Abstract
The Guide for the Care and Use of Laboratory Animals (Guide) recommends minimum floor space per mouse based on weight, with no other factors considered. We conducted a randomized experiment to evaluate the effect of housing density on reproductive indices and corticosterone levels in lactating mice. Female mice matched for age, strain, and date-of-pregnancy were housed individually. At parturition the dams were randomly allocated to have litters culled or remain intact. The experimental group had litters culled to meet the Guide’s space density requirement. Litters of the second group were maintained as the numbers born to each dam. Fecal corticosterone levels (first-generation mice only), growth, and weaning weights were measured for mice in all cages; in addition, the reproductive behavior of progeny generated under both housing conditions was assessed to determine whether a space × litter size interaction affected subsequent reproduction. The growth rates for pups from culled litters were significantly greater than those from intact litters. The first-generation pups showed no statistically significant differences in fecal corticosterone or reproductive parameters. The second-generation pups showed no statistically significant differences in growth rates. The results of the study suggest that a strict interpretation of space requirements as listed in Table 2.1 of the Guide is not warranted for lactating dams with litters.
The welfare of animals housed at different population densities continues to be a topic of debate. Opinions differ on the effect, if any, on the welfare of mice housed in cages where space per mouse is markedly less than that recommended in the Guide for the Care and Use of Laboratory Animals24 (the Guide). Published literature on the minimum floor space required per mouse is contradictory; few studies have examined the minimum space a lactating dam with litter requires. Early studies (1950 to 1970s) did not seek to establish minimum space requirements per mouse but rather used crowding to study a particular physiologic aspect of stress.14 Further, comparison across studies is difficult due to differences in experimental design. Some breeding operations advocate removing the male mouse after conception;3 others advocate adding a second female mouse to assist in nursing for ‘difficult’ lines;15 and still others permit housing 2 or 3 pregnant female mice in a cage without separation after parturition.10
Currently, in the United States and the United Kingdom, guidelines for space per mouse are based solely on weight of the animal, with the allowable number of mice dependent on meeting the space requirement per animal. Neither strain (nor stock), age, gender, social behavior, group composition, nor health status are considered within the recommendations. According to Van Loo,36 both the British Code of Practice for the Housing of Animals35 and the Report of the Rodent Refinement Working Party19 identify cage size as an issue that should be studied in greater detail. New studies and better understanding of rodent social behavior support this view.
Breeding is performed for many purposes: commercial production; experimental studies; and creation of genetically engineered mice. The marked increase in numbers of breeding colonies has increased the pressure on facilities for sufficient space for breeding colony maintenance. This pressure creates a desire for maintaining maximal housing density within cages in a room as well as accommodating the maximal number of cages in a room. The advent of individually ventilated caging systems removed one barrier to increased room density because individual cage ventilation dries the bedding and removes waste gases from each cage, augmenting cage ventilation. Racks of individually ventilated cages typically hold more cages than do nonventilated racks for a given footprint of floor space. Here we describe a study conducted to examine one small subset of the cage density debate, that is, lactating dams with litters.
In the United States, the principal space recommendations used for mice housed in research facilities are described in the Guide.24 The amount of floor space recommended per mouse ranges from 6 to more than 15 in.2 (38.7 to 96.75 cm2), with no distinction made between nursing pups and weaned animals. Commercial cages for mice typically provide floor space in the range of 60 to 75 in.2 (387 to 451.5 cm2) per ‘single’ cage. Although the Guide stresses the value of professional judgment, the appropriate rodent housing density within a cage is a debated topic with little consensus. With strict application of engineering standards to housing density, large litters would not comply with space recommendations at the time of birth, and noncompliance would be exacerbated if individual pup body weights exceed 10 g at weaning. Strict interpretation of Table 2.1 in the Guide is therefore clearly at odds with the goal of maximizing the production of mouse breeding colonies.
This study was undertaken to compare litters that were culled to meet a strict engineering interpretation of space provision for mouse litters with litters that were not culled, which creates a deviation from the Guide's recommendations. We hypothesized that providing less than the recommended amount of floor space for dams with litters would not adversely affect fecal corticosterone, growth rates, or reproductive performance. Fecal corticosterone levels and the growth and weaning rates of pups housed in compliance with, or more densely than, Guide recommendations were evaluated to determine whether housing density affected these parameters. The reproductive behavior of progeny raised under both conditions also was evaluated to determine whether a space × litter size interaction prior to weaning affected subsequent reproductive performance. ICR mice were used for this study because of their genetic diversity and large litter size.
Materials and Methods
Animals.
Twenty-eight primiparous female ICR mice (Harlan Sprague Dawley, Frederick, MD) arrived on day E13 of gestation. Animals were housed in individually ventilated cages (Tecniplast, Milan, Italy) that provided 65 in.2 (419.25 cm2) floor space. The mice were housed on either hardwood chip bedding (Beta Chip, Nepco, Warrensburg, NY) with cotton nesting material (Nestlets, Ancare, Bellmore, NY) or wire-grid–floor inserts without nesting material above absorbent paper bedding material (Iso-pads, Harlan-Teklad, Madison, WI). Mice were fed NIH-07 diet (Zeigler Brothers, Gardners, PA) and provided with reverse-osmosis water ad libitum. Environmental temperature and relative humidity were maintained at 21.1 to 22.2 °C and 35% to 55 %, respectively. A 12:12-h light cycle, with the dark phase lasting from 0200 until 1400, was used. Sentinel animals were not placed with this particular colony; regular surveillance of colonies in this facility show that mice remain negative for 15 pathogens (Ectromelia, mouse rotavirus, Theiler murine encephalomyelitis virus, lymphocytic choriomeningitis virus, mouse cytomegalovirus, mouse hepatitis virus, mouse adenovirus, mouse minute virus, polyoma virus, pneumonia virus of mice, reovirus 3, Sendai virus, cilia-associated respiratory bacillus, Mycoplasma pulmonis, and Salmonella spp.) and external and intestinal parasites. The institutional animal care and use committee approved the reported experiments. Mice were euthanized with CO2 gas at the end of the experiments.
Experimental protocol.
All first-generation mice were housed on wire-grid–floor inserts over absorbent paper material. On the first postnatal day, pups were counted, and litters were randomly assigned to intact or cull conditions. In culled litters, the dams were left with 6 nursing pups. Feces (0.1 g/cage) were collected from the cage bottom below the wire-grid–floor inserts on postnatal days 7, 14, and 21. Collections were performed 2 h after the end of the dark cycle, and 1.5 h after placement in a clean cage. When available, small pellets also were collected, which were assumed to reflect the pups rather than the dam. Feces were stored at –70 °C until analyzed. Body weights of pups were measured after the fecal collections were completed.
To examine potential effect of rearing conditions on subsequent reproductive performance, 1 male and 2 female mice from each litter were retained in the colony. Matings were established between nonsiblings reared under the same conditions (culled versus intact litters). On the first postnatal day, pups were counted. These litters were not culled. Pups were weighed on days 7, 14, and 21 for 40 litters (20 from mice raised in culled litters, 20 from mice from intact litters); days 8, 15, and 22 for 9 litters (6 from culled rearing, 3 from intact); and days 9, 16, and 23 for 1 litter from a dam raised in an intact litter. After completion of the study, mice were either euthanized with CO2 or transferred to other approved animal studies.
Corticosterone assay.
Feces collected from cages with different rearing conditions were analyzed for corticosterone. Corticosterone levels were determined by competitive enzyme immunoassay by using a commercially available kit (Cayman Chemical Company, Ann Arbor, MI). Briefly, samples were halved and weighed. To each half, 10 volumes of 90% methanol were added (for example, 570 μl was added to a 57-mg sample). One sample was left as is, and the other was spiked with 10 ng/ml corticosterone. All samples then were homogenized by shaking 3 times in a Bertin–Precellys homogenizer (Mo Bio Laboratories, Carlsbad, CA) at 6500 revolutions/min for 60 s. The homogenate was spun at 5000 × g for 10 min, and the supernatant transferred to a clean 1.5-ml tube. The supernatant was dried under nitrogen and the residue reconstituted in a volume of enzyme immunoassay buffer equal to the volume of methanol used above. Samples were diluted with enzyme immunoassay buffer to bring the analyte concentration within the linear range (that is, 20% to 80% B/B0, where B is the absorbance of the well with sample or standard, the B0 is the maximal absorbance [no sample or standard in the well].) of the standard curve. Standard curves were constructed by serial dilution between 10,000 and 16.4 pg/ml by using enzyme immunoassay buffer as the diluent. The concentrations of the samples were calculated from a log–regression line fit to a curve of the standard concentrations versus the logit (that is, ln[x/{1 – x}])-transformed B/B0 values.
Statistical design and analysis.
Sample size for the experiment was based on detecting at least a 30% difference in corticosterone levels between the 2 groups over the 3 wk of observation, with type 1 error no greater than 0.05 and minimum power 0.80. nQuery Advisor 5.0 software (Statistical Solutions, Saugus, MA) was used to estimate sample size in context of repeated-measures analysis of variance models. Baseline characteristics (dam weight, number of pups, sex) between the culled and the intact cages were evaluated by using t tests or χ2 tests as appropriate for both the first and second generations. For the first generation, mean weight and corticosterone levels for the 2 groups were compared over time with repeated-measures analysis of variance models. To account for excess availability for nursing in the culled cages, the number of pups weaned was introduced as a covariate for comparison of growth between the 2 groups. As for the first generation, the growth of the second generation of culled and intact matings was compared by using a repeated-measures analysis of variance model for mean weight over a period of 21 d.
Results
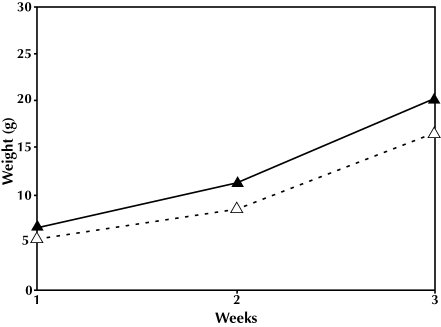
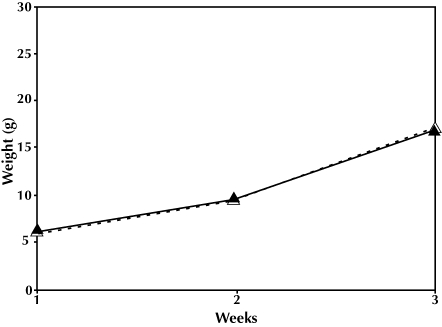
In the first generation, the dams’ body weights after delivery, size of litters, percentage of female pups, and the percentage of pups weaned were not significantly different for the 2 experimental groups (Table 1). Litter size varied from 5 to 16 pups in the intact group and originally ranged from 8 to 15 in the culled group. The pup growth rate for the culled cages was significantly greater than for the intact cages (F2,25 = 23.35, P = 0.001; Figure 1). However, after adjusting for the number of pups weaned, the pup growth rates of the 2 groups were not significantly different (F2,24 = 0.42, P = 0.57; Figure 2).
Table 1.
Summary of results: means (SDs) and percentages
| First generation |
Second generation |
|||
| Intact | Culled | Intact | Culled | |
| Number of cages | 14 | 14 | 24 | 26 |
| Weight of dama (g) | 39.9 (4.1) | 40.8 (3.4) | 41.9 (5.3) | 49.0 (10.4) |
| Number of pups born per litter | 11.4 (2.7) | 11.4 (2.2) | 11.8 (2.2) | 12.8 (2.4) |
| % female pups | 47.1 | 50.0 | not done | not done |
| % of pups weaned | 96.3 | 100.0 | 95.8 | 100.0 |
| Weight of individual pups in each litter (g) | ||||
| Week 1 | 5.46 (1.01) | 6.57 (0.53) | 5.33 (0.80) | 5.25 (0.73) |
| Week 2 | 8.59 (1.83) | 11.28 (0.97) | 8.26 (1.38) | 8.07 (0.95) |
| Week 3 | 16.52 (2.88) | 20.20 (1.63) | 14.24 (2.35) | 14.57 (1.54) |
| Corticosterone (pg/mg) | ||||
| Week 1 | 3177.7 (2281.6) | 2925.6 (1134.7) | not done | not done |
| Week 2 | 6350.3 (4001.2) | 4383.5 (2438.3) | not done | not done |
| Week 3 | 2194.9 (1701.0) | 1699.1 (1375.2) | not done | not done |
First-generation dams were weighed on the day of parturition; second-generation dams were weighed on the day of weaning.
Figure 1.
First generation, weight × time. F1,26 = 22.948, P = 0.001. Intact litter, ▵; culled litter, ▴
Figure 2.
First generation, weight × time, adjusted by number weaned. F2,25 = 2.935, P = 0.853. Intact litter, ▵; culled litter, ▴
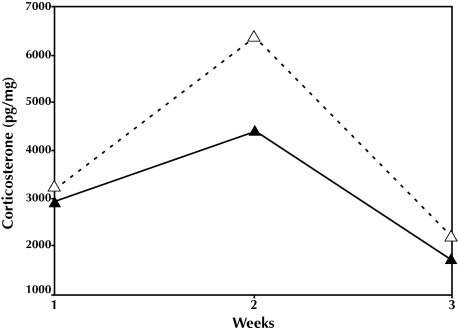
Fecal corticosterone levels (Figure 3) were slightly higher for the intact cages than for the culled cages, but differences between the 2 groups were not significant (F2,25 = 0.89, P = 0.42). Fecal corticosterone levels were highest in both groups at day 14 but had decreased to similar levels by day 21. However, these temporal fluctuations were not statistically significant.
Figure 3.
Corticosterone levels × time. F1,26 = 1.696, P = 0.797. Intact litter, ▵; culled litter, ▴
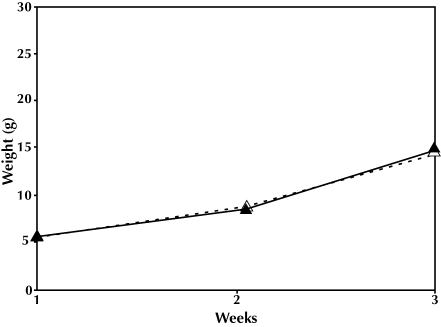
In the second generation, dams originating from culled litters weighed significantly more (P = 0.005) than did dams from intact litters. Age at delivery of the first litter was 67.3 d for dams from culled rearing and 67.6 d from those from intact rearing conditions. The second-generation litters from dams raised under intact versus culled conditions were similar in size and growth rate (F2,46 = 2.10, P = 0.13; Figure 4). Offspring from the culled cohort produced 26 litters ranging in size from 8 to 16 (mean, 13.0) pups per litter. Offspring from the intact group produced 24 litters ranging in size from 6 to 16 (mean, 11.8) pups.
Figure 4.
Second generation, weight × time. F1,47 = 1.36, P = 0.257. Intact litter, ▵; culled litter, ▴
In the first generation, all 84 pups from culled litters were weaned, as were 154 of 159 pups (96.8%) from intact litters. The 5 pups that died prior to weaning all came from 3 intact litters—1 litter had 3 deaths between postnatal days 0 and 7; the second litter had 1 death between postnatal days 8 and 14; and the last litter had 1 death between postnatal days 15 and 21. The finding of perinatal deaths in a large litter is not surprising, but the dam who had 3 pups die had delivered only 8 pups, suggesting that her fecundity was below average. If this litter is excluded from analysis, the weaning figures for intact litters rise to 149 pups weaned of 151 total (98.7%).
The percentage of pups weaned was 94.5% for the second-generation progeny of mice raised in intact litters compared with 99.8% for litters from mice raised in under culled condition. However this comparison is skewed by the fact that in one of the litters from a dam raised in an intact litter, 9 of the 16 pups died between postnatal days 0 and 7. When this litter was excluded, the weaning rate for mice from intact litters rose to 97.2%. Of the 13 pups that died among litters from dams raised in intact litters, 12 of the 13 pups died between postnatal days 0 and 7; the remaining pup died between postnatal days 8 and 14.
Discussion
Pups from culled litters grew faster than did those from intact litters. When corrected for litter size to account for competition for ability to nurse, growth rate did not differ between pups from intact versus culled litters. In addition, corticosterone levels did not differ significantly between cages containing culled versus intact litters. Subsequent reproductive performance was not significantly different between mice raised in culled or intact litters.
Serum corticosterone historically has been used for assessment of stress in mice. Obtaining serum samples for analysis of stress hormones can be problematic in itself because handling and anesthesia can elevate serum corticosterone levels in rodents. Analysis of feces for stress hormones offers a noninvasive sampling method that avoids the potential for handling-associated stress. The use of fecal corticosterone metabolite levels as a proxy for serum corticosterone levels was validated in a study34 in which fecal corticosterone levels accurately paralleled serum levels of exogenously administered hormone after a variable delay. When corticosterone was given during the dark phase, the peak of fecal corticosterone metabolites occurred 4 h after administration. This same assay could follow normal diurnal variation and changes in adrenocortical activity in response to adrenocorticotropic hormone stimulation and dexamethasone suppression.33
Fecal corticosterone levels did not differ statistically between pups from intact and culled litters. Assuming corticosterone reflects systemic stress, these results suggest that the provision of less floor space per pup is not stressful. However, concentrations of corticosterone do not fully reflect the animals’ physiology.
Reproductive performance and pup growth yielded no significant differences between intact and culled litters. Our results are similar to data reported by others14,20,23,29 who examined various physiologic parameters (testosterone, adrenal weight, growth, reproduction, immune measures, aggression, and mortality) as reflections of animal welfare of mice provided significantly less floor space per mouse than Guide recommendations. These results suggest that a strict interpretation of the current Guide’s cage-space guidelines, in which each pup must be provided a certain number of square inches based on body weight, is unwarranted.
Other studies using corticosterone levels and lymphocyte function (or counts) as measures of stress in ‘crowded’ conditions reported results similar to ours. Among mice housed in cages with 3 different floor spaces (48.7, 97.5, or 195 cm2 [7.54, 15.11, or 30.23 in.2]), mice in the smallest cages had higher corticosterone levels on days 1 and 7 of the study than did the other 2 groups.25 However, peripheral lymphocyte counts were lower in the mice housed in the smallest and largest cages on day 1, and the effect continued only for mice housed in the smallest cages on day 7. Blood collection may have influenced the results. A study of mice housed at 43.3 cm2 (6.71 in2) and 86.6 cm2 (13.42 in2) per mouse with controls housed per the Guide found no significant physiologic effects (body weight, organ weights, plasma corticosterone, total and differential leukocyte count, gastric histology, and so forth) in mice housed in various densities, with the exception of heart weight, which was greater in mice housed at higher density.23 However, the white blood cell count and corticosterone values may have been confounded by the stress of CO2 narcotization followed by cardiac puncture. Acute stress of handling and CO2 narcotization may have overshadowed a modest difference between groups for these parameters. Similarly, growth rates and adrenal weights did not differ significantly among male and female mice (BALB/c and MF1) housed at 27 cm2 (4.19 in.2) versus 60 cm2 (9.3 in2),26 which is the space provision recommended by the UK Home Office35 in 1995.
A study29 that measured levels of testosterone secretion in C57BL/6J mice found no significant differences among male mice housed for 8 wk at various cage densities (5.6 to 12.9 in.2 [36.1 to 83.2 cm2] per mouse) and in 3 different cage types. In a follow-up study in mice housed at different densities (down to 3.2 in.2 [20.64 cm2] per mouse) and assessed for 4 wk, testosterone was higher in male than female mice, but density was not related to differences.29 In another study,16 C57BL/10J and A/J mice housed at different densities first were subjected to heat stress and then were placed into new groups of various population densities and observed for aggressive behavior (biting), but a significant difference in number of bites was not observed. The most crowded condition in the study16 provided each mouse 18 in.2 (116.18 cm2) of floor space, which is more generous than current Guide recommendations.
The authors of the studies cited in the previous paragraph16,29 seem to imply that significantly reduced area per mouse does not introduce a welfare issue. However, a limited review of the literature, including the previously cited studies and ours, suggest that these results should not be considered concrete evidence against the area requirements stated in the Guide because of the complexity of the question and difficulty in global application of a ‘standard.’ Assumptions that physiologic factors such as growth, body weight, hormone concentration, and immune status accurately describe an animal's stress status may incompletely characterize stress. Mench21 suggests that the Guide's goal of “maximizing species-specific behaviors and minimizing stress-induced behaviors,24 while laudable, may be difficult to achieve.” The laboratory animal community usually measures physiologic parameters associated with stress when testing a cage design, enrichment, and so forth, but these parameters have limitations and have proven difficult to interpret.21
As others have noted,14 many early studies on crowding were done not to establish a minimum area for an animal but rather to study the physiologic basis of stress. However, review of these studies, some of which followed animals for months, document interesting observations (for example, decreased aggression, no difference in corticosterone, adrenal weights) that were interpreted differently across studies.14,20,23,30,36 Recognizing that different explanations may be proposed for similar observations is important, because the basis for a change in behavior may be more complex than a simple conclusion that less floor space elicits no significant change in the animal.21 Behavioral changes in response to changes in the environment may reflect subtle signs of stress (or distress) not easily detected. Failure to consider the effect of such changes could unknowingly confound data collected from the animal model.
Several authors have tried to determine threshold of space limitation that triggers aggression. Deer mice (Peromyscus maniculatus) apparently were more aggressive in ‘crowded’ conditions compared with uncrowded mice.17 However, the floor space allotted the ‘crowded’ mice met Guide recommendations (12 in.2; 30.48 cm2) compared with that for the uncrowded mice (38 in.2; 96.52 cm2). In 2 separate studies,29,30 mice in various cage-density settings were followed for 8 wk, after which the authors concluded that BALB/c male, NOD/LtJ male, and C57BL/6 male and female mice, but not FVB/NJ male mice, could be housed at significantly less floor space per mouse than specified in the Guide. Initial individual or group weights of mice at the beginning of the experiment were not reported, but significant differences on rate of growth between groups were noted.29,30 In another study,36 cohorts of 3, 5, or 8 male BALB/c mice were housed at 2 cage densities; aggressive behavior increased with age and was higher in mice provided larger cages for the same group size.
Results of studies examining territorial size and aggression appear conflicting. When male mice are housed in groups, some aggression occurs, but the level and intensity depend on strain and age plus environmental factors. Reduced aggression in populations of increased density29,30 is generally viewed as a beneficial effect, but review of the literature does not reveal a simple relationship between aggression and population density. A study assessing the interaction of cage and group size (males) on aggression identified 3 types of interaction: age, group size, and frequency of aggression.36 Behavioral scores showed significant age × aggression effects—aggression increased with age, whereas corticosterone levels rose and fell. The authors suggested that the initial grouping was stressful (rise in corticosterone), but as a hierarchy was established, corticosterone levels decreased, reaching a nadir at 11 to 12 wk of age. However, corticosterone began to rise again, perhaps secondary to the increasing aggression seen with age. Similarly, a significant cage size × group size interaction was observed with agonistic behavior. Effects of cage size on aggression were small but significant; in larger cages, duration and intensity of aggression was greater. Authors of other studies14,30 made similar observations, concluding that smaller cages had a positive effect on the animals, although mouse strain did make a difference. However, the authors of the earlier cited work36 suggest that a ‘crowding effect’ revealed a curvilinear relationship between aggression and cage size. This relationship was interpreted to indicate that larger groups had more aggression, but high density provides insufficient space to defend as territory, and aggression subsists. A third interaction of cage size × group size revealed significant differences in frequency of aggression between groups of 8 versus 5 mice and significant differences in duration of aggression in groups of 8 versus 3 mice.36 Behavioral analysis revealed agonistic encounters between nondominant animals occurred more often in groups of 8 than 3. As group size increased, the average social activity decreased.
Another investigator5,6 made similar observations when studying a mouse population in unconventional laboratory housing. Square pens (101 in. [256.4 cm]) were constructed with considerable cage complexity (tunnels and nesting boxes) and housed a variable number of mice, which peaked at 2200 animals. As population density increased, alterations in maternal behavior manifested, with female mice biting males and neglecting their pups. Male mice did not display increased aggressive activity but had difficulty finding female partners to mate. The same author observed that as population size increased, average social activity decreased; when overcrowding became severe, all aggression ceased, and younger mice were rejected from the group. Surprisingly, female mice assumed the role of aggressor and attacked males with the behavior ultimately generalizing to their own young. The pups, if not fatally injured, were forced from the nest prematurely and failed to develop affective bonds. The population eventually died of old age with no signs of recovery because the disaffected pups, now adults, never tried to mate. Another study of 7 strains of mice 7 made similar observations: female mice became aggressive as the population increased, and breeding eventually stopped; in 2 of the strains, female mice also developed ovarian lesions.
Studies focusing on reproduction and territory size in wild mice revealed that mice self-regulate density through social interactions, termed spacing behavior, that include territoriality, presaturation dispersal, breeding inhibition, and various forms of social mortality.32 A laboratory cage environment does not allow density regulation as occurs in the wild, but mouse behavior in the wild can provide perspective about potential effects of increasing cage density in the laboratory. In the wild-mouse study,32 as the population increased, the median size of mice decreased (despite aging and food availability). Adult females were more likely than males or juveniles to exhibit behavioral changes as population density increased. As population increased, females initially became pregnant at an earlier age, but eventually they became more aggressive and breeding decreased, finally ceasing. Another study6 showed that in noncrowded situations, conception rarely occurred before 80 d of age in laboratory mice; the author suggested that frequency of earlier conceptions can be construed as indicating disruption of normal behavior. Other experiments8 prompted similar conclusions. A study of cycles of abundance and limitations in mouse populations indicated that with increasing population, the mean number of embryos per pregnancy was reduced significantly.8 One relevant factor was increased adrenocorticotropic hormone secretion in female mice in response to increasing density. Adrenocorticotropic hormone had little effect on male reproduction but suppressed reproduction in female mice. With increasing density, female mice delayed implantation of embryos, which increased the length of time between litters and increased the length of estrous cycles. Increasing density was inversely correlated with duration or quantity of lactation, resulting in permanent stunting of pups. The population was limited by inhibition of maturation of pups rather than by inhibition of reproduction in mature mice.
Crowding is a technique used extensively in the fields of neurophysiology, neuropathology, and neuropharmacology—as models for psychosocial mental stress. One study measured body weight gain, food and water intake, muscle strength on an inclined plane, and urinary corticosterone:creatinine ratios in rats.31 Differences in corticosterone:creatinine ratio between groups were insignificant, but the large pen environment seemed to stimulate physical activity, decrease weights, and increase muscle strength, which the authors concluded reflected improved health and therefore judged to be beneficial.31 An economic demand study demonstrated that mice value space.2 Small cages not only limit their ability to explore and exercise, but do not provide sufficient space for some hygiene behaviors. Mice do not defecate randomly if provided sufficient space. Experiments using ‘crowding’ as the stressor to examine skin barrier function found that mice housed at high density for 2 wk were significantly slower to heal than mice in lower population densities.13 This observation was confirmed in a study1 that found a negative correlation between population density and skin barrier function. This same study1 also found significant decreases in body weight in mice housed in large groups. Other reported effects of crowding include suppressed hematopoiesis. Mice housed in dense populations that then are irradiated for bone marrow transplants had significantly lower cell counts than did mice housed alone or in small groups.4 Similarly, a study that used overcrowding to simulate psychosocial stress followed intracytoplasmic calcium concentrations for 3 mo in young and adult CBA/CA mice and found a significant effect in older animals. 11 The authors concluded that overcrowding negatively affected the calcium metabolism of T lymphocytes.
Differences in housing conditions may contribute to difficulties in reproducing results across laboratories.9 A study of various population densities of mice for 18 mo revealed an indirect correlation between increasing density and food consumption and a statistically significant increased incidence of gastritis as housing density increased. 9 Another study also reported increased incidence of gastritis with increasing population of mice.6 D-amphetamine toxicity and adrenal weight can be altered significantly by changing group size in a standard cage.38 In addition, higher levels of catecholamines were present in the adrenal medulla of mice housed long-term under crowded conditions.38 Therefore pharmacologic, physiologic, and behavioral effects vary with the level of stimulation provided in the environment in which the animal lives.
Assuming that one component of animal welfare is representative of total animal wellbeing can be misleading. Typical measures used to assess animal welfare either were not valid or their validity has not been determined.22,27 Fewer studies have examined the relationship of litter size and floor area. Some noted no effect on preweaning growth or survival rate in litters averaging 17 pups.28 Another study individually housed pregnant dams, reducing litters to a standard size of 8 pups with equal numbers of male and females (4 each).18 Weanlings were housed in 3 different sized cages with 1, 2, or 4 mice per cage. No differences between the different caging regimes were found until mice were 4 to 5 wk old. Those maintained at 4 per cage began to fall behind in growth and body weight, which became significant at 9 wk of age. The authors concluded that 4 mice per cage were too many for normal growth and that the number of animals in a cage affects growth rate and ultimately body size and weight. Another study compared average weights at weaning from 20 strains of mice (1 inbred, 17 knockout, 2 transgenic) housed in standard shoebox cages as breeding pairs or trios.37 Differences in pup weight at weaning for the 2 housing conditions were not significant. In another study, timed-pregnant ICR mice were housed individually in 3 different cage sizes (small, standard [Guide], and large), and pup weights at weaning and behavioral measures of anxiety were examined.12 Again, significant differences between the 3 housing situations were not noted, consistent with our findings.
Table 2.1 of the Guide24 provides a useful baseline for cage density decisions. Similarly, Appendix A of the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes10 provides guidance on floor space for mice housed in European research institutions. This document recommends allowing 2 (either inbred or outbred) or 3 (inbred only) adult mice, and potentially 2 litters, in cages providing 330 cm2 (51.15 in.2). Adult mice are allowed 60 to 100 cm2 (9.3 to 15.5 in.2), depending on their weight. Unlike performance standards, the prescriptive approach does not allow the use of measurable criteria to accomplish a specific goal. In our study, the goal was to wean healthy pups that meet generally accepted physiologic milestones in body growth and weight from intact litters, with no adverse effect on subsequent reproductive performance. Studies examining the interaction of space × litter size provide scientific information regarding lactating dams with intact litters. The Guide24 clearly states that space allocation may be modified as necessary to meet “animal needs (for example, for prenatal and postnatal care …),” as long as performance indices (health, reproduction, growth, behavior, activity, and use of space) have been assessed.
In summary, determination of minimum space requirements for weanling and adult mice is a complex subject that does not easily lend itself to generalizations necessary for guidelines. A review of the literature reveals the complex nature of the question. Interactions between group size and space have many dimensions, ranging from positive health effects (increased exercise, leaner animals) to negative health effects (gastric ulcers, decreased immune response in older animals, and subtle interactions of chronic stimulation on the central nervous system). Increased density appears to affect physiologic functions over time and thus may provide justification for limiting housing density in experimental animals. The identification of general measures of welfare that reflect total welfare may be impossible. The Guide24 clearly advocates the use of professional judgment and experience by the institutional animal care and use committee in specific situations. Deviations from the Guide24 are allowed as long as the scientist, veterinarian, and institutional animal care and use committee establish clear performance indices with documentation of performance outcomes. Nevertheless, the Report of the Rodent Refinement Working Party19 identified determination of an optimal practical cage size as a needed research topic. In the interim, the Working Party Report suggests that cage size be considered minimal and additional space provided when possible—and that the increased space should include all 3 dimensions, floor area and volume, so the animals can perform a wide range of normal behaviors and activities.
In the absence of spontaneous or genetically induced aberrations in social behavior, the literature indicates that potential for aggression among a nursing dam and her litter is negligible until population size is markedly increased.5-8,12,33,38 Because significant elevation of corticosterone levels was not demonstrated in the intact litters in which the dam accounted for 15 of the available 65 in.2 (491.25 cm2), we conclude that the remaining 50 in.2 (322.5 cm2) was adequate for the needs of the litters, which had a mean of 11 pups and mean body weight of 16.52 g, each, at weaning. Additional studies would be required to determine the point at which limited floor space becomes a stressor for a nursing mouse and her litter. Current information supports viewing a lactating dam with litter as a single biologic unit. The dam can rear her pups successfully in a standard commercial cage (65 in.2; 419.25 cm2), and Table 2.1 of the Guide24 should be amended appropriately, similar to the Report of the Rodent Refinement Working Party19 recommendations, which views the dam and her litter as a single biologic unit.
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute of Neurological Disorders and Stroke.
References
- 1.Aioi A, Okuda M, Matsui M, Tonogaito H, Hamada K. 2001. Effect of high population density environment on skin barrier function in mice. J Dermatol Sci 25:189–197 [DOI] [PubMed] [Google Scholar]
- 2.Balcombe JP. 2006. Laboratory environments and rodents’ behavioural needs: a review. Lab Anim 40:217–235 [DOI] [PubMed] [Google Scholar]
- 3.Bennett JP, Vickery BH. 1970. Rats and mice. In: Hafez ESE.Reproduction and breeding techniques for laboratory animals. Philadelphia: Lea and Febiger; p 325 [Google Scholar]
- 4.Boranic M, Poliak-Blazi M. 1983. Effect of the overcrowding stress on hemopoietic colony formation in mice. Exp Hematol 11:873–877 [PubMed] [Google Scholar]
- 5.Calhoun JB. 1973. Death squared: the explosive growth and demise of a mouse population. Proc R Soc Med 66:80–88 [PMC free article] [PubMed] [Google Scholar]
- 6.Calhoun JB. 1973. From mice to men. Trans Stud Coll Physicians Phila 41:92–118 [PubMed] [Google Scholar]
- 7.Chapman JC, Christian JJ, Pawlikowski MA, Yasukawa N, Michael SD. 2000. Female house mice develop a unique ovarian lesion in colonies that are at maximum population density. Proc Soc Exp Biol Med 225:80–90 [DOI] [PubMed] [Google Scholar]
- 8.Christian JJ. 1971. Population density and reproductive efficiency. Biol Reprod 4:248–294 [DOI] [PubMed] [Google Scholar]
- 9.Chvedoff M, Clarke MR, Irisarri E, Faccini JM, Monro AM. 1980. Effect of housing conditions on food intake, body weight and spontaneous lesions in mice. A review of the literature and results of an 18-month study. Food Cosmet Toxicol 18:517–522 [DOI] [PubMed] [Google Scholar]
- 10.Council of Europe 1998. European convention for the protection of vertebrate animals used for experimental and other scientific purposes, appendix A. Strasbourg: Council of Europe [Google Scholar]
- 11.Csermely P, Penzes I, Toth S. 1995. Chronic overcrowding decreases cytoplasmic free calcium levels in T lymphocytes of aged CBA/Ca mice. Experientia 51:976–979 [DOI] [PubMed] [Google Scholar]
- 12.Davidson LP, Chedester AL, Cole MN. 2007. The effects of cage density on behavior in young adult mice. Comp Med 57:355–359 [PubMed] [Google Scholar]
- 13.Denda M, Tsuchiya T, Hosoi J, Koyama J. 1998. Immobilization-induced and crowded environment-induced stress delay barrier recovery in murine skin. Br J Dermatol 138:780–785 [DOI] [PubMed] [Google Scholar]
- 14.Fullwood S, Hicks TA, Brown JC, Norman RL, McGlone JJ. 1998. Floor space needs for laboratory mice: C57BL/6 males in solid-bottom cages with bedding. ILAR J 39:29–36 [DOI] [PubMed] [Google Scholar]
- 15.Gandelman R, Paschke RE, Zarrow MX, Denenberg VH. 1970. Care of young under communal conditions in the mouse (Mus musculus). Dev Psychobiol 3:245–250 [DOI] [PubMed] [Google Scholar]
- 16.Greenberg G. 1972. The effects of ambient temperature and population density on aggression in two inbred strains of mice, Mus musculus. Behaviour 42:119–130 [DOI] [PubMed] [Google Scholar]
- 17.Gregor GL, Smith RF, Simons LS, Parker HB. 1972. Behavioral consequences of crowding in the deermouse (Peromyscus maniculatus). J Comp Physiol Psychol 79:488–493 [DOI] [PubMed] [Google Scholar]
- 18.Hughes PCR, Nowak M. 1973. The effect of the number of animals per cage on the growth of the rat. Lab Anim 7:293–296 [DOI] [PubMed] [Google Scholar]
- 19.Jennings M, Batchelor GR, Brain PF, Dick A, Elliott H, Francis RJ, Hubrecht RC, Hurst JL, Morton DB, Peters AG, Raymond R, Sales GD, Sherwin CM, West C. 1998. Refining rodent husbandry: the mouse. Report of the Rodent Refinement Working Party. Lab Anim 32:233–259 [DOI] [PubMed] [Google Scholar]
- 20.Mcglone JJ, Anderson DL, Norman RL. 2001. Floor space needs for laboratory mice: BALB/cJ males and females in solid-bottom cages with bedding. Contemp Top Lab Anim Sci 40:21–25 [PubMed] [Google Scholar]
- 21.Mench J. 1998. Why it is important to understand animal behavior. ILAR J 39:20–26 [DOI] [PubMed] [Google Scholar]
- 22.Mendl M. 1991. Some problems with the concept of a cut off point for determining when an animal's welfare is at risk. Appl Anim Behav Sci 31:139–146 [Google Scholar]
- 23.Naidu S, Winget CM, Jenner JW, Mele G, Holley DC. 1995. Effects of housing density on mouse physiology and behavior in the NASA Animal Enclosure Module simulators. J Gravit Physiol 2:140. [PubMed] [Google Scholar]
- 24.National Research Council 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academy Press [Google Scholar]
- 25.Peng X, Lang CM, Drozdowicz CK, Ohlsson-Wilhelm BM. 1989. Effect of cage population density on plasma corticosterone and peripheral lymphocytic populations of laboratory mice. Lab Anim 23:302–306 [DOI] [PubMed] [Google Scholar]
- 26.Peters A, Festing M. 1990. Population density and growth rate in laboratory mice. Lab Anim 24:273–279 [DOI] [PubMed] [Google Scholar]
- 27.Rushen J. 1991. Problems associated with the interpretation of physiological data in the assessment of animal welfare. Appl Anim Behav Sci 28:381–386 [Google Scholar]
- 28.Schüler L. 1990. Postnatal loss and the possible effect of overcrowding in a high fertility mouse population. Z Versuchstierkd 33:146–148 [PubMed] [Google Scholar]
- 29.Smith AL, Mabus SL, Stockwell JD, Muir C. 2004. Effects of housing density and cage floor space on C57BL/6J mice. Comp Med 54:656–663 [PubMed] [Google Scholar]
- 30.Smith AL, Mabus SL, Muir C, Woo Y. 2005. Effects of housing density and cage floor space on three strains of young adult inbred mice. Comp Med 55:368–376 [PubMed] [Google Scholar]
- 31.Spangenberg EMF, Augustsson H, Dahlborn K, Essen-Gustavsson B, Cvek K. 2005. Housing-related activity in rats: effects on body weight, urinary corticosterone levels, muscle properties and performance. Lab Anim 39:45–57 [DOI] [PubMed] [Google Scholar]
- 32.Sutherland DR, Singleton GR. 2006. Self-regulation within outbreak populations of feral house mice: a test of alternative models. J Anim Ecol 75:584–594 [DOI] [PubMed] [Google Scholar]
- 33.Touma C, Palme R, Sachser N. 2004. Analyzing corticosterone metabolites in fecal samples of mice: a noninvasive technique to monitor stress hormones. Horm Behav 45:10–22 [DOI] [PubMed] [Google Scholar]
- 34.Touma C, Sachser N, Mostl E, Palme R. 2003. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen Comp Endocrinol 130:267–278 [DOI] [PubMed] [Google Scholar]
- 35.United Kingdom Home Office 1995. Code of practice for the housing of animals in designated breeding and supplying establishments. London: HMSO [Google Scholar]
- 36.Van Loo PLP, Mol JA, Koolhaas JM, Van Zutphen BFM, Baumans V. 2001. Modulation of aggression in male mice: influence of group size and cage size. Physiol Behav 72:675–683 [DOI] [PubMed] [Google Scholar]
- 37.Weed J. 2005. Personal communication.
- 38.Welch BL, Welch AS. 1966. Graded effect of social stimulation upon d-Amphetamine toxicity, aggressiveness and heart and adrenal weight. J Pharmacol Exp Ther 151:331–338 [PubMed] [Google Scholar]