) Purified I2PP2A (50 nM) was incubated with various concentrations of d-e-C6-Pyr-Cer (0, 0.25, 0.5, 2, or 5 μM), and its roles in the regulation of PP2A trimer activity were determined in vitro as described above. Results represent at least three independent experiments performed in duplicate. Error bars = sd. P < 0.05 was considered significant.
This view was then examined using in vitro protein phosphatase assays performed in the presence of the purified trimeric PP2A, which contains all three components of the enzyme, referred to as PP2A (ABC) or the catalytic subunit of PP2A (PP2Ac) and 32P-labeled MBP as a substrate. First, the optimum concentration of I2PP2A that inhibited trimeric PP2A by ∼50% was determined using increasing concentrations of I2PP2A. The results showed that the presence of I2PP2A at 50 nM inhibited PP2A activity ∼50% compared with that in untreated controls (Fig. 4C). Then the effects of C6-Pyr-Cer at various concentrations (0.5–5 μM) on PP2A activity in the absence or presence of I2PP2A were examined. It was shown that ceramide alone at 0.25 and 0.5 μM did not have any detectable effects, but it induced PP2A (ABC) activity ∼15–30% at 2–5 μM (Fig. 4D). More importantly, whereas I2PP2A alone inhibited the enzyme activity by ∼50%, the presence of ceramide significantly prevented the inhibitory effects of I2PP2A in a dose-dependent manner (Fig. 4D).
To determine whether protection of PP2A activity by ceramide from the inhibitor 2 requires the catalytic domain of PP2A, we repeated the experiments above using PP2Ac. Consistent with the data shown in Fig. 4D, ceramide also reduced the inhibition of PP2Ac by 10 nM I2PP2A, at which concentration of I2PP2A alone inhibited PP2Ac activity by 50% (Fig. 5A). Interestingly, I1PP2A at 10 nM also inhibited PP2A activity by ∼50%; however, in this case, ceramide did not prevent the inhibition of PP2Ac activity by I1PP2A (Fig. 5A), which did not bind ceramide. The biologically inactive analog of exogenous ceramide, dihydro (dh)-C6-Pyr-Cer did not affect the inhibition of PP2Ac by I2PP2A or I1PP2A in vitro (Fig. 5A). The prevention of the inhibition of PP2Ac activity from the inhibitor 2 by ceramide was also dose-dependent when increasing concentrations of ceramide was used (0.05–1 μM) (Fig. 5B). In addition, sphingosine, sphingosine 1-phosphate, ceramide 1-phosphate, or d-e-1-methyl-C6-Cer did not affect the inhibition of PP2A activity by I2PP2A (data not shown), demonstrating the specificity of ceramide in the prevention of I2PP2A-dependent inhibition of PP2A.
Figure 5.
Regulation of PP2Ac activity by the I2PP2A-ceramide interaction. A) Role of ceramide in the inhibition of PP2A activity by purified I2PP2A or I1PP2A in vitro was examined using PP2A activity assays in the absence or presence of d-e-C6-Pyr-Cer or dh-C6-Pyr-Cer (0.5 μM), as described in Materials and Methods. B) Effects of ceramide at 0.05, 0.1, 0.5, and 1 μM on PP2Ac activity were determined in the absence or presence of purified I2PP2A. C) Purified I2PP2A was preincubated with d-e-, l-e-, d-threo-, or l-threo-C6-Pyr-ceramides, and their effects on PP2A activity in vitro were assessed as described above. D) Effects of ceramide on the regulation of PP2A activity in vitro by purified wt and VIK/SSS mutants of I2PP2A were examined as described in A–C. Results represent at least three independent experiments performed in duplicate. Error bars = sd. P < 0.05 was considered significant.
Then the effects of l-e-C6-Pyr-Cer, which did not bind I2PP2A, and other stereoisomers, such as l-threo- or d-threo-C6-Pyr-Cer, on the inhibition of PP2A activity by I2PP2A were also examined. The results demonstrated that whereas d-e-C6-Pyr-Cer prevented inhibition, l-e-, l-threo-, or d-threo-C6-Pyr-Cer did not have any detectable effects on the inhibition of PP2Ac activity by I2PP2A (Fig. 5C). These results suggest that prevention of the inhibitory function of I2PP2A against PP2A activity by ceramide is stereoisomer-specific for the d-erythro- configuration of ceramide, consistent with its binding with I2PP2A. Notably, although the inhibition of PP2A activity by wt I2PP2A was prevented by ceramide, the VIK/SSS mutant of I2PP2A, which does not bind ceramide, did not affect PP2A activity in the absence or presence of ceramide (Fig. 5D). These data suggest that direct interaction between I2PP2A and ceramide might be involved in the regulation of PP2A activity in vitro.
I2PP2A-ceramide binding is involved in the regulation of PP2A activity and signaling
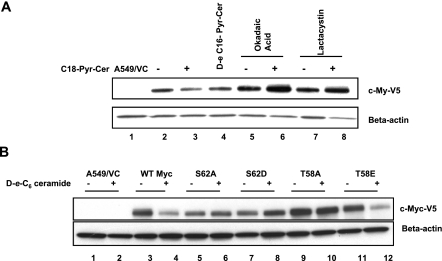
It became important to examine whether I2PP2A-ceramide interaction plays any biological roles via regulation of PP2A activity. It has been well documented that there are orchestrated phosphorylation and dephosphorylation events at S62/T58 residues of c-Myc that regulate its proteasomal degradation (5, 6). Because ceramide has been shown to induce the ubiquitination and proteasome-dependent degradation of c-Myc in A549 cells (19) by an as yet unknown mechanism, it is hypothesized that the I2PP2A-ceramide interaction might play a role in the degradation of c-Myc at least in part by regulating its dephosphorylation at S62 by PP2A. To test this hypothesis, we first examined whether ceramide-mediated degradation of c-Myc requires PP2A. A549 cells that express c-Myc-V5 were treated with d-e-C18-ceramide (10 μM for 24 h) in the absence or presence of okadaic acid (10 nM) (an inhibitor of PP2A) or lactacystin (20 nM) (an inhibitor of the proteasome), and then their roles in c-Myc expression were determined. The results showed that treatment with C18-ceramide caused significant degradation of c-Myc, which was almost completely prevented by okadaic acid and also by lactacystin (Fig. 6A, lanes 2 and 3, 5 and 6, and 7 and 8, respectively). Similar data were also obtained after treatment with d-e-C6-ceramide, whose inhibitory effects on endogenous c-Myc protein were prevented by okadaic acid and lactacystin in these cells (data not shown). Interestingly, treatment with C16-ceramide, which did not bind I2PP2A, was less effective for the degradation of c-Myc compared with that in controls than C18-ceramide in these cells (Fig. 6A, lanes 4, 2, and 3, respectively). Thus, these data suggest that ceramide-mediated proteasome degradation of c-Myc might be regulated by PP2A.
Figure 6.
Ceramide mediates degradation of c-Myc via PP2A-dependent dephosphorylation. A) Expression of c-Myc-V5 in response to C18-Cer in A549 cells was detected in the absence or presence of okadaic acid or lactacystin (lanes 2–8) by Western blot analysis using the anti-V5 antibody. B) wt c-Myc, and its mutants containing S62A, S62D, T58A, and T58E conversions with the V5 tag were expressed in A549 cells as described previously (6). Then their expression levels in the absence or presence of ceramide (lanes 2–12) were examined by Western blotting using the anti-V5 antibody (top panel). Lane 1 contains extracts obtained from vector-transfected A549 cells, used as vector controls (VC).
To further delineate the role of S62 dephosphorylation of c-Myc by PP2A in ceramide-mediated degradation, expression of wt and the S62 mutants (S62A or phospho-mimicking S62D) of c-Myc containing a V5-tag on their C termini in the absence or presence of ceramide in A549 cells was examined. Treatment with ceramide reduced the expression of wt c-Myc-V5 ∼75% compared with that in untreated controls as expected (Fig. 6B, lanes 4 and 3), whereas the phospho-mimicking S62D mutant of c-Myc, which cannot be dephosphorylated by PP2A, was resistant to ceramide-mediated degradation (Fig. 6B, lanes 8 and 7). These data reveal the importance of S62 dephosphorylation in ceramide-mediated c-Myc degradation.
Interestingly, mutation of S62A also prevented the ceramide-mediated degradation of c-Myc (Fig. 6B, lanes 6 and 5). These data are consistent with results of a previous study that showed that S62 phosphorylation is required for T58 phosphorylation by GSK3-β, which then leads to the dephosphorylation of c-Myc at S62 by PP2A for its degradation (5). Therefore, the role of T58 phosphorylation in ceramide-mediated degradation of c-Myc was also examined in A549 cells. Expression of c-Myc containing the T58A mutation was also resistant to ceramide-mediated degradation (Fig. 6B, lanes 10 and 9), consistent with previous reports (5, 6), which showed that T58 phosphorylation by GSK3-β is important for PP2A-mediated dephosphorylation of S62, and thus T58A mutant is resistant to S62 dephosphorylation and degradation. Interestingly, expression of the phospho-mimicking T58E mutant of c-Myc was nearly abolished by ceramide treatment (Fig. 6B, lanes 12 and 10), suggesting that, unlike the T58A mutant, the T58E phospho-mimic can support PP2A-mediated dephosphorylation of c-Myc at S62. These data, taken together with the protective effects of okadaic acid and lactacystin, suggest that dephosphorylation of c-Myc at S62 by PP2A, plays important roles in its degradation in response to ceramide, and the S62 dephosphorylation of c-Myc is dependent on T58 phosphorylation, as described previously (5).
After establishing the importance of S62 dephosphorylation by PP2A in ceramide-mediated degradation of c-Myc, we next determined whether the I2PP2A-ceramide interaction plays any role in the regulation of this process. The effects of knockdown of I2PP2A in A549 cells using siRNAs on the degradation of c-Myc in the absence or presence of ceramide were examined. The data showed that down-regulation of I2PP2A expression alone, which was decreased ∼90% (Fig. 7A, third panel, lanes 4 and 5), resulted in decreased levels of total c-Myc in the absence of ceramide compared with that in controls (Fig. 7A, top panel, lanes 4, 3, and 1, respectively). Moreover, although ceramide decreased total c-Myc levels in controls (Fig. 7A, top panel, lanes 2 and 1, respectively), down-regulation of I2PP2A using siRNA further enhanced the ceramide-mediated degradation of total c-Myc compared with that in controls (Fig. 7A, top panel, lanes 5 and 4 and 2 and 1, respectively). Interestingly, examination of the T58 phosphorylation of c-Myc showed that treatment with ceramide (10 μM for 24 h) in the absence or presence of I2PP2A siRNA enhanced the phosphorylation of c-Myc at T58 (Fig. 7A, second panel). This may be due to the effects of ceramide on the activation of GSK3-β, which was reported previously (24). Thus, these data (decreased total c-Myc and induced T58 phosphorylation in response to ceramide, particularly in the presence of I2PP2A-siRNA) indicate that the I2PP2A-ceramide interaction may play key roles in ceramide-mediated c-Myc degradation via PP2A signaling in A549 cells, and this regulation is further increased in response to the down-regulation of I2PP2A.
Figure 7.
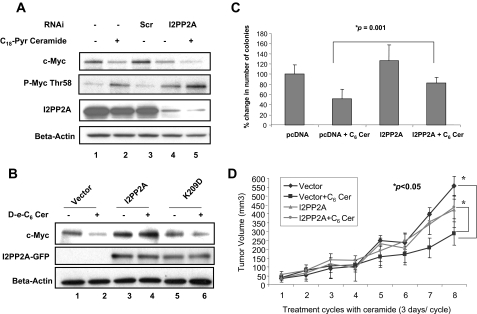
I2PP2A-ceramide binding regulates PP2A signaling and modulates ceramide-mediated cancer growth and tumor suppression in A549 cells both in situ and in vivo. A) Effect of down-regulation of I2PP2A on ceramide-mediated degradation of c-Myc in A549 cells was examined using siRNAs by Western blotting using antibodies that recognize total c-Myc or T58 phosphorylation of c-Myc (second panel). Levels of endogenous I2PP2A in response to scrambled (lane 3) or I2PP2A-specific siRNAs in the absence or presence of ceramide (lanes 4 and 5, respectively) were also detected by Western blotting using anti-I2PP2A antibody (third panel). β-Actin levels were used as loading controls (bottom panel). B) A549 cells were transiently transfected with plasmids that contain wt I2PP2A and its K209D mutant that is defective in ceramide binding. Roles of the wt or mutant I2PP2A-GFP in the expression of total c-Myc in response to ceramide (lanes 4 and 6, respectively) compared with that of their untreated controls (lanes 3 and 5, respectively) were determined by Western blotting using the anti-c-Myc antibody. Vector-transfected A549 cells were used as controls (lanes 1 and 2). Equal expression levels of wt and mutant I2PP2A-GFP proteins were confirmed using the anti-GFP and anti-β-actin antibodies (second and third panels, lanes 3–6 or 1–6, respectively). C) Roles of ceramide in the inhibition of anchorage-independent cell growth using A549 cells that express I2PP2A compared with vector transfected controls were assessed using soft agar assays. Results represent at least three independent experiments performed in duplicate. D) Effects of overexpression of wt I2PP2A in ceramide-mediated tumor suppression was also examined in vivo using SCID mice implanted with A549 cells in their flanks as described in Materials and Methods. Results were obtained using at least 6 tumors in each group. Error bars = sd. P < 0.05 was considered significant.
In reciprocal experiments, I2PP2A was overexpressed in A549 cells, and its roles in ceramide-induced c-Myc degradation were assessed. Results showed that overexpression of wt I2PP2A significantly reduced ceramide-induced c-Myc degradation compared with that in vector controls (Fig. 7B, lanes 4 and 2). On the other hand, overexpression of the mutant I2PP2A (K209D), which does not bind ceramide, did not have any significant protective effects on ceramide-induced c-Myc degradation (Fig. 7B, lanes 6 and 5). Thus, these data suggest that overexpressed I2PP2A might trap ceramide, reducing ceramide-mediated PP2A activation and signaling, which leads to decreased c-Myc dephosphorylation at S62 and protects it from degradation.
The results presented in Fig. 7B also suggested that overexpression of I2PP2A that sequesters ceramide might protect growth-inhibitory roles of ceramide. To test this view, roles of I2PP2A overexpression in the regulation of anchorage-independent growth of A549 cells on soft agar or tumor progression of A549 xenografts in SCID mice in response to ceramide were examined. As shown in Fig. 7C, although ceramide inhibited the growth of A549 cells by ∼50% compared with that in controls on soft agar, overexpression of wt I2PP2A significantly (∼40%) protected ceramide-mediated inhibition of anchorage-independent growth of A549 cells.
In addition, treatment with ceramide obviated the growth of A549 xenograft-driven tumors compared with that of controls in SCID mice, and I2PP2A overexpression almost completely protected tumor suppression in response to ceramide in vivo (Fig. 7D). More specifically, as shown in Fig. 7D, the data showed that ceramide significantly reduced the growth of A549 xenograft tumors compared with that in untreated controls, from 557 to 288 (P<0.05), and overexpression of I2PP2A prevented growth-inhibitory effects of ceramide in these tumor xenografts. The tumor volumes in A549/I2PP2A cells in the absence or presence of ceramide were 424 and 438 (P>0.1). More important, the difference between volumes of A549/vector and A549/12PP2A xenograft tumors in the presence of ceramide was statistically significant (P<0.05). However, the difference between the growth of A549/I2PP2A xenograft tumors in the absence or presence of ceramide was not significant. Therefore, these data suggest that I2PP2A plays key roles in the regulation of antiproliferative signaling of ceramide both in situ and in vivo.
DISCUSSION
These results identify I2PP2A as one of the novel ceramide-binding proteins that specifically recognizes the natural d-e-ceramide and show that it may exhibit fatty acid chain length preference for C18- over C16-ceramide in A549 cells. Notably, data provided here also suggest that the I2PP2A-ceramide interaction is involved in the regulation of PP2A activity and signaling, controlling the dephosphorylation and degradation of c-Myc and thus defining a novel mechanism for transducing the antiproliferative roles of ceramide (Fig. 8).
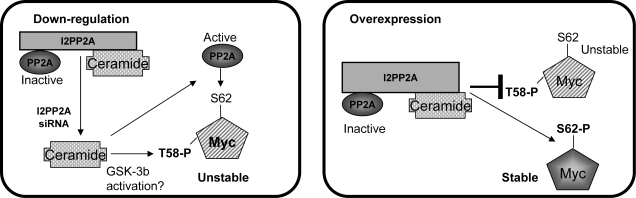
Figure 8.
Potential biological roles of the I2PP2A-ceramide interaction in the regulation of c-Myc degradation via control of PP2A. Results provided in this study indicate that I2PP2A-ceramide binding reveals a novel mechanism for ceramide-mediated activation (relief of repression) of PP2A activity when I2PP2A expression is decreased (left panel), leading to c-Myc dephosphorylation and degradation. It is still not known whether ceramide-mediated activation of GSK3-β is also involved in this process. On the other hand, overexpressed I2PP2A traps ceramide, which leads to repression of PP2A, resulting in decreased dephosphorylation of c-Myc at S62 (right panel), increased stability of c-Myc, and protection of cells from the antiproliferative roles of ceramide.
I2PP2A belongs to the SET domain containing acidic proteins, which is mainly localized to the nucleus (25). It is associated with acute myeloid leukemia and has been regarded as an oncoprotein (9, 10). The SET domain [Su(var), Enhancer-of-zeste, Trithorax] proteins function as methyltransferases that target specific lysine residues in the N-terminal tails of histones (26, 27). A number of reports have indicated that SET proteins are also involved in the modulation of gene transcription or histone acetylation (17, 27). However, whether the interaction between ceramide and I2PP2A controls these diverse biological roles, especially nuclear vs. cytoplasmic functions of I2PP2A in different disease models, is unknown and needs to be further explored.
We determined here that I2PP2A preferentially associates with exogenous C6-ceramide and endogenous C18-ceramide generated in A549 cells. This degree of fatty acid chain length preference of I2PP2A for ceramide binding is interesting; however, molecular mechanisms for this recognition and/or interaction need to be further determined. Nevertheless, there are various proteins and metabolic enzymes that can recognize, associate, and/or generate ceramides/lipids with a high degree of fatty acid chain length specificity, such as the newly discovered ceramide synthases 1–6 (CerS1-6) (28, 29) and CERT (30, 31), which preferentially binds C16-ceramide, as well as C6- and C18-ceramides but not very long-chain C24-ceramide (14, 31).
Interestingly, our data suggest that, similar to CERT, I2PP2A also preferentially interacts with C6- and C18-ceramides, but, unlike CERT, it does not interact with C16-ceramide. The crystal structure of ceramide-bound CERT suggests that the hydrophobicity and the size of the residues formed within the StART domain may play a role in fatty acid specificity and recognition (31). The globular domain of I2PP2A exhibits strong structural similarities to the recently solved CERT StART domain (23, 31). These domains form a fold analogous to a clenched fist, in which the “palm” is formed from a β-sheet and the “fingertips” are defined by helices. In the StART domain, the “fingers” are formed by continuation of the palm β-strands, whereas in the I2PP2A structure, this portion is made up of helices. Nevertheless, on the basis of these data, we propose that the central globular domain of I2PP2A, which includes VIK 207-209 residues, might be involved in interaction with ceramide.
It has been well established that ceramide induces PP2A activity; however, mechanisms of this induction remain enigmatic. The data presented in this article showed that ceramide reduces the association between I2PP2A and PP2Ac, which helped relieve its inhibition by the inhibitor 2 and not the inhibitor 1, which did not bind ceramide in vitro. These data suggest then that the I2PP2A-ceramide association might provide an alternative and novel mechanism for ceramide-mediated regulation of PP2A (32, 33).
Notably, loss- and gain-of-function studies using siRNA and overexpression of I2PP2A demonstrated that I2PP2A-ceramide binding plays important biological roles in ceramide-mediated regulation of PP2A, which then controls c-Myc dephosphorylation and degradation (Fig. 8). More specifically, when I2PP2A expression is down-regulated, ceramide can further induce the degradation of c-Myc via PP2A activity, whereas when I2PP2A is overexpressed, such as in cancer tissues and cells, it inhibits PP2A activity, leading to the protection of ceramide-mediated c-Myc degradation. Therefore, overexpression of I2PP2A might present a biological sink for proapoptotic ceramide (34, 35), and when I2PP2A is overexpressed, it prevents PP2A-dependent c-Myc dephosphorylation and degradation (Fig. 8), consistent with the protection of A549 cells or xenograft-driven tumor tissues from antiproliferative roles of ceramide.
Nuclear localization and metabolism of sphingolipids, including ceramide, are known to play important functional roles (1, 36, 37), such as the regulation of SP3-HDAC1 suppressor function for the repression of human telomerase reverse transcriptase in A549 cells (38, 39) and regulation of nucleocytoplasmic trafficking of various proteins, including the prevention of nuclear localization of glyceraldehyde-3-phosphate dehydrogenase that protects telomeres in A549 cells (40) or the inhibition of classic protein import in smooth muscle cells (41). Therefore, it is important to determine the subcellular regulation of how exogenous and endogenous ceramides reach nuclear I2PP2A for binding in cells and whether this interaction regulates only the nuclear functions of PP2A (42, 43).
Supplementary Material
Acknowledgments
We thank Dr. J.G. Schnellmann for critically reviewing the manuscript. This work was supported by research grants from the U.S. National Institutes of Health (CA-088932 and CA-097132). S.A.S. was supported by scholarships obtained from they Abney and Wachovia Foundations.
References
- Ogretmen B, Hannun Y A. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- Hannun Y A, Obeid L M. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- Chalfant C E, Kishikawa K, Mumby M C, Kamibayashi C, Bielawska A, Hannun Y A. Long chain ceramides activate protein phosphatase-1 and protein phosphatase-2A: activation is stereospecific and regulated by phosphatidic acid. J Biol Chem. 1999;274:20313–20317. doi: 10.1074/jbc.274.29.20313. [DOI] [PubMed] [Google Scholar]
- Dobrowsky R T, Kamibayashi C, Mumby M C, Hannun Y A. Ceramide activates heterotrimeric protein phosphatase 2A. J Biol Chem. 1993;268:15523–15530. [PubMed] [Google Scholar]
- Yeh E, Cunningham M, Arnold H, Chasse D, Monteith T, Ivaldi G, Hahn W C, Stukenberg P T, Shenolikar S, Uchida T, Counter C M, Nevins J R, Means A R, Sears R. A signalling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells. Nat Cell Biol. 2004;6:308–318. doi: 10.1038/ncb1110. [DOI] [PubMed] [Google Scholar]
- Arnold H K, Sears R C. Protein phosphatase 2A regulatory subunit B56α associates with c-myc and negatively regulates c-myc accumulation. Mol Cell Biol. 2006;26:2832–2844. doi: 10.1128/MCB.26.7.2832-2844.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Damuni Z. I1PP2A and I2PP2A: two potent protein phosphatase 2A-specific inhibitor proteins. Methods Mol Biol. 1998;93:59–66. doi: 10.1385/0-89603-468-2:59. [DOI] [PubMed] [Google Scholar]
- Li M, Makkinje A, Damuni Z. The myeloid leukemia-associated protein SET is a potent inhibitor of protein phosphatase 2A. J Biol Chem. 1996;271:11059–11062. doi: 10.1074/jbc.271.19.11059. [DOI] [PubMed] [Google Scholar]
- Wang G G, Cai L, Pasillas M P, Kamps M P. NUP98-NSD1 links H3K36 methylation to Hox-A gene activation and leukaemogenesis. Nat Cell Biol. 2007;9:804–812. doi: 10.1038/ncb1608. [DOI] [PubMed] [Google Scholar]
- Neviani P, Santhanam R, Trotta R, Notari M, Blaser B W, Liu S, Mao H, Chang J S, Galietta A, Uttam A, Roy D C, Valtieri M, Bruner-Klisovic R, Caligiuri M A, Bloomfield C D, Marcucci G, Perrotti D. The tumor suppressor PP2A is functionally inactivated in blast crisis CML through the inhibitory activity of the BCR/ABL-regulated SET protein. Cancer Cell. 2005;8:355–368. doi: 10.1016/j.ccr.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Snook C F, Jones J A, Hannun Y A. Sphingolipid-binding proteins. Biochim Biophys Acta. 2006;1761:927–946. doi: 10.1016/j.bbalip.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Heinrich M, Wickel M, Schneider-Brachert W, Sandberg C, Gahr J, Schwandner R, Weber T, Saftig P, Peters C, Brunner J, Kronke M, Schutze S. Cathepsin D targeted by acid sphingomyelinase-derived ceramide. EMBO J. 1999;18:5252–5263. doi: 10.1093/emboj/18.19.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Silva J, Krishnamurthy K, Tran E, Condie B G, Bieberich E. Direct binding to ceramide activates protein kinase Czeta before the formation of a pro-apoptotic complex with PAR-4 in differentiating stem cells. J Biol Chem. 2005;280:26415–26424. doi: 10.1074/jbc.M501492200. [DOI] [PubMed] [Google Scholar]
- Hanada K, Kumagai K, Yasuda S, Miura Y, Kawano M, Fukasawa M, Nishijima M. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 2003;426:803–809. doi: 10.1038/nature02188. [DOI] [PubMed] [Google Scholar]
- Vaesen M, Barnikol-Watanabe S, Gotz H, Awni L A, Cole T, Zimmermann B, Kratzin H D, Hilschmann N. Purification and characterization of two putative HLA class II associated proteins: PHAPI and PHAPII. Biol Chem Hoppe Seyler. 1994;375:113–126. doi: 10.1515/bchm3.1994.375.2.113. [DOI] [PubMed] [Google Scholar]
- Nagata K, Kawase H, Handa H, Yano K, Yamasaki M, Ishimi Y, Okuda A, Kikuchi A, Matsumoto K. Replication factor encoded by a putative oncogene, set, associated with myeloid leukemogenesis. Proc Natl Acad Sci U S A. 1995;92:4279–4283. doi: 10.1073/pnas.92.10.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S B, McNamara P, Heo S, Turner A, Lane W S, Chakravarti D. Regulation of histone acetylation and transcription by INHAT, a human cellular complex containing the set oncoprotein. Cell. 2001;104:119–130. doi: 10.1016/s0092-8674(01)00196-9. [DOI] [PubMed] [Google Scholar]
- Ogretmen B, Kraveka J M, Schady D, Usta J, Hannun Y A, Obeid L M. Molecular mechanisms of ceramide-mediated telomerase inhibition in the A549 human lung adenocarcinoma cell line. J Biol Chem. 2001;276:32506–32514. doi: 10.1074/jbc.M101350200. [DOI] [PubMed] [Google Scholar]
- Ogretmen B, Pettus B J, Rossi M J, Wood R, Usta J, Szulc Z, Bielawska A, Obeid L M, Hannun Y A. Biochemical mechanisms of the generation of endogenous long chain ceramide in response to exogenous short chain ceramide in the A549 human lung adenocarcinoma cell line: role for endogenous ceramide in mediating the action of exogenous ceramide. J Biol Chem. 2002;277:12960–12969. doi: 10.1074/jbc.M110699200. [DOI] [PubMed] [Google Scholar]
- Ogretmen B, Schady D, Usta J, Wood R, Kraveka J M, Luberto C, Birbes H, Hannun Y A, Obeid L M. Role of ceramide in mediating the inhibition of telomerase activity in A549 human lung adenocarcinoma cells. J Biol Chem. 2001;276:24901–24910. doi: 10.1074/jbc.M100314200. [DOI] [PubMed] [Google Scholar]
- Senkal C E, Ponnusamy S, Rossi M J, Sundararaj K, Szulc Z, Bielawski J, Bielawska A, Meyer M, Cobanoglu B, Koybasi S, Sinha D, Day T A, Obeid L M, Hannun Y A, Ogretmen B. Potent antitumor activity of a novel cationic pyridinium-ceramide alone or in combination with gemcitabine against human head and neck squamous cell carcinomas in vitro and in vivo. J Pharmacol Exp Ther. 2006;317:1188–1199. doi: 10.1124/jpet.106.101949. [DOI] [PubMed] [Google Scholar]
- Szulc Z M, Bielawski J, Gracz H, Gustilo M, Mayroo N, Hannun Y A, Obeid L M, Bielawska A. Tailoring structure-function and targeting properties of ceramides by site-specific cationization. Bioorg Med Chem. 2006;14:7083–7104. doi: 10.1016/j.bmc.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Muto S, Senda M, Akai Y, Sato L, Suzuki T, Nagai R, Senda T, Horikoshi M. Relationship between the structure of SET/TAF-Ibeta/INHAT and its histone chaperone activity. Proc Natl Acad Sci U S A. 2007;104:4285–4290. doi: 10.1073/pnas.0603762104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C F, Chen C L, Chiang C W, Jan M S, Huang W C, Lin Y S. GSK-3β act as down-stream of PP2A and the PI 3-kinase-Akt pathway, and upstream of caspase-2 in ceramide-induced mitochondrial apoptosis. J Cell Sci. 2007;120:2935–2943. doi: 10.1242/jcs.03473. [DOI] [PubMed] [Google Scholar]
- Ten Klooster J P, Leeuwen I, Scheres N, Anthony E C, Hordijk P L. Rac1-induced cell migration requires membrane recruitment of the nuclear oncogene SET. EMBO J. 2007;26:336–345. doi: 10.1038/sj.emboj.7601518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H B, Guo H. Mechanism of histone methylation catalyzed by protein lysine methyltransferase SET7/9 and origin of product specificity. Proc Natl Acad Sci U S A. 2007;104:8797–8802. doi: 10.1073/pnas.0702981104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein R, Cui X, Huie P, Cleary M L. Set domain-dependent regulation of transcriptional silencing and growth control by SUV39H1, a mammalian ortholog of Drosophila Su(var)3–9. Mol Cell Biol. 2000;20:4900–4909. doi: 10.1128/mcb.20.13.4900-4909.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pewzner-Jung Y, Ben-Dor S, Futerman A H. When do Lasses (longevity assurance genes) become CerS (ceramide synthases)? Insights into the regulation of ceramide synthesis. J Biol Chem. 2006;281:25001–25005. doi: 10.1074/jbc.R600010200. [DOI] [PubMed] [Google Scholar]
- Venkataraman K, Riebeling C, Bodennec J, Riezman H, Allegood J C, Sullards M C, Merrill A H, Jr, Futerman A H. Upstream of growth and differentiation factor 1 (uog1), a mammalian homolog of the yeast longevity assurance gene 1 (LAG1), regulates N-stearoyl-sphinganine (C18-(Dihydro)ceramide) synthesis in a fumonisin B1-independent manner in mammalian cells. J Biol Chem. 2002;277:35642–35649. doi: 10.1074/jbc.M205211200. [DOI] [PubMed] [Google Scholar]
- Kawano M, Kumagai K, Nishijima M, Hanada K. Efficient trafficking of ceramide from the endoplasmic reticulum to the Golgi apparatus requires a VAMP-associated protein-interacting FFAT motif of CERT. J Biol Chem. 2006;281:30279–30288. doi: 10.1074/jbc.M605032200. [DOI] [PubMed] [Google Scholar]
- Kudo N, Kumagai K, Tomishige N, Yamaji T, Wakatsuki S, Nishijima M, Hanada K, Kato R. Structural basis for specific lipid recognition by CERT responsible for nonvesicular trafficking of ceramide. Proc Natl Acad Sci U S A. 2008;105:488–493. doi: 10.1073/pnas.0709191105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfant C E, Szulc Z, Roddy P, Bielawska A, Hannun Y A. The structural requirements for ceramide activation of serine-threonine protein phosphatases. J Lipid Res. 2004;45:496–506. doi: 10.1194/jlr.M300347-JLR200. [DOI] [PubMed] [Google Scholar]
- Ruvolo P P, Clark W, Mumby M, Gao F, May W S. A functional role for the B56 α-subunit of protein phosphatase 2A in ceramide-mediated regulation of Bcl2 phosphorylation status and function. J Biol Chem. 2002;277:22847–22852. doi: 10.1074/jbc.M201830200. [DOI] [PubMed] [Google Scholar]
- Koybasi S, Senkal C E, Sundararaj K, Spassieva S, Bielawski J, Osta W, Day T A, Jiang J C, Jazwinski S M, Hannun Y A, Obeid L M, Ogretmen B. Defects in cell growth regulation by C18:0-ceramide and longevity assurance gene 1 in human head and neck squamous cell carcinomas. J Biol Chem. 2004;279:44311–44319. doi: 10.1074/jbc.M406920200. [DOI] [PubMed] [Google Scholar]
- Senkal C E, Ponnusamy S, Rossi M J, Bialewski J, Sinha D, Jiang J C, Jazwinski S M, Hannun Y A, Ogretmen B. Role of human longevity assurance gene 1 and C18-ceramide in chemotherapy-induced cell death in human head and neck squamous cell carcinomas. Mol Cancer Ther. 2007;6:712–722. doi: 10.1158/1535-7163.MCT-06-0558. [DOI] [PubMed] [Google Scholar]
- Ledeen R W, Wu G. Sphingolipids of the nucleus and their role in nuclear signaling. Biochim Biophys Acta. 2006;1761:588–598. doi: 10.1016/j.bbalip.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Ledeen R W, Wu G. Nuclear sphingolipids: metabolism and signaling. J Lipid Res. 2008;49:1176–1186. doi: 10.1194/jlr.R800009-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooten L G, Ogretmen B. Sp1/Sp3-dependent regulation of human telomerase reverse transcriptase promoter activity by the bioactive sphingolipid ceramide. J Biol Chem. 2005;280:28867–28876. doi: 10.1074/jbc.M413444200. [DOI] [PubMed] [Google Scholar]
- Wooten-Blanks L G, Song P, Senkal C E, Ogretmen B. Mechanisms of ceramide-mediated repression of the human telomerase reverse transcriptase promoter via deacetylation of Sp3 by histone deacetylase 1. FASEB J. 2007;21:3386–3397. doi: 10.1096/fj.07-8621com. [DOI] [PubMed] [Google Scholar]
- Sundararaj K P, Wood R E, Ponnusamy S, Salas A M, Szulc Z, Bielawska A, Obeid L M, Hannun Y A, Ogretmen B. Rapid shortening of telomere length in response to ceramide involves the inhibition of telomere binding activity of nuclear glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 2004;279:6152–6162. doi: 10.1074/jbc.M310549200. [DOI] [PubMed] [Google Scholar]
- Faustino R S, Cheung P, Richard M N, Dibrov E, Kneesch A L, Deniset J F, Chahine M N, Lee K, Blackwood D, Pierce G N. Ceramide regulation of nuclear protein import. J Lipid Res. 2008;49:654–662. doi: 10.1194/jlr.M700464-JLR200. [DOI] [PubMed] [Google Scholar]
- Arnold H K, Sears R C. A tumor suppressor role of PP2A-B56α through negative regulation of c-Myc and other key oncoproteins. Cancer Metastasis Rev. 2008;27:147–158. doi: 10.1007/s10555-008-9128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Lavin V A, Moser L R, Cui Q, Kanies C, Yang E. PP2A regulates the pro-apoptotic activity of FOXO1. J Biol Chem. 2008;283:7411–7420. doi: 10.1074/jbc.M708083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.