Abstract
Bidirectional synaptic plasticity during development ensures that appropriate synapses in the brain are strengthened and maintained while inappropriate connections are weakened and eliminated. This plasticity is well illustrated in mouse visual cortex, where monocular deprivation during early postnatal development leads to a rapid depression of inputs from the deprived eye and a delayed strengthening of inputs from the non-deprived eye. The mechanisms that control these bidirectional synaptic modifications remain controversial. Here we demonstrate, both in vitro and in vivo, that genetic deletion or reduction of the NR2A NMDA receptor subunit impairs activity-dependent weakening of synapses and enhances the strengthening of synapses. Although brief monocular deprivation in juvenile WT mice normally causes a profound depression of the deprived-eye response without a change in the non-deprived eye response, NR2A-knockout mice fail to exhibit deprivation-induced depression and instead exhibit precocious potentiation of the non-deprived eye inputs. These data support the hypothesis that a reduction in the NR2A/B ratio during monocular deprivation is permissive for the compensatory potentiation of non-deprived inputs.
Keywords: metaplasticity, synaptic homeostasis, synaptic scaling, visual evoked potential, BCM theory
The circuitry of primary visual cortex is susceptible to changes in sensory experience during early postnatal development, as evidenced by the well studied paradigm of monocular deprivation (MD) (1). MD and reverse occlusion studies demonstrate that the strength of synapses is bidirectionally modifiable (2–4). A detailed time course of the synaptic events following MD in mice shows that the initial consequence is a rapid depression of the deprived-eye inputs followed by a delayed strengthening of the non-deprived eye inputs (5). However, little is known about the molecular mechanisms that regulate the susceptibility of synapses to bidirectional modifications in their strength.
Bidirectional synaptic plasticity has been studied in slice recordings of visual cortex in the form of long-term potentiation (LTP) and long-term depression (LTD), whereby synapses strengthen and weaken in response to stimulation (6). These activity-dependent modifications can be modeled by a learning rule whereby high levels of post-synaptic activation (evoked electrically by high-frequency stimulation) induce LTP and smaller levels of post-synaptic activation (evoked electrically by lower-frequency stimulation) induce LTD (7). The crossover point from synaptic weakening to strengthening is called the modification threshold (θm). An important feature of this model is that the value of θm is not fixed; rather, its value can “slide” as a function of the history of post-synaptic activation. According to the BCM theory, closing the dominant contralateral eye first leads to depression of the deprived synapses, followed by a leftward shift in θm caused by the reduction in average cortical activity. This shift in θm is permissive for the subsequent increase in the responses to the non-deprived, ipsilateral eye (5).
A wealth of data now indicate that deprivation and experience during early postnatal development can indeed modify the plasticity threshold. For example, a period of complete darkness lowers the plasticity threshold such that LTP is enhanced and LTD is attenuated across a range of stimulation frequencies (8–10). These observations demonstrate that the susceptibility of synapses to plastic changes in visual cortex modifies in relation to their history of experience-driven activity.
Data suggest that the shift in the θm is caused by a change in NMDA receptor (NMDAR) function (9), and regulation of the molecular composition of the NMDA receptor provides a powerful means to achieve this change. The NMDA receptor is a heteromer that contains the obligatory NR1 subunit and a mixture of NR2A-D subunits that alter receptor properties (11, 12). At birth, most cortical NMDARs contain the NR2B subunit (11). NR2A subunit levels gradually increase with development and reach a maximal expression between the peak and end of juvenile plasticity (13, 14). This switch from predominantly NR2B to NR2A subtypes is experience-dependent and reflects the recent history of visual experience (15–17). During MD, after the initial depression of deprived-eye responses, there is a transient reduction in the NR2A/B ratio that slightly precedes open-eye response potentiation (18). Because lowering the NR2A/B ratio reduces the threshold for inducing LTP in mouse visual cortex (10), it has been proposed that activity-dependent regulation of NR2A and/or NR2B receptor expression is the molecular basis for the “sliding” θm.
In the current study we examined the connection between NMDAR subunit composition and the qualities of bidirectional synaptic plasticity in the visual cortex of NR2A KO, heterozygote (Het), and WT mice. We confirm in layer IV that reducing NR2A expression shifts to lower frequencies both the LTP threshold and the optimal stimulation for LTD. In response to MD, visually evoked potentials (VEPs) evoked in vivo through the deprived eye fail to depress normally in NR2A mutants. Instead, an ocular dominance shift occurs by precocious potentiation of responses through the non-deprived eye. These data support the hypothesis that experience-dependent modifications in the NR2A/B ratio at synapses provides a powerful in vivo mechanism for regulating subsequent induction of plasticity.
Results
Effect of NR2A Gene Dosage on the Synaptic θm in Layer IV of Mouse Visual Cortex.
The goal of this study was to determine how decreasing the NR2A/B ratio alters the LTD/LTP θm in vitro and compare this with changes in the properties of naturally occurring plasticity in the visual cortex in vivo as a consequence of MD. We examined this question using mice with targeted disruption of one or both alleles of the NR2A gene (19). Because NR2A mutant mice do not display compensatory alterations in NR1 or NR2B subunit expression in visual cortex at the ages of interest, reducing NR2A expression effectively changes the NR2A/B ratio (10).
The bidirectional changes in visual responsiveness that occur after MD were established using VEPs recorded in layer IV of visual cortex (5). Current source density (CSD) analysis in vivo has confirmed that changes in the amplitude of layer IV VEPs reflect changes in synaptic current sinks in this layer (20, 21). However, previous studies of LTP and LTD in NR2A mutant mice were performed in layer III (10), and it is now understood that there are significant laminar differences in the mechanisms of visual cortical plasticity in mice (21, 22). Therefore, our study began with an analysis of the effect of NR2A gene dosage on synaptic plasticity in layer IV of slices of mouse visual cortex. A CSD analysis confirmed that the negative extracellular field potential (FP) evoked in layer IV of mouse visual cortex by white matter (WM) stimulation in vitro reflects a current sink qualitatively similar to the VEP in vivo [supporting information (SI) Fig. S1]. Therefore, we attempted to replicate, in layer IV FPs, the effects previously described in layer III of reducing NR2A on the LTD/LTP threshold (10).
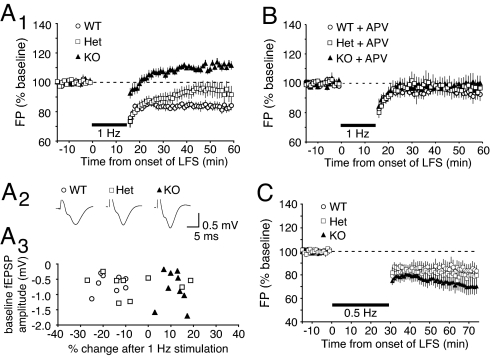
To test whether the plasticity threshold was altered by reducing NR2A gene dosage, we examined the consequences of a stimulation protocol (1 Hz for 15 min) in KO and Het mutants that typically result in LTD in normally reared WT mice (aged between postnatal days 21 and 28). Following collection of a baseline, 1 Hz stimulation produced reliable depression in WT mice (Fig. 1A; 83.03% ± 2.56% of baseline, n = 8 slices from 7 mice). However, as previously shown in layer III of the KO mouse, we discovered that 1 Hz stimulation causes LTP of layer IV FP amplitudes in mice lacking NR2A (Fig. 1A; 111.39% ± 2.33% of baseline, n = 9 slices from 7 mice). Moreover, in the NR2A Het mice, 1 Hz stimulation resulted in a modest depression of synapses (Fig. 1A; 93.77% ± 5.33% of baseline, n = 9 slices from 7 mice) that was intermediate between the WT (P = 0.034) and NR2A KO (P = 0.001) values. Importantly, basal synaptic transmission was comparable between genotypes (Fig. 1A), and there was no correlation between baseline FP amplitude and the percent change in synaptic transmission following 1 Hz stimulation (Fig. 1A). There was also no effect of genotype on AMPA receptor-mediated miniature excitatory post-synaptic currents (mEPSCs) in layer IV neurons (NR2A KO, 18.58 pA ± 1.76, n = 10 neurons from 3 mice; WT, 18.90 pA ± 1.30, n = 13 neurons from 3 mice). Together, these results support previous conclusions that that the LTD/LTP threshold is proportional to the level of NR2A expression in mouse visual cortex (10).
Fig. 1.
Loss of NR2A lowers the LTP threshold and the optimal LTD stimulation frequency. (A1) Averaged data (±SEM) demonstrate that 1 Hz stimulation (900 pulses) induces LTD in WT and Het mice and LTP in NR2A KO mice. (A2) Baseline waveforms averaged across all individual experiments summarized in A1 (WT, n = 8; Het, n = 9; KO, n = 7). Note that averaged waveforms are of comparable size and shape in all 3 genotypes. (A3) Scatter plot of individual baseline FP amplitudes of each genotype and percent change of synaptic transmission following 1 Hz stimulation. Note that the effect of 1 Hz stimulation is not correlated with initial response amplitude. (B) Averaged data demonstrating that bath-applied APV prevents the effects of 1 Hz stimulation in all genotypes. (C) Averaged data demonstrate that stimulation at 0.5 Hz (900 pulses) yields LTD in all genotypes, with maximal effect in NR2A KO mice.
To confirm that the plasticity observed in the NR2A mutants was still NMDAR-dependent, we repeated the experiment in the presence of the competitive NMDAR antagonist D-2-amino-5-phosphonopentanoic acid (APV; 50 μM). In addition to the expected effect of APV on LTD in WT mice (n = 5 slices from 3 mice) (22), we found that blocking NMDARs prevented both the residual LTD in the Het mice (n = 5 slices from 3 mice) and the LTP induced by 1 Hz stimulation in the KO animals (n = 5 slices from 3 mice; Fig. 1D).
Finally, to confirm that reducing NR2A caused a change in the induction requirements for LTD/P rather than a dose-dependent loss of LTD, we repeated the experiment using 0.5 Hz stimulation—a frequency that was shown previously to be optimal for LTD induction in visually deprived animals (9). We found that 900 pulses at 0.5 Hz elicited reliable and statistically significant depression in mice of all genotypes, with the greatest effect in the NR2A KO mice (Fig. 1; NR2A KO, 70.56% ± 6.41% of baseline, n = 10 slices from 5 mice; Het, 79.05% ± 6.86%, n = 6 slices from 3 mice; WT, 84.43% ± 5.64%, n = 6 slices from 3 mice). Taken together, these data lead us to conclude that lowering the NR2A/B ratio shifts the stimulation-response curve to the left, and the degree of this shift is proportional to the amount of NR2A present in visual cortex.
Effect of NR2A Gene Dosage on the Ocular Dominance Shift Following MD in Layer IV of Mouse Visual Cortex.
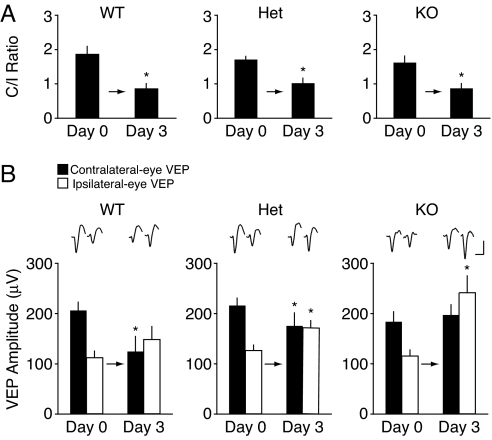
We next examined the impact of altered NR2A and synaptic plasticity on ocular dominance plasticity in layer IV of mouse visual cortex. Electrodes were chronically implanted in layer IV of the binocular zone in primary visual cortex. Baseline VEPs were measured at postnatal days 27 to 29, and the eyelid of the eye contralateral to the experimental hemisphere was sutured closed. After 3 days of MD, the sutured eye was opened, the animal was allowed to recover from anesthesia, and VEPs were again recorded. We assessed ocular dominance plasticity by determining the ratio of contralateral to ipsilateral eye responses (C/I ratio), which is normally approximately 2:1 at baseline and decreases after MD to approximately 1:1. Our results show that NR2A KO and Het mice, as well as their WT litter-mates, exhibit a normal shift in the C/I ratio (Fig. 2A; day 0, 1.67 ± 0.21; day 3, 0.89 ± 0.13 in KO, n = 8, P = 0.01; day 0, 1.75 ± 0.09; day 3, 1.05 ± 0.14 in Het, n = 9, P < 0.01; day 0, 1.95 ± 0.22; day 3, 0.90 ± 0.13 in WT, n = 10, P < 0.001), similar to what has been reported previously (23). The degree of the shift is indistinguishable among the 3 genotypes (Kruskal-Wallis test, n = 27; P = 0.81).
Fig. 2.
The ocular dominance shift following 3 days of MD is qualitatively different in NR2A KO, Het, and WT mice. (A) There is a significant decrease in C/I ratios of WT (n = 10), Het (n = 9), and KO (n = 8) mice following 3 days of MD. Average values of C/I ratios (±SEM) are plotted. Asterisks indicate P < 0.01. (B) Day 0 and day 3 waveforms, averaged across all individual experiments. (Scale bar: 100 ms, 100 μV.) Average trough-to-peak amplitude (±SEM, n = 10) of VEPs in WT mice in response to deprived eye (filled bars) and non-deprived eye (open bars) stimulation during baseline (day 0) and after 3 days of MD. There is a significant decrease in the deprived-eye VEP amplitude and no change in the non-deprived eye VEP amplitude (Left). Average amplitude (±SEM, n = 9) of VEPs in Het mice in response to deprived-eye (filled bars) and non-deprived eye (open bars) stimulation during baseline (day 0) and after 3 days of MD. There is a significant decrease in the deprived-eye VEP amplitude and significant increase in the non-deprived eye VEP amplitude (Middle). Average amplitude (±SEM, n = 8) of VEPs in KO mice in response to deprived-eye and non-deprived eye stimulation during baseline (day 0) and after 3 days of MD. No change in deprived-eye VEP amplitude is observed, but the non-deprived eye VEP amplitude is significantly increased (Right). Average amplitude (±SEM) of VEPs in WT mice (Left), Het (Middle), and KO mice (Right) in response to deprived-eye and non-deprived eye stimulation after 3 days of normal visual experience are comparable to baseline values. Asterisks indicate P < 0.05.
However, upon closer examination of the deprived and non-deprived eye responses, we discovered profound differences in the qualities of the ocular dominance shift between the genotypes (Fig. 2B). As previously reported (5), we found that deprived-eye responses in WT mice were significantly depressed (Fig. 2B; day 0, 211.2 ± 17.1 μV; day 3, 127.6 ± 30.5 μV, n = 10, P = 0.002), and non-deprived eye responses remained at baseline levels (day 0, 114.5 ± 13.2 μV; day 3, 151.9 ± 26.4 μV, n = 10, P = 0.07). In stark contrast to WT mice, the deprived-eye responses in NR2A KO mice were unchanged (Fig. 2B; day 0, 188.2 ± 19.7 μV; day 3, 202.6 ± 21.4 μV, n = 8, P = 0.55), whereas the non-deprived eye responses dramatically potentiated (day 0, 118.5 ± 11.6 μV; day 3, 247.9 ± 34.3 μV, n = 11, P = 0.005). Results in the Het mice were intermediate: there was still a significant depression of the deprived eye (Fig. 2B; day 0, 221.3 ± 14.7 μV; day 3, 180.0 ± 26.5 μV, n = 9, P = 0.04) and a slight but statistically significant potentiation of the non-deprived eye responses (day 0, 129.1 ± 10.4 μV; day 3, 175.2 ± 14.9 μV, n = 9, P = 0.03).
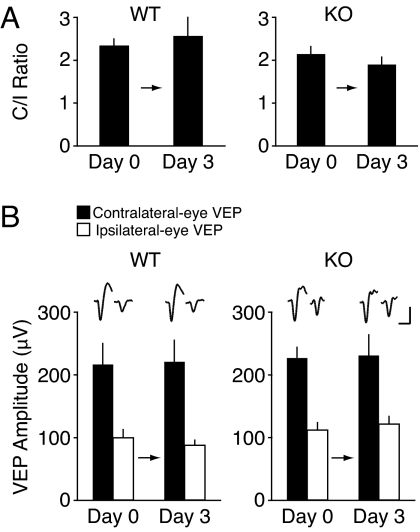
These findings are consistent with the idea that reducing the NR2A/B ratio promotes the deprivation-induced adjustment of the BCM θm, and thereby enhances open-eye response potentiation and reduces deprived-eye response depression in vivo, similar to what we observed in the slice experiments. However, an alternative explanation is that the shift occurs normally, but is superimposed on an exaggerated global upward scaling of responses caused by visual deprivation. To investigate the possibility of enhanced synaptic scaling in response to deprivation, we recorded VEPs before and after 3 days of binocular lid suture in NR2A KO and WT litter-mates. An increased homeostatic scaling response should lead to substantially increased visual responses after binocular deprivation (BD).
Our results show that the C/I ratios of both NR2A KO and WT do not change following this visual manipulation (Fig. 3A; day 0, 2.16 ± 0.19; day 3, 1.92 ± 0.20 in KO, n = 7; day 0, 2.34 ± 0.15; day 3, 2.57 ± 0.43 in WT, n = 7; P = 0.31). More importantly, BD did not affect the VEP amplitudes of contralaterally projecting eyes (Fig. 3B; day 0, 228.4 ± 16.2 μV; day 3, 232.0 ± 35.5 μV in KO, n = 7; day 0, 236.5 ± 35.7 μV; day 3, 220.8 ± 35.1 μV in WT, n = 7; P = 0.72) nor the VEP amplitudes of ipsilaterally projecting eyes (Fig. 3B; day 0, 111.9 ± 11.5 μV; day 3, 121.6 ± 12.0 μV in KO, n = 7; day 0, 99.2 ± 12.2 μV; day 3, 87.0 ± 6.8 μV in WT, n = 7; P = 0.26). These data indicate that reduction of NR2A does not promote synaptic scaling in response to 3 days of visual deprivation.
Fig. 3.
No evidence of synaptic scaling is seen following 3 days of BD in NR2A KO and WT mice. (A) Three days of BD fail to modify the C/I ratio of VEPs in either WT or NR2A KO mice. (B) Neither the ipsilateral nor contralateral eye VEP responses are modified by 3 days of BD in WT and NR2A KO mice. The scale for the averaged waveforms is 100 ms, 100 μV.
Open-Eye Potentiation in WT Mice Requires NMDAR Activation.
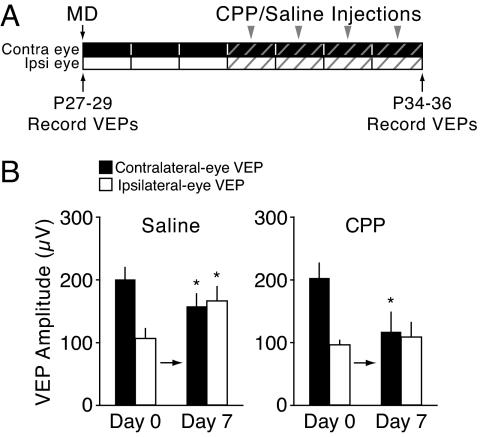
In WT mice, MD for >5 days causes potentiation of visual responses that we hypothesize is enabled by a deprivation-induced decrease in the NR2A/B ratio (18). This hypothesis rests on the assumption that response potentiation is an NMDAR-dependent form of Hebbian synaptic plasticity (24, 25). The alternative hypothesis is that responses increase by global upward scaling (26), a process that has been shown to be independent of NMDAR activation (27). To distinguish among these hypotheses, we designed experiments in which NMDARs were blocked pharmacologically during the time span when response potentiation occurs (Fig. 4A).
Fig. 4.
Ipsilateral-eye response potentiation following 7 days of monocular deprivation is NMDAR-dependent in juvenile WT mice. (A) Juvenile mice were treated with saline solution or CPP for the last 4 days of a 7-day MD. (B) Ipsilateral eye potentiation was blocked in mice treated with CPP.
Following 3 days of MD, which allowed for deprived-eye depression, either (R, S)-3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid (CPP; 10 mg/kg) or saline solution were injected i.p. every 6 h over the course of 4 additional days of MD. The saline solution controls showed a normal response to 7 days of MD. First, deprived-eye responses were significantly depressed relative to baseline (Fig. 4B; day 0, 198.2 ± 16.6 μV; day 7, 155.4 ± 17.7 μV, n = 7, P = 0.007), but as described in previous studies (5), this depression was less than that observed after 3 days of MD (cf. Fig. 2B, WT). Second, open-eye responses were significantly potentiated (Fig. 4B; day 0, 104.7 ± 13.0 μV; day 7, 163.0 ± 21.0 μV, n = 7, P = 0.035). In contrast, the mice that received CPP injections exhibited deprived-eye depression similar to what is obtained after 3 days of MD (Fig. 4B; day 0, 200.4 ± 22.8 μV; day 7, 115.7 ± 31.8 μV, n = 7, P = 0.004), and the ipsilateral eye responses remained unchanged (Fig. 4B; day 0, 94.8 ± 6.1 μV; day 7, 106.3 ± 24.9 μV, n = 7, P = 0.62). The blockade of response potentiation with an NMDAR antagonist is not consistent with the scaling hypothesis.
Discussion
Our data show that even a graded reduction of the NR2A subunit can dramatically alter the qualities of NMDAR-dependent bidirectional synaptic plasticity in layer IV of visual cortex. Reduced NR2A expression shifts the LTD/LTP threshold to the left; consequently, some stimulation frequencies that would normally lead to LTD cause LTP instead. In vivo, the patterns of synaptic activity that normally cause depression of responses from the deprived eye no longer have that effect, and the patterns of synaptic activity through the open eye that normally have no effect cause precocious potentiation of responses instead. Our results are consistent with the hypothesis that the NR2A/B ratio specifies the value of the synaptic θm that choreographs the bidirectional cortical response to monocular deprivation.
A considerable body of work in the visual cortex has shown how the subunit composition of NMDARs varies during the course of early postnatal development and after periods of visual deprivation. As the cortex matures, the NR2A/B ratio progressively increases, reaching an asymptote at approximately the time of adolescence. This developmental profile is at least partially experience-dependent, as even brief episodes of visual deprivation can reversibly lower the NR2A/B ratio. Changes in NR2A and NR2B expression also occur during the course of MD. In the hemisphere contralateral to the deprived eye, the NR2A/B ratio is significantly reduced after 5 days of MD (18).
Three primary theories have been advanced in the literature regarding the possible significance of NMDAR subunit composition changes: (i) the subunit switch might bring the classically defined critical period for ocular dominance plasticity to a close (28), (ii) an increase in NR2A might favor the induction of LTP versus LTD (29, 30), or (iii) the activity-dependent increase in the NR2A/B ratio adjusts the threshold for synaptic plasticity and facilitates the refinement of receptive field properties in juvenile subjects (15–17, 31).
The idea that the NMDAR subunit switch might bring the critical period to a close was attractive because the timing of the NMDAR subunit switch seemed to coincide with a reduction in NMDAR function and the end of the critical period. However, closer examination demonstrated that NR2A levels in layer IV are maximal during the period of maximal plasticity, not at the end, suggesting that the increase in NR2A is not the ultimate signal for terminating juvenile ocular dominance plasticity (13; however, see ref. 32). Moreover, NR2A KO mice continue to exhibit an age-dependent decline in ocular dominance plasticity (23), corroborating findings in the somatosensory cortex (33).
The second putative role for NMDAR subunits was that NR2A-containing receptors were a requirement for the induction of LTP, whereas NR2B receptors were a requirement for the induction of LTD (29, 30). This possibility was attractive because it provided a simple mechanism to describe the developmental loss of NMDAR-dependent LTD observed in many regions of the brain. However, the validity of these findings is now being questioned because these studies were conducted using non-specific concentrations of NR2A-selective antagonists (34). Moreover, recent data contradict the initial findings that NR2A and NR2B play distinct roles in regulating the polarity of synaptic plasticity (35–39). Finally, accumulating evidence (10, 33, 36, 40), including findings from the present study, demonstrate that LTP can be induced in NR2A KO mice, suggesting that a synaptic requirement of NR2A for LTP is overly simplistic.
The current findings best fit the theory that NMDAR subunit composition regulates a sliding threshold for bidirectional synaptic plasticity (7, 31). As previously demonstrated in layer III (10), we find in layer IV that reducing NR2A expression shifts the optimal LTD stimulation frequency leftward and enables LTP at low stimulation frequencies. It has been suggested previously that the decrease in NR2A/B protein that normally occurs between 3 and 5 days of MD enables the potentiation of the non-deprived eye by shifting the θm to the left (18). Our finding of reduced deprived-eye depression and precocious open-eye potentiation after 3 days of MD in the Het and KO animals are consistent with this theory. However, rather than setting the threshold per se, reducing NR2A appears to remove a constraint on how fast it can adjust, so that 3 days of contralateral-eye MD is sufficient to cause potentiation of the ipsilateral eye responses. Additional mechanisms for adjusting the threshold independently of NR2A could include regulation of NR2B (18) and/or the total number of NMDARs at the synapse (10), among other possibilities (41, 42).
The current data are relevant to the recent debate over whether the compensatory potentiation of the non-deprived eye after MD reflects a process analogous to input-specific LTP enabled by metaplastic adjustment of the θm (5, 20), or a cell-wide process of homeostatic synaptic scaling (26, 43). Scaling is a phenomenon that does not require NMDAR activation (27), so the OD plasticity phenotype in the NR2A mutant mice is unlikely to result from altered scaling. Moreover, consistent with findings in adult mice (20, 44), we find that the response potentiation caused by 7 days of MD in juvenile mice requires NMDAR activation. Therefore, the current findings implicate metaplasticity rather than scaling as the mechanism for deprivation-induced response potentiation, at least in layer IV.
In conclusion, our data support the hypothesis that the experience-dependent regulation of the NR2A/B ratio is critical for adjusting the threshold for synaptic modifications, both in vitro and in vivo. These data suggest that lowering the NR2A/B ratio might provide a permissive milieu for strengthening weak cortical inputs. An exciting possibility is that manipulation of this ratio, either experientially or pharmacologically, could be exploited therapeutically to promote synaptic rewiring after brain injury or disease.
Materials and Methods
Subjects.
Mice deficient in NR2A were supplied by S. Nakanishi (Kyoto, Japan). The mice were developed by replacing the region spanning the M2 transmembrane segment of NR2A subunits with the neomycin resistance gene as previously described (19). A pathogen-free line was re-derived on a C57BL/6 background by Charles River Laboratories. WT (+/+), heterozygote (+/-), and NR2A-KO (-/-) mice were used between postnatal days 21 and 28 for in vitro experiments and between postnatal days 24 and 36 for in vivo experiments. Subjects were fed ad libitum and reared in normal lighting conditions (12 h/12 h light/dark cycle). There was no significant difference in AMPA receptor-mediated responses across genotypes, as evidenced by the facts that (i) the baseline VEP amplitudes were not different (Figs. 2B and 3B), (ii) the baseline FPs evoked in layer IV by WM stimulation were not different (Fig. 1A), and (iii) the stimulation intensities required to evoke a half-maximal FPs was not different. Whole-cell recordings of AMPA/NMDAR ratios in layer IV cells revealed no difference between KO and WT, suggesting a normal level of NMDAR expression at these ages (data not shown). As described previously, changes in NR2A gene dosage systematically alter NR2A protein and the properties of NMDAR-mediated synaptic currents in visual cortex (10).
Cortical Slice Preparation.
Following an overdose of barbiturates (i.p.), mice were decapitated upon disappearance of corneal reflexes in compliance with the US Department of Health and Human Services. The brain was rapidly removed and immersed in ice-cold dissection buffer (composition in mM, NaCl, 87; KCl, 2.5; NaH2PO4, 1.25; NaHCO3, 25; sucrose, 75; dextrose, 10; ascorbic acid, 1.3; MgCl2, 7; and CaCl2, 0.5) bubbled with 95% O2 and 5% CO2. The visual cortex was rapidly removed and 350-μm coronal slices were cut using a vibrating microtome (model VT100S; Leica). Slices recovered for 15 min in a submersion chamber at 32 °C filled with warmed artificial cerebral spinal fluid (ACSF; 124 mM NaCl, 5 mM KCl, 1.25 mM Na2PO4, 26 mM NaHCO3, 1 mM MgCl2, 2 mM CaCl2, and 10 mM dextrose, saturated with 95% O2, 5% CO2) and then cooled gradually to room temperature until use.
Extracellular Electrophysiology.
Slices were transferred to an interface recording chamber maintained at 30 °C and perfused with ACSF at a rate of 2.5 mL/min. A stimulation electrode (concentric bipolar tungsten) was positioned in layer 6/WM, and a glass recording electrode (≈1 MΩ) filled with ACSF was positioned in layer IV. The magnitude of responses evoked by a 200-μs pulse was monitored by the amplitude of the FP. Stimulation intensity was adjusted to elicit half the maximal response, and stable baseline responses were elicited every 30 s. The resulting signals were filtered between 0.1 Hz and 3 kHz, amplified 1,000 times, and captured at 10 kHz on an IBM-compatible computer using pCLAMP 9.2 software (Molecular Devices). After achieving a stable baseline (<5% drift) for 15 min, slices were stimulated with 900 pulses at 1 Hz or with 900 pulses at 0.5 Hz. FP amplitudes were recorded every 30 seconds for 45 min following the cessation of the stimulation protocol. The concentration used for bath application of D-APV was 50 μM. Control and experimental subjects were run in an interleaved fashion. Objective criteria (baseline drifts ≤5% and proper waveform alignment) were applied as inclusion criteria for further analysis. The data were normalized, averaged, and reported as mean ± SEM. Changes in synaptic strength were measured by comparing the average response amplitude 35 to 45 min after conditioning stimulation to the preconditioning baseline response.
CSD Analysis.
CSD analysis was performed to determine the spatio-temporal pattern of current sinks and sources evoked in layer IV by biphasic stimulation at the layer VI/WM boundary of primary visual cortex. The glass recording electrode (≈1 MΩ) filled with ACSF was tracked down through the layers in 100-μm steps. At each recording depth, ten 200-μsec pulses were delivered by biphasic stimulation (Isolated Pulse Stimulator model 2100; A-M Systems) and the responses were averaged. At the completion of the recording session, the recording electrode was lifted along the z-plane and its tip immersed in FluoSphere polystyrene microspheres and returned to its recording site to verify layer IV localization. The section was then mounted on gelatin-coated slides and fluorescently stained for Nissl substance (Neurotrace; Molecular Probes).
From the FPs collected, the corresponding one-dimensional (i.e., depth) CSD profile was constructed according to the method described by Mitzdorf (45) by using a spatial differentiation grid of 200 μm. A full account of the theoretical basis of CSD analysis has previously been presented (45, 46).
mEPSC Recordings.
Slices were maintained in ACSF containing (in mM) 124 NaCl, 3 KCl, 1.25 Na2PO4, 26 NaHCO3, 1 MgCl2, 2 CaCl2, and 20 D-glucose, saturated with 95% O2 and 5% CO2 (315 mOsm, pH 7.25). Recording electrodes were filled with internal containing (in mM) 20 KCl, 100 (K)gluconate, 10 Hepes, 4 (Mg)ATP, 0.3 (Na)GTP, and 10 (Na)phosphocreatine with pH adjusted to 7.25 and osmolarity adjusted to 300 mOsm. AMPA receptor-mediated mEPSCs were recorded in the presence of blockers for voltage-gated sodium channels (tetrodotoxin; 200 nM), GABAA receptors (picrotoxin; 50 μM), and NMDARs (D,L-APV; 100 μM). To further block NMDAR currents, the internal recording solution contained 1 μM MK801 and mEPSCs were recorded at negative holding potentials (−80 mV). Events were first identified using an automatic template detection program (pCLAMP; Molecular Devices) and then manually verified so that only events with a monotonic rise time and exponential decay were included in the analysis. More than 100 events were analyzed for each data point for each cell.
In Vivo Electrophysiology.
VEP recordings were conducted in awake mice as described previously (5). Mice were anesthetized with 50 mg/kg ketamine and 10 mg/kg xylazine i.p. Tungsten micro-electrodes (FHC) were chronically implanted into binocular visual cortex at postnatal day 24. Reference electrodes were placed bilaterally into prefrontal cortex. All electrodes were secured in place with cyanoacrylate and the entire exposure was covered with dental cement.
For MD and BD, mice at postnatal day 27 to 29 were anesthetized by inhalation of isoflurane (IsoFlo 2%−3%). Lids were sutured using 6–0 Vicryl. Animals were monitored daily to ensure a full seal. Mice whose eyelids did not remain fully shut for the entire duration of MD were excluded from the study. For CPP experiments, CPP (Tocris Bioscience) or saline solution was delivered i.p. every 6 h at 10 mg/kg (47, 48).
Visual stimuli consisted of full-field sine-wave gratings of 0% and 100% contrast, square reversing at 1 Hz, and presented at 0.05 cycles/degree. VEPs were evoked by either horizontal or vertical stimuli. As described previously, stimuli of orthogonal orientations were presented before and after MD to avoid the phenomenon of stimulus-selective response potentiation (5, 48). Visual display occupied 92°× 66° of the animal's visual field. Visual stimuli were presented to left and right eyes randomly. A total of 100 to 200 stimuli were presented per each condition. VEP amplitude was quantified by measuring trough-to-peak response amplitude, as described previously (20).
Statistics.
Global ANOVAs with a repeated measures factor were run with post-hoc analyses (Fisher protected least significant difference) to test for statistical significance among multiple groups. Data are expressed as mean ± SEM, and significance was placed at P < 0.05.
Drugs.
Unless otherwise noted, drugs were purchased from Sigma.
Supplementary Material
Acknowledgments.
The authors thank R. Corlew for contributing data on AMPA mEPSCs. We also thank J. deMarchena, J. Coleman, E. Sklar, and S. Meagher for assistance. This work was supported by the Howard Hughes Medical Institute, National Institutes of Health (NIH) (M.F.B.), NIH Grant R01 EY018323–01 (to N.E.I.), and the Whitehall Foundation (B.D.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808104106/DCSupplemental.
References
- 1.Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blakemore C, Van Sluyters RC. Reversal of the physiological effects of monocular deprivation in kittens: further evidence for a sensitive period. J Physiol. 1974;237:195–216. doi: 10.1113/jphysiol.1974.sp010478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Movshon JA. Reversal of the physiological effects of monocular deprivation in the kitten's visual cortex. J Physiol. 1976;261:125–174. doi: 10.1113/jphysiol.1976.sp011551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mioche L, Singer W. Chronic recordings from single sites of kitten striate cortex during experience-dependent modifications of receptive-field properties. J Neurophysiol. 1989;62:185–197. doi: 10.1152/jn.1989.62.1.185. [DOI] [PubMed] [Google Scholar]
- 5.Frenkel MY, Bear MF. How monocular deprivation shifts ocular dominance in visual cortex of young mice. Neuron. 2004;44:917–923. doi: 10.1016/j.neuron.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Kirkwood A, Dudek SM, Gold JT, Aizenman CD, Bear MF. Common forms of synaptic plasticity in the hippocampus and neocortex in vitro. Science. 1993;260:1518–1521. doi: 10.1126/science.8502997. [DOI] [PubMed] [Google Scholar]
- 7.Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirkwood A, Rioult MC, Bear MF. Experience-dependent modification of synaptic plasticity in visual cortex. Nature. 1996;381:526–528. doi: 10.1038/381526a0. [DOI] [PubMed] [Google Scholar]
- 9.Philpot BD, Espinosa JS, Bear MF. Evidence for altered NMDA receptor function as a basis for metaplasticity in visual cortex. J Neurosci. 2003;23:5583–5588. doi: 10.1523/JNEUROSCI.23-13-05583.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Philpot BD, Cho KK, Bear MF. Obligatory role of NR2A for metaplasticity in visual cortex. Neuron. 2007;53:495–502. doi: 10.1016/j.neuron.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 12.McBain CJ, Mayer ML. N-methyl-D-aspartic acid receptor structure and function. Physiol Rev. 1994;74:723–760. doi: 10.1152/physrev.1994.74.3.723. [DOI] [PubMed] [Google Scholar]
- 13.Roberts EB, Ramoa AS. Enhanced NR2A subunit expression and decreased NMDA receptor decay time at the onset of ocular dominance plasticity in the ferret. J Neurophysiol. 1999;81:2587–2591. doi: 10.1152/jn.1999.81.5.2587. [DOI] [PubMed] [Google Scholar]
- 14.Flint AC, Maisch US, Weishaupt JH, Kriegstein AR, Monyer H. NR2A subunit expression shortens NMDA receptor synaptic currents in developing neocortex. J Neurosci. 1997;17:2469–2476. doi: 10.1523/JNEUROSCI.17-07-02469.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinlan EM, Olstein DH, Bear MF. Bidirectional, experience-dependent regulation of N-methyl-D-aspartate receptor subunit composition in the rat visual cortex during postnatal development. Proc Natl Acad Sci USA. 1999;96:12876–12880. doi: 10.1073/pnas.96.22.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quinlan EM, Philpot BD, Huganir RL, Bear MF. Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nat Neurosci. 1999;2:352–357. doi: 10.1038/7263. [DOI] [PubMed] [Google Scholar]
- 17.Philpot BD, Sekhar AK, Shouval HZ, Bear MF. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron. 2001;29:157–169. doi: 10.1016/s0896-6273(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 18.Chen WS, Bear MF. Activity-dependent regulation of NR2B translation contributes to metaplasticity in mouse visual cortex. Neuropharmacology. 2007;52:200–214. doi: 10.1016/j.neuropharm.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Kadotani H, et al. Motor discoordination results from combined gene disruption of the NMDA receptor NR2A and NR2C subunits, but not from single disruption of the NR2A or NR2C subunit. J Neurosci. 1996;16:7859–7867. doi: 10.1523/JNEUROSCI.16-24-07859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sawtell NB, et al. NMDA receptor-dependent ocular dominance plasticity in adult visual cortex. Neuron. 2003;38:977–985. doi: 10.1016/s0896-6273(03)00323-4. [DOI] [PubMed] [Google Scholar]
- 21.Liu CH, Heynen AJ, Shuler MG, Bear MF. Cannabinoid receptor blockade reveals parallel plasticity mechanisms in different layers of mouse visual cortex. Neuron. 2008;58:340–345. doi: 10.1016/j.neuron.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 22.Crozier RA, Wang Y, Liu CH, Bear MF. Deprivation-induced synaptic depression by distinct mechanisms in different layers of mouse visual cortex. Proc Natl Acad Sci USA. 2007;104:1383–1388. doi: 10.1073/pnas.0609596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fagiolini M, et al. Separable features of visual cortical plasticity revealed by N-methyl-D-aspartate receptor 2A signaling. Proc Natl Acad Sci USA. 2003;100:2854–2859. doi: 10.1073/pnas.0536089100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bear MF. Bidirectional synaptic plasticity: from theory to reality. Philos Trans R Soc Lond B Biol Sci. 2003;358:649–655. doi: 10.1098/rstb.2002.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith GB, Heynen AJ, Bear MF. Review. Bidirectional synaptic mechanisms of ocular dominance plasticity in visual cortex. Philos Trans R Soc Lond B Biol Sci. 2009;364:357–367. doi: 10.1098/rstb.2008.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mrsic-Flogel TD, et al. Homeostatic regulation of eye-specific responses in visual cortex during ocular dominance plasticity. Neuron. 2007;54:961–972. doi: 10.1016/j.neuron.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 27.Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- 28.Nase G, Weishaupt J, Stern P, Singer W, Monyer H. Genetic and epigenetic regulation of NMDA receptor expression in the rat visual cortex. Eur J Neurosci. 1999;11:4320–4326. doi: 10.1046/j.1460-9568.1999.00859.x. [DOI] [PubMed] [Google Scholar]
- 29.Liu L, et al. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- 30.Massey PV, et al. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Philpot BD, Bear MF, Abraham WC. Metaplasticity: the plasticity of. synaptic plasticity. In: Katz PS, editor. Beyond Neurotransmission: Neuromodulation and Its Importance for Information Processing. Oxford: Oxford Univ Press; 1999. pp. 160–197. [Google Scholar]
- 32.Erisir A, Harris JL. Decline of the critical period of visual plasticity is concurrent with the reduction of NR2B subunit of the synaptic NMDA receptor in layer 4. J Neurosci. 2003;23:5208–5218. doi: 10.1523/JNEUROSCI.23-12-05208.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu HC, Gonzalez E, Crair MC. Barrel cortex critical period plasticity is independent of changes in NMDA receptor subunit composition. Neuron. 2001;32:619–634. doi: 10.1016/s0896-6273(01)00501-3. [DOI] [PubMed] [Google Scholar]
- 34.Neyton J, Paoletti P. Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J Neurosci. 2006;26:1331–1333. doi: 10.1523/JNEUROSCI.5242-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berberich S, et al. Lack of NMDA receptor subtype selectivity for hippocampal long-term potentiation. J Neurosci. 2005;25:6907–6910. doi: 10.1523/JNEUROSCI.1905-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weitlauf C, et al. Activation of NR2A-containing NMDA receptors is not obligatory for NMDA receptor-dependent long-term potentiation. J Neurosci. 2005;25:8386–8390. doi: 10.1523/JNEUROSCI.2388-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao MG, et al. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron. 2005;47:859–872. doi: 10.1016/j.neuron.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 38.Morishita W, et al. Activation of NR2B-containing NMDA receptors is not required for NMDA receptor-dependent long-term depression. Neuropharmacology. 2007;52:71–76. doi: 10.1016/j.neuropharm.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 39.de Marchena J, et al. NMDA receptor antagonists reveal age-dependent differences in the properties of visual cortical plasticity. J Neurophysiol. 2008;100:1936–1948. doi: 10.1152/jn.90290.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakimura K, et al. Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor epsilon 1 subunit. Nature. 1995;373:151–155. doi: 10.1038/373151a0. [DOI] [PubMed] [Google Scholar]
- 41.Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008;9:387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- 42.Yoshimura Y, et al. Involvement of T-type Ca(2+) channels in the potentiation of synaptic and visual responses during the critical period in rat visual cortex. Eur J Neurosci. 2008;28:730–743. doi: 10.1111/j.1460-9568.2008.06384.x. [DOI] [PubMed] [Google Scholar]
- 43.Kaneko M, Stellwagen D, Malenka RC, Stryker MP. Tumor necrosis factor-alpha mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron. 2008;58:673–680. doi: 10.1016/j.neuron.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sato M, Stryker MP. Distinctive features of adult ocular dominance plasticity. J Neurosci. 2008;28:10278–10286. doi: 10.1523/JNEUROSCI.2451-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol Rev. 1985;65:37–100. doi: 10.1152/physrev.1985.65.1.37. [DOI] [PubMed] [Google Scholar]
- 46.Freeman JA, Nicholson C. Experimental optimization of current source-density technique for anuran cerebellum. J Neurophysiol. 1975;38:369–382. doi: 10.1152/jn.1975.38.2.369. [DOI] [PubMed] [Google Scholar]
- 47.Heynen AJ, et al. Molecular mechanism for loss of visual cortical responsiveness following brief monocular deprivation. Nat Neurosci. 2003;6:854–862. doi: 10.1038/nn1100. [DOI] [PubMed] [Google Scholar]
- 48.Frenkel MY, et al. Instructive effect of visual experience in mouse visual cortex. Neuron. 2006;51:339–349. doi: 10.1016/j.neuron.2006.06.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.