Abstract
Environmental enrichment for laboratory animals is a widely accepted practice for many species, but few studies address the periods of preadolescence and adolescence. Provision of igloos, tunnels, nesting materials, and social or communal housing are commonly used enrichment strategies in rat cages. In the present study, the effects of individual, pair, and trio housing and the presence or absence of physical cage enrichment on the growth rate, food consumption, and locomotor behavior of juvenile male rats through adolescence were examined. The results indicated that social and physical enrichment decreased the growth and feeding rates and locomotor activity of developing rats as compared with rats living in an impoverished environment. The results show that the growth rates are dependent predominantly on environmental enrichment and that social enrichment alone has no effect. These results demonstrate that enrichment can have significant effects on growth and behavior of male rats.
Abbreviations: ANOVA, analysis of variance; PND, postnatal day
Environmental enrichment for laboratory animals is a widely accepted practice for many species and is mandated for nonhuman primates. Various approaches can be used to provide environmental enrichment to rodents, including, for example, structure and substrate changes to cages, addition of manipulanda, novelty food, and social contact (from conspecifics or human handling).8 Provision of igloos, tunnels, nesting materials, and the like are commonly used physical enrichment items in rat and mouse cages. Used alternatively or in conjunction with physical manipulation of the cage environment are increased handling of rats and social enrichment (for example, multiple housing of rats).1,13 Recently, interest in the potential effects of introducing enrichment on research variables has increased.2 Consequently, careful consideration should be given to the types of enrichment, schedule of enrichment, and possible effects on research results.1
Although several studies have examined the effects of ‘impoverished’ versus ‘enriched’ housing conditions on growth and feeding, many compared singly housed animals without environmental enrichment to group-housed animals with enrichment.4,6,9,10 Often the enriched condition involved much larger cages than the impoverished condition, as well as the presence of other animals, various objects, or both. Therefore, little information is available regarding the respective roles of social and environmental factors on altering body weight and feeding. The present study was designed to assess systematically whether the addition of physical and social environmental enrichment causes different effects on the growth and behavior of male rats from weaning through adolescence (postnatal days [PNDs] 23 through 45). The results indicate that social and physical enrichment have different effects on growth and feeding rates of juvenile rats and locomotor activity during adolescence, depending on housing conditions.
Materials and Methods
Rats.
Crl:Sprague–Dawley male rats (Charles River Labs, Wilmington, MA) arrived on PND 23 and were assigned randomly to 1 of 6 housing conditions (see following section). All procedures were conducted in an AAALAC-accredited facility under a care and use protocol that follows guidelines established for the humane care and use of rats and was approved by the University of Miami Institutional Animal Care and Use Committee, which adheres to the Guide for the Care and Use of Laboratory Animals.11
Housing.
Rats were maintained on a 12:12-h light:dark schedule, with lights on at 0700 and off at 1900. Temperature was maintained at 19.4 to 23.3 °C and a relative humidity of 30% to 70%. All rats were housed in the same room, which was attached to the testing rooms. Rat cages consisted of standard polycarbonate shoebox caging (9 in. × 12 in. × 16 in.) containing standard aspen chip bedding and covered with microisolation lids. The rats were fed standard rodent chow (Purina 5001 Maintenance Diet, Purina Mills International, St Louis, MO).
On their arrival on PND 23, rats were housed in 1 of 6 conditions. First, they were housed as either 1 rat per cage (isolated housing) or with 2 or 3 rats per cage (social housing). In addition, the environments were different. Some rats lived in an enriched environment in which objects were placed in the cages, and different objects were rotated in and out at each cage change (twice per week). Plastic tunnels and balls that the rats could move into and out of or climb over were used in addition to objects that they could chew and paper nestlets that they could scratch and chew. The objects were rotated systematically through the cages such that for each rat, the same objects were never presented during subsequent switches. Other rats experienced an environment in which no objects were provided. The 6 housing conditions were thus isolated/impoverished (II; n = 21), isolated/enriched (IE; n = 8), social/impoverished with 2 rats per cage (SI2, standard housing conditions; n = 16), social/enriched with 2 rats per cage (SE2; n = 8), social/impoverished with 3 rats per cage (SI3; n = 12), and social/enriched with 3 rats per cage (SE3; n = 27).
Physiologic parameters.
Body weight.
Body weight was measured daily Monday through Friday for all groups, but otherwise the rats were not handled for the first 3 wk. Thereafter, the locomotor activity of the rats was tested for 1 h daily for 2 d during weeks 4 and 5 (see description following).
Feeding.
Preweighed aliquots of food were placed on the lids of the cages upon arrival and housing of the rats. The amount of food consumed by the II (n = 32) and SE3 (n = 39) rats was determined several times a week at approximately 1500 by weighing the remaining food and subtracting it from the original amount. To determine the amount of food consumed by a single rat in the rats in the SE3 group, the total amount of food eaten per cage was divided by 3, based upon the assumption that each rat within a cage ate the same amount of food.
Data were analyzed by 2-way analysis of variance (ANOVA) (SuperAnova, Abacus), with housing condition and week as the variables, followed by post hoc analysis with the Fisher protected least significant difference test. P values less than 0.05 were considered significant.
Locomotor activity testing.
All behavioral testing was done during the light period between 0900 and 1700, with each group tested at the same hour each day and the groups randomized over the course of the day. Each rat was placed in a locomotor activity chamber, and activity was measured for 1 h subsequent to a 15-min habituation period during which activity was not measured. Rats were placed in clear acrylic chambers (40.64 × 40.64 cm) inside Digiscan activity monitors (Accuscan, Columbus, OH) that were equipped with infrared light-sensitive detectors mounted 2.5 cm apart along 2 perpendicular walls. Mounted along the opposing walls were infrared light beams that were directed at the detectors. One count of horizontal activity was registered each time the subject interrupted a beam. Activity was monitored for a total of 60 min, during which beam breaks were measured over 12 consecutive 5-min time periods. Rats were tested on PNDs 38 and 39 and then again on PNDs 45 and 46.
Data were analyzed by 2-way ANOVA (SuperAnova, Abacus), with housing condition and test session as the variables, followed by post hoc analyses with Fisher's protected least significant difference test. P values less than 0.05 were considered significant.
Results
Body weight.
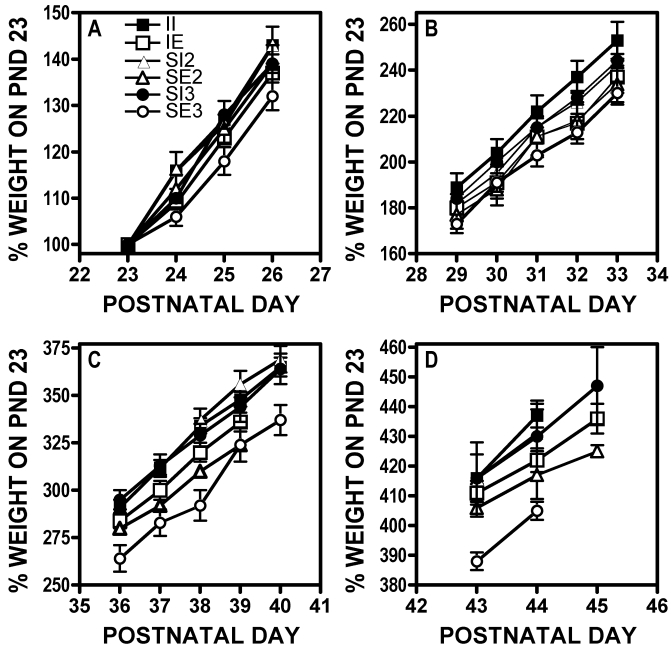
Mean body weights on PND 23 were: II, 51 ± 1 g (n = 21); IE, 50 ± 1 g (n = 8); SI2, 51 ± 1 g (n = 16); SE2, 50 ± 1 g (n = 8); SI3, 48 ± 1 g (n = 12); and SE3, 51 ± 1 g (n = 27), and an overall ANOVA of these initial body weights showed that these weights were not significantly different from one another. Over the course of the next 4 wk, the rats were weighed daily from Monday to Friday, and all of the rats gained weight daily. Figure 1 shows mean weight, expressed as a percentage of that on PND 23, by week across the 4-wk period. A 2-way ANOVA of group × day showed a significant effect of group (F(5,750) = 67.21, P < 0.0001) and of day (F(14,750) = 2798, P < 0.0001). In addition, there was a significant group × day interaction (F(68,750) = 2.37, P < 0.0001). Posthoc analyses of all groups showed that across the entire 4-wk period, the groups all differed from one another, except that the SI2 group was not significantly different from either the II or SI3 groups (Figure 1). Within 24 h of housing (PND 24), the SE3 group weighed an average of 4 g less than the II group, and this difference was maintained for the next several days (Figure 1 A). By PND 31, both the II and SI2 rats weighed significantly more than the SE3 rats, and this pattern was maintained through the last day of the experiment (PND 44). In contrast, the divergence in weight between the SI3 and SE3 rats was not significant until PND 39, after which time the SI3 group maintained a significantly higher weight (Figure 1 C, D). There were no significant differences among the weights of animals housed in the same cages (data not shown).
Figure 1.
Body weight (g, mean ± SEM) of rats housed under different conditions beginning on postnatal (PND) 23. (A) PNDs 23 through 27. (B) PNDs 28 through 34. (C) PNDs 35 through 41. (D) PNDs 42 through 46. Data are given as a percentage of the group mean body weight on PND 23: II, 51 ± 1 g; IE, 50 ± 1 g; SI2, 51 ± 1 g; SE2, 50 ± 1 g; SI3, 48 ± 1 g; and SE3, 51 ± 1 g. II, isolated impoverished housing condition (that is, 1 rat/cage with no objects); SI2, social impoverished (2 rats/cage with no objects; the standard housing condition at our institution); IE, isolated enriched (1 rat/cage with objects); SE2, social enriched 2 (2 rats/cage with objects); SE3, social enriched 3 (3 rats/cage with objects).
Feeding.
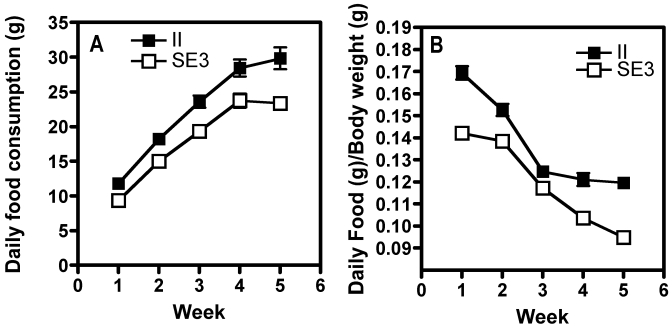
In a separate group of rats, analysis of the amount of food consumed under the 2 most extreme housing conditions (that is, II and SE3) showed that the II rats (n = 32) consumed more food than the average amount of food eaten by the SE3 housed rats (n = 39; Figure 2 A). A 2-way ANOVA of housing × week showed a significant effect of housing (F(1,239) = 56.8, P < 0.0001) and of week (F(3,239) =207, P < 0.001), but there was no significant interaction between these parameters. Post hoc tests showed that the II rats ate more than the SE3 rats during each of the 4 wk of the experiment.
Figure 2.
(A) Daily food consumption per rat (g, mean ± SEM) averaged weekly and (B) food consumed (g) per body weight (g) of rats in the isolated, impoverished housing condition (II group; filled squares) versus those of rats socially housed (3/cage) with environmental enrichment (SE3 group; open squares). Measurements began on PND 23, when the rats arrived and were housed. The SE3 rats ate less food (P ≤ 0.001) than did the II rats, according to both criteria.
Because the II rats weighed more than the SE3 rats, we wondered whether they were eating more because of their greater weight. To control for differential food consumption based on differing body weights, the amount of food consumed by each rat was corrected by body weight. These results indicated that at any given body weight, the II rats consumed more food per body weight than did the SE3 rats (Figure 2 B). An overall ANOVA of housing × week for the food:body weight ratio showed a significant effect of housing (F(1,230) = 58.6, P < 0.0001) and of week (F(4,230) = 89.2, P < 0.0001), with a significant housing × week interaction (F(4,230) = 4.26, P < 0.002). Post hoc analyses showed that although the grams of food per body weight decreased with time for both groups, they differed from one another during each of the 4 wk of the study.
Sufficient food was provided such that neither food limitation nor competition for food was likely to influence the amount of food eaten. Rats in the II housing condition showed little variation in the daily amount of food eaten. Similarly, enriched rats showed little difference in the amount of food eaten on a given day across cages.
Locomotor activity.
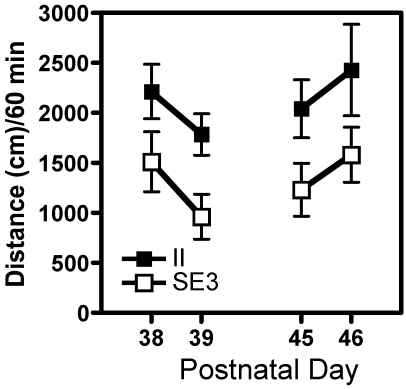
To determine whether the various housing conditions altered a behavioral measure, locomotor activity in the II and SE3 adolescent rats was examined. The rats were tested on PNDs 38 and 39 then again on PNDs 45 and 46. The data show that the distance traveled during 60-min test sessions was significantly less for the SE3 rats than for the II rats during all 4 test sessions (Figure 3). A 2-way ANOVA of housing × test session showed a significant effect of housing (F(1,128) = 16.58, P < 0.0001). There were no differences across test sessions and no interaction effect.
Figure 3.
Locomotor activity in II (filled squares) and SE3 (open squares) adolescent rats. Data shown are the distance traveled during four 60-min test sessions during which the rats were placed individually in a testing chamber. Testing began after a 15-min habituation session during which activity was not recorded. Rats were tested on PNDs 38 and 39 and again on PNDs 45 and 46. The SE3 rats had significantly (P ≤ 0.0001) less activity than did the II rats across days.
Discussion
This study was designed to assess whether the addition of physical and social environmental enrichment results in differential effects in growth and behavior of adolescent male rats. The results indicate that physiologic and behavioral differences are evident soon after differential housing of the rats, and the effects of physical enrichment vary with the number of animals in the cage.
Body weight.
Housing conditions altered body weight and the increase in body weight during development. In previous studies, isolated rats living in small cages gained weight more rapidly than did group-housed animals with access to objects and novel environments.4,6,9,10 In the present study, the 2 groups housed under extreme conditions (that is, rats housed singly without enrichment [II group] and rats housed 3 to a cage with enrichment [SE3 group]) gained weight at different rates, with the II rats gaining more rapidly. The data show that this effect is predominantly due to environmental enrichment, which interacts with the number of rats housed in the cage. The initial effects of housing on body weight appear to be due to physical enrichment (objects), because by the end of the first week the rats with impoverished environments (that is, II, SI2, and SI3) all had weights that were greater than those of the corresponding rats housed with environmental enrichment (that is, IE, SE2, SE3). After 2 wk, the groups the rats living in impoverished housing all essentially weighed the same, and this similarity was maintained throughout the remainder of the experiment. In contrast, the IE, SE2, and SE3 rats had lower weights than did those in impoverished environments, with the II and SE3 having weights that were significantly different by PND 31. With time, the effects of enrichment grew, such that by PND 39, the SI3 rats had weights that were significantly greater than those of the SE3 rats. Similarly, by the end of the third week, the growth rates of the SE2 and SI2 rats had separated significantly. Interestingly, the number of rats in the cage appeared to have no effect on the rats in impoverished environments (no objects), whereas the housing density did alter the body weights of the enriched rats, with a greater number of rats leading to lower body weights in the presence of environmental enrichment. Therefore, social and environmental factors interacted, and the effects of these 2 factors became evident at different times during development. These data also show that social enrichment alone can be used without altering the growth patterns of the rats.
Primary considerations for decreased weight gain include competition for food, increased activity, altered metabolism, stress, illness, and decreased food consumption. In the present study, all rats remained healthy with good body condition and bright eyes and coats throughout the study. Ample food was available such that excess food was present on top of the cage each time new food was replaced. Daily informal observations of the group housed and environmentally enriched rats suggested increased and more frequent home cage activity during the light period than for individually housed or impoverished rats. The data suggest that decreased food consumption contributes at least in part to the weight differences.
Feeding.
The group-housed, environmentally enriched (SE3) rats consumed less food per gram of body weight than did the rats housed individually in an impoverished environment (II group). Several studies, using various housing paradigms, have demonstrated that group-housed rats have slower weight gain or weigh less or both. The current data show that the weight change is due to the environmental, not the social, enrichment. In an elaborate study in which rats were housed at either 12/cage in a large cage with daily access to objects and an open-field environment or in isolation with no enrichment, the isolated rats in small cages ate more food and gained weight at a more rapid rate than did the rats with enriched environments.6,9,10 The present data show that the same effects occur under much less disparate conditions (in the present case, all of the rats were housed in the same size cages and other than the number of cagemates and availability of objects, the experiences of the rats were not different across conditions). Further, rehousing considerably older rats (PND 45 at start of experiment) from isolated to paired conditions after 21 d suppressed feeding for several days.6,9,10 Therefore, the effects of social enrichment may differ depending on age, because in the present study, body weight did not differ between rats housed singly or doubly in the absence of environmental enrichment. Evidence that the environmental enrichment may be driving the differences in feeding is obtained from a study showing that in fasting adult rats, access to an activity wheel decreased feeding when food was made available.6,9,10 One caveat is that the study was done in adult rats; therefore although those findings are consistent with the present data, whether the same results would be obtained in younger animals is unknown.
A dramatic decrease in food consumption per body weight occurred during week 3 and was most noticeable in the II rats. The reason underlying this change is unknown, but week 3 is the week when locomotor activity testing was performed. Perhaps the testing was a form of enrichment for the rats and has a greater effect on the animals in impoverished environments than on those in enriched ones. Additional studies will need to be done to determine the cause of this decrease.
Drawing consistent conclusions from the literature is difficult, given that the conditions of the group housing (for example, number of cagemates and cage sizes), extent of enrichment (for example, type and number of objects, length of time enriched), and age range when housed or tested all vary across studies. However, the present data suggest that environmental enrichment reduces feeding and weight gain. The present results show that reduced weight gain, in part due to reduced food intake per rat, occurs when preadolescent and adolescent rats are group-housed and enriched between PNDs 23 and 45 and concur with the preponderance of data that indicate that group-housed adult rats typically demonstrate a decrease in weight.6,9,10 The data further show that, as time goes on, an interaction between the social and environmental factors influences body weight and feeding behavior and that the number of rats in the cage is important only in the presence of enrichment.
Locomotor activity.
Locomotor or open-field activity testing is a common means to assess standard neurologic processes such as habituation and adaptation. Environmental enrichment is well known to affect cerebral and cellular morphology, neuronal plasticity, and gene expression.1 Decreased locomotor activity in the adolescent rats housed in a socially and environmentally enriched environment is consistent with previous findings in adult rats. For example, adult rats living in an impoverished environment exhibited an increase in locomotor activity and rearing,7 whereas an enriched environment decreased exploration and basal locomotor activity.3,12,15 Further, rats reared in isolation exhibited more exploratory behavior in open-field testing as adults than did socially reared rats.5 The decreased open-field activity values were interpreted as an index of increased habituation to a novel environment and stimuli and were believed to reflect improved information processing and adaptation to new environments.14,15 Therefore, the current data extend the findings of previous studies on adult rats to adolescent rats and show that the effects of housing conditions occur rapidly (in the present case, within 2 wk of housing).
More research is needed to assess long-term affects of group-housing and enriched environments. In the current study, an enriched environment appears to have a greater effect on body weight change than did group housing. However, the interaction of these variables is likely more important, because it better mirrors actual situations in social rats and in humans. Questions remain regarding whether metabolic differences are present among the differentially housed rats and whether the food consumption differences account totally for the weight changes. Data like these, in addition to ensuring environmental conditions for optimal rodent housing and care, suggest that both the social and physical environments are important factors in development. In addition care should be used when applying environmental enrichment strategies to rodents, because unpredictable variables may alter or increase the variability among and reproducibility of experimental results. Any effects that increase variability in the data will result in the use of more rats.16 Pilot studies are recommended highly to define whether any differences in results will be created when changing rodent housing conditions during or between experiments.
Acknowledgments
This work was supported by the National Institute on Drug Abuse (grants DA 015119 and DA 024584).
References
- 1.Bayne K. 2005. Potential for unintended consequences of environmental enrichment for laboratory animals and research results. ILAR J 46:129–139 [DOI] [PubMed] [Google Scholar]
- 2.Benefiel AC, Dong WK, Greenough WT. 2005. Mandatory “enriched” housing of laboratory animals: the need for evidence-based evaluation. ILAR J 46:95–105 [DOI] [PubMed] [Google Scholar]
- 3.Bowling SL, Rowlett JK, Bardo MT. 1993. The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis, and dopamine release. Neuropharmacology 32:885–893 [DOI] [PubMed] [Google Scholar]
- 4.Diamond M, Rosenzweig MR, Krech D. 1965. Relationships between body weight and skull development in rats raised in enriched and impoverished conditions. J Exp Zool 160:29–35 [DOI] [PubMed] [Google Scholar]
- 5.Faith RE, Hessler JR. 2006. Housing and environment. In: Suckow MA, Weisbroth SH, Franklin CL.The laboratory rat Amsterdam: Academic Press; p. 303–338 [Google Scholar]
- 6.Fiala B, Snow FM, Greenough WT. 1977. “Impoverished” rats weigh more than “enriched” rats because they eat more. Dev Psychobiol 10:537–541 [DOI] [PubMed] [Google Scholar]
- 7.Heidbreder CA, Weiss IC, Domeney AM, Pryce C, Homberg J, Hedou G, Feldon J, Moran MC, Nelson P. 2000. Behavioral, neurochemical, and endocrinological characterization of the early social isolation syndrome. Neuroscience 100:749–768 [DOI] [PubMed] [Google Scholar]
- 8.Hutchinson E, Avery A, Vandewoude S. 2005. Environmental enrichment for laboratory rodents. ILAR J 46:148–161 [DOI] [PubMed] [Google Scholar]
- 9.Levitsky DA. 1970. Feeding patterns of rats in response to fasts and changes in environmental conditions. Physiol Behav 5:291–300 [DOI] [PubMed] [Google Scholar]
- 10.Lopak V, Eikelboom R. 2000. Pair-housing–induced feeding suppression: individual housing not novelty. Physiol Behav 71:329–333 [DOI] [PubMed] [Google Scholar]
- 11.National Research Council 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academy Press [Google Scholar]
- 12.Neugebauer NM, Cunningham ST, Zhu J, Bryant RI, Middleton LS, Dwoskin LP. 2004. Effects of environmental enrichment on behavior and dopamine transporter function in medial prefrontal cortex in adult rats prenatally treated with cocaine. Brain Res Dev Brain Res 153:213–223 [DOI] [PubMed] [Google Scholar]
- 13.Smith AL, Corrow DJ. 2005. Modifications to husbandry and housing conditions of laboratory rodents for improved well-being. ILAR J 46:140–147 [DOI] [PubMed] [Google Scholar]
- 14.Van Waas M, Soffie M. 1996. Differential environmental modulations on locomotor activity, exploration, and spatial behaviour in young and old rats. Physiol Behav 59:265–271 [DOI] [PubMed] [Google Scholar]
- 15.Varty GB, Paulus MP, Braff DL, Geyer MA. 2000. Environmental enrichment and isolation rearing in the rat: effects on locomotor behavior and startle response plasticity. Biol Psychiatry 47:864–873 [DOI] [PubMed] [Google Scholar]
- 16.Weed JL, Raber JM. 2005. Balancing animal research with animal well-being: establishment of goals and harmonization of approaches. ILAR J 46:118–128 [DOI] [PubMed] [Google Scholar]