Abstract
In this manuscript, the remarkable NMR signal enhancement that can be provided by dynamic nuclear polarization (DNP) was combined with the reactivity of acetic anhydride with amines to perform rapid, high SNR analyses of amino acid mixtures and to hyperpolarize new biomolecules of interest. [1,1-13C] acetic anhydride is an excellent substrate for DNP hyperpolarization because it can be well polarized in only 30 min and has a relatively long T1 relaxation time (33.9 s at 11.7 T and 37 °C). The secondary hyperpolarization approach developed in this project takes advantage of the preferential reaction of acetic anhydride with amine nucleophiles, which occurs much more rapidly than hydrolysis to produce hyperpolarized N-acetyl adducts. This new approach was used to reproducibly and near-quantitatively (mean yield − 89.8%) resolve a mixture of amino acids Gly, Ser, Val, Leu, and Ala in a single acquisition (3 s) with a signal enhancement of up to 1,400-fold as compared with thermal equilibrium. Secondary hyperpolarization was performed for several small peptides and N-acetylcysteine, a drug administered intravenously to treat acetaminophen overdose. Although, in general the T1 of the N-acetyl adducts decreased with increasing molecular weight of the biomolecules, the T1 values were still on the order of 10 s, and the correlation of T1 with molecular weight was not exact suggesting the potential of secondarily polarizing relatively large biomolecules. This study demonstrates the feasibility of using prepolarized [1,1-13C] acetic anhydride and rapid chemical reactions to provide high SNR NMR spectra of amino acid derivatives and other biomolecules.
Keywords: acetylation, metabolism, peptides, spectroscopy
Recent development of techniques to retain highly polarized spins in solution via dynamic nuclear polarization (DNP) has enabled 13C NMR spectroscopy and MR spectroscopic imaging studies with very high signal to noise in short acquisition times (1, 2). Generally, the technique requires polarization of a low molecular-weight 13C labeled chemical probe in a highly concentrated sample, for example [1-13C] pyruvic acid or 13C-urea (3–5). For molecules having limited solubility, crystalline properties at low temperature, or high molecular weights, the DNP technique may not provide sufficient polarization for in vitro or in vivo applications. For molecules with limited solubility, the polarization rate can also be prohibitively long. To address this limitation, this study was designed to develop a method of “secondary hyperpolarization,” whereby a reactive molecule that hyperpolarizes well could be used to chemically “tag” biomolecules of interest. Recently, chemical derivatization of amines in biofluids with [1-13C] acetic anhydride, and their 500-MHz 13C NMR spectra was reported (6). In this project, the secondary hyperpolarization approach was applied using prepolarized [1-13C] acetic anhydride and rapid chemical reactions to provide high SNR NMR spectra of amino acid derivatives and other biomolecules.
This secondary hyperpolarization technique could be of great value to the emerging field of metabolomics or metabolic profiling, where molecular processes in the living cell are assessed, and the chemical content of biofluids resolved (7–12). Currently, only a limited number of biomarkers are used in clinical practice. These biomarkers include the levels of an expressed protein in health or disease, for example CA-121 expressed in ovarian cancer, or of a given metabolite concentration in biofluids, for example catecholamines found in the urine of patients with pheochromocytoma (13, 14). Increasingly, medical diagnostics will incorporate fast methods of obtaining a patient's disease profile, through genomic, proteomic and metabolic evaluations. Effective screening methods could allow earlier disease identification and treatment and improve outcomes. The most established technologies in this field are NMR and mass spectroscopy, both of which have been used to identify errors in metabolism and the accumulation of specific metabolites in disease (11, 15–17). Very recently, DNP NMR has been used to obtain metabolic data with very short acquisition times, in animal and cell culture models of human disease (2, 3, 18–21). Most importantly, this data can be acquired in seconds at high spatial resolution. This improved speed of acquisition is clearly of benefit in the development of medical diagnostics, with information often needed quickly in acutely ill patients.
The goal of this project was to combine the remarkable NMR signal enhancement provided by DNP with the reactivity and good polarization characteristics of [1,1-13C] acetic anhydride to resolve a mixture of secondarily hyperpolarized N-acetylated amino acid adducts in aqueous solution in a matter of seconds. Additionally, we initiated investigations of the utility of this secondary hyperpolarization technique to polarize important biomolecules that may not be well polarized using direct DNP methods.
Results
Secondary hyperpolarization of amino acid mixtures with hyperpolarized [1,1-13C]-Acetic anhydride.
[1,1-13C] acetic anhydride is a good substrate for DNP hyperpolarization because it is a liquid at room temperature that dissolves the trityl radical, forms a glass at 1.4 T, and reaches a maximum solution state polarization in 30 min yielding 5.9% polarization. To avoid premature reaction of the hyperpolarized acetic anhydride during dissolution, initial studies used 1,4 dioxane as the dissolution media. Acetic anhydride was stable to dissolution conditions in this solvent, with only the hyperpolarized acetylcarbonyls observed in the 13C spectrum. At 11.7 T and 37 °C, the calculated T1 for hyperpolarized carbonyl of [1,1-13C] acetic anhydride in dioxane was 33.9 s.
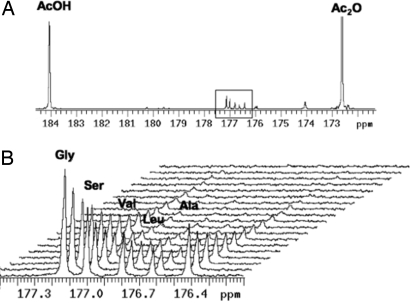
In a mixed dioxane/aqueous solvent, derivatization of amines with hyperpolarized [1,1-13C] acetic anhydride takes place rapidly, before acquisition of the first hyperpolarized spectrum, to form the corresponding acetylated products as shown in Fig. 1. This method takes advantage of the preferential reaction of acetic anhydride with amine nucleophiles, which occurs much more rapidly than hydrolysis. In triplicate studies it was also determined that a mixture of hyperpolarized [1- 13C] N-acetylated Gly, Ser, Val, Leu, and Ala could be well resolved with excellent signal-to-noise, in a single acquisition (Fig. 2). The ability to obtain sufficient spectral resolution to resolve the amino acid mixture required stabilizing the reaction solution temperature and preshimming the 10-mm NMR probe using the same mixture of amino acids before acquiring the hyperpolarized 13C data. By the time of the first hyperpolarized spectrum (≈ 11 s after mixing), the acetylation reaction had already gone to completion, because the hyperpolarized [1-13C] N-acetylated amino acid peak areas were at a maximum at T0 and decayed because of T1 relaxation (Fig. 2B). The calculated T1 values for the acetylated amino acids correlated with their corresponding molecular weights with glycine and alanine having the longest T1 (14.7 ± 0.4 s) and leucine having the shortest T1 (8.9 ± 0.6 s, Table 1).
Fig. 1.
General strategy for secondary hyperpolarization of amino acids using prepolarized [1,1-13C] acetic anhydride.
Fig. 2.
Hyperpolarized spectrum obtained when a buffered solution (100 mM phosphate, pH = 7.8) containing the amino acids gly, ser, val, leu, ala (3.5 mM amino acid) is reacted with a 2-fold excess of hyperpolarized acetic anhydride. (A) Full spectrum (t = 0) including large peaks corresponding to unreacted acetic anhydride and hydrolysis product acetic acid, and acetylated amino acid products (outlined). (B) Dynamic hyperpolarized spectrum with resolved acetylated amino acid products.
Table 1.
Chemical shifts, spin-lattice relaxation constants (T1), percentage of yield, and hyperpolarized signal enhancement for amino acid mixture data (3.5 mM amino acid)
| Product | Chemical shift, ppm | T1, s | Yield, % | Enhancement |
|---|---|---|---|---|
| N-acetylglycine | 177.13 | 14.7 (0.4) | 91.7 (4.5) | 1,391 (220) |
| N-acetylserine | 177.00 | 10.9 (0.2) | 97.0 (3.0) | 775 (86) |
| N-acetylvaline | 176.79 | 10.0 (0.2) | 93.3 (2.3) | 551 (43) |
| N-acetylleucine | 176.62 | 8.9 (0.6) | 85.0 (4.6) | 412 (70) |
| N-acetylalanine | 176.41 | 14.7 (0.4) | 82.0 (3.6) | 654 (96) |
Data was obtained in triplicate, with averages reported and the corresponding SD indicated in parentheses. Chemical shifts were referenced to acetic anhydride (172.60 ppm).
Because there was a 2-fold excess of acetic anhydride relative to the amino acids, there was a large residual acetic anhydride peak in the hyperpolarized 13C spectra and a large acetic acid peak because of hydrolysis. There was an additional doublet up-field from acetylalanine, at 176.0 ppm, that was present in control experiments using only an aqueous buffered solution, without any amine nucleophiles present. Therefore, this additional doublet most likely represents a breakdown product of the hyperpolarized [1,1-13C] acetic anhydride in aqueous solution.
A representative fully-relaxed thermal spectrum used to calculate the hyperpolarized signal enhancement factors is shown in Fig. 3. Unlike the hyperpolarized acetylated amino acid spectra, in which different amino acids had significantly different peak areas (Fig. 2B), all of the amino acids had approximately equal peak areas in the fully-relaxed thermal spectra. Signal enhancement in the first hyperpolarized spectrum reflected differences in T1 signal decay during the ≈11 s required for mixing, temperature equilibration and insertion into the NMR tube. N-acetylglycine, with the longest T1 demonstrated the largest signal enhancement (1.4 × 103) and leucine, with the shortest T1, demonstrated the smallest enhancement (4.1 × 102). Acetylalanine was slightly anomalous in this respect demonstrating a lower peak area in the dynamic hyperpolarized spectrum despite its longer measured T1.
Fig. 3.
Thermal spectrum corresponding to the hyperpolarized data presented in Fig. 2. (A) After addition of Magnevist Gd-chelate to a final concentration of 5 mM, the only peaks observed correspond to [1-13C]-AcOH and the previously observed acetylated amino acid products (outlined). (B) Expanded region of interest in the 13C spectrum.
As anticipated, the yields for the acetylation reaction were high, ranging from 82 to 97% with excellent reproducibility between the replicate studies (Table 1), and were similar to those published previously for the reaction of amino acids and acetic anhydride in aqueous media (6).
Even at physiologic concentrations of amino acids (70 μM) excellent signal to noise hyperpolarized 13C spectra can be acquire in a single acquistion. Specifically, the acetylcarbonyl of acetylated glycine, serine, valine, leucine, and alanine had a peak area to noise of 43.1 ± 8.5, 27.8 ± 5.9, 16.0 ± 4.6, 9.1 ± 3.8, and 13.5 ± 3.1, respectively. The relative peak areas in the hyperpolarized spectrum were the same as in the higher amino acid concentration study (Fig. 2B), i.e., scaled by their T1 relaxation rates, however, the chemical shifts were slightly different because of the higher pH of the reaction solution because less acetic acid is produced.
Secondary Hyperpolarization of Glycine, Diglycine, and Triglycine.
A second set of experiments focused on a chemically similar set of substrates of increasing molecular weight, namely glycine, diglycine and triglycine to assess the effect of molecular size on T1. A representative dataset is depicted in Fig. 4, which demonstrates peaks corresponding to 1-13C acetic acid, 13C acetylglycine, and [1,1-13C] acetic anhydride. The data for glycine confirmed that the calculated T1 value correlated well with that seen in the previous, mixed amino acid experiment. The T1 relaxation time for diglycine was 35% less than glycine and the T1 triglycine was 9% less than the diglycine. Results are summarized in Table 2.
Fig. 4.
Sample dynamic dataset for reaction of hyperpolarized (1,1-13C) acetic anhydride with glycine, with T1 values calculated from the corresponding acetylated product.
Table 2.
Chemical shifts and spin-lattice relaxation constants for glycine peptides
| Product | Chemical shift, ppm | T1, s |
|---|---|---|
| N-acetyl glycine | 177.13 | 15.0 (0.5) |
| N-acetyl diglycine | 178.05 | 9.8 (1.2) |
| N-acetyl triglycine | 178.25 | 8.5 (0.2) |
In each case, the average T1 value is reported with the standard deviation in parentheses. For all experiments, a 2-fold excess of hyperpolarized [1,1-13C] acetic anhydride was applied to a buffered solution (100 mM phosphate, pH = 7.8) of the corresponding glycine peptide at 17.5 mM.
Secondary Hyperpolarization of N-Acetyllysine, and C Terminus of α-Melanocyte Stimulating Hormone (α-MSH).
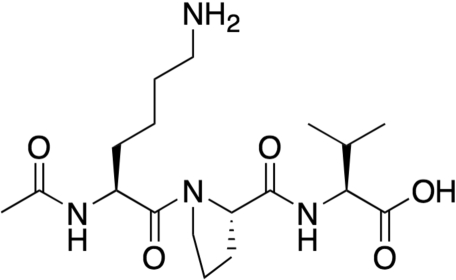
Peptides bearing lysine side-chains represent an excellent opportunity to label larger macromolecular structures. To investigate this application, we used hyperpolarized [1,1-13C] acetic anhydride to label an N-acetylated version of lysine, with its nucleophilic side-chain amine, and a short peptide corresponding to the C terminus of α-melanocyte stimulating hormone (α-MSH). N-acetyllysine and N-acetyl (11–13) α-MSH have molecular weights of 188.23 g/mol and 383.5 g/mol respectively. The structure of this tripeptide is shown in Fig. 5. After secondary labeling via the hyperpolarized anhydride, the acetylcarbonyl of both compounds were found to have the same chemical shift (177.11 ppm), and the T1 values of the acetylcarbonyls scaled with molecular weight, with N-acetyllysine having and N-acetyl (11–13) α-MSH having T1 values of 12.2 and 9.5 s.
Fig. 5.
Chemical structure of N-acetyl MSH (11–13), a short peptide corresponding to the C terminus of α-MSH. Only the lysine side-chain is available for acetylation.
Secondary Hyperpolarization of N-Acetylcysteine (Fig. 4).
For in vivo applications it will be necessary to demonstrate that a hyperpolarized, anhydride-depleted, aqueous solution of a molecule of interest could be generated using this technique. Instead of using toxic dioxane as a dissolution media, a basic solution of cysteine was used to generate N-acetylcysteine during the dissolution process. Fig. 6 demonstrates successful secondary labeling of this small molecular weight drug. This experiment also demonstrated the ability to perform the secondary hyperpolarization in aqueous solution during dissolution in the Hypersense DNP polarizer. Moreover, the reaction went to completion as evidenced by the presence of N-acetylcysteine (172.6 ppm) and acetic acid, and absence of acetic anhydride in the hyperpolarized 13C spectrum.
Fig. 6.
Generation of hyperpolarized acetylcysteine by secondary labeling. Note the total absence of AcsO at 172.6 ppm, indicating that the reaction has been successfully driven to completion.
Discussion
These studies demonstrated the feasibility of a “secondary hyperpolarization” approach combining the remarkable NMR signal enhancement provided by DNP with the high reactivity of [1,1-13C] acetic anhydride with amino acids to perform rapid metabolic analyses of amino acid mixtures and to hyperpolarize biomolecules of interest. [1,1-13C] acetic anhydride is an excellent substrate for DNP hyperpolarization because it is well polarized (5.9%) in 30 min and has a relatively long T1 relaxation time (33.9 s at 11.7 T and 37 °C). This approach also takes advantage of the preferential reaction of acetic anhydride with amine nucleophiles, which occurs much more rapidly than hydrolysis, as was evidenced by the reaction being complete by the first hyperpolarized 13C spectra acquired 11 s after mixing the reactants.
This study demonstrated that this secondary hyperpolarization approach could be used to reproducibly and near-quantitatively resolve a mixture of amino acids at physiologic concentrations in a single acquisition. The amino acid acetylcarbonyl resonances demonstrated excellent signal-to-noise ratios (SNR) ranging 43 to 9. This difference in SNR was due to T1 relaxation during the 11 second delay between the mixing of the reactants and the NMR experiment. This delay was required to mix the reactants outside of the spectrometer to minimize air bubbles and allow for temperature equilibration, and obtain sufficient magnetic field homogeneity to resolve the N-acetylcarbonyls of the amino acids that had very similar chemical shifts. The secondary hyperpolarization approach also allows the calculation of the T1 relaxation of the products providing a means to correct for T1 necessary for quantification of the spectral results.
A recent study demonstrated that, by using high pressure liquid chromatography techniques, the hyperpolarized solution can be injected directly into the NMR spectrometer with good magnetic field homogeneity allowing for good quality spectra to be obtained seconds after injection (22). This study suggests that in the near future, the mixing of reactants involved in a secondary hyperpolarization could be performed directly in the NMR spectrometer. This would provide a way to capitalize on the full signal-to-noise enhancement provided by DNP and negate the need for T1 corrections.
The secondary hyperpolarization approach also benefits the study of biologically interesting compounds that either hyperpolarize poorly or have such short T1 values that they need to be polarized and immediately studied within the NMR spectrometer. Several findings from this study support the feasibility of accomplishing this. Specifically, it was possible to secondarily polarize polymers of glycine (N-acetyl di- and tri-glycine) and 2 small peptides N-acetyllysine, and N-acetyl α-MSH. Although, in general the T1 of the acetylcarbonyl decreased with increasing molecular weight of the biomolecule, the T1 values were on the order of 10 s, and the correlation of T1 with molecular weight was not exact. This was exemplified by the fact that N-acetyl α-MSH with a molecular weight that was 2 times larger than N-acetyldiglycine, had the same T1. The ability to acetylate peptides with lysine side-chains also provides an excellent opportunity to label a number of important biomolecules.
For in vivo applications, excess acetic anhydride and dioxanes cannot be used for reactions involved in secondary hyperpolarization because of their toxic effects. In the current study, it was possible to generate hyperpolarized N-acetylcysteine in aqueous solution during the dissolution process within the hyperpolarizer. N-acetylcysteine is a drug administered IV to treat Tylenol overdose, so this method could be used to investigate the biodistribution and metabolism of this drug in ex-vivo and in vivo models of disease.
To our knowledge, this secondary hyperpolarization approach has not been reported in the DNP literature. However, similar strategies have been used for years in the field of positron emission tomography (PET), which often involves incorporation of a radioactive nucleus into a ligand of interest shortly before administration to a patient. For example, biological peptides of interest bearing artificial amino acid side-chains as chelating groups can be “tagged” with positron-emitting isotopes such as technecium-99 and delivered to a patient (23, 24). These labeled tracers can then be assessed in vivo, using a gamma camera. Potential advantages of DNP-NMR over this technique include direct spectroscopic evidence of both uptake and metabolism, lack of ionizing radiation dose, short acquisition times, and direct correlation with MR images with superior soft-tissue contrast. However, evaluation of hyperpolarized 13C substrates in vivo will be limited by relatively short 13C T1 values of very large biomolecules, which can be quite short (on the order of seconds) for carbon atoms of interest in large proteins and drugs. The next step involves determining whether secondary hyperpolarization approach can be used to analyze amino acid levels in human biofluids and used to follow the biodistribution and metabolism of a small molecular weight drug such as N-acetylcysteine in vivo.
Materials and Methods
Polarization of [1,1-13C]-Acetic Anhydride.
Neat samples containing 10 microliters of [1,1-13C] acetic anhydride (1.06 × 10−4 mol) (Isotec) containing 15 mM Finland trityl radical OX076 (Oxford Instruments) were polarized at 94.106 MHz and 1.2–1.4K), for 30 min and subsequently dissolved in 2.5 mL of anhydrous 1,4 dioxane (Sigma–Aldrich), using a Hypersense DNP polarizer (Oxford Instruments). The hyperpolarized [1,1-13C] acetic anhydride/dioxane solution was subsequently injected from the DNP polarizer directly into the amino acid solutions (described in the following sections), manually mixed in a 500-cc teardrop flask, and injected using a 5-cc syringe into a previously-shimmed 10-mm NMR tube into at 37 °C. This process required ≈10 s. Percentage polarizations were quantified in solution by measuring the signal enhancement obtained by DNP polarization compared with the signal at thermal equilibrium (25).
Secondary Hyperpolarization of Amino Acid Mixtures with Hyperpolarized [1,1-13C]-Acetic Anhydride.
Initial studies (n = 3) involved mixing the dissolution solution containing dioxane and hyperpolarized [1,1-13C]-acetic anhydride with 3 mL of a buffered (100 mM phosphate, 0.3 mM EDTA pH = 7.8) solution of amino acids Glycine (Gly), Serine (1), Valine (Val), Leucine (Leu), and Alanine (Ala) at 3.5 mM each, or 17.5 mM total amino acid concentration. A 2-fold excess of acetic anhydride with respect to the total amino acid concentration was used in all labeling studies. The final pH of the resulting solutions was ≈ 5.5 (5.55, 5.54, and 5.61 respectively). To confirm the chemical shifts of each of the acetylated amino acids, the same hyperpolarized experiment was performed for each amino acid individually at a concentration of 17.5 mM. To determine whether or not this technique could produce reasonable SNR for a solution containing amino acids at physiologic concentrations, a buffered, aqueous solution containing 70 μM amino acids (Gly, Ser, Val, Leu, Ala) was acetylated under the same conditions used for the higher amino acid concentration studies. The peak area to noise for the acetyl carbon of the acetylated amino acids was measured in triplicate studies. The final pH of the reaction mixtures was higher (pH ≈ 7.33) than that observed for the higher concentration studies because of the lower concentration of hyperpolarized [1,1-13C]-acetic anhydride used and subsequent lower production of acetic acid.
Secondary Polarizarion of Glycine, Diglycine, and Triglycine.
A second set of labeling experiments (n = 3) using hyperpolarized [1,1-13C]-acetic anhydride was conducted with a chemically similar set of substrates, namely glycine (MW = 75.07), diglycine (MW = 132.12) and triglycine (MW = 191.14). The reaction conditions in these studies were identical to those described for the labeling of the mixture of amino acids (17.5 mM). The pHs of the resulting solutions were ≈ 5.5.
Secondary Hyperpolarization of N-Acetyllysine, and C Terminus of α-Melanocyte Stimulating Hormone (α-MSH).
The reaction conditions in these studies were identical to those described for the labeling of the mixture of amino acids, except for mixing a 6-fold excess of the hyperpolarized [1,1-13C] acetic anhydride/dioxane solution with 5 mM N-acetyllysine, and 5 mM α-melanocyte stimulating hormone (α-MSH), resulting in a final reaction solution pH of 6.0.
Secondary Hyperpolarization of N-Acetylcysteine (Fig. 3).
Twenty microliters of [1,1-13C] acetic anhydride (2.12 × 10−4 mol) were polarized using the HyperSense DNP polarizer at 94.106 MHz for 30 min. Five milliliters of the following dissolution media were used: 200 mM cysteine, 150 mM NaOH, 0.3 mM EDTA. The final concentration of cysteine using this volume corresponds to ≈5-fold excess of cysteine with respect to acetic anhydride with a resulting pH of 6.8.
Hyperpolarized 13C Spectroscopic Studies.
All NMR studies were performed on a 11.7 T Varian INOVA spectrometer (125MHz 13C, Varian Instruments), using a 10-mm 15N/31P/13C triple-tuned direct detect probe. For the acquisition of hyperpolarized 13C spectra of the hyperpolarized acetylated amino acids eighty proton-decoupled (WALTZ-16, 9,000 Hz bandwidth, decoupling during acquisition only) pulse and acquire hyperpolarized 13C NMR spectra (1 average, spectral window = 4,000 Hz, number of points = 16,000, TR = 3.5 s, acq time = 2 s, total acq time = 2 min 55 s) were acquired every 3 s, using a 5° pulse. For the low concentration amino acid secondary hyperpolarization study, a single 90° pulse was used to acquired the hyperpolarized 13C Spectrum. Spin-lattice (T1) relaxation times were determined by performing a monoexponential fit to the signal decay curve of the hyperpolarized compounds. In all cases the r2 value for the fit was >0.997 where r = the Pearson product moment correlation coefficient. All spectral measurements were collected at ≈ 37 °C.
After each hyperpolarized experiment; a small quantity of Magnevist Gd-chelate (Bayer Healthcare Pharmaceuticals, Inc.) was added to the reaction solution, resulting a final Magnevist concentration of 5 mM. A proton decoupled 13C thermal spectrum was acquired using the same acquisition parameters used for the hyperpolarized studies except for a 90° pulse and obtaining 256 averages to calculate signal enhancements and reaction yields. A repetition time of 3 s was >3T1 because the T1 of acetic acid and the acetyl-amino acids were determined to be <1 s after addition of 5 mM Magnevist. Signal enhancements due to hyperpolarization were calculated by integrating acetyl-amino acid peaks in the first spectrum of the hyperpolarized dynamic experiment, and comparing these to the corresponding peaks in the thermal spectrum, accounting for differences in gain, tip angle, and the number of transients obtained. Because the reaction goes to completion (all of the acetic anhydride was converted to either acetic acid or the acetylated amino acids), yields were calculated using the acetic acid peak in fully relaxed thermal spectra as an internal standard.
Acknowledgments.
This work was supported by National Institutes of Health Grants R21 EB005363 and R01 EB007588, University of California Discovery Grants LSIT01-10107 and ITL-BIO04-10148, National Institute of Biomedical Imaging and Bioengineering Training Grant 1 T32 ED001631 (to D.M.W.), and GE Healthcare.
Footnotes
Conflict of interest statement: R.E.H. and A.P.C. are employees of GE Healthcare.
This article is a PNAS Direct Submission. A.S.E. is a guest editor invited by the Editorial Board.
See Commentary on page 5453.
References
- 1.Ardenkjaer-Larsen JH, et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci USA. 2003;100:10158–10163. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Golman K, Zandt RI, Lerche M, Pehrson R, Ardenkjaer-Larsen JH. Metabolic imaging by hyperpolarized 13C magnetic resonance imaging for in vivo tumor diagnosis. Cancer Res. 2006;66:10855–10860. doi: 10.1158/0008-5472.CAN-06-2564. [DOI] [PubMed] [Google Scholar]
- 3.Kohler SJ, et al. In Vivo 13 carbon metabolic imaging at 3T with hyperpolarized 13C-1-pyruvate. Magn Reson Med. 2007;58:65–69. doi: 10.1002/mrm.21253. [DOI] [PubMed] [Google Scholar]
- 4.Mayer D, Levin YS, Hurd RE, Glover GH, Spielman DM. Fast metabolic imaging of systems with sparse spectra: Application for hyperpolarized 13C imaging. Magn Reson Med. 2006;56:932–937. doi: 10.1002/mrm.21025. [DOI] [PubMed] [Google Scholar]
- 5.Merritt ME, et al. Hyperpolarized 13C allows a direct measure of flux through a single enzyme-catalyzed step by NMR. Proc Natl Acad Sci USA. 2007;104:19773–19777. doi: 10.1073/pnas.0706235104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shanaiah N, et al. Class selection of amino acid metabolites in body fluids using chemical derivatization and their enhanced 13C NMR. Proc Natl Acad Sci USA. 2007;104:11540–11544. doi: 10.1073/pnas.0704449104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bollard ME, Stanley EG, Lindon JC, Nicholson JK, Holmes E. NMR-based metabonomic approaches for evaluating physiological influences on biofluid composition. NMR Biomed. 2005;18:143–162. doi: 10.1002/nbm.935. [DOI] [PubMed] [Google Scholar]
- 8.Lindon JC, Holmes E, Bollard ME, Stanley EG, Nicholson JK. Metabonomics technologies and their applications in physiological monitoring, drug safety assessment and disease diagnosis. Biomarkers. 2004;9:1–31. doi: 10.1080/13547500410001668379. [DOI] [PubMed] [Google Scholar]
- 9.Lindon JC, Holmes E, Nicholson JK. Metabonomics and its role in drug development and disease diagnosis. Expert Rev Mol Diagn. 2004;4:189–199. doi: 10.1586/14737159.4.2.189. [DOI] [PubMed] [Google Scholar]
- 10.Lindon JC, Nicholson JK. Toxicological applications of magnetic resonance. Proc Nucl Magn Reson Spectrosc. 2004;45:109–143. [Google Scholar]
- 11.Nicholson JK, Wilson ID. Opinion: Understanding “global” systems biology: Metabonomics and the continuum of metabolism. Nat Rev Drug Discov. 2003;2:668–676. doi: 10.1038/nrd1157. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, et al. Metabonomic investigations in mice infected with Schistosoma mansoni: An approach for biomarker identification. Proc Natl Acad Sci USA. 2004;101:12676–12681. doi: 10.1073/pnas.0404878101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bast RC, Jr, et al. New tumor markers: CA125 and beyond. Int J Gynecol Cancer 15 Suppl. 2005;3:274–281. doi: 10.1111/j.1525-1438.2005.00441.x. [DOI] [PubMed] [Google Scholar]
- 14.Young WF., Jr Clinical practice. The incidentally discovered adrenal mass. N Engl J Med. 2007;356:601–610. doi: 10.1056/NEJMcp065470. [DOI] [PubMed] [Google Scholar]
- 15.Gartland KP, Beddell CR, Lindon JC, Nicholson JK. Application of pattern recognition methods to the analysis and classification of toxicological data derived from proton nuclear magnetic resonance spectroscopy of urine. Mol Pharmacol. 1991;39:629–642. [PubMed] [Google Scholar]
- 16.Pauling L, Robinson AB, Teranishi R, Cary P. Quantitative analysis of urine vapor and breath by gas-liquid partition chromatography. Proc Natl Acad Sci USA. 1971;68:2374–2376. doi: 10.1073/pnas.68.10.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vandergreef J, Tas AC, Bouwman J, Debrauw MCT, Schreurs WHP. Evaluation of field-desorption and fast atom-bombardment mass-spectrometric profiles by pattern-recognition techniques. Anal Chim Acta. 1983;150:45–52. [Google Scholar]
- 18.Chen AP, et al. Hyperpolarized C-13 spectroscopic imaging of the TRAMP mouse at 3T-initial experience. Magn Reson Med. 2007;58:1099–1106. doi: 10.1002/mrm.21256. [DOI] [PubMed] [Google Scholar]
- 19.Chen AP, et al. Feasibility of using hyperpolarized [1-13C]lactate as a substrate for in vivo metabolic (13)C MRSI studies. Magn Reson Imaging. 2008 doi: 10.1016/j.mri.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day SE, et al. Detecting tumor response to treatment using hyperpolarized (13)C magnetic resonance imaging and spectroscopy. Nat Med. 2007;13:1382–1387. doi: 10.1038/nm1650. [DOI] [PubMed] [Google Scholar]
- 21.Keshari KR, et al. ISMRM 16th Scientific Meeting; Toronto: ISMRM; 2008. [Google Scholar]
- 22.Bowen S, Hilty C. 49th ENC. Santa Fe, NM: ENC; 2008. [Google Scholar]
- 23.Bakker WH, et al. [111In-DTPA-d-Phe1]-octreotide, a potential radiopharmaceutical for imaging of somatostatin receptor-positive tumors: Synthesis, radiolabeling and in vitro validation. Life Sci. 1991;49:1583–1591. doi: 10.1016/0024-3205(91)90052-d. [DOI] [PubMed] [Google Scholar]
- 24.Van de Wiele C, et al. Biodistribution and dosimetry of (99m)Tc-RP527, a gastrin-releasing peptide (GRP) agonist for the visualization of GRP receptor-expressing malignancies. J Nucl Med. 2001;42:1722–1727. [PubMed] [Google Scholar]
- 25.Van de Ven FJM. Multidimensional NMR in Liquids. Weinheim, Germany: Vch-Wiley; 1995. [Google Scholar]