Abstract
Studies on humans and rodents indicate possible long-term cognitive impairment after surgery or general anesthesia. The goal of this study was to evaluate the effect of various anesthetic concentrations on spatial learning in adult mice. The behavior of adult mice in a T-maze was assessed 28 h after anesthesia (control [0%], low [1%], or high [2%] isoflurane concentration). The mice anesthetized with 1% isoflurane had a significantly poorer performance than did the other 2 groups. The performance of the mice anesthetized with 2% isoflurane was not statistically different from that of the control group. Therefore, low, not high, isoflurane concentration impaired spatial learning in mice.
Concern regarding animal welfare and the quality of data obtained from research projects using animals is increasing.10 Therefore, optimizing anesthesia protocols and understanding the extent to which these can affect the behavior and cognitive function of animals is important. Anesthesia is used in diverse procedures, including neurobiology research (for example, implantation of electrodes, neurotoxin administration, induction of traumatic brain injury and other experimental lesions) in which animals subsequently are studied in behavioral tests and where their performance may be influenced by persistent effects of anesthetic drugs. Volatile anesthesia has been used widely to anesthetize animals because it is safe and associated with rapid recovery. Anesthesia often involves halogenated drugs, which act on the central nervous system and produce reversible immobility and amnesia.11 However, studies in humans and rodents indicate that these effects may persist, leading to impairment of cognitive functions.3 Several studies on rodents reported differing results with isoflurane. Performance of a previously learned spatial task was impaired after anesthesia in aged rats but improved in young rats,7 whereas acquisition of a new spatial task was impaired in both age groups over several weeks.8,9 In contrast learning improved in adult rats 2 wk after anesthesia,6 and repetitive exposure to enflurane after training enhanced spatial memory in mice.15 Mice submitted to repeated or single anesthetic procedures showed no immediate or prolonged spatial memory impairment when tested in the Barnes maze.4 Whereas the cited studies compared anesthetized and nonanesthetized animals, the aim of the present study was to evaluate the effect of anesthesia at various concentrations on spatial learning performance of adult mice.
Materials and Methods
All procedures were performed under personal and project licenses approved by the national regulatory office of Portugal (Direcção Geral de Veterinária).
Animals.
We randomly allocated 44 10-wk-old, locally bred, male C57BL/6J mice among 11 cages (Makrolon type II cage, Tecniplast, Dias de Sousa, Alcochete, Portugal) such that each contained 3 to 5 animals. Each cage was provided with standard corncob litter (Probiológica, Lisbon, Portugal), a piece of tissue paper, and a cardboard tube. Water was provided ad libitum. Before restriction of the food supply, the mice were fed with rodent pellets (4RF25-GLP, Mucedola SRL, Settimo Milanese, Italy) ad libitum. Food restriction began 1 wk before T-maze habituation. A limited amount of food was provided by distributing pellets, previously split into small pieces, over the cage floor once daily. Initially, each mouse received 2 g daily; this amount was adjusted such that mice were maintained at 80% to 95% of free-feeding weight. The animals were weighed daily throughout the experiment to verify they maintained this weight. The animals were kept in a room with controlled temperature (21 °C) and humidity (55%). Lights were kept on a 12:12-h cycle, with lights off at 5 p.m.
Anesthesia.
Mice were randomly assigned into 3 treatment groups: the control group (no anesthesia, n = 8 in batch A and n = 6 in batch B), group I (1% isoflurane, n = 9 in batch A and n = 6 in batch B), and group II (2% isoflurane, n = 9 in batch A and n = 6 in batch B). Mice were anesthetized 28 h before the T-maze test. The animals were placed individually in an induction chamber, and anesthesia was induced with 5% isoflurane (Isoflo, Esteve Farma, Carnaxide, Portugal) in 100% oxygen with a delivery rate of 5 l/min until loss of righting reflex. In batch A, after induction, the animals were moved to a homeothermic blanket (N-HB101-S-402, Panlab, Barcelona, Spain) and placed in dorsal recumbence. Anesthesia then was maintained with isoflurane in 100% oxygen with a flow of 1.5 l/min administered by means of a facemask connected to a coaxial circuit (Fluovac anesthetic mask, Harvard Apparatus, Reagente 5, Porto, Portugal). The procedures for batch A replicated typical clinical practice when laboratory mice are anesthetized for surgical procedures. For precise control of the isoflurane concentration, the animals in batch B were maintained inside the chamber throughout anesthesia; a pulse oximeter was placed on the upper, right hindleg of each mouse. Pulse and respiratory rate were recorded over intervals of 10 min. In both batches, body temperature was maintained at 37 °C by a homeothermic blanket connected to a rectal thermal probe (N-HB101-S-402, Panlab). Animals remained anesthetized for 1 h. Isoflurane concentration was monitored with an agent gas monitor (Datex Capnomac Ultima, Helsinki, Finland), and no stimuli were applied during that hour (for example, background noise was reduced to a minimum). In batch A, a gas sample was taken from within the mask, 5 mm to the back from the transition gas in - gas scavenging in the coaxial anesthesia circuit. The isoflurane concentration from the vaporizer was 1% and 2%. With this technique, the anesthetic may mix with environmental air at the gas scavenging, diluting its concentration. Therefore the concentration inhaled by the mice may have varied between the higher value emitted from the vaporizer and the lower value measured by the agent gas monitor, that is, 0.8% to 1% and 1.8% to 2%. In batch B, isoflurane concentration was controlled in the exhausted air; 1% and 2% of isoflurane were used. At recovery, all animals received 100% oxygen until recovery of righting reflex. No mice were restrained during anesthesia. Animals from the control group were not anesthetized; however, they were manipulated and placed inside the induction chamber for 1 min (average time until loss of the righting reflex in anesthetized animals). To avoid isolation stress in the nonanesthetized control animals, they were returned to their home cage.
T-maze.
Apparatus.
The T-maze was made of gray acrylic sheets and consisted of a starting arm and 2 choice arms, each measuring 10 × 60 × 20 cm (width × length × height). A ceramic food pot, in which a reward could be placed, was located in the far end of each choice arm. The mouse could also take further cues from surrounding features of the room, because the T-maze was not moved during the study. The reward was a 2-mm piece of cereal (Cheerios, Nestlé Portugal SA, Linda-a-Velha, Portugal).
Habituation.
The day before anesthesia, all animals in each cage were placed individually in the starting arm of the T-maze and allowed to explore it for 15 min with no food reward present; the mice then were returned to their home cages. Five hours later, the mice were placed individually in the starting arm of the T-maze and allowed to explore it in 15 trials of 2 min each; this time the ceramic pots at the end of each choice arm were baited with pieces of cereal. The first choice arm entered by each animal in each trial was recorded for analysis of the presence of lateralization bias. Initial bias for a particular side was corrected by baiting the opposite arm in the test. Bias was considered to be present if a mouse chose a particular side 10 times or more in 15 trials. For mice that did not present a bias, the arm to contain the reward during the test (the ‘correct arm’) was selected randomly.
Test.
T-maze sessions began 28 h after the anesthetic procedure. All tests were performed by the same researcher, who was blind to the anesthetic procedures that the animals had undergone. During the test, only 1 of the choice arms was baited, and this arm was considered as the correct arm. Each animal was placed in the starting arm, with a sliding door blocking access to the choice arms. The trials started when the experimenter lifted the door. The first 3 trials were learning trials wherein the mouse was allowed to go to both pots and eat the reward from the correct arm. After these learning trials the next tests trials ended when 1 of the following situations occurred: the mouse entered the unbaited arm (wrong choice), 2 min passed without the mouse making any choice (no choice; never observed), or the mouse chose the correct arm and ate the reward (correct choice). Entrance was recorded as when the animal first had all 4 paws in the selected arm. When a trial ended, the mouse was pushed gently back to the starting position. The test ended when the learning criterion was achieved, that is, when the mouse entered the correct arm 9 times out of 10 consecutive tests trials (excluding the 3 learning trials); therefore every mouse had to perform a minimum of 10 trials. The number of correct and incorrect trials was recorded.
Statistical analysis.
The number of trials needed to complete the task was analyzed to evaluate the cognitive performance of the mice. Hemodynamic data and demographic information (such as the animals’ weights) also were analyzed. Data were expressed as mean ± SD, with inclusion of median, quartiles and 95% limits of confidence when relevant. Pulse and respiratory rate were analyzed by using the dependent t test. Other data were analyzed by using univariate analysis of variance with Bonferroni posthoc correction and 2 factors: treatment (groups: control, low, and high isoflurane concentration) and isoflurane delivery methodology (batches A and B). Extreme cases were defined has a value positioned more than 3 interquartile ranges outside the box range of the group. All results were analyzed by using SPSS 13.0 for Windows (Apache Software Foundation, Forest Hill, MD).
Results
One animal from batch B (chamber anesthesia) that received the low isoflurane concentration was identified as an extreme value and excluded from analysis. The results from the remaining 43 animals were analyzed.
Before food restriction, the mice weighed 24.54 ± 3.19 g (mean ± SD) in the control group (no anesthesia), 24.58 ± 1.79 g in group I (1% isoflurane), and 23.97 ± 1.65 g in group II (2% isoflurane). One week after the start of food restriction, mean body weight had decreased to 85% ± 3.62% of the initial weight in group I, 86% ± 3.82% in group II and 86% ± 3.19% in the control group. Weight loss did not differ significantly between groups or batches.
Group I from batch B (chamber anesthesia) showed a significantly (P ≤ 0.01) higher pulse rate 20 min after the start of anesthesia than did group II. The respiratory rate was significantly (P ≤ 0.01) lower in group II than in group I (data not shown).
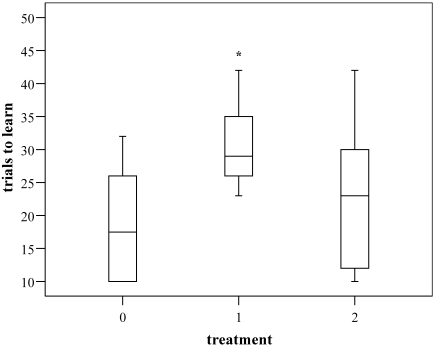
No significant differences were detected between batches, and no statistical interactions between groups and batches were detected (Figure 1).
Figure 1.
Number of trials necessary to complete the T-maze task in the various treatment groups (0, control group [not anesthetized]; I, anesthetized with 0.8% to 1% of isoflurane; II, anesthetized with 1.8% to 2% of isoflurane) 28 h after administration of anesthesia. *, P ≤ 0.05 (analysis of variance) between values for control group and group I (P = 0.002) and between values for groups I and II (P = 0.041). Data are presented as a box plot (the median is indicated by the horizontal bar inside the box; the 25th and 75th percentile are the boxes' borders; and the whiskers are the lowest and highest values for the 5th and 95th percentiles, respectively).
The animals from group I (1% isoflurane) showed impaired T-maze performance. These animals required significantly more trials to complete the task (30.57 ± 5.81) than did mice in the control group (18.79 ± 8.02; P = 0.002) and group II (2% isoflurane; 22.40 ± 10.85 trials; P = 0.041). Number of trials did not differ between the control group and group II.
No clinical responses indicative of stress (movement, sudden increase in heart rate or respiratory rate) occurred during anesthesia.
Discussion
In this study, light but not deep isoflurane anesthesia caused impairment in spatial learning in adult mice when compared with animals that had not been anesthetized. Previous studies showed age-related effects, with anesthesia improving cognition in young and adult6,7 rats and mice15 but causing impairment in aged animals.6,7,9 Our study introduced a different variable: the depth of anesthesia. Considering anesthesia as a reversible intoxication process that affects the central nervous system and several brain regions, we expected higher isoflurane concentration to have a greater effect on learning; however, this effect was not confirmed. Instead, our results showed the opposite scenario. Similarly, recent data from studies on humans, showed that patients maintained under higher anesthetic concentration demonstrated improved cognitive function and less postoperative cognitive dysfunction than did patients anesthetized at lower levels.12 Although the cognitive capacity affected in the cited study, processing speed, was different than the spatial orientation tested in our experiment, it is an indicator of how different depths of anesthesia may influence brain processes.
The learning impairment in the mice with 1% isoflurane anesthesia may be explained by the influence of general anesthesia on the neurotransmitter system.5,6,13,14 However, this influence does not explain the lack of effect of the higher isoflurane concentration anesthesia on learning. Results from group II (2% isoflurane) may suggest a neuroprotective effect of a high concentration of isoflurane.2,17 Another explanation may be based on the electroencephalographic similarities between states of sleep and general anesthesia.16 Deep anesthesia may better mimic natural slow-wave sleep and therefore have a less disruptive effect on cognition than does light anesthesia.
The possibility of some cognition processing and stress during light anesthesia procedures should not be excluded during standard clinical setups, especially when surgery is performed. However in our experimental study without surgery, we took care to reduce stress (the only manipulation in both treatments was placement of the rectal probe and pulse oxymeter immediately after induction of anesthesia), and all the stimuli, including background noise, was reduced to a minimum.
To create a contrast between the different treatments, we opted for comparing a low level of anesthesia (sufficient for mice to lose the righting reflex and to maintain them unconscious if stimuli were not applied) to a higher level sufficient for mouse clinical anesthesia. We previously showed the importance that the selection of the anesthetic agent may have in research projects in which electroencephalogram records are made during inhalation anesthesia.1 In such studies, halothane would be preferable to isoflurane because isoflurane is associated with stronger brain depression.1 The objectives of these studies are to be applicable to routine anesthesia of mice and rats used in research, and the techniques were chosen accordingly. For procedures to be carried out on anesthetized animals, anesthesia cannot be maintained in the chamber; therefore anesthesia was induced in a chamber, and animals then were transferred to the facemask. This practice is the approach routinely used for clinical anesthesia of mice. Readjustment of the inhaled isoflurane being delivered inside a chamber would be another approach but would only be possible for experimental purposes because it does not allow surgery. This approach was used for the second batch of animals, and it produced similar results to those obtained with the face mask.
As this is a preliminary study, our main goal was not to analyze the existence of a concentration–response curve but to determine whether different depths of anesthesia had any effect on cognition. For that reason and to minimize the number of animals used, we only studied 2 concentrations: surgical anesthesia and a concentration sufficient for loss of consciousness. Now that a concentration-dependent effect on learning has been established, further studies should be performed with different anesthetic concentrations and different cognitive tasks. Furthermore, to ensure that the depth of anesthesia remains similar between subjects, careful monitoring of the reflexive state and electroencephalographic titration of anesthetic could be used in larger species.
In conclusion, our results indicate impairment of spatial learning in adult mice after light anesthesia, whereas deep anesthesia had no effect on performance. The effect of 2 different anesthetic doses on spatial learning performance in adult mice may have particular importance for research projects involving anesthesia.
Acknowledgments
We thank Hanno Würbel (Division of Animal Welfare and Ethology, Justus Liebig University, Giessen, Germany) for sharing his experience of food restriction of group-housed mice. The research was funded in part by research grant POCTI/CVT/59056/2004 from the Fundação para a Ciência e Tecnologia (FCT) and by the Fundo Europeu de Desenvolvimento Regional (FEDER).
References
- 1.Antunes LM, Golledge HD, Roughan JV, Flecknell PA. 2003. Comparison of electroencephalogram activity and auditory evoked responses during isoflurane and halothane anaesthesia in the rat. Vet Anaesth Analg 30:15–23 [DOI] [PubMed] [Google Scholar]
- 2.Bekker A, Shah R, Quartermain D, Li YS, Blanck T. 2006. Isoflurane preserves spatial working memory in adult mice after moderate hypoxia. Anesth Analg 102:1134–1138 [DOI] [PubMed] [Google Scholar]
- 3.Bekker AY, Weeks EJ. 2003. Cognitive function after anaesthesia in the elderly. Best Pract Res Clin Anaesthesiol 17:259–272 [DOI] [PubMed] [Google Scholar]
- 4.Butterfield NN, Graf P, Ries CR, MacLeod BA. 2004. The effect of repeated isoflurane anesthesia on spatial and psychomotor performance in young and aged mice. Anesth Analg 98:1305–1311 [DOI] [PubMed] [Google Scholar]
- 5.Caramanos Z, Shapiro ML. 1994. Spatial memory and N-methyl-D-aspartate receptor antagonists APV and MK-801: memory impairments depend on familiarity with the environment, drug dose, and training duration. Behav Neurosci 108:30–43 [DOI] [PubMed] [Google Scholar]
- 6.Crosby C, Culley DJ, Baxter MG, Yukhananov R, Crosby G. 2005. Spatial memory performance 2 weeks after general anesthesia in adult rats. Anesth Analg 101:1389–1392 [DOI] [PubMed] [Google Scholar]
- 7.Culley DJ, Baxter M, Yukhananov R, Crosby G. 2003. The memory effects of general anesthesia persist for weeks in young and aged rats. Anesth Analg 96:1004–1009, table of contents [DOI] [PubMed] [Google Scholar]
- 8.Culley DJ, Baxter MG, Yukhananov R, Crosby G. 2004. Long-term impairment of acquisition of a spatial memory task following isoflurane-nitrous oxide anesthesia in rats. Anesthesiology 100:309–314 [DOI] [PubMed] [Google Scholar]
- 9.Culley DJ, Baxter MG, Crosby CA, Yukhananov R, Crosby G. 2004. Impaired acquisition of spatial memory 2 weeks after isoflurane and isoflurane-nitrous oxide anesthesia in aged rats. Anesth Analg 99:1393–1397 [DOI] [PubMed] [Google Scholar]
- 10.Demers G, Griffin G, De Vroey G, Haywood JR, Zurlo J, Bedard M. 2006. Animal research. Harmonization of animal care and use guidance. Science 312:700–701 [DOI] [PubMed] [Google Scholar]
- 11.Eger EI, 2nd, Sonner JM. 2006. Anaesthesia defined (gentlemen, this is no humbug). Best Pract Res Clin Anaesthesiol 20:23–29 [DOI] [PubMed] [Google Scholar]
- 12.Farag E, Chelune GJ, Schubert A, Mascha EJ. 2006. Is depth of anesthesia, as assessed by the bispectral index, related to postoperative cognitive dysfunction and recovery? Anesth Analg 103:633–640 [DOI] [PubMed] [Google Scholar]
- 13.Fodale V, Santamaria LB. 2003. The inhibition of central nicotinic nAch receptors is the possible cause of prolonged cognitive impairment after anesthesia. Anesth Analg 97:1207. [DOI] [PubMed] [Google Scholar]
- 14.Franks NP, Lieb WR. 1994. Molecular and cellular mechanisms of general anaesthesia. Nature 367:607–614 [DOI] [PubMed] [Google Scholar]
- 15.Komatsu H, Nogaya J, Kuratani N, Ueki M, Yokono S, Ogli K. 1998. Repetitive post-training exposure to enflurane modifies spatial memory in mice. Anesthesiology 89:1184–1190 [DOI] [PubMed] [Google Scholar]
- 16.Sleigh JW, Andrzejowski J, Steyn-Ross A, Steyn-Ross M. 1999. The bispectral index: a measure of depth of sleep? Anesth Analg 88:659–661 [DOI] [PubMed] [Google Scholar]
- 17.Warner DS. 2000. Isoflurane neuroprotection: a passing fantasy, again? Anesthesiology 92:1226–1228 [PubMed] [Google Scholar]