Abstract
Nitric oxide (NO) may limit myocardial ischemia-reperfusion injury by slowing the mitochondrial metabolism. We examined whether rat heart contains catalysts potentially capable of reducing nitrite to NO during an episode of regional myocardial ischemia produced by temporary coronary artery occlusion. In intact Sprague-Dawley rats, a 15-min coronary occlusion lowered the nitrite concentration of the myocardial regions exhibiting ischemic glucose metabolism to ∼50% that of nonischemic regions (185 ± 223 vs. 420 ± 203 nmol/l). Nitrite was rapidly repleted during subsequent reperfusion. The heart tissue tested in vitro acquired a substantial ability to consume nitrite when made hypoxic at neutral pH, and this ability was slightly enhanced by simultaneously lowering the pH to 5.5. More than 70% of this activity could be abolished by flushing the coronary circulation with crystalloid to remove trapped erythrocytes. Correspondingly, erythrocytes demonstrated the ability to reduce exogenous nitrite to NO under hypoxic conditions in vitro. In erythrocyte-free heart tissue, the nitrite consumption increased fivefold when the pH was lowered to 5.5. Approximately 40% of this pH-sensitive increase in nitrite consumption could be blocked by the xanthine oxidoreductase inhibitor allopurinol, whereas lowering the Po2 sufficiently to desaturate myoglobin accelerated it further. We conclude that rat heart contains several factors capable of catalyzing ischemic nitrite reduction; the most potent is contained within erythrocytes and activated by hypoxia, whereas the remainder includes xanthine oxidoreductase and other pH-sensitive factors endogenous to heart tissue, including deoxymyoglobin.
Keywords: myocardial ischemia, nitric oxide, erythrocyte, xanthine oxidoreductase
nitric oxide (NO), generated locally within the heart, regulates a large number of normal physiological processes (11). NO is also produced within regions of the heart damaged by ischemia or infarction, and its physiological role in this context has been controversial. Under some conditions ischemic NO appears to contribute to pathological injury (3, 22, 26), whereas under others it appears to limit myocardial injury by delaying the onset of the mitochondrial calcium permeability transition (1, 4, 8, 10, 12, 20, 25). Given the evanescent nature of NO in living tissues, a cytoprotective action would presumably require the formation of NO locally within the ischemic heart. Identifying mechanisms capable of generating NO within the ischemic heart may be an important step toward the development of new therapies to limit myocardial ischemic injury.
Under aerobic conditions, the primary source of cardiac NO is thought to be NO synthases (NOS) expressed by the coronary endothelium and other cells resident within the myocardium. The requirement of NOS for physiological pH and molecular oxygen makes it unlikely that these enzymes would be a major source of NO in the ischemic heart. A plausible alternative means of generating NO under the conditions of acidosis and hypoxia prevailing in ischemic tissue is the disproportionation reactions involving nitrite ion. The hypothesis that the nitrite ion can be chemically converted to cytoprotective NO within the ischemic heart was suggested by Webb et al. (25) and is supported by both the earlier experiments of Johnson et al. (9) and the more recent observations of Duranski et al. (4) that the intravenous administration of relatively small quantities of sodium nitrite to experimental animals protected their hearts against ischemia-reperfusion injury in a manner associated with the accumulation of tissue NO metabolites. Tiravanti et al. (24) correspondingly demonstrated that rat hearts perfused with nitrite accumulate nitrosyl-heme complexes (primarily NO-myoglobin) progressively during ischemia associated with the activation of guanylate cyclase, suggesting a role for nitrite in the activation of NO signaling pathways during ischemia or reperfusion.
The processes theoretically capable of converting nitrite to NO in the ischemic heart include nonenzymatic disproportionation reactions (5, 28), enzymatic catalysis by xanthine oxidoreductase (XOR) (7, 14, 18, 28), or other oxygenases, and nonenzymatic, nonacidic reduction catalyzed by heme-containing proteins in blood and myocardial tissue (2, 19, 21, 23). Little is known about the degree to which these various processes contribute to ischemic nitrite reduction in vivo.
In this study, we attempted to identify the catalysts potentially active against nitrite in the ischemic heart by examining 1) whether rat heart consumes nitrite in vivo during a clinically relevant episode of regional ischemia-reperfusion produced by transient coronary occlusion; 2) whether the catalysts for this reaction are blood borne, resident in cardiac tissue, or both; and 3) the roles of acidosis and hypoxia in this process.
MATERIALS AND METHODS
Experimental animals.
Experiments were performed in overnight, fasted male Sprague-Dawley rats weighing 300–350 g. All experiments were approved by the Animal Care and Use Committee of Pennsylvania State University and performed in accordance with the guidelines published by the American Physiological Society.
Experimental protocols.
In the first experiment, rats (n = 16) anesthetized with halothane and mechanically ventilated with room air underwent a 15-min snare occlusion of the left coronary artery. Ischemia and reperfusion were confirmed by visual inspection, as previously described (15, 17). Hearts were excised either at the end of the 15-min ischemic period while the left coronary artery was still occluded (n = 6) or after 1 min (n = 5) or 15 min (n = 5) subsequent open-artery reperfusion. Excised hearts were quickly rinsed in nitrite-free phosphate-buffered saline and blotted dry. The central regions of the ischemic anterior and nonischemic posterior left ventricle were then biopsied and immediately frozen in liquid nitrogen for subsequent analysis.
In a second experiment, rats (n = 16) were deeply anesthetized with halothane and mechanically ventilated with room air. Hearts were excised, rinsed in nitrite-free phosphate-buffered saline, blotted dry, and then treated in one of two ways. In sanguinous preparations (n = 8), the left ventricular tissue was finely minced with scissors under nitrite-free phosphate-buffered saline and then homogenized in a glass Dounce homogenizer prerinsed with nitrite-free buffer, and the resulting homogenates were placed on ice for immediate nitrite consumption assay. Asanguinous hearts (n = 8) were treated identically with the exception that red blood cells trapped within the coronary circulation were removed by physiological (perfusion pressure, ∼60 mmHg) antegrade perfusion with nitrite-free phosphate-buffered saline through an aortic cannula before mincing and homogenization.
Four rats were euthanized as blood donors for the experiments testing whether erythrocytes convert nitrite ion to NO under reducing conditions in vitro.
Chemical analyses.
The frozen anterior and posterior myocardial tissue samples (∼100 mg) were ground to powder under liquid nitrogen and the weighed aliquots extracted into 5 volumes nitrite-free water by incubating in hermetically sealed plastic tubes at 4°C for 24 h. To confirm that this procedure quantitatively removed the tissue nitrite and that the extracted nitrite was stable in solution for >24 h, six samples of powdered frozen tissue (∼100 mg) from one rat heart were extracted into nitrite-free water at 4°C. After 24 h, the supernatants were separated by centrifugation and their nitrite concentrations measured immediately and again after being stored an additional 24 h at 4°C. After the original supernatants were removed at 24 h, the tissue pellets were resuspended in another five volumes nitrite-free water and extracted for an additional 24 h at 4°C. These second supernatants were then recovered by centrifugation, and their nitrite concentration was measured immediately. The results of this pilot experiment are shown in Table 1.
Table 1.
Validation of method for recovering nitrite from frozen heart tissue samples
| Extract 1 (24 h) | Extract 1 (48 h) | Extract 2 (24 h) | |
|---|---|---|---|
| Nitrite concentration, nmol/l | 121±25 | 114±29 | 11±6* |
Values are means ± SE. Samples (∼100 mg each, n = 6) of one rat heart frozen at −80°C were powdered in the cold and extracted for 24 h into 0.5 ml nitrite-free water at 4°C. Supernatents were separated from tissue pellets and their nitrite concentrations measured immediately (extract 1, 24 h) and again after storage at 4°C for an additional 24 h (extract 1, 48 h). Tissue pellets recovered at 24 h were reextracted into fresh nitrite-free water for another 24 h (extract 2, 24 h) and measurements repeated. Extraction into water for 24 h quantitatively recovers nitrite from frozen heart tissue.
P < 0.01 vs. extract 1 at 24 h.
The extract nitrite concentration was measured in a Sievers 280i NO analyzer under potassium iodide-acetic acid reducing conditions using procedures recommended by the manufacturer, as previously described (16) and normalized to sample volume, assuming 1 ml/g tissue wt. To confirm that anterior tissue samples were taken from the regions of the myocardium subjected to ischemia (and conversely that posterior samples were not), the glycogen concentration was measured by the amyloglucosidase digestion method (17). Myocardial glycogen is rapidly consumed during ischemia and rapidly repleted during reperfusion (15, 17); its tissue concentration is therefore a sensitive and specific marker of ischemic metabolism.
Nitrite consumption assays were performed to measure the ability of freshly prepared sanguinous and asanguinous heart tissue to consume nitrite in vitro under conditions simulating individual components of the ischemic condition. First, the tissue homogenates were suspended in nitrite-free phosphate-buffered saline at pH of 7.4 and purged for 10 min via a glass cannula with either oxygen or nitrogen. In pilot experiments using whole blood, this procedure yielded erythrocyte hemoglobin saturations of 99 ± 1% (Po2 = 254 ± 37 mmHg) and 25 ± 4% (Po2 = 18 ± 3 mmHg), respectively. Concentrated sodium nitrite solution was then added to produce a slightly supraphysiological (2,000 nmol/l) final concentration in each sample, and the samples were incubated uncapped for 20 min at room temperature. In a subsets of samples, the pH was lowered to 5.5 by an addition of concentrated HCl before incubation; to some of these acidic samples, 100 μmol/l of the XOR inhibitor allopurinol (Sigma Chemical) were also added. Aliquots of each reaction mixture were removed at intervals during the incubation, tissue was removed by brief centrifugation at 10,000 g, and clear supernatants were immediately assayed for residual nitrite concentration.
To examine whether heart tissue contains endogenous nitrite reductase activity that becomes active under more severe hypoxia, parallel assays were performed in which nitrite consumption was measured in sanguinous and asanguinous homogenates after a prolonged degassing with nitrogen to achieve a Po2 of 8 ± 1 mmHg. Based on the oxygen dissociation curve of myoglobin, this level of hypoxia would be expected to produce myoglobin oxygen saturation of ∼60% versus >90% at a Po2 of 18 ± 3 mmHg.
To confirm that nitrite consumption in this assay was specific to heart tissue, parallel control experiments were performed to demonstrate that nitrite was not consumed when the aliquots of a 200 nmol/l nitrite solution not containing heart tissue were subjected to identical treatment. In still other control experiments, it was confirmed that substituting argon for nitrogen as the deoxygenating purge gas had no effect on the nitrite consumption rate of heart homogenates.
Finally, to establish that nitrite consumption by sanguinous heart homogenates reflected its chemical reduction to NO by erythrocyte deoxyhemoglobin, dilute suspensions (hematocrit, ∼5%) of freshly collected rat erythrocytes suspended in nitrite-free phosphate-buffered saline (pH 7.4) were loaded into the reaction chamber of the Sievers 280i NO analyzer and deoxygenated by gassing slowly with helium. The suspensions were allowed to equilibrate until a stable baseline chemiluminescence signal was obtained, and then 100 μl of 200 nmol/l sodium nitrite were added by pipetting through an airtight gasket. The chemiluminescence signal generated by the addition of sodium nitrite was compared with that generated by the addition of isovolumic controls (nitrite-free water and 200 nmol/l sodium nitrate); this experiment was repeated with the order of addition varied to exclude ordering bias.
RESULTS
Tissue glycogen and nitrite consumption in vivo.
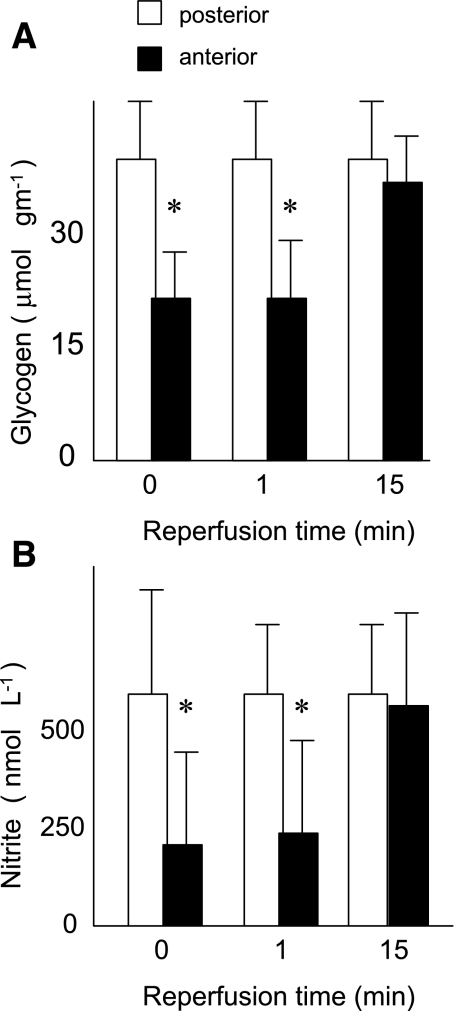
As shown in Fig. 1, a 15-min period of left coronary occlusion reduced the glycogen concentration in the ischemic anterior myocardium by ∼40% relative to control nonischemic posterior regions of the same hearts (23 ± 6 vs. 41 ± 8 μmol/g, P < 0.01), indicating the development of ischemic metabolism in the anterior region. In these same anterior regions, the tissue nitrite concentration simultaneously fell >50% (185 ± 223 vs. 420 ± 203 nmol/l, P < 0.05). During subsequent reperfusion, both the glycogen and nitrite concentration of ischemic myocardial regions returned to the level of control nonischemic regions.
Fig. 1.
Rats underwent a 15-min left coronary occlusion followed by 15 min reperfusion. Concentrations of glycogen (A) and nitrite (B) were measured in ischemic anterior (black bars) and nonischemic posterior (white bars) left ventricle at the end of the 15-min ischemic period (0 min reperfusion) or after 1 or 15 min reperfusion. *P < 0.05 vs. nonischemic posterior myocardium at the same time.
Nitrite consumption by ischemic heart tissue in vitro.
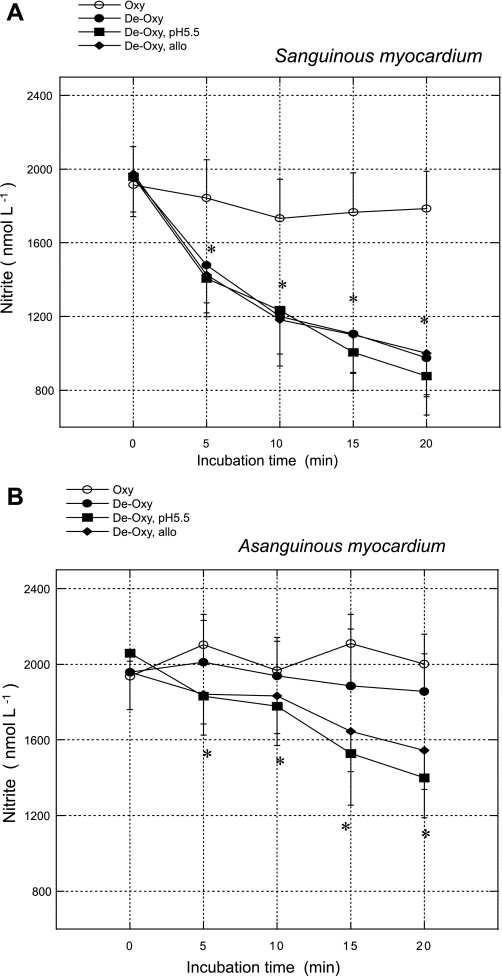
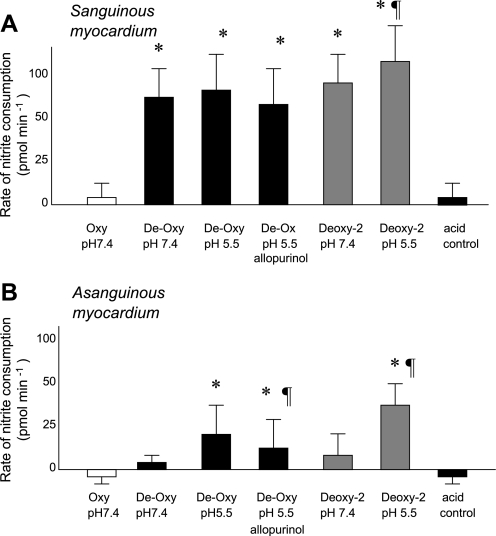
As shown in Figs. 2 and 3, neither sanguinous nor asanguinous myocardium consumed significant amounts of nitrite during the incubation in well-oxygenated medium at a neutral pH. The imposition of simulated tissue hypoxia (purging samples with nitrogen or argon to remove oxygen) accelerated nitrite consumption by sanguinous tissue >15-fold, whereas the additional imposition of simulated tissue acidosis (pH = 5.5) had a minor additional effect. In contrast, in asanguinous samples, moderate hypoxia had little effect on the rate of nitrite consumption, but the lowering pH to 5.5 dramatically accelerated it. Pretreatment with allopurinol blocked ∼40% of the pH effect on nitrite consumption by asanguinous tissue. In both sanguinous and asanguinous homogenates, nitrite consumption proceeded faster under severe hypoxia (Po2 = 8 ± 1 mmHg) than under moderate hypoxia (Po2 = 18 ± 3 mmHg).
Fig. 2.
Residual nitrite plotted against incubation time for experiments in which homogenates of sanguinous (A) or asanguinous (B) heart tissue were incubated with 2,000 nmol/l sodium nitrite at 25°C under conditions listed. Oxy, incubation medium purged with oxygen; De-Oxy, purged with nitrogen; De-Oxy pH 5.5, pH of medium adjusted to 5.5 with HCl and then purged with nitrogen; De-Oxy allo, pH of medium adjusted to 5.5 with HCl and then purged with nitrogen in presence of 100 μM allopurinol. *P < 0.05 vs. time = 0.
Fig. 3.
Average rates of nitrite consumption in vitro during first 10 min incubation by homogenates of sanguinous (A) or asanguinous (B) rat heart incubated with 2,000 nmol/l sodium nitrite under conditions listed. Deoxygenating the incubation medium to Po2 of 18 ± 3 mmHg (Deoxy, black bars) accelerated nitrite consumption by sanguinous heart tissue, whereas acidifying it accelerated nitrite consumption by asanguinous tissue, and 40% of the acidification effect could be blocked by allopurinol. Deoxygenating the medium further to Po2 of 8 ± 1 mmHg (Deoxy-2, gray bars) accelerated acidic nitrite consumption still further in both sanguinous and asanguinous assays. *P < 0.05 vs. De-Oxy (pH 7.4); ¶P < 0.05 vs. De-Oxy (pH 5.5).
Nitrite reduction by erythrocytes.
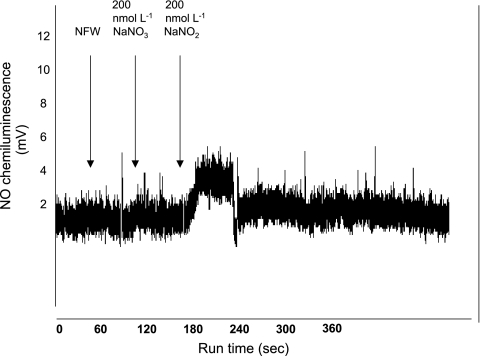
As shown in Fig. 4, the addition of 100 μl of 200 nmol/l sodium nitrite to deoxygenated erythrocytes suspended within the reaction chamber of the NO analyzer resulted in a prompt chemiluminescence signal consistent with the formation and release of NO gas. This signal was not observed after the addition of an equal amount of nitrite-free water or 200 nmol/l sodium nitrate. The quantification of the NO signal, using peak-integration software supplied by the manufacturer, indicated that 12 ± 7% (average of 4 experiments) of the sodium nitrite added to erythrocyte suspensions was released as NO gas.
Fig. 4.
Nitric oxide (NO) chemiluminescence signal generated by addition of 100 μl aliquots of the substances indicated to deoxygenated rat erythrocytes suspended in nitrite-free phosphate-buffered saline within the reaction chamber of a Sievers 280i NO analyzer. Addition of sodium nitrite, but not sodium nitrate or nitrite-free water (NFW), to fresh erythrocytes produced a prompt chemiluminescence signal consistent with formation of NO gas within the reaction chamber.
DISCUSSION
Rat hearts loaded with supplemental nitrite accumulate nitrosly heme complexes during subsequent ischemic perfusion, associated with the activation of guanylate cyclase (24). Likewise, in mice subjected to myocardial hypoxia or ischemia, the administration of sodium nitrite at concentrations only moderately above the physiological range downregulated cardiac energy consumption and substantially limited the development of myocardial necrosis and apoptosis in association with the accumulation of products of NO metabolism (4). These observations suggest the mammalian heart contains an endogenously functioning system for reducing nitrite to cytoprotective NO during ischemia-reperfusion. The results of the present study are consistent with that suggestion. Thus rats subjected to a left coronary artery occlusion at ambient tissue nitrite concentration exhibited a substantial loss of nitrite during ischemia from myocardial regions that exhibited ischemic metabolism (i.e., glycogen depletion).
Several plausible inferences regarding the mechanism involved can be made by comparing the effects of hypoxia and acidosis on sanguinous and asanguinous heart tissue in vitro. The observation that the accelerating effect of hypoxia on nitrite consumption by sanguinous heart tissue homogenates could be substantially abolished by flushing the coronary circulation with crystalloid solution implicates erythrocyte deoxyhemoglobin as the major catalyst for ischemic nitrite reduction in vivo. Indeed, an examination of the data in Figs. 2A and 3A suggests that erythrocytes were responsible for >70% of nitrite consumption when the heart tissue containing the trapped blood was exposed to simulated ischemia (i.e., hypoxia plus acidosis). Figure 4 suggests that at least a portion of the nitrite so consumed was chemically reduced to NO.
A second inference concerns the relative importance of the two principal components of the ischemic condition (hypoxia and acidosis). Based on the comparisons in Figs. 2A and 3A, hypoxia would appear to be a more potent stimulus for nitrite reduction by erythrocyte-containing heart tissue than acidosis, at least under the conditions of moderate hypoxia. In sanguinous heart tissue, deoxygenating the assay medium at pH 7.4 increased the nitrite consumption from 5 to 70 pmol/min, whereas lowering the pH of the deoxygenated assay medium to 5.5 increased it only slightly further to 81 pmol/min. This indicates that hypoxia alone was responsible for ∼85% of the combined effect.
When the erythrocytes were removed, the heart tissue retained a modest ability to consume nitrite. In this circumstance, the effect of moderate hypoxia was small (Figs. 2B and 3B) but the simultaneous imposition of acidosis accelerated nitrite consumption approximately threefold. Pretreatment with the XOR inhibitor allupurinol reduced the rate at which the asanguinous heart tissue incubated at pH 5.5 consumed nitrite by ∼40%, suggesting that XOR is responsible for at least 40% of the endogenous acid-dependent nitrite reductase activity of the asanguinous rat heart tissue. Because XOR mediates nitrite reduction to NO via an electron-transfer reaction resulting from its action on its substrates xanthine and hypoxanthine, we acknowledge that our experiments may underestimate the contribution of XOR to ischemic nitrite reduction if the rate of the parent reaction was constrained by the substrate dilution in the assay medium or other factors. However, our estimate is consistent with the observation of Webb et al. (25) that the treatment of human heart homogenates at pH 5.5 with allopurinol was ∼50% as effective at blocking their nitrite reductase activity as boiling the samples, whereas the NOS inhibitor NG-nitro-l-arginine methyl ester was ineffective.
We note that several authors have recently observed that deoxymyoglobin is a nitrite reductase in heart, generating NO that interacts with mitochondrial cytochromes to inhibit respiration and downregulate cardiac energy production (21, 23–25). These observations were made in experimental preparations largely lacking erythrocytes, specifically the isolated crystalloid-perfused mouse (21) and rat (24–25) heart and dilute rat heart homogenates (23). Our observation that most of the hypoxia-dependent nitrite consumption exhibited by the freshly excised rat heart can be removed by flushing erythrocytes from the coronary circulation distinguishes the apparently major contribution of erythrocyte deoxyhemoglobin from the apparently smaller (at least under the conditions of our experiments) contributions of other heme-containing proteins resident in the heart. This observation is not, however, discordant with the hypothesis that deoxymyoglobin is an important catalyst for the formation of cytoprotective NO in ischemic heart. We observed an approximately threefold increase in allopurinol-insensitive nitrite consumption when the pH of the asanguinous heart tissue assays was lowered from 7.4 to 5.5 (Fig. 3). This is consistent with previous observations that deoxymyoglobin-mediated nitrite reduction also accelerates approximately threefold upon acidification (21, 23) and suggests that deoxymyoglobin may represent the major non-XOR nitrite reductase activity of the asanguinous rat heart under the conditions of moderate hypoxia and acidosis. Moreover, our observation (Fig. 3) that lowering the oxygen partial pressure to a level which should produce significant myoglobin desaturation accelerated acidic nitrite consumption by asanguinous tissue still further suggests that deoxymyoglobin may be an even more important catalyst for NO formation in severely hypoxic cardiac tissue. Taken together, our observations are consistent with those of Tiravanti et al. (24) in the isolated-perfused heart and suggest that the deoxymyoglobin may account for most of the nondeoxyhemoglobin, non-XOR nitrite reductase activity in ischemic heart, at least in cardiomyocytes in which the oxygen partial pressure falls as low as 8 mmHg.
The deoxymyoglobin-mediated nitrite reduction would furthermore result in the formation of intracellular NO, presumably more immediately available to mitochondria and therefore presumably more capable of immediately influencing energy metabolism, than NO generated within the coronary lumen by erythrocytes, which would need to diffuse into tissue before it could influence energy metabolism. These presumptions are supported by the observation of Shiva et al. that deoxymyoglobin is ∼2.5 times more effective than erythrocytes at inhibiting mitochondrial respiration in rat heart homogenates containing exogenous nitrite (23).
Glycogen constitutes a myocardial store of glycolytic substrate that is consumed during ischemia to generate the energy necessary to preserve cellular viability and then rapidly repleted during subsequent reperfusion via the activation of the synthetic enzyme glycogen synthase (17). The observation in this study that myocardial nitrite concentration also recovered quickly after the relief of the coronary occlusion suggests that, in addition to a mechanism for consuming nitrite during ischemia, the rat heart must also contain a mechanism for repleting nitrite during reperfusion when oxygen again becomes available. The identification of this mechanism, including whether it involves an oxygen-dependent NOS, will require additional study.
This study has a number of limitations. The procedure we used to assess nitrite consumption by ischemic heart in vivo (snare coronary occlusion in the rat) also occludes the draining coronary vein, preventing the washout of protons from the ischemic region. This likely resulted in a greater reduction in the ischemic region pH than would be seen in a clinical setting where only the coronary artery would be occluded, and this may have exaggerated the magnitude of the ischemic region nitrite reduction. For the sake of reproducibility, in vitro nitrite consumption assays were conducted at room temperature (25°C) rather than at physiological temperature. Although we measured the ability of the heart tissue to consume nitrite under the conditions simulating tissue ischemia, we did not measure the appearance of NO or any NO metabolic product and so cannot prove that the only metabolic fate of nitrite under these conditions was a chemical reduction to NO.
In summary, rats subjected to a transient coronary occlusion demonstrate a net consumption of nitrite at its ambient concentration, and this phenomenon is limited to the myocardial regions exhibiting ischemic glucose metabolism. Considered along with previous observations that nitrite can be reduced to nitric oxide under conditions of hypoxia and acidosis (4, 24–25), the current observation suggests that the chemical reduction of nitrite to NO may be a characteristic feature of regional ischemic metabolism in the rat heart. Among the potential catalysts for this reaction, the most potent is activated by hypoxia and removed by the flushing of trapped erythrocytes from the coronary circulation and is therefore probably deoxyhemoglobin, whereas other catalysts appear to include both XOR and other pH-sensitive factors endogenous to cardiac tissue, including deoxymyoglobin. To the extent that nitrite-derived NO is cytoprotective in heart tissue, these catalysts may be potential targets for therapies aimed at ameliorating myocardial ischemia-reperfusion injury.
GRANTS
This study was supported by an American Heart Association Grant 0350681N and General Clinical Research Center (Penn State Milton S. Hershey Medical Center) Grants MO1-RR-10732 and C06-RR-016499.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bolli R Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J Mol Cell Cardiol 33: 1897–1918, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO 3rd, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med 9: 1498–1505, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Dimmeler S, Zeiher AM. Nitric oxide and apoptosis: another paradigm for the double-edged role of nitric oxide. Nitric Oxide 1: 275–281, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG, Gladwin MT, Lefer DJ. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest 115: 1232–1240, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrari R, Cargnoni A, Bernocchi P, Pasini E, Curello S, Ceconi C, Ruigrok TJ. Metabolic adaptation during a sequence of no-flow and low-flow ischemia. A possible trigger for hibernation. Circulation 94: 2587–2596, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Gladwin MT Role of the red blood cell in nitric oxide homeostasis and hypoxic vasodilation. Adv Exp Med Biol 588: 189–205, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Godber BL, Doel JJ, Sapkota GP, Blake DR, Stevens CR, Eisenthal R, Harrison R. Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J Biol Chem 275: 7757–7763, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Hataishi R, Rodrigues AC, Neilan TG, Morgan JG, Buys E, Shiva S, Tambouret R, Jassal DS, Raher MJ, Furutaine E, Ichinose F, Gladwin MT, Rosenzweig A, Zapol WM, Bloch KD, Scherrer-Crosbie M. Inhaled nitric oxide decreases infarction size and improves left ventricular function in a murine model of myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 291: H379–H384, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Johnson G, Tsao PS, Mulloy D, Lefer AM. Cardioprotective effects of acidified sodium nitrite in myocardial ischemia with reperfusion. J Phamacol Exp Ther 252: 35–41, 1990. [PubMed] [Google Scholar]
- 10.Jones SP Endothelial nitric oxide synthase overexpression attenuates myocardial reperfusion injury. Am J Physiol Heart Circ Physiol 286: H276–H282, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Jones SP, Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol 40: 16–23, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Kanno S Attenuation of myocardial ischemia/reperfusion injury by superinduction of inducible nitric oxide synthase. Circulation 101: 2742–2748, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Lefer DJ, Nakanishi K, Johnston WE, Vinten-Johansen J. Antineutrophil and myocardial protecting actions of a novel nitric oxide donor after acute myocardial ischemia and reperfusion in dogs. Circulation 88: 2337–2350, 1993. [DOI] [PubMed] [Google Scholar]
- 14.Li H, Samouilov A, Liu X, Zweier JL. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrite reduction. Evaluation of its role in nitric oxide generation in anoxic tissues. J Biol Chem 276: 24482–24489, 2001. [DOI] [PubMed] [Google Scholar]
- 15.McNulty PH, Jagasia D, Cline G, Whiting JM, Ng C, Garg P, Shulman GI, Soufer R. Persistent changes in myocardial glucose metabolism in vivo during reperfusion of a limited-duration coronary occlusion. Circulation 101: 917–922, 2000. [DOI] [PubMed] [Google Scholar]
- 16.McNulty PH, King N, Hartman G, McCann J, Kozak M, Chambers CE, Sinoway LI. Effect of supplemental oxygen administration on coronary blood flow in patients undergoing cardiac catheterization. Am J Physiol Heart Circ Physiol 288: H1057–H1062, 2005. [DOI] [PubMed] [Google Scholar]
- 17.McNulty PH, Luba MC. Transient ischemia induces regional myocardial glycogen synthase activation and glycogen synthesis in vivo. Am J Physiol Heart Circ Physiol 268: H364–H370, 1995. [DOI] [PubMed] [Google Scholar]
- 18.Millar TM, Stevens CR, Benjamin N, Eisenthal R, Harrison R, Blake DR. Xanthine oxidoreductase catalyzes the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett 427: 225–228, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Nagababu E, Ramasamy S, Abernethy DR, Rifkind JM. Active nitric oxide produced in the red cell under hypoxic conditions by deoxyhemoglobin-mediated nitrite reduction. J Biol Chem 278: 11065–11073, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Rakhit RD, Mojer MH, Marber MS, Duchen MR. Mitochondria as targets for nitric-oxide-induced protection during simulated ischemia and reoxygenation in isolated neonatal cardiomyocytes. Circulation 103: 2617–2623, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Rassaf T, Flagel U, Drexhage C, Hengden-Cotta U, Kelm M, Schrader J. Nitrite reductase function of deoxymyoglobin. Oxygen sensor and regulator of cardiac energetics and function. Circ Res 100: 1749–1754, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Schulz R, Wambolt R. Inhibition of nitric oxide synthesis protects the isolated working rabbit heart from ischemia-reperfusion injury. Cardiovasc Res 30: 432–439, 1995. [PubMed] [Google Scholar]
- 23.Shiva S, Huang Z, Grubina R, Sun J, Ringward LA, MacArthur PH, Xu X, Murphey E, Darley-Usman VM, Gladwin MT. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res 100: 654–661, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Tiravanti E, Samouliov A, Zweier JL. Nitrosyl-heme complexes are formed in the ischemic heart: evidence of nitrite-derived nitric oxide formation, storage, and signaling in post-ischemic tissues. J Biol Chem 279: 11065–11073, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemic-reperfusion damage. Proc Natl Acad Sci USA 101: 13683–13688, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woolfson RG, Patel VC, Neild GH, Yellon DM. Inhibition of nitric oxide synthesis reduces infarct size by an adenosine-dependent mechanism. Circulation 91: 1545–1551, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z, Naughton DP, Blake DR, Benjamin N, Stevens CR, Winyard PG, Symons MC, Harrison R. Human xanthine oxidase converts nitrite ions to nitric oxide (NO). Biochem Soc Trans 25: 524S, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med 1: 804–809, 1995. [DOI] [PubMed] [Google Scholar]