Abstract
Class switch recombination (CSR) involves a DNA rearrangement in the Ig heavy chain (IgH) gene that allows the same variable (V) region to be expressed with any one of the downstream constant region (C) genes to encode antibodies with many different effector functions. One hypothesis for how CSR is targeted to different C region genes is that histone modifications increase accessibility and/or recruit activation-induced cytosine deaminase (AID) and its associated processes to particular donor and recipient switch regions. In this work, we identified H3 acetyl K9 and H3 trimethyl K9 as histone modifications that correlate with the recombining pair of donor and recipient switch regions. The appearance of H3 trimethyl K9 is surprising because usually it is thought to mark silent genes and heterochromatin. Nevertheless, the time course of appearance of these histone modifications, the regions in IgH they associate with, and their appearance independent of AID damage suggest that both modifications play a role in targeting CSR.
Keywords: activation-induced cytoside deaminase, B cells, ChIP, immunoglobulin
Antibodies are critical for the organism's defense against the many pathogens and toxins that it encounters. A diverse repertoire of antibodies is created by V(D)J rearrangement. Upon activation by their cognate antigen and T cells, B cells enter germinal centers where they express activation-induced cytosine deaminase (AID) that initiates somatic hypermutation (SHM) and class switch recombination (CSR) (1). SHM results in point mutations in the Ig heavy chain and light chain variable region genes, which code for the antigen-binding site, and with selection, these mutations lead to increased affinity for antigen (2). In CSR, the AID-induced mutations lead to double-stranded DNA breaks in the switch regions (SR) that are upstream of the antibody constant regions, and DNA rearrangements result in the apposition of different downstream constant region segments with the same heavy chain variable (V) region (3). Clonal progeny of an IgM-expressing B cell can express a particular V region with any one of the four IgG subclasses, IgE, or IgA. These isotypes have different effector functions and tissue localizations facilitating an effective response to a single antigenic determinant.
The rearranged and productive IgH locus in mice and humans is organized in units consisting of a VDJ region, an intronic enhancer, followed by repeating units of intervening exons (I) and SRs that are noncoding, and the constant region (C) coding exons (Fig. 1A). Non-class-switched IgM- (IgD)-expressing B cells make the VDJ-Cμ/Cδ transcript that is spliced and translated into a protein, and a sterile transcript that is not encoded into a protein and begins 5′ of the Iμ exon and continues through the μSR and μC regions. Upon receipt of the appropriate signals to begin CSR, sterile RNA transcripts originate 5′ of one or more of the recipient downstream γ, ε, or α I-SRs. Sterile transcription is required for CSR, perhaps because it creates regions of single-stranded DNA that are the substrate for AID (3, 4). Independently, AID is expressed and introduces mutations at the donor μSR and recipient SRs by deamination of cytosines to uracil. Various enzymes from the base excision repair, mismatch repair, and nonhomologous end-joining pathways convert the single-strand breaks created by AID into double-strand breaks and form a new hybrid SR consisting of parts of the donor SR (Sμ) and recipient SR (3). Unlike VDJ recombination, CSR is a region-specific rather than a sequence-specific event, which means that the junction site can be anywhere along the 1- to 10-kb tract that defines each SR (5).
Fig. 1.
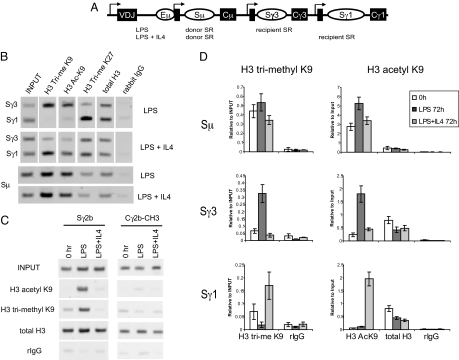
H3 trimethyl K9 and H3 acetyl K9 associate with pairs of active SRs. (A) Diagram of the mouse IgH locus with a rearranged VDJ and the three SRs examined in this study (not to scale). Arrows, sterile transcription initiation sites; black boxes, I exons. (B) ChIP performed on chromatin derived from splenic B cells treated with LPS or LPS + IL4 for 72 h, using rabbit antibodies against the indicated modified histones or control rabbit IgG. PCR was performed by using primers that identify Sγ1 and Sγ3, or Sμ, or (C) Sγ2b and Cγ2b. These gels are representative of 2–6 independent experiments. (D) Real-time quantitative PCR was performed in triplicate, and error bars indicate the standard error. This figure is representative of 2 independent experiments.
Because AID is so mutagenic, it is critical that it be restricted to the SRs, sparing the other parts of the IgH gene and the rest of the genome. One hypothesis suggests that cytokine-induced sterile transcripts at recipient SRs provide the necessary specificity and create transcriptional accessibility (3, 4). AID, perhaps traveling in a complex with RNA pol II and other factors, accesses and mutates the ssDNA regions in the transcribed SRs, triggering CSR (6, 7). Although sterile transcription is necessary for CSR (3), transcription alone is not sufficient because it occurs in many other genes in the B cell and at the unaffected downstream C region of the IgH gene, and thus additional elements must contribute to the targeting of CSR to SRs.
Another, not mutually exclusive, hypothesis is that histone modifications in the SRs play a role in CSR targeting. The amino-terminal tails of the four core histones are covalently modified in a variety of ways, such as acetylation, phosphorylation, and methylation (reviewed in refs. 8 and 9). The “histone code” hypothesis suggests that histone modifications direct or facilitate various biological processes through recognition of the modified residues by DNA binding or regulatory proteins. These modifications may also result in relaxation of the nucleosome structure, increasing the accessibility of the targeted DNAs (8). The combinatorial possibilities provided by histone modifications offer a plausible mechanism to explain how targeting of specific regions of the IgH gene is achieved. Similar hypotheses are proposed for VDJ recombination in both Ig and TCR genes (10), and histone modifications play a role in Rag2 recruitment to the IgH locus to carry out V(D)J rearrangement (11, 12).
Previous studies have shown that donor and recipient SRs undergoing CSR are associated with histone modifications, including: H3 and H4 hyperacetylation, γ-H2AX, and H2B Ser-14 phosphorylation (6, 13–16). In multiple studies, stimulation of naïve splenic B cells with LPS alone leads to the production of sterile transcripts from Sγ3, but not Sγ1, and switching to IgG3. Even though stimulation with LPS + IL4 leads to switching to IgG1 and represses switching to IgG3, there is sterile transcription of both Sγ3 and Sγ1, although the abundance of sterile transcripts is greater in Sγ1 (14, 17). Because previous studies examined H3 or H4 hyperacetylation (6, 13, 14), this discordance of sterile transcription and histone modifications with the actual site of recombination, and the recent appreciation that combinations of modifications regulate different DNA transactions (9), led us to examine individual lysine acetylations and other histone modifications to determine whether there were marks that would better predict which SR would undergo CSR. In this work, we have identified two histone modifications, H3 acetyl K9 and H3 trimethyl K9, that are associated with the donor and recipient SRs that ultimately undergo recombination, suggesting that these two modifications may play a role in targeting CSR.
Results
H3 Trimethyl K9 and Acetyl K9 Are Associated with SRs That Will Undergo Recombination.
The finding that the histones associated with both Sγ3 and Sγ1 were hyperacetylated with LPS + IL4, whereas switching occurred only to IgG1 (13, 14), led us to examine other histone modifications to determine whether some marks would better predict which SR would undergo CSR. Primary naïve splenic murine B cells are cultured either with LPS to stimulate them to switch from IgM to IgG3, or with LPS + IL4 to stimulate them to switch from IgM to IgG1 (18). Typically, by 96 h with LPS treatment, 8–10% of the IgM-producing cells have switched to IgG3, whereas with LPS + IL4 treatment, 30–40% have switched to IgG1 [Fig. S1 in the supporting information (SI) Appendix]. Recombination can be detected at 48 h (19) and continues through 96 h. We selected 72 h to examine chromatin modifications because by then most cells have undergone at least the two divisions required to initiate switching, and CSR is still ongoing in a significant percentage of them (20). Chromatin was prepared from B cells treated with LPS or LPS + IL4 for 72 h and subject to ChIP by using commercial antibodies that specifically recognize various modified histones (see SI Appendix). We discovered that H3 trimethyl K9 and H3 acetyl K9 preferentially associated with the SRs corresponding to the isotype that underwent recombination (Fig. 1B).
H3 acetyl K9 in active SRs was not wholly unexpected because H3 and H4 hyperacetylation have been reported (6, 13, 14). However, H3 trimethyl K9 was surprising because this modification is usually associated with heterochromatin or silent euchromatin (8), whereas here it is associated with actively transcribed SRs. To confirm this observation, we used anti-H3 trimethyl K9 antibodies from two different commercial sources, and the findings were the same. We also examined H3 trimethyl K27, which is another modification usually associated with heterochromatin (8). In LPS-treated cells, it had the opposite pattern of association with the SRs as H3 trimethyl K9, whereas with LPS + IL4 treatment, H3 trimethyl K27 was associated with both SRs (Fig. 1B). The relative absence of H3 trimethyl K9 on the histones associated with the unused recipient SR was not the result of a lack of nucleosomes at those sites because histone density, as measured by total H3 ChIP, remained constant with different treatments and between SRs (Fig. 1B). Because all of the naïve splenic B cells start out making IgM and Sμ is a CSR participant in both LPS and LPS + IL4-treated cells, it was not surprising that Sμ was modified with both trimethyl K9 and acetyl K9 with both treatments. H3 monomethyl and dimethyl K9 modifications did not correlate with the SR that would undergo recombination (Fig. S2 in the SI Appendix). We did not find a correlation with CSR and H3 trimethyl K79, and H3 trimethyl K4 and K36 largely correlated with presence of sterile transcription.
To determine whether our findings could be generalized to other isotypes, we assayed another SR. LPS stimulation also leads to class switching to IgG2b (21). PCR performed on ChIP DNAs demonstrated that both H3 acetyl K9 and H3 trimethyl K9 were similarly associated with this SR under LPS treatment but not under LPS + IL4 treatment (Fig. 1C Left). In summary, H3 trimethyl K9 and H3 acetyl K9 were the only modifications that we examined that were associated with pairs of SRs (donor and recipient) that undergo recombination.
Having screened by using end point PCR, we quantified H3 trimethyl K9 and acetyl K9 association at the various SRs by using real-time PCR on ChIPed DNA from both kinds of treated cells at 72 h and untreated cells at 0 h (Fig. 1D). Sμ, the common donor SR, was constitutively marked with H3 acetyl K9 and H3 trimethyl K9 in untreated B cells at 0 h, and at 72 h under both treatments. This probably reflects its participation in switching to both γ3 and γ1 and its constitutive transcription from both its own Iμ promoter and the V region promoter. At the recipient SRs (Sγ3, Sγ1) in untreated B cells at the 0-h time point, there was a variable but small degree of H3 trimethyl K9 and no H3 acetyl K9. With LPS stimulation to switch to IgG3, there was an increase in H3 trimethyl K9- and acetyl K9-modified histones associated with Sγ3 that was not seen with Sγ1 (Fig. 1D). With LPS + IL4 stimulation to switch to IgG1, there was an increase of these two histone modifications associated with Sγ1 and not with Sγ3 (Fig. 1D).
Trimethylation and Acetylation at Recipient SRs Are Prominent 48 h After Stimulation.
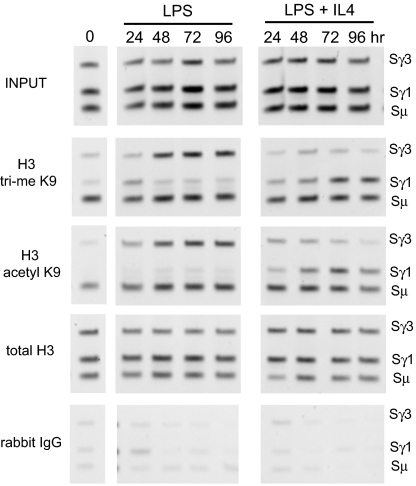
Time course ChIP experiments were carried out to discover when chromatin modifications occur after stimulation. As shown in Fig. 2, both H3 acetyl K9 and H3 trimethyl K9 were constitutively present on the histones associated with the Sμ common donor SR, whereas these modifications were barely detectable in Sγ3 and Sγ1 at 0 h. With LPS stimulation, Sγ3 became H3 K9 trimethylated and acetylated between 24 and 48 h, and these modifications persisted through to 96 h (Fig. 2, LPS). Neither of these modifications was increased at the Sγ1 region. Conversely, with LPS + IL4 stimulation, there was an increase in both H3 trimethyl K9 and H3 acetyl K9 at Sγ1 between 24 and 48 h, and these modifications persisted through 96 h, whereas these modifications were not increased at Sγ3, which does not undergo recombination (Fig. 2, LPS + IL4). The relative abundance of total H3 was constant throughout the course of the experiments (Fig. 2).
Fig. 2.
Time course studies of H3 trimethyl K9 and H3 acetyl K9 in CSR. ChIP was performed on chromatin derived from splenic B cells untreated at 0 h or treated with LPS or LPS + IL4 for the specified times, using rabbit antibodies against the indicated histone modifications, or control rabbit IgG. PCR was performed by using primers that identify Sμ, Sγ1, and Sγ3. These gels are representative of 2 independent time course experiments.
H3 Trimethyl K9 Is Associated Predominantly with SRs.
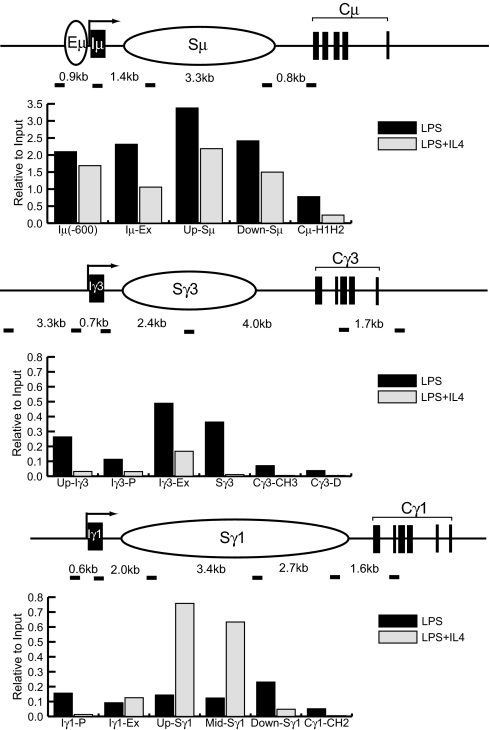
CSR is selectively targeted to the SRs and their immediate flanking sequences and not to the constant regions (3). Because the domains of total H3 hyperacetylation in IgH, which presumably included H3 acetyl K9, have been studied (14), we investigated the domain marked by H3 trimethyl K9 in 72 h LPS or LPS + IL4-treated cells. PCR fragments representative of the I exon promoters; the I exon itself; various parts of the of μ, γ3, and γ1 SRs; and the corresponding constant region exons were amplified after ChIP for H3 trimethyl K9 (Fig. 3). In the μ region, H3 trimethyl K9 was constitutively associated with both Iμ regions and Sμ segments of the gene under both stimulations, consistent with its participation in both reactions (Fig. 3 Top).
Fig. 3.
H3 trimethyl K9 domains in IgH. ChIP was performed on chromatin derived from splenic B cells treated with LPS or LPS + IL4 for 72 h, using rabbit antibodies against H3 trimethyl K9 histones or control rabbit IgG. PCR was performed by using primers that identify the indicated regions on each locus (not to scale). Numbers indicate genomic distances between amplified fragments (short dark bars). Densitometry was performed as described in SI Appendix. Bar graphs indicate specific IP for trimethyl K9 as a percentage of the input band. These data are representative of 2 independent experiments.
In chromatin from LPS-treated cells, there was an increase in H3 trimethyl K9 association with Iγ3 and Sγ3 compared with cells treated with LPS + IL4 (Fig. 3 Middle). H3 trimethyl K9 was not appreciably associated with the IgG3 constant region (Cγ3) in either treatment. Conversely, in chromatin derived from LPS + IL4-treated B cells, there was an increase in H3 trimethyl K9 association with most parts of Sγ1 compared with cells treated with LPS (Fig. 3 Bottom). The modification was not significantly associated with the IgG1 constant region (Cγ1) with either treatment. Additionally, both modifications were not significantly associated with the IgG2b constant region (Cγ2b) under either treatment (Fig. 1C Right).
Chromatin Changes Do Not Require AID Action.
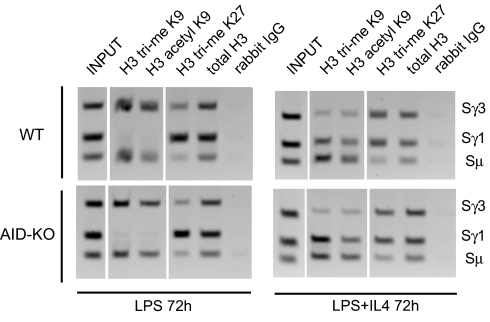
To determine whether these chromatin changes were the direct or indirect result of AID mutagenic action on SRs, splenic B cells were purified from AID-deficient and wild-type littermate mice and stimulated in ex vivo culture to switch to IgG3 or to IgG1, and chromatin was harvested at 72 h. ChIP was performed by using antibodies against H3 trimethyl K9 and H3 acetyl K9, the two marks associated with SRs that undergo recombination, and against H3 trimethyl K27, a mark associated with heterochromatin (Fig. 4). AID-deficient B cells showed the same patterns of H3 K9 and K27 trimethylation and H3 K9 acetylation as were seen above (Fig. 1) and in wild-type stimulated B cells, and this was confirmed by semiquantitative PCR. These findings show that H3 trimethyl and acetyl K9 are not dependent on prior AID mutation or recombination.
Fig. 4.
Chromatin changes are independent of AID-induced DNA damage. ChIP was performed on chromatin derived from wild-type (Upper) or AID-deficient (Lower) splenic B cells treated with LPS (Left) or LPS + IL4 (Right) for 72 h, using rabbit antibodies against indicated modified histones, or control rabbit IgG. PCR was performed by using primers that identify Sμ, Sγ1, and Sγ3. These gels are representative of 2 independent experiments.
H3 Trimethyl K9 and Acetyl K9 Are Both Found on Functional Switch Regions.
It is difficult to explain the presence of both activating and silencing histone modifications in recombining SRs. Not all B cells in the ex vivo system complete isotype switching, and it is unknown whether that is because of selective expression of AID or other factors. One explanation for our data could be that “active” H3 acetyl K9 is associated with SRs in the cells that succeed in switching, whereas the “repressive” H3 trimethyl K9 association is in the cells that fail to switch. To answer that question, we examined chromatin from 96-h LPS + IL4-stimulated B cells that were FACS-sorted into IgG1+ and IgG1− populations (Fig. S3A in the SI Appendix). Both H3 trimethyl K9 and acetyl K9 modifications were associated with SRs in cells that had undergone recombination (IgG1+ cells) and those that had not (IgG1−, IgM+) (Fig. S3B in the SI Appendix). This indicated that both the active and repressive marks were associated with the SRs of both populations of cells.
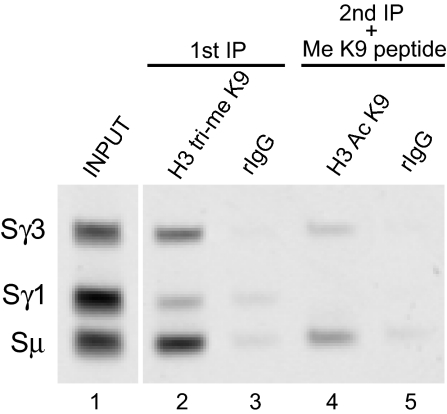
To address whether the active H3 acetyl K9 and silent H3 trimethyl K9 were present on the same or neighboring nucleosomes, we used sequential ChIP to determine whether there were molecules of SR DNA that were simultaneously associated with both modifications. ChIP was first performed with anti-H3 trimethyl K9 or control rabbit IgG antibodies on chromatin derived from LPS-treated B cells. Immunocomplexes were dissociated from the beads, and 10% of the first IP reaction and 100% of the control IP reaction were reverse cross-linked and subjected to PCR analysis (Fig. 5, lanes 2 and 3). The remaining eluate of the first IP reaction was subject to a second ChIP by using anti-H3 acetyl K9 or control rabbit IgG in the presence of H3 trimethyl K9 peptide to inhibit the activity of the first IP antibody. PCR analysis was performed on the second IP eluates. This revealed that in chromatin derived from LPS-treated cells, there were Sγ3 and Sμ DNAs that were associated with both H3 trimethyl K9 and H3 acetyl K9 (Fig. 5, lane 4). Sequential ChIP performed in the opposite direction also confirmed that H3 trimethyl K9 and H3 acetyl K9 coassociate on some molecules of SR DNAs as determined by end point and quantitative PCR (Fig. S4 in the SI Appendix).
Fig. 5.
H3 acetyl K9 and H3 trimethyl K9 coassociate on SR DNAs. Sequential ChIP was performed on chromatin from B cells treated for 72 h with LPS by using anti-H3 trimethyl K9 antibodies, followed by anti-H3 acetyl K9 antibodies or rabbit IgG. The second IP reaction was carried out in the presence of 1 μg/mL H3 trimethyl K9 peptide. PCR was performed by using SR primers. These data are representative of 2 independent experiments.
Because H3 trimethyl K9 is thought to be a repressive mark, we examined whether associated SR DNAs were targets of AID-induced mutations by sequencing a ≈330-nt DNA fragment of Sμ. The Sμ DNAs associated with H3 trimethyl K9 from untreated B cells at 0 h had very few mutated sequences and a very low frequency of mutations in H3 trimethyl K9 (Table 1). Significantly more mutations were detected in Sμ marked by H3 trimethyl K9 after LPS or LPS + IL4 treatment for 96 h. The majority of the mutations were also in AID hot-spot (RGYW/WRCH) motifs. Slightly more transversion mutations were detected, but this did not reach the level of statistical significance. This was compared with the input DNA that represents the total population of Sμ DNAs. Untreated B cells at 0 h had very few mutated sequences and very low levels of mutations (Table 1). With LPS or LPS + IL4 treatment for 96 h, significantly more mutated sequences were detected in input Sμ, with mutation frequencies similar to that reported (13, 22). The majority of mutations were transition mutations at G or C and were in AID hot-spot motifs. Although we tried to do similar experiments with the recipient SRs, they have many fewer mutations (23), and there were too few for us to analyze. Nevertheless, these data indicated that AID-induced mutations were being targeted to Sμ DNAs marked by H3 trimethyl K9 in a stimulation-dependent manner.
Table 1.
Mutation analysis of Sμ sequences
| Treatment | Chromatin fraction | No. of sequences, nt | No. of mutated sequences, % | No. of mutations | Mutation frequency, × 10−4/nt | GC:AT | Ts:Tv |
|---|---|---|---|---|---|---|---|
| Untreated 0 h | Input | 47 (15,698) | 2 (4.3) | 2 | 1.24 | 2:0 | 2:0 |
| Untreated 0 h | H3 tri-me K9 | 49 (16,366) | 3 (6.1) | 3 | 1.83 | 3:0 | 3:0 |
| LPS 96 h | Input | 86 (28,724) | 18* (20.9) | 21 | 7.31* | 11:0 | 11:0 |
| LPS 96 h | H3 tri-me K9 | 90 (30,060) | 21† (23.3) | 26 | 8.65† | 16:1 | 11:6 |
| LPS + IL4 96 h | Input | 92 (30,728) | 12* (13.0) | 18 | 5.85* | 8:2 | 9:1 |
| LPS + IL4 96 h | H3 tri-me K9 | 87 (29,058) | 17† (19.5) | 17 | 5.85 | 6:1 | 5:2 |
Input or anti-H3 trimethyl (tri-me) K9 immunoprecipitated DNAs were subject to high-fidelity PCR with primers for Sμ amplifying a 330-nt fragment. Total mutated sequences are indicated. Mutation frequency is calculated based on total mutations divided by total nucleotides sequenced. Mutations were scored for position at G:C or at A:T. Ts, transitions; Tv, transversions. Fisher's exact test was used to compare the total number of mutated vs. nonmutated sequences between untreated and 96-h-treated cells, and to compare total number of mutated nucleotides vs. nonmutated nucleotides between untreated and 96-h-treated cells.
*P < 0.05 compared with corresponding INPUT fraction at 0 h.
†P < 0.05 compared with corresponding H3 trimethy K9 fraction at 0 h.
Discussion
We set out to determine whether there are chromatin modifications that mark the SRs destined for recombination, and we discovered two such chromatin modifications, H3 acetyl K9 and trimethyl K9. In unstimulated cells and throughout CSR, Sμ is both methylated and acetylated at H3 K9, perhaps serving to identify it as the common donor SR. The individual recipient SR that ultimately recombines is dynamically modified depending on the cytokine treatment: Sγ3 and Sγ2b with LPS stimulation, Sγ1 with LPS + IL4 stimulation. Both modifications become prominent at 48 h, which is coincident with AID expression and the onset of switch recombination (24), and both persist through 96 h. Sterile transcripts alone do not seem sufficient to explain these modifications because in LPS + IL4-treated cells, sterile transcripts are also made at Sγ3, but neither H3 acetyl K9 nor trimethyl K9 modifications of H3 were detected there. In stimulated cells, H3 trimethyl K9 is associated predominantly with the SRs, which are areas that are subject to AID mutation and subsequent recombination (25). In stimulated AID-deficient B cells, both H3 acetyl K9 and H3 trimethyl K9 were associated with the same SRs as in AID-expressing cells. This indicates that the presence of these modifications does not require the mutagenic action of AID or the subsequent repair processes it elicits. It suggests, but does not prove, that these modifications could precede mutation and recombination.
The presence of both activating and silencing histone modifications is not the result of heterogeneous cell populations in the ex vivo culture system, because it is found on both the switched and unswitched cells. Allelic exclusion could be an alternate explanation with H3 acetyl K9 associated with SRs of the productive allele, and H3 trimethyl K9 associated with SRs at the nonproductive locus. However, previous studies show that sterile transcription and isotype switching occur on both alleles and usually to the same isotype (26, 27). In addition, if H3 trimethyl K9 was marking only the excluded allele, one would have expected it to be seen in the constant regions and to be constitutively present rather than induced as it is in the recipient SRs. This suggests that both IgH alleles are accessible and active for CSR, and we are observing histone modifications that are common to both alleles. Importantly, we have also demonstrated that there are SR DNA molecules that are dually modified with H3 acetyl K9 and H3 trimethyl K9. Another possibility is that H3 trimethyl K9 is associated with the SRs within the excised circles, byproducts of successful CSR, and serve to shut down what was originally part of an active gene, whereas H3 acetyl K9 is associated with the SR in the H chain locus that will remain. Our finding of both modifications in the SRs of stimulated AID-deficient B cells that do not isotype switch and thus do not have such excised circles rules out this possibility.
After stimulation, more mutations were detected in H3 trimethyl K9-associated Sμ DNAs, as compared with untreated 0 h, supporting the idea that this histone modification plays a role in promoting CSR. However, this does not prove that H3 trimethyl K9 is a prerequisite for AID mutations. Sμ mutations were not enriched in the H3 trimethyl K9-associated chromatin fraction when compared with the input fraction. This may be because all of the H3 associated with the Sμ regions is H3 K9 trimethylated and is undergoing preparation to serve as a donor in all of the stimulated B cells, but only some of the Sμ regions actually recombine with a particular recipient SR. This is consistent with the finding that many Sμ undergo internal deletion and recombination, perhaps while waiting to recombine with any one of the downstream constant regions (28).
It seems paradoxical to find that both activating and silencing chromatin modifications constitutively mark the active donor μSR and conditionally mark the recipient γ3 or γ1 SRs. H3 acetyl K9 is generally associated with regions of active transcription (8), so its presence at actively transcribed SRs seems appropriate. In contrast, H3 mono-, di-, and trimethyl K9 are generally associated with heterochromatin or inactive genes. However, a number of exceptions have been reported in both primary cells and cancer cells (29, 30). In a study that was just published (31), H3 trimethyl K9 was also found in the SRs of human tonsillar B cells after stimulation to undergo CSR. The trimethylation and acetylation cannot be on a single K9 because they are mutually exclusive. Because the average DNA fragment size in the chromatin that was analyzed was ≈500 bp, more than one nucleosome was usually immunoprecipitated. The two modifications could be present on the two separate H3 molecules within a single nucleosome or on neighboring nucleosomes. In a study using mass spectrometric analysis of H3 peptides from various organisms, H3 trimethyl K9 and acetyl K14, an active mark, were detected on the same H3 peptide in higher organisms such as mouse and human, but not in yeast or tetrahymena (32). In activated T cells in which genome-wide modifications have recently been examined on mononucleosomes, methylation and acetylation of H3 K9 have been observed on a common subset of genes (33).
Based on this study, we propose that both H3 acetyl K9 and trimethyl K9 play a role in targeting the CSR machinery to the donor SR and the correct recipient SR under appropriate cytokine treatments. Their molecular function could be to create accessibility through chromatin relaxation or, perhaps in combination with other modifications, to provide an unusual binding motif for one or more of the factors involved in CSR (34). Recent work in VDJ recombination provides a precedent for such a hypothesis. The PHD domain of RAG2 was found to bind H3 trimethyl K4 peptides and mutation of that PHD domain decreases VDJ recombination efficiency (11, 12). Alternately, the pairing of H3 acetyl K9 and trimethyl K9 may serve to target those subregions of the IgH gene to a special area of the nucleus where many or all of the CSR protein factors are present.
Ascribing cause and effect to histone modifications in mammalian cells is difficult because mutant histones are poorly tolerated. Manipulation of the responsible enzymes would be critical to assess the relative biological importance of these modifications in CSR. Histone acetylases frequently modify multiple lysines, but histone methytransferases tend to be more specific (8). The main contributor to H3 trimethyl K9 at heterochromatin is Suv39h (35). We were unable to identify Suv39h1 by ChIP in our ex vivo primary culture system. Mice deficient in Suv39h1 alone or combined with Suv39h2 deficiency are proficient for switching to most isotypes (36), although it is not known whether H3 trimethyl K9 is associated with SRs in those mice. G9a is the main contributor of H3 K9 methylation in silenced euchromatin (37), and we also attempted ChIP for G9a at SRs and did not find it present. A lymphocyte-specific conditional G9a-deficient mouse was recently generated; and despite significant global and IgH-specific reduction in H3 dimethyl K9, lymphocyte development and VDJ recombination were relatively unperturbed (38). Because each particular state of histone methylation reflects a balance between methylation and demethylation that could be responsible for the H3 trimethyl K9 in SRs, there are many possibilities, including some of which that have yet to be discovered (39). In conclusion, the studies reported here extend earlier findings and begin to reveal the complex pattern of chromatin modifications (9) in the IgH gene that could facilitate the correct targeting for CSR in a context-dependent manner.
Materials and Methods
Animals and Cell Culture.
All animal experiments were approved by the Albert Einstein College of Medicine Animal Use Committee. Barrier facility-housed 8- to 12-week-old wild-type C57BL/6 or AID-deficient (backcrossed to C57BL/6) mice were used. Ex vivo CSR experiments were carried out as in ref. 13 and detailed in the SI Appendix.
Fluorescence Analysis and Sorting.
Isotype switch efficiency was assayed at 96 h by double-staining cultured cells with goat anti-mIgM-FITC, and anti-mIgG1-PE, or anti-mIgG3-PE (1020-02, 1070-09, and 1100-09, respectively; Southern Biotech). For FACS sort experiments, at least 4 × 108 cells in culture were Ficoll-treated, and the live fraction was formaldehyde cross-linked for ChIP. Cross-linked cells were double-stained as above and sorted by using a MoFlo high-speed cell sorter (DakoCytomation). At least 5 × 107 cells were collected per cell population.
ChIP.
ChIP experiments were performed as described in ref. 13 and detailed in the SI Appendix. Sequential ChIP experiments were performed by using 3 times the usual amount of chromatin and ChIP antibody. After ChlP washes, immunocomplexes from the first ChIP were eluted for 30 min at 37 °C by using 10 mM DTT in reverse cross-linking buffer (29). The reaction was quenched with 10 mM iodoacetamide, and the supernatant was transferred to a fresh tube. The second ChIP reaction was performed in the presence of 5 mg/mL BSA (Sigma), 50 μg/mL yeast tRNAs (Sigma), and 25 μg/mL λ phage DNA (NEB) (40) and sometimes in the presence of a 1 μg/mL modified H3 peptide (Upstate and Abcam) that inhibits the binding of the first ChIP antibody. ChIP washes and DNA purification were performed as described in SI Appendix.
Sequencing Sμ Regions.
PCR was performed on ChIP DNA or input chromatin fractions by using a high-fidelity PfuTurbo Cx DNA polymerase (Strategene) and the method described in ref. 13. PCR products were gel-purified (MinElute; Qiagen), cloned (TOPO Blunt; Invitrogen), and sequenced by the Albert Einstein College of Medicine Cancer Center DNA Facility.
Supplementary Material
Acknowledgments.
We thank members of the Scharff and Birshtein laboratories, M. Sadofsky, H. Ye, and B. Diamond for helpful discussions. We thank B. K. Birshtein and A. Skoultchi for critical reading of the manuscript, and T. Honjo for AID-deficient mice. This work was supported by the Albert Einstein College of Medicine Cancer Center Flow Cytometry Core Facility Grant P30CA013330. F.L.K. and Z.L. were supported by the Medical Scientist Training Program T32GM007288; and M.D.S. was supported by National Institutes of Health Grants R01CA72649 and R01CA102705 and by the Harry Eagle Chair provided by the National Women's Division of Albert Einstein College of Medicine.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901368106/DCSupplemental.
References
- 1.Muramatsu M, et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 2.Peled JU, et al. The biochemistry of somatic hypermutation. Annu Rev Immunol. 2008;26:481–511. doi: 10.1146/annurev.immunol.26.021607.090236. [DOI] [PubMed] [Google Scholar]
- 3.Stavnezer J, Guikema JE, Schrader CE. Mechanism and regulation of class switch recombination. Annu Rev Immunol. 2008;26:261–292. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang CL, Wabl M. DNA acrobats of the Ig class switch. J Immunol. 2004;172:5815–5821. doi: 10.4049/jimmunol.172.10.5815. [DOI] [PubMed] [Google Scholar]
- 5.Dunnick W, Hertz GZ, Scappino L, Gritzmacher C. DNA sequences at immunoglobulin switch region recombination sites. Nucleic Acids Res. 1993;21:365–372. doi: 10.1093/nar/21.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nambu Y, et al. Transcription-coupled events associating with immunoglobulin switch region chromatin. Science. 2003;302:2137–2140. doi: 10.1126/science.1092481. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhuri J, et al. Transcription-targeted DNA deamination by the AID antibody diversification enzyme. Nature. 2003;422:726–730. doi: 10.1038/nature01574. [DOI] [PubMed] [Google Scholar]
- 8.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Latham JA, Dent SY. Cross-regulation of histone modifications. Nat Struct Mol Biol. 2007;14:1017–1024. doi: 10.1038/nsmb1307. [DOI] [PubMed] [Google Scholar]
- 10.Cobb RM, Oestreich KJ, Osipovich OA, Oltz EM. Accessibility control of V(D)J recombination. Adv Immunol. 2006;91:45–109. doi: 10.1016/S0065-2776(06)91002-5. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Subrahmanyam R, Chakraborty T, Sen R, Desiderio S. A plant homeodomain in RAG-2 that binds hypermethylated lysine 4 of histone H3 is necessary for efficient antigen receptor gene rearrangement. Immunity. 2007;27:561–571. doi: 10.1016/j.immuni.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthews AG, et al. RAG2 PHD finger couples histone H3 lysine 4 trimethylation with V(D)J recombination. Nature. 2007;450:1106–1110. doi: 10.1038/nature06431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z, Luo Z, Scharff MD. Differential regulation of histone acetylation and generation of mutations in switch regions is associated with Ig class switching. Proc Natl Acad Sci USA. 2004;101:15428–15433. doi: 10.1073/pnas.0406827101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L, Whang N, Wuerffel R, Kenter AL. AID-dependent histone acetylation is detected in immunoglobulin S regions. J Exp Med. 2006;203:215–226. doi: 10.1084/jem.20051774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen S, et al. AID is required to initiate Nbs1/γ-H2AX focus formation and mutations at sites of class switching. Nature. 2001;414:660–665. doi: 10.1038/414660a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Odegard VH, Kim ST, Anderson SM, Shlomchik MJ, Schatz DG. Histone modifications associated with somatic hypermutation. Immunity. 2005;23:101–110. doi: 10.1016/j.immuni.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Wuerffel R, et al. S-S synapsis during class switch recombination is promoted by distantly located transcriptional elements and activation-induced deaminase. Immunity. 2007;27:711–722. doi: 10.1016/j.immuni.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Layton JE, Vitetta ES, Uhr JW, Krammer PH. Clonal analysis of B cells induced to secrete IgG by T cell-derived lymphokine(s) J Exp Med. 1984;160:1850–1863. doi: 10.1084/jem.160.6.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ChuCC, Max EE, Paul WE. DNA rearrangement can account for in vitro switching to IgG1. J Exp Med. 1993;178:1381–1390. doi: 10.1084/jem.178.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roa S, et al. Ubiquitylated PCNA plays a role in somatic hypermutation and class switch recombination and is required for meiotic progression. Proc Natl Acad Sci USA. 2008;105:16248–16253. doi: 10.1073/pnas.0808182105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lutzker S, Rothman P, Pollock R, Coffman R, Alt FW. Mitogen- and IL4-regulated expression of germ-line Igγ2b transcripts: Evidence for directed heavy chain class switching. Cell. 1988;53:177–184. doi: 10.1016/0092-8674(88)90379-0. [DOI] [PubMed] [Google Scholar]
- 22.Nagaoka H, Muramatsu M, Yamamura N, Kinoshita K, Honjo T. Activation-induced deaminase (AID)-directed hypermutation in the immunoglobulin Sμ region: Implication of AID involvement in a common step of class switch recombination and somatic hypermutation. J Exp Med. 2002;195:529–534. doi: 10.1084/jem.20012144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schrader CE, et al. Mutations occur in the Ig Sμ region but rarely in Sγ regions prior to class switch recombination. EMBO J. 2003;22:5893–5903. doi: 10.1093/emboj/cdg550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schrader CE, Linehan EK, Mochegova SN, Woodland RT, Stavnezer J. Inducible DNA breaks in Ig S regions are dependent on AID and UNG. J Exp Med. 2005;202:561–568. doi: 10.1084/jem.20050872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xue K, Rada C, Neuberger MS. The in vivo pattern of AID targeting to immunoglobulin switch regions deduced from mutation spectra in msh2−/− ung−/− mice. J Exp Med. 2006;203:2085–2094. doi: 10.1084/jem.20061067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delpy L, Le Bert M, Cogne M, Khamlichi AA. Germ-line transcription occurs on both the functional and the nonfunctional alleles of immunoglobulin constant heavy chain genes. Eur J Immunol. 2003;33:2108–2113. doi: 10.1002/eji.200323969. [DOI] [PubMed] [Google Scholar]
- 27.Radbruch A, Muller W, Rajewsky K. Class switch recombination is IgG1-specific on active and inactive IgH loci of IgG1-secreting B cell blasts. Proc Natl Acad Sci USA. 1986;83:3954–3957. doi: 10.1073/pnas.83.11.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dudley DD, et al. Internal IgH class switch region deletions are position-independent and enhanced by AID expression. Proc Natl Acad Sci USA. 2002;99:9984–9989. doi: 10.1073/pnas.152333499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vakoc CR, Mandat SA, Olenchock BA, Blobel GA. Histone H3 lysine 9 methylation and HP1γ are associated with transcription elongation through mammalian chromatin. Mol Cell. 2005;19:381–391. doi: 10.1016/j.molcel.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Wiencke JK, Zheng S, Morrison Z, Yeh RF. Differentially expressed genes are marked by histone 3 lysine 9 trimethylation in human cancer cells. Oncogene. 2008;27:2412–2421. doi: 10.1038/sj.onc.1210895. [DOI] [PubMed] [Google Scholar]
- 31.Chowdhury M, et al. Analysis of intergenic transcription and histone modification across the human immunoglobulin heavy-chain locus. Proc Natl Acad Sci USA. 2008;105:15872–15877. doi: 10.1073/pnas.0808462105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia BA, et al. Organismal differences in post-translational modifications in histones H3 and H4. J Biol Chem. 2007;282:7641–7655. doi: 10.1074/jbc.M607900200. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, et al. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat Genet. 2008;40:897–903. doi: 10.1038/ng.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ta VT, et al. AID mutant analyses indicate requirement for class switch-specific cofactors. Nat Immunol. 2003;4:843–848. doi: 10.1038/ni964. [DOI] [PubMed] [Google Scholar]
- 35.Peters AH, et al. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell. 2001;107:323–337. doi: 10.1016/s0092-8674(01)00542-6. [DOI] [PubMed] [Google Scholar]
- 36.Bradley SP, Kaminski DA, Peters AH, Jenuwein T, Stavnezer J. The histone methyltransferase Suv39h1 increases class switch recombination specifically to IgA. J Immunol. 2006;177:1179–1188. doi: 10.4049/jimmunol.177.2.1179. [DOI] [PubMed] [Google Scholar]
- 37.Tachibana M, et al. G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev. 2002;16:1779–1791. doi: 10.1101/gad.989402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas LR, et al. Functional analysis of histone methyltransferase g9a in B and T lymphocytes. J Immunol. 2008;181:485–493. doi: 10.4049/jimmunol.181.1.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi Y. Histone lysine demethylases: Emerging roles in development, physiology and disease. Nat Rev Genet. 2007;8:829–833. doi: 10.1038/nrg2218. [DOI] [PubMed] [Google Scholar]
- 40.Geisberg JV, Struhl K. Current Protocols in Molecular Biology. New York: Wiley; 2005. Analysis of protein cooccupancy by quantitative sequential chromatin immunoprecipitation. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.