Abstract
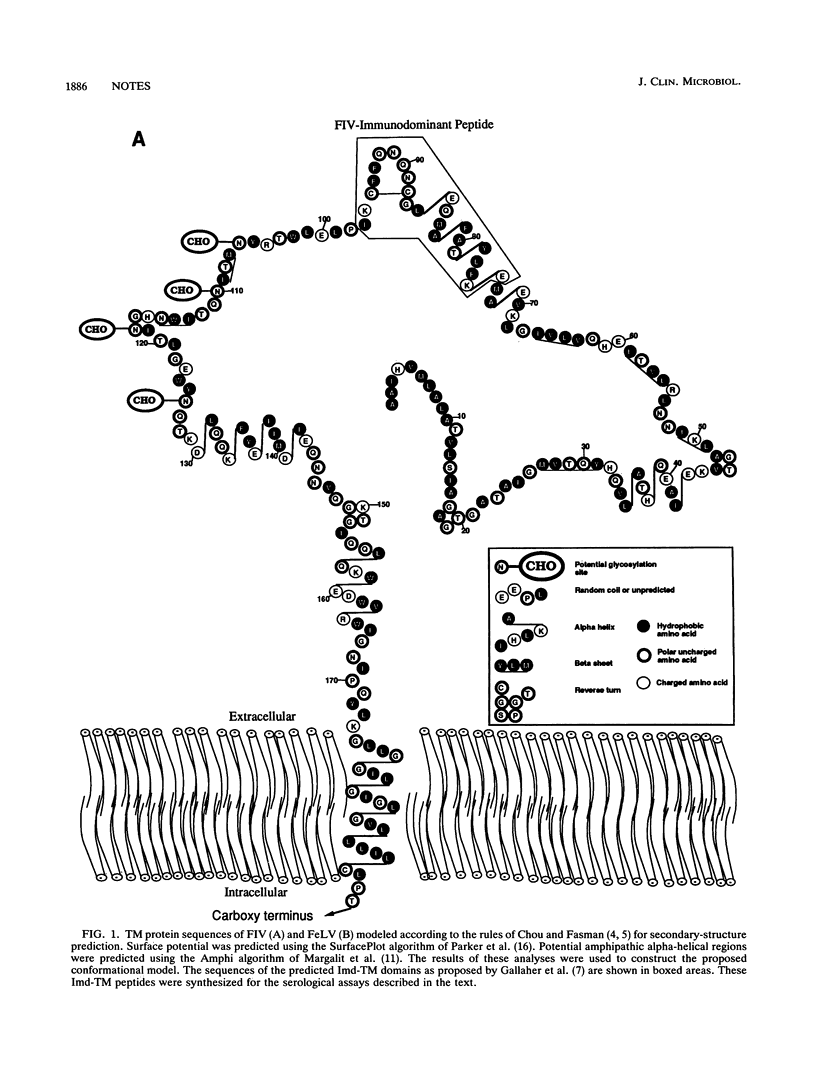
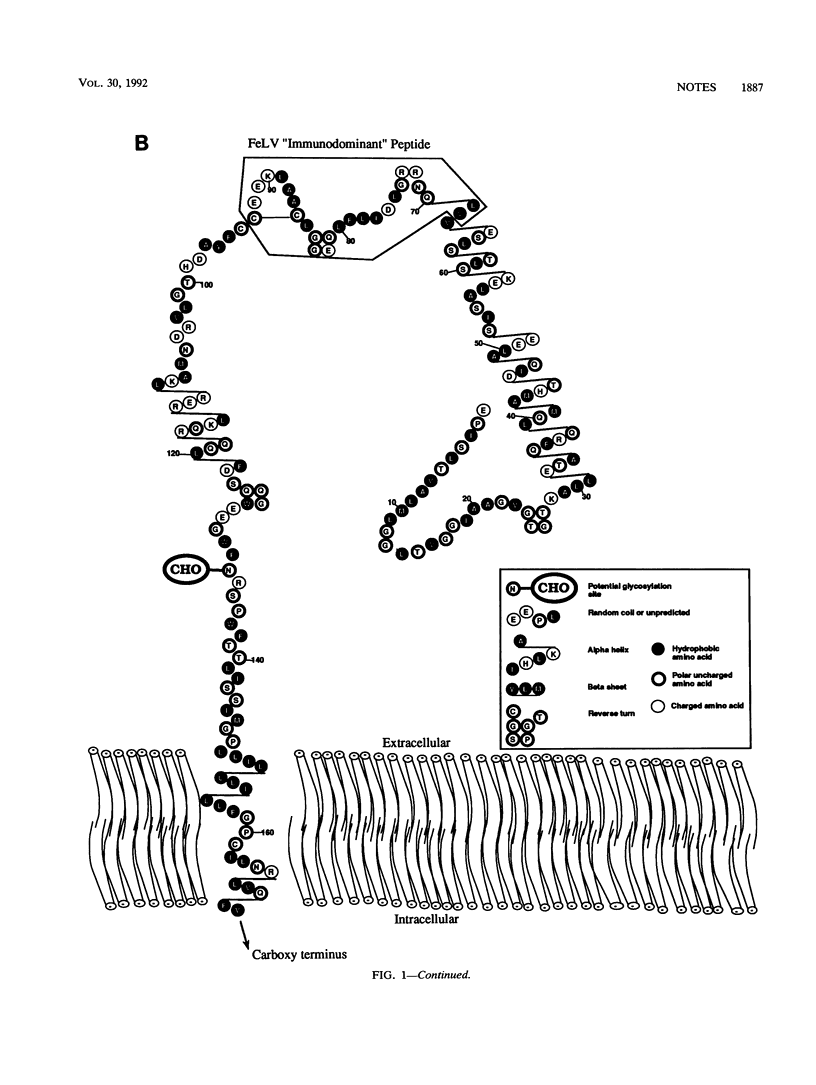
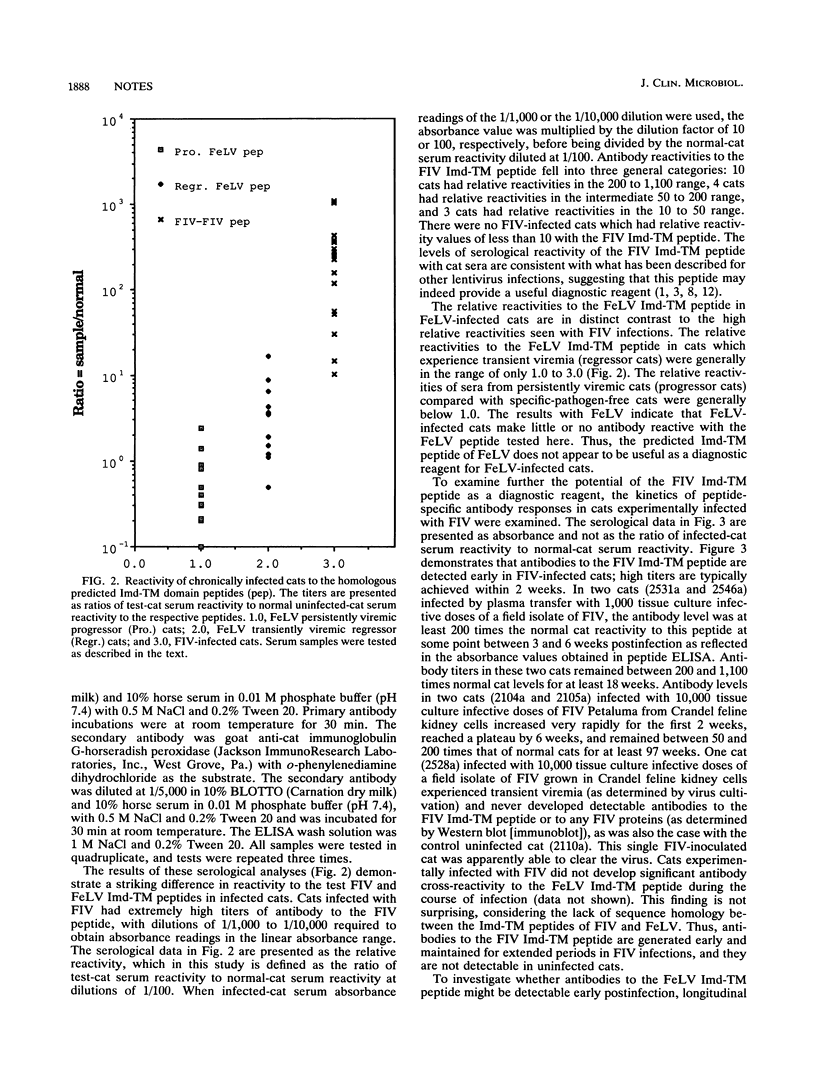
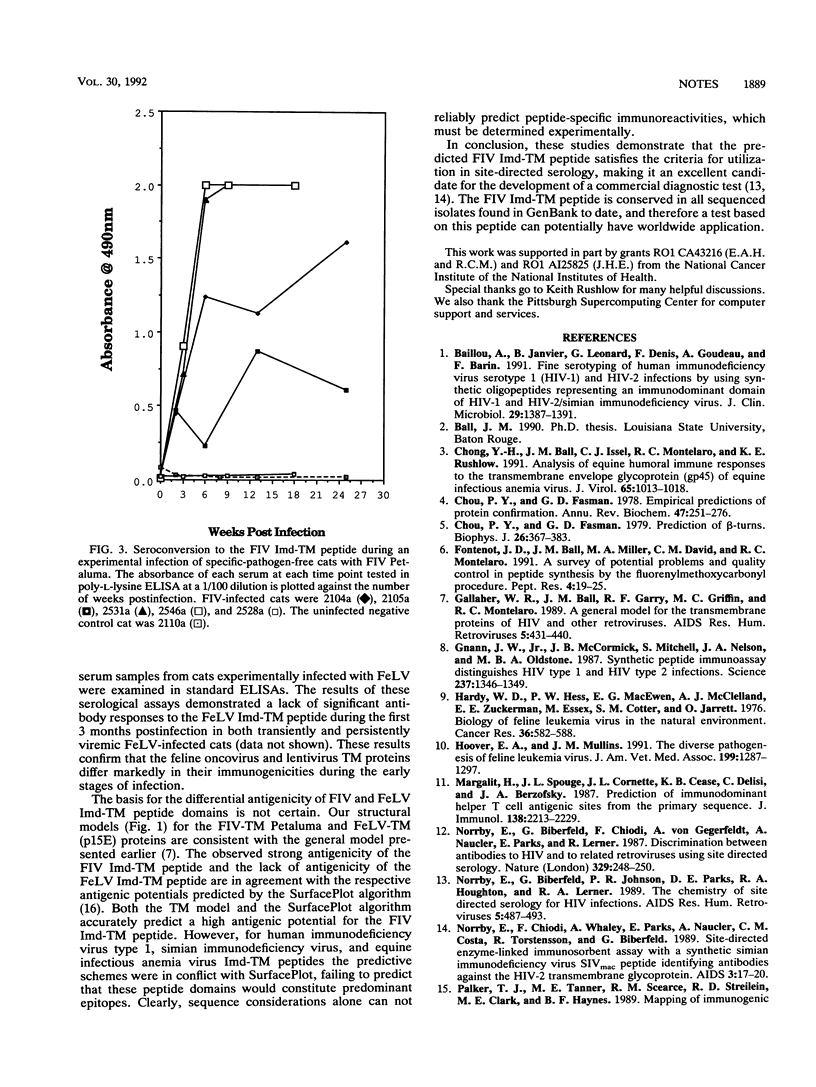
The general model for retrovirus transmembrane (TM) proteins proposed by Gallaher et al. (W. R. Gallaher, J. M. Ball, R. F. Garry, M. C. Griffin, and R. C. Montelaro, AIDS Res. Hum. Retroviruses 5:431-440, 1989) suggests that all retrovirus TM proteins may contain an immunodominant domain (Imd-TM peptide) located at the apex of the TM polypeptide. Although this Imd-TM peptide has been shown to be immunodominant in a variety of lentivirus infections, there has not been a detailed serological analysis of an oncovirus Imd-TM peptide as a diagnostic agent. We describe here an analysis of the antigenic properties and diagnostic potentials of the predicted Imd-TM peptides of feline immunodeficiency virus (FIV) and feline leukemia virus (FeLV) in serological assays of sera from infected cats. The results of these studies demonstrate that antibodies specific to the FIV Imd-TM peptide are detected within 2 weeks postinfection, are maintained at high levels for extended periods, and are not detectable in uninfected or FeLV-infected cats. In marked contrast, the FeLV Imd-TM peptide displayed only negligible levels of serological reactivity in FeLV-infected cats. These studies indicate that the peptide is a useful reagent for the detection of antibodies to FIV.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baillou A., Janvier B., Leonard G., Denis F., Goudeau A., Barin F. Fine serotyping of human immunodeficiency virus serotype 1 (HIV-1) and HIV-2 infections by using synthetic oligopeptides representing an immunodominant domain of HIV-1 and HIV-2/simian immunodeficiency virus. J Clin Microbiol. 1991 Jul;29(7):1387–1391. doi: 10.1128/jcm.29.7.1387-1391.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong Y. H., Ball J. M., Issel C. J., Montelaro R. C., Rushlow K. E. Analysis of equine humoral immune responses to the transmembrane envelope glycoprotein (gp45) of equine infectious anemia virus. J Virol. 1991 Feb;65(2):1013–1018. doi: 10.1128/jvi.65.2.1013-1018.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of beta-turns. Biophys J. 1979 Jun;26(3):367–383. doi: 10.1016/S0006-3495(79)85259-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot J. D., Ball J. M., Miller M. A., David C. M., Montelaro R. C. A survey of potential problems and quality control in peptide synthesis by the fluorenylmethoxycarbonyl procedure. Pept Res. 1991 Jan-Feb;4(1):19–25. [PubMed] [Google Scholar]
- Gallaher W. R., Ball J. M., Garry R. F., Griffin M. C., Montelaro R. C. A general model for the transmembrane proteins of HIV and other retroviruses. AIDS Res Hum Retroviruses. 1989 Aug;5(4):431–440. doi: 10.1089/aid.1989.5.431. [DOI] [PubMed] [Google Scholar]
- Gnann J. W., Jr, McCormick J. B., Mitchell S., Nelson J. A., Oldstone M. B. Synthetic peptide immunoassay distinguishes HIV type 1 and HIV type 2 infections. Science. 1987 Sep 11;237(4820):1346–1349. doi: 10.1126/science.2888192. [DOI] [PubMed] [Google Scholar]
- Hardy W. D., Jr, Hess P. W., MacEwen E. G., McClelland A. J., Zuckerman E. E., Essex M., Cotter S. M., Jarrett O. Biology of feline leukemia virus in the natural environment. Cancer Res. 1976 Feb;36(2 Pt 2):582–588. [PubMed] [Google Scholar]
- Hoover E. A., Mullins J. I. Feline leukemia virus infection and diseases. J Am Vet Med Assoc. 1991 Nov 15;199(10):1287–1297. [PubMed] [Google Scholar]
- Margalit H., Spouge J. L., Cornette J. L., Cease K. B., Delisi C., Berzofsky J. A. Prediction of immunodominant helper T cell antigenic sites from the primary sequence. J Immunol. 1987 Apr 1;138(7):2213–2229. [PubMed] [Google Scholar]
- Norrby E., Biberfeld G., Chiodi F., von Gegerfeldt A., Nauclér A., Parks E., Lerner R. Discrimination between antibodies to HIV and to related retroviruses using site-directed serology. Nature. 1987 Sep 17;329(6136):248–250. doi: 10.1038/329248a0. [DOI] [PubMed] [Google Scholar]
- Norrby E., Biberfeld G., Johnson P. R., Parks D. E., Houghten R. A., Lerner R. A. The chemistry of site-directed serology for HIV infections. AIDS Res Hum Retroviruses. 1989 Oct;5(5):487–493. doi: 10.1089/aid.1989.5.487. [DOI] [PubMed] [Google Scholar]
- Norrby E., Chiodi F., Whalley A., Parks E., Nauclér A., Costa C. M., Torstensson R., Biberfeld G. Site-directed enzyme-linked immunosorbent assay with a synthetic simian immunodeficiency virus SIVmac peptide identifying antibodies against the HIV-2 transmembrane glycoprotein. AIDS. 1989 Jan;3(1):17–20. [PubMed] [Google Scholar]
- Parker J. M., Guo D., Hodges R. S. New hydrophilicity scale derived from high-performance liquid chromatography peptide retention data: correlation of predicted surface residues with antigenicity and X-ray-derived accessible sites. Biochemistry. 1986 Sep 23;25(19):5425–5432. doi: 10.1021/bi00367a013. [DOI] [PubMed] [Google Scholar]
- Pedersen N. C., Ho E. W., Brown M. L., Yamamoto J. K. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987 Feb 13;235(4790):790–793. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- Pedersen N. C., Torten M., Rideout B., Sparger E., Tonachini T., Luciw P. A., Ackley C., Levy N., Yamamoto J. Feline leukemia virus infection as a potentiating cofactor for the primary and secondary stages of experimentally induced feline immunodeficiency virus infection. J Virol. 1990 Feb;64(2):598–606. doi: 10.1128/jvi.64.2.598-606.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier R. D., Gnann J. W., Jr, Langlois A. J., Shriver K., Nelson J. A., Oldstone M. B. B- and T-lymphocyte responses to an immunodominant epitope of human immunodeficiency virus. J Virol. 1988 Aug;62(8):2531–2536. doi: 10.1128/jvi.62.8.2531-2536.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott R. L., Sparger E. E., Lovelace K. M., Fitch W. M., Pedersen N. C., Luciw P. A., Elder J. H. Nucleotide sequence and genomic organization of feline immunodeficiency virus. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5743–5747. doi: 10.1073/pnas.86.15.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. J., Steel S., Wisniewolski R., Wang C. Y. Detection of antibodies to human T-lymphotropic virus type III by using a synthetic peptide of 21 amino acid residues corresponding to a highly antigenic segment of gp41 envelope protein. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6159–6163. doi: 10.1073/pnas.83.16.6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto J. K., Hansen H., Ho E. W., Morishita T. Y., Okuda T., Sawa T. R., Nakamura R. M., Pedersen N. C. Epidemiologic and clinical aspects of feline immunodeficiency virus infection in cats from the continental United States and Canada and possible mode of transmission. J Am Vet Med Assoc. 1989 Jan 15;194(2):213–220. [PubMed] [Google Scholar]
- Yamamoto J. K., Sparger E., Ho E. W., Andersen P. R., O'Connor T. P., Mandell C. P., Lowenstine L., Munn R., Pedersen N. C. Pathogenesis of experimentally induced feline immunodeficiency virus infection in cats. Am J Vet Res. 1988 Aug;49(8):1246–1258. [PubMed] [Google Scholar]


