Abstract
We describe a case of methicillin-resistant Staphylococcus non-aureus infection in a rhesus macaque (Macaca mulatta). The nonhuman primate described was part of a research project that involved whole-body gamma irradiation and subsequently developed acute generalized dermatitis with skin dryness, peeling, and erythema around the eyes. After initial evaluation, which included microbiologic culture and 6 d of medical treatment, the animal was euthanized due to concern regarding a possible outbreak of infectious or zoonotic disease. On the basis of skin culture, diagnosis of methicillin-resistant Staphylococcus non-aureus was confirmed. This report underscores the importance of the occupational risk of methicillin-resistant Staphylococcus non-aureus to research and animal care staff in a research animal facility setting.
Abbreviation: MRSA, methicillin-resistant Staphylococcus aureus
Methicillin-resistant Staphylococcus aureus (MRSA) infection and colonization has occurred in various domestic animals, including horses, dogs, cats, birds, and cattle.3 Transmission of infection from animals to humans and from humans to animals has been reported.2,8,10,15,17,19 MRSA is more common in humans with severe illness, comorbid conditions, and immunosuppression.1 MRSA infection in a rhesus macaque in a research animal facility setting and showing signs of respiratory distress has been reported.13
Similar to MRSA, methicillin-resistant Staphylococcus non-aureus appears to be an emerging pathogen in veterinary medicine. Methicillin-resistant Staphylococcus non-aureus species such as S. epidermidis are seen commonly in the human hospital setting and are sometimes even more resistant than and displace the growth of MRSA.7,11,16 This report describes an acute case of methicillin-resistant Staphylococcus non-aureus in an immunosuppressed rhesus macaque whose infection was characterized by skin dryness, dermatitis, hyperkeratosis, and erythema in the facial area.
Case Report
A 3-y-old male rhesus macaque (Macaca mulatta) was reported to the on-duty clinical veterinarian because of erythema of the face and periocular edema. The animal had been procured from an approved vendor and underwent an uneventful quarantine prior to receiving full-body gamma irradiation 35 d prior to presentation. The animal was part of an IACUC-approved study of stem cell reconstitution after irradiation exposure. All procedures were performed in accordance with US Department of Agriculture Animal Welfare Act regulations and the Guide for the Care and Use of Laboratory Animals.9
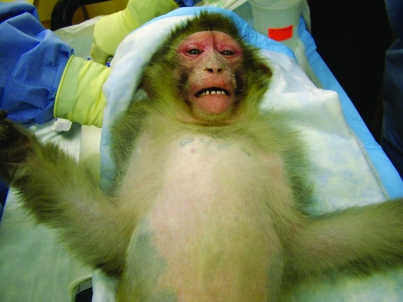
On initial observation, the animal was bright, alert, and active with dry skin, epithelial sloughing, skin thickening, and scaling on cheeks, with erythema on the face and periocular edema. Skin dryness with scaling was also present in the chest area (Figure 1). The animal appeared to be eating normally and had normal urine and feces in the cage pan. The monkey was sedated with ketamine (10 mg/kg IM, Ketaset, Fort Dodge Animal Health, Fort Dodge, IA) for examination.
Figure 1.
Photograph of the skin. Erythema of the face and periocular edema, with skin thickening and scaling on cheeks.
On physical examination, the animal weighed 5.45 kg and had a heart rate of 180 beats per minute, rectal temperature of 38.7 °C (101.7 °F), and a respiration rate of 38 breaths per minute. No abnormal heart or lung sounds were ausculted. The peripheral lymph nodes and the remainder of the physical examination were normal. Initial differential diagnoses included postirradiation cutaneous hypersensitivity, food or drug reaction, contact dermatitis, bacterial pyoderma, and viral infection. Blood was drawn for blood chemistry, complete blood count and differentials, and culture. Swabs were taken from facial lesions and several different parts of the skin over the anterior thoracic area. A 4-mm skin biopsy from anterior thoracic area was performed for microscopic examination, because skin dryness similar to that in the facial area was present on the chest.
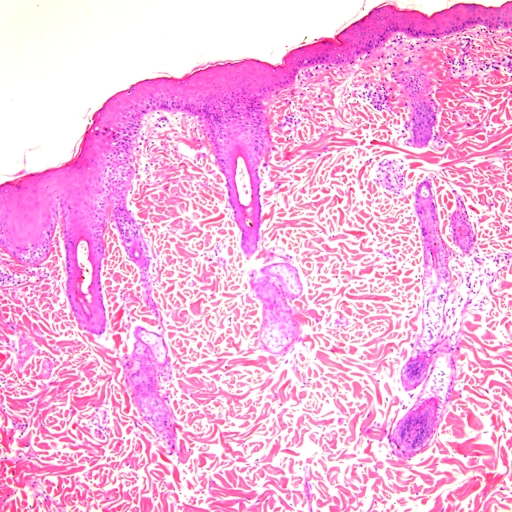
Initially the animal was treated for pyoderma or hypersensitivity–allergic reaction with 2 mg/kg diphenhydramine maleate (Baxter Healthcare, Deerfield, IL) and 22 mg/kg cefazolin (West-Ward Pharmaceutical, Eatontown, NJ) given IM twice daily. Evaluation of facility husbandry records and the principal investigator's experimental records revealed no recent drug administration or change in diet, which made drug or food reaction an unlikely cause of the dermatopathy. The animal was monitored closely for clinical response to treatment over the next 6 d, while awaiting laboratory results. Throughout the observation period, the animal remained bright, alert, and responsive with no signs of pruritis or distress. During this time, results of skin culture (Antech Diagnostics, Lake Success, NY) by tube coagulase test6 indicated that the culture was positive for methicillin-resistant Staphylococcus non-aureus. Sensitivity testing indicated that the organism was sensitive to chloramphenicol, erythromycin, gentamycin, neomycin, and tetracycline but resistant to ampicillin, amoxicillin trihydrate–clavulanate potassium, clindamycin, cephalothin, methicillin, enrofloxacin, trimethoprim–sulfamethoxazole, and marbofloxacin (Table 1). No other bacteria or fungi were isolated. Blood cultures were negative for aerobic and anaerobic bacteria. Fecal samples showed moderate numbers of Giardia cysts (11 to 50 cysts per slide) and were ELISA-positive for Giardia spp. Histopathology showed that skin was affected by epidermal hyperplasia and chronic dermal inflammation. Perivascular plasma cells and lymphocytes were identified and the dermis showed a background of mucinosis (Figure 2)
Table 1.
Results of antibiotic sensitivity and resistance testing of the bacterial isolate
| Antibiotic | Sensitive | Resistant |
| Amoxicillin trihydrate–clavulanate | X | |
| Potassium | ||
| Ampicillin | X | |
| Cephalothin | X | |
| Chloramphenicol | X | |
| Clindamycin | X | |
| Enrofloxacin | X | |
| Erythromycin | X | |
| Gentamycin | X | |
| Marbofloxacin | X | |
| Methicillin | X | |
| Neomycin | X | |
| Tetracycline | X | |
| Trimethoprim–sulfamethoxazole | X |
Figure 2.
Photomicrograph of skin. Epidermal hyperplasia and chronic dermal inflammation. Perivascular plasma cells and lymphocytes with mucinosis background in dermis.
Clinical records showed marked decreases in leukocyte, erythrocyte, mononuclear, and absolute lymphocyte counts (96%, 40%, 96% and 96%, respectively, compared with baseline preirradiation values) on day 10 after irradiation, indicating severe immunosuppression; these counts gradually increased to original baseline values by day 35 after irradiation, due to blood transfusion and stem cell reconstitution. During treatment, the animal had no visible improvement in clinical signs, indicating that the methicillin-resistant Staphylococcus non-aureus was a likely contributor to (if not cause of) the dermatopathy. After 6 d with no response to symptomatic treatment and given the positive laboratory finding of methicillin-resistant Staphylococcus non-aureus, the animal was euthanized with sodium pentobarbital (100 mg/kg IV). Euthanasia was elected after communication with the principal investigator in light of concerns of a potential infectious (or zoonotic) disease outbreak, based on the highly resistant nature of the organism and the animal's proximity to other immunosuppressed nonhuman primates. A timeline of the case from the monkey's entrance into the animal facility to final disposition appears in Figure 3.
Figure 3.
Timeline of case progression from animal facility entry to final disposition.
Discussion
To our knowledge, this case report is the first description of methicillin-resistant Staphylococcus non-aureus infection in a nonhuman primate. The diagnosis was made on the basis of skin culture, gross pathology, and histopathologic findings. This case indicates the susceptibility of immunosuppressed rhesus macaques to infection with methicillin-resistant Staphylococcus non-aureus strains and may provide a model for human infection with this organism.
MRSA infection has occurred in various animals, including dogs and cats, in recent years, with increasing incidence of transmission of disease between animals and humans.18,20 MRSA has been isolated from animals and veterinary personnel in veterinary hospitals, indicating the spread of infection from animals to humans and vice versa.10 Another clinical case of MRSA in pigs used for experimental purposes in a research animal setting was associated with spread of infection to research staff, highlighting the possible risk of spread of infection from laboratory animals to animal care staff.14 MRSA was diagnosed in rhesus macaques showing signs of respiratory distress and pneumonia at a national primate research center; bacterial culture of lung parenchyma was positive for MRSA.13 Recognizing the possibility of and diagnosing methicillin-resistant Staphylococcus non-aureus bacterial infection is important to prevent the spread of infection to other animals, and possibly humans, in research facilities.
The risk factors associated with methicillin-resistant Staphylococcus non-aureus infection of laboratory animals are not clearly understood. However, immunosuppression was a likely contributor to infection in the case we described. Immunosuppressed mice inoculated intranasally with MRSA showed substantive growth of organisms in ceca and feces.5 A similar risk for MRSA occurs in humans with immunosuppression.1,12
We hypothesize that the animal we report developed methicillin-resistant Staphylococcus non-aureus infection secondary to radiation-induced immunosuppression. The animal had received a radiation dose of approximately 600 cGy as a central beam over the trunk while in a dorsoventral position, leading to immunosuppression after depletion of bone marrow cells. This immunosuppressive effect has been shown in other studies.22 The animal likely had a subclinical infection that became clinically apparent 35 d after irradiation, when the immune system was being restored by blood transfusion and other treatments such as stem cell reconstitution. The animal had moderate giardiasis but lacked diarrhea and other gastrointestinal signs, so the giardiasis was deemed to be a subclinical infection. More studies should be performed to determine the mechanisms of spread of this group of bacteria as well as appropriate measures for prevention of spread of the infection to other animals and research and animal care staff. Routine screening and testing for antibiotic-resistant organisms such as MRSA and methicillin-resistant Staphylococcus non-aureus are performed in the human hospital setting 4. Although laborious and expensive in the lab animal setting, these measures should be considered in the future to protect research and animal care staff in research animal facilities. In vitro studies from clinical isolates have demonstrated that fluoroquinolones such as ciprofloxacin are effective in the treatment of methicillin-resistant Staphylococcus non-aureus,11,16 but a treatment option should be adopted with caution and strict isolation of the affected animal along with antibiotic sensitivity tests. Isolation or quarantine and enhanced Animal Biosafety Level 2 precautions should be taken in addition to antibiotic therapy if methicillin-resistant Staphylococcus non-aureus is identified in a nonhuman primate, particularly if experimental or other factors necessitate maintaining the animal in the facility. Maintaining a nonhuman primate with bacterial subspecies resistant to methicillin antibiotics likely poses a considerable threat to other animals in the colony as well as an unknown zoonotic and potential harmful effect on veterinary care and research staff. This potential also is based on the fact that the gene for methicillin resistance can be transferred horizontally to other Staphylococcus spp in humans and nonhuman primates.21 This case report provides evidence of the susceptibility of rhesus macaques to antibiotic-resistant bacteria and indicates that this animal can serve as a model for natural infection with methicillin-resistant Staphylococcus non-aureus organisms.
Acknowledgments
We thank Charles McLeod for histopathology assistance. We also thank Liliana Rao and Elisa Luna for technical assistance in tissue processing.
References
- 1.Fridkin SK, Gaynes R. 1999. Antimicrobial resistance in intensive care units. Clin Chest Med 20:303–316 [DOI] [PubMed] [Google Scholar]
- 2.Guardabassi L, Schwartz S, Llyod DH. 2004. Pet animals as reservoirs of antimicrobial-resistant bacteria. J Antimicrob Chemother 54:321–332 [DOI] [PubMed] [Google Scholar]
- 3.Hanselman BA, Kruth SA, Rousseau J, Low DE, Willey BM, McGeer A, Weese JS. 2006. Methicillin-resistant Staphylococcus aureus colonization in veterinary personnel. Emerg Infect Dis 12:1933–1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson JK, Khoie T, Shurland S, Kreisel K, Stine OC, Roghmann MC. 2007. Skin and soft tissue infections caused by methicillin-resistant Staphylococcus aureus USA 300 clone. Emerg Infect Dis 13:1195–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kato-Matsunaga N, Okonogi K. 1996. Gastrointestinal colonization by methicillin-resistant Staphylococcus aureus in immunosuppressed mice. Infect Immun 64:4231–4235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kloos WE, Zambe JDW. In: Murray PR, Baron EJ, Jorgensen JH, Pfaller MA, Yolken RH.Manual of clinical microbiology. Washington (DC): ASM Press [Google Scholar]
- 7.Lina G, Boutite F, Tristan A, Bes M, Etienne J, Vandenesch F. 2003. Bacterial competition for human nasal cavity colonization: role of staphylococcal agr alleles. Appl Environ Microbiol 69:18–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manian FA. 2003. Asymptomatic nasal carriage of mupirocin-resistant, methicillin-resistant Staphylococcus aureus (MRSA) in a pet dog associated with MRSA infection in household contacts. Clin Infect Dis 36:e26–e28 [DOI] [PubMed] [Google Scholar]
- 9.National Research Council 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academy Press [Google Scholar]
- 10.O'Mahony R, Abbott Y, Leonard FC, Markey BK, Quinn PJ, Pollock PJ, Fanning S, Rossney AS. 2005. Methicillin-resistant Staphylococcus aureus (MRSA) isolated from animals and veterinary personnel in Ireland. Vet Microbiol 109:285–296 [DOI] [PubMed] [Google Scholar]
- 11.Rabagliati AM, Fiorio P, Cavallo R, Mills V, Penna R. 1991. In-vitro activity of quinolones against different species of staphylococci of recent clinical isolation. J Chemother 3Suppl 1:176–178 [PubMed] [Google Scholar]
- 12.Rao GG. 1998. Risk factor for the spread of antibiotic-resistant bacteria. Drugs 55:323–330 [DOI] [PubMed] [Google Scholar]
- 13.Rivas K, Garcia A, Morales PR, Matchett CA, Saucedo A, Wagner JL. 2007. Methicillin-resistant Staphylococcus aureus (MRSA) pneumonia in a rhesus macaque [abstract]. J Am Assoc Lab Anim Sci 46:102 [Google Scholar]
- 14.Sergio DB, Koh T, Hsu L, Ogden BE, Goh A, Chow P. 2007. Investigation of methicillin-resistant Staphylococcus aureus (MRSA) in pigs (Sus scrofa) used for research [abstract]. J Am Assoc Lab Anim Sci 46:108 [Google Scholar]
- 15.Simoons-Smit AM, Savelkoul PH, Stoof J, Starink TM. 2000. Transmission of Staphylococcus aureus between humans and domestic animals in a household. Eur J Clin Microbiol Infect Dis 19:150–152 [DOI] [PubMed] [Google Scholar]
- 16.Sposini T, Bastianini L, D'Alo F, Verducci N, Sbaraglia G. 1991. Methicillin-resistant staphylococcal strains isolated from clinical samples. J Chemother 3Suppl 1:169–171 [PubMed] [Google Scholar]
- 17.van Duijkeren E, Ikawaty R, Broekhuizen-Stins MJ, Jansen MD, Spalburg EC, de Neeling AJ, Allaart JG, van Nes A, Wagenaar JA, Fluit AC. 2008. Transmission of methicillin-resistant Staphylococcus aureus strains between different kinds of pig farms. Vet Microbiol 126:383–389 [DOI] [PubMed] [Google Scholar]
- 18.van Duijkeren E, Wolfhagen MJ, Box AT, Heck ME, Wannet WJ, Fluit AC. 2004. Human-to-dog transmission of methicillin-resistant Staphylococcus aureus. Emerg Infect Dis 10:2235–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Duijkeren E, Wolfhagen MJ, Heck ME, Wannet WJ. 2005. Transmission of a Panton–Valentine leucocidin-positive, methicillin-resistant Staphylococcus aureus strain between humans and a dog. J Clin Microbiol 43:6209–6211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walther B, Friedrich AW, Brunnberg L, Wieler LH, Lubke-Becker A. 2006. Methicillin-resistant Staphylococcus aureus (MRSA) in veterinary medicine: a “new emerging pathogen”? [In German] Berl Munch Tierarztl Wochenschr 119:222–232 [PubMed] [Google Scholar]
- 21.Walther C, Perreten V. 2007. Methicillin-resistant Staphylococcus epidermidis in organic milk production. J Dairy Sci 90:5351. [DOI] [PubMed] [Google Scholar]
- 22.Waselenko JK, MacVittie TJ, Blakely WF, Pesik N, Wiley AL, Dickerson WE, Tsu H, Confer DL, Coleman CN, Seed T, Lowry P, Armitage JO, Dainiak N. 2004. Medical management of the acute radiation syndrome: recommendations of the strategic national stockpile radiation working group. Ann Intern Med 140:1037–1051 [DOI] [PubMed] [Google Scholar]