Abstract
Nonhuman primates are commonly used in cardiovascular research. Increased arterial stiffness is a marker of subclinical atherosclerosis and higher CV risk. We determined the augmentation index (AI) using applanation tonometry in 61 healthy monkeys (59% female, age 1–25 years). Technically adequate studies were obtained in all subjects and required 1.5 ± 1.3 minutes. The brachial artery provided the highest yield (95%). AI was correlated with heart rate (HR) (r = −0.65, P < .001), crown rump length (CRL) (r = 0.42, P = .001), and left ventricular (LV) mass determined using echocardiography (r = 0.52, P < .001). On multivariate analysis, HR (P < .001) and CRL (P = .005) were independent predictors of AI (R2 = 0.46, P < .001). Body Mass Index (BMI) and AI were independent predictors of higher LV mass on multivariate analysis (P < .001 and P = .03). In conclusion, applanation tonometry is feasible for determining AI. Reference values are provided for AI in bonnet macaques, in whom higher AI is related to HR and CRL, and in turn contributes to higher LV mass.
1. Background
Nonhuman primates are commonly used in biomedical research. Macaques and baboons are the most widely studied species [1–8]. Macaques are anatomically similar to humans and exhibit similar cardiovascular (CV) physiology and metabolism [9–16]. Increased arterial stiffness is a marker of subclinical atherosclerosis and a predictor of higher CV risk. Higher stiffness also contributes to isolated systolic hypertension [17]. Human studies have shown an association between increased arterial stiffness and left ventricular hypertrophy, carotid, and coronary artery disease [18–20]. Recent advances in applanation tonometry have enhanced assessment of arterial stiffness/wave reflection and have lead to its frequent use in human cardiovascular research [17, 18]. However, there are few data using these techniques in nonhuman primates as only one prior study has utilized applanation tonometry in 6 rhesus monkeys [6]. The objective of this study was to determine the feasibility of performing applanation tonometry in apparently healthy bonnet macaques. Bonnet macaques (Macaca radiata) are similar to rhesus in size and appearance and have anecdotally been observed to develop cardiovascular events.
2. Methods
The characteristics of the SUNY Downstate Medical Center primate colony have been described previously [16]. In summary, there are 250 laboratory-born and raised bonnet macaques living in social groups of 6 to 10 and maintained on commercial monkey chow. The SUNY Downstate Medical Center Division of Laboratory Animal Research approved this prospective study. All procedures were performed in careful conformance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. We studied 61 bonnet macaques without recent or ongoing participation in physiological or pharmacological studies.
3. Laboratory Methods
Anesthesia was administered using ketamine (15 mg/kg) via intramuscular injection as clinically indicated to achieve sedation throughout the procedure. Immediately after sedation, each monkey was weighed, crown rump length (CRL) was measured, and blood pressures were recorded by sphygmomanometry of the right lower extremity. Monkey body mass index (BMI) was calculated by dividing weight in kilograms by CRL2 in meters [21]. Monkey body surface area (BSA) was calculated using the formula of Liu and Higbee [22].
We used a pulse wave analysis system (Sphygmocor applanation tonometer interfaced with SphygmoCor software, version 8.0 (AtCor Medical, New South Wales, Australia)). The tonometry transducer was applied to the brachial artery in all subjects and to the axillary (AxA) and carotid artery (CA) in some of the monkeys. After BA and AxA, arterial waveforms were recorded and the central aortic pressure waveform and augmentation index (AI) were derived from the pressure waveform by means of a generalized transfer function, which has been validated in humans [18]. As performed in humans, carotid waveforms were not transformed. AI was defined as the proportional increase in systolic pressure due to the reflected wave and was expressed as a percentage of the pulse pressure (PP) [17]. Arterial waveforms were considered technically adequate if all of the following criteria were met: large amplitude and consistency of pulse height, diastolic decay, and morphology maintained for 10 seconds. Repeat BA studies were performed in 8 randomly selected monkeys.
Two experienced echocardiographers performed transthoracic echocardiographic studies (JL, LS). Each study was carefully inspected to assure adequate endocardial definition. Left ventricular (LV) dimensions were measured from M-mode images according to American Society of Echocardiography standards [23]. Two dimensional images were used when the scanning axis was not perpendicular to the axis of the heart. LV mass and ejection fraction (EF) were calculated by the American Society of Echocardiography-corrected cube formula and indexed by CRL [23].
4. Statistics
Data were expressed as mean ± standard deviation. Univariate associations between variables were analyzed by using Spearman's correlation coefficients. Partial correlation controlling for age was used to assess the relationship between LV mass and AI. Multiple linear regression analysis was performed to determine independent predictors of AI and of LV mass. 95% CI were calculated for indices. Repeatability was expressed as the percentage of the coefficient of variation (SD of the paired differences/the overall mean)/√2 × 100. All statistical analyses were achieved using the Statistical Package for Social Sciences (SPSS) 15.0 software (SPSS Inc., Chicago, Ill, USA). A P < .05 was considered to be statistically significant.
5. Results
Clinical and echocardiographic values including mean values, 95% confidence intervals (CIs), and minimum to maximum ranges are reported for all 61 macaques in Table 1. There were 61 macaques, 36 females, and 25 males with mean age 10 ± 5 years. Mean BMI was 33.2 ± 11.8 kg/m2 and CRL was 0.46 ± 0.05 meters. Mean HR was 167 ± 33 beats/min. Technically adequate arterial waveforms were obtained from the BA in 58 of 61 (95%), from the AxA in 37 of 50 (74%), and from the CA in 18 of 36 (50%) monkeys tested. Brachial artery waveform recording required 1.5 ± 0.3 minutes of interrogation time. Figure 1 shows an example of a BA recording from a 9.5 kg male. Mean AI values were 8.2 ± 18.2% at the BA, 9.9 ± 20.0% at the AxA, and 6.1 ± 20.0% at the CA, P = .21. The coefficient of variation of AlBA was 4.0%.
Table 1.
Clinical and echocardiographic measurements in 61 bonnet macaques.
| Mean ± SD | 95% CI | Range | |
|---|---|---|---|
| Age (years) | 10.3 ± 5.2 | 9.0, 11.7 | 1.0–25.0 |
| CRL (meters) | 0.46 ± 0.05 | 0.44, 0.47 | 0.30–0.57 |
| Weight (kg) | 7.5 ± 3.9 | 6.5, 8.5 | 1.4–19.7 |
| Body mass index (kg/m2) | 33.2 ± 11.8 | 30.1, 36.2 | 14.5–70.0 |
| Body surface area | 0.26 ± 0.07 | 0.24, 0.28 | 0.10–0.45 |
| Heart rate (beats/min) | 167 ± 33 | 158, 175 | 97–244 |
| Septal WT (cm) | 0.39 ± 0.07 | 0.37, 0.41 | 0.26–0.54 |
| Posterior WT (cm) | 0.38 ± 0.06 | 0.36, 0.40 | 0.27–0.51 |
| LVEDD (cm) | 1.76 ± 0.29 | 1.67, 1.85 | 1.1–2.4 |
| LVESD (cm) | 0.89 ± 0.26 | 0.82, 0.97 | 0.49–1.9 |
| Relative wall thickness | 0.45 ± 0.08 | 0.42, 0.47 | 0.28–0.74 |
| EF (%) | 72 ± 13 | 69, 76 | 30–92 |
| LV mass (gm) | 10.2 ± 3.9 | 9.0, 11.4 | 4.4–20.6 |
| LVMI (LV mass/BSA) | 37 ± 9 | 34.3, 39.6 | 17.5–56.4 |
| LVMI (LV mass/CRL) | 21 ± 6.7 | 19.6, 23.7 | 8.8–39.5 |
| Systolic BP | 111 ± 17 | 102, 121 | 82–142 |
| Diastolic BP (mmHg) | 68 ± 11 | 61, 74 | 44–91 |
| Pulse pressure (mmHg) | 43 ± 7 | 39, 48 | 33–56 |
| AI-brachial artery (%) | 8.2 ± 18.2 | 3.4, 13 | −35–43 |
| AI-axillary artery (%) | 9.9 ± 20 | 3.3, 17 | −41–41 |
| AI-carotid artery (%) | 6.1 ± 21 | −4.4, 16 | −55–36 |
Figure 1.
Pulse wave recording of a 13-year-old male bonnet macaque weighing 9.5 kg.
Brachial artery AI was correlated with HR (r = −0.65, P < .001), weight (r = 0.36, P = .006), and CRL (r = 0.42, P = .001). AI was not correlated with age (r = 0.16, P = 0.21). AI was higher in males (16 ± 19 versus 2.6 ± 15%, P = .004). Males were similar in age (10.1 ± 5.1 versus 10.50 ± 5.3 years, P = .98), but were greater in weight (9.9 ± 5.0 versus 5.7 ± 1.5 kg, P < .001), BMI (39.4 ± 16.0 versus 28.8 ± 6.2, P < .001), and CRL (.48 ± .07 versus .44 ± .031, P = .002). Males had lower HRs (142 ± 27 versus 183 ± 25 beats/min, P < .001).
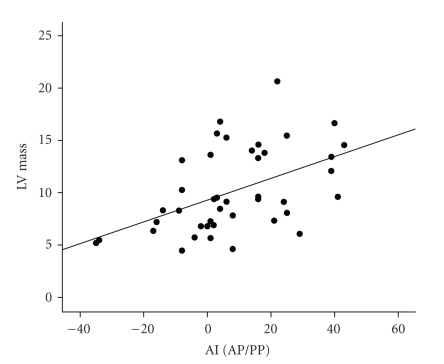
AI was also correlated with the following echocardiographic indices: LVEDD (r = 0.56, P < .001), PWT (r = 0.35, P = .02), LVESD (r = 0.60, P < .001), LV mass (r = 0.52, P < .001) (Figure 2), and LVMI (CRL) (r = 0.41, P = .007). LV mass was significantly correlated with age (r = 0.44, P < .001). Upon partial correlation adjusting for age, AI remained significantly correlated with LV mass (r = 0.45, P = .003) (Figure 2) and with LVMI (CRL) (r = 0.36, P = .01). On multivariate analysis, using age, HR, systolic BP, gender and CRL, and weight as independent variables, HR (P < .001) and CRL (P = .005) were independent predictors of AI with a trend toward age (P = .10) (R2 = 0.46, P < .001). On multiple linear regression using age, gender, BMI, systolic BP, and AI as independent variables, BMI (P < .001) and AI (P = .03) were independent predictors of LV mass (R2 = 0.46, P < .001).
Figure 2.
Relation between AI and LV mass.
6. Discussion
The present study determined the feasibility of applanation tonometry for the noninvasive recording of arterial waveforms and deriving AI from a large cohort of adult bonnet macaques. The subjects represent a wide range of ages and weights in animals of both sexes with a mean HR of 167 beats/min, thereby reflecting differences in access to arteries in relatively small animals. Interpretable waveforms were obtained from the BA in 95% of cases, from the AxA in 80%, and from the CA in 50% of macaques studied.
The BA provided the highest yield likely because of its close proximity to the surface of the skin and its location superficial to humerus bone. This allows the vessel to be flattened with the transmural arterial forces perpendicular to the tonometry probe. In humans, the radial artery is easily accessible and is supported by bony tissue; however, it was too small to evaluate pulse waves in macaques. These findings are likely applicable to rhesus monkeys, which are the most commonly studied macaques and are similar in size to bonnets. In support of this, LV dimensions obtained by echocardiography were similar to those previously reported for rhesus monkeys [7]. The AxA, which is located several cm from the BA, appeared to be a suitable alternative in the majority of animals. We were able to adequately record waveforms from the CA in only half of the animals probably because of their depth and overlying redundant skinfold. Although the femoral artery was not interrogated in this study, our group has been successful in acquiring femoral artery waveforms using this technique in other bonnets, vervet, and cynomologous monkeys.
Indices of arterial wave reflection derived by these techniques are frequently used in human cardiovascular research, but have not been well studied in primates [17]. Although several studies have evaluated arterial stiffness in primates [24, 25], only one prior study utilized applanation tonometry to assess arterial stiffness in monkeys [6]. Vaitkevicius measured the AI in 6 older Rhesus monkeys in order to determine the effects of a synthesized thiazolium compound on arterial properties in old, healthy Macaca mulatta primates. This prior study underscores the value and utility of a noninvasive measure of cardiovascular function. Of note, AI values obtained in the present study are similar to those obtained without a transfer function from the carotid artery in the prior study (6.1–8.1% versus 8.8%) which used telazol anesthesia [6].
The AI is a measure of the magnitude of arterial wave reflection, which is highly correlated well with invasive and noninvasive measures of arterial stiffness in humans [17]. In the present study, AI was easily determined in the vast majority of macaques and was related to HR, CRL, and possibly age. Age and HR dependency of AI has been reported in humans [26]. AI was directly related to CRL. This finding is seemingly contradictory to human data in which AI has been found to be inversely correlated with height [27]. The reason for this discrepancy is not known, but may relate to the CRL being used as a measure of length in macaques or to the possibility that the relation between AI and length differs between anthropoids of shorter and taller stature. The relation between LV mass and AI is also consistent with prior human data [28]. In humans, women exhibit higher AI values than men, even after adjustment for shorter stature [27]. Although males had higher AI than female bonnets on univariate analysis, gender was not an independent predictor of AI on multivariate analysis.
The strong dependency of AI on HR was observed at HRs higher than typically observed in humans. These findings extend the findings of prior studies showing the AI to vary with HRs between 60 and 110 beats/min [26]. The observed slope of the regression line between AI and HR is quite similar to that reported in humans (−.33 versus −.39) [26]. In humans, correction of AI to an arbitrary value of 75 beats/min (AI75) has been proposed and reported extensively [27]. The device automatically calculates the AI75 up to an HR of 110 beats/min, the upper level of HR previously studied. Mean HR of the macaques in the present study was 100 beats/min higher than in humans in whom AI75 was first proposed (167 versus 65 beats/min). Accordingly, correction of AI to 175 beats/min may be a reasonable convention for use in primates. Correction of AI to 175 beats/min resulted in mean values of AI175 of BA 4.8 ± 15%, AxA 6.3 ± 17%, and CA 2.9 ± 16%. Although a rapid HR could be expected to complicate the assessment, reflective wave characteristics were measurable in the vast majority of subjects. The tonometry device records data points at a sampling rate of 128 cycles/s and can easily record arterial waveforms occurring at a rate of 200 beats/min. Higher HRs would limit the sampling to fewer than 40 points per cardiac cycle.
7. Limitations
This is a cross-sectional study of apparently healthy bonnet macaques. All measurements were obtained during sedation with ketamine. Although anesthetic agents are known to influence LV performance, ketamine has been shown to have minimal effects on cardiac contractility and HR [29]. Mean HR of our macaques was similar to that observed in other monkey experiments. Similar LV wall thicknesses have been obtained in rhesus monkeys sedated with ketamine and with a combination of Telazol and Isoflurane [7]. LV mass was based on measurements from the parasternal view, which was easily obtained in all of the animals. Although pulse wave velocity may also be measured by this technique, it was not evaluated in the present study. Similarly, other indices derived from applanation tonometry reflecting cardiac workload were not assessed. The AI was derived using a general transfer function, which has been validated in humans but not yet in nonhuman primates. This merits further study.
In conclusion, this study demonstrates the feasibility and supports the applicability of applanation tonometry for characterizing arterial wave reflection in bonnet macaques. It provides preliminary reference values of AI in a group of macaques, which may be useful for identification of cardiovascular abnormalities in similar primates. The success of applanation tonometry performed at various arterial sites might differ slightly among colony breeds. Validation by comparison of calculated indices to those obtained invasively merits further study.
References
- 1.Haider B, Yeh CK, Thomas G, Oldewurtel HA, Lyons MM, Regan TJ. Altered myocardial function and collagen in diabetic rhesus monkeys on atherogenic diet. Transactions of the Association of American Physicians. 1978;91:197–203. [PubMed] [Google Scholar]
- 2.Swindle MM, Blum JR, Lima SD, Weiss JL. Spontaneous mitral valve prolapse in a breeding colony of rhesus monkeys. Circulation. 1985;71(1):146–153. doi: 10.1161/01.cir.71.1.146. [DOI] [PubMed] [Google Scholar]
- 3.Crawford MH, Walsh RA, Cragg D, Freeman GL, Miller J. Echocardiographic left ventricular mass and function in the hypertensive baboon. Hypertension. 1987;10(3):339–345. doi: 10.1161/01.hyp.10.3.339. [DOI] [PubMed] [Google Scholar]
- 4.Wallrabe D, Wolter F, Heine H, Martin G, Urmantscheeva T, Storrer W. The application of the doppler echocardiography for the determination of the prompt hemodynamic reactors in the rhesus monkeys. Zeitschrift für Klinische Medizin. 1989;44:483–492. [Google Scholar]
- 5.Williams JK, Anthony MS, Clarkson TB. Coronary heart disease in rhesus monkeys with diet-induced coronary artery atherosclerosis. Archives of Pathology and Laboratory Medicine. 1991;115(8):784–790. [PubMed] [Google Scholar]
- 6.Vaitkevicius PV, Lane M, Spurgeon H, et al. A cross-link breaker has sustained effects on arterial and ventricular properties in older rhesus monkeys. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(3):1171–1175. doi: 10.1073/pnas.98.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korcarz CE, Padrid PA, Shroff SG, Weinert L, Lang RM. Doppler echocardiographic reference values for healthy rhesus monkeys under ketamine hydrochloride sedation. Journal of Medical Primatology. 1997;26(6):287–298. doi: 10.1111/j.1600-0684.1997.tb00057.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Tang H, Huang H. Echocardiography of Rhesus monkeys (Macaca mulatta) Chinese Journal of Zoology (Peking) 2005;40(3):95–98. [Google Scholar]
- 9.Swindle MM, Blum JR, Weiss JL. Morphometric studies of the heart in normal rhesus monkey (Macaca mulatta) Journal of Medical Primatology. 1986;15(6):433–440. [PubMed] [Google Scholar]
- 10.Lazar JM, Qureshi G, Qureshi MR, et al. Left ventricular systolic and diastolic function in healthy adult bonnet macaques (Macaca radiata): new echocardiographic indices in old world monkeys. Cardiology. 2008;113(2):116–121. doi: 10.1159/000176065. [DOI] [PubMed] [Google Scholar]
- 11.Buss DD, Hyde DM, Poulos PW., Jr. Coronary artery distribution in bonnet monkeys (Macaca radiata) Anatomical Record. 1982;203(3):411–417. doi: 10.1002/ar.1092030311. [DOI] [PubMed] [Google Scholar]
- 12.Sandhyamani S. Cardiovascular effects in bonnet monkeys (Macaca radiata) of a cassava-based protein-deficient diet. Veterinary and Human Toxicology. 1991;33(5):429–430. [PubMed] [Google Scholar]
- 13.Sandhyamani S. Vasculopathic and cardiomyopathic changes induced by low-protein high-carbohydrate tapioca based diet in Bonnet monkey: vasculopathic and cardiomyopathic changes in induced malnutrition. American Journal of Cardiovascular Pathology. 1992;4(1):41–50. [PubMed] [Google Scholar]
- 14.Kawashima T, Sato K, Akita K, Sasaki H. Comparative anatomical study of the autonomic cardiac nervous system in macaque monkeys. Journal of Morphology. 2005;266(1):112–124. doi: 10.1002/jmor.10371. [DOI] [PubMed] [Google Scholar]
- 15.Nussmeier NA, Benthuysen JL, Steffey EP, et al. Cardiovascular, respiratory, and analgesic effects of fentanyl in unanesthetized rhesus monkeys. Anesthesia and Analgesia. 1991;72(2):221–226. doi: 10.1213/00000539-199102000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Kaufman D, Smith ELP, Gohil BC, et al. Early appearance of the metabolic syndrome in socially reared bonnet macaques. The Journal of Clinical Endocrinology & Metabolism. 2005;90(1):404–408. doi: 10.1210/jc.2004-0452. [DOI] [PubMed] [Google Scholar]
- 17.Nichols WW. Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. American Journal of Hypertension. 2005;18(1, part 2):3S–10S. doi: 10.1016/j.amjhyper.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Chen C-H, Nevo E, Fetics B, et al. Estimation of Central aortic pressure waveform by mathematical transformation of radial tonometry pressure: validation of generalized transfer function. Circulation. 1997;95(7):1827–1836. doi: 10.1161/01.cir.95.7.1827. [DOI] [PubMed] [Google Scholar]
- 19.Boutouyrie P, Tropeano AI, Asmar R, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39(1):10–15. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 20.Qureshi G, Brown R, Salciccioli L, et al. Relationship between aortic atherosclerosis and non-invasive measures of arterial stiffness. Atherosclerosis. 2007;195(2):e190–e194. doi: 10.1016/j.atherosclerosis.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 21.Jen K-L, Hansen BC, Metzger BL. Adiposity, anthropometric measures, and plasma insulin levels of rhesus monkeys. International Journal of Obesity. 1985;9(3):213–224. [PubMed] [Google Scholar]
- 22.Liu CT, Higbee GA. Determination of body surface area in the rhesus monkey. Journal of Applied Physiology. 1976;40(1):101–104. doi: 10.1152/jappl.1976.40.1.101. [DOI] [PubMed] [Google Scholar]
- 23.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's guidelines and standards committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. Journal of the American Society of Echocardiography. 2005;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Farrar DJ, Green HD, Bond MG, Wagner WD, Gobbeé RA. Aortic pulse wave velocity, elasticity, and composition in a nonhuman primate model of atherosclerosis. Circulation Research. 1978;43(1):52–62. doi: 10.1161/01.res.43.1.52. [DOI] [PubMed] [Google Scholar]
- 25.Koenig SC, Ludwig DA, Reister C, Fanton JW, Ewert D, Convertino VA. Left ventricular, systemic arterial, and baroreflex responses to ketamine and TEE in chronically instrumented monkeys. Comparative Medicine. 2001;51(6):513–517. [PubMed] [Google Scholar]
- 26.Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. Journal of Physiology. 2000;525(1):263–270. doi: 10.1111/j.1469-7793.2000.t01-1-00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gatzka CD, Kingwell BA, Cameron JD, et al. Gender differences in the timing of arterial wave reflection beyond differences in body height. Journal of Hypertension. 2001;19(12):2197–2203. doi: 10.1097/00004872-200112000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Hashimoto J, Imai Y, O'Rourke MF. Indices of pulse wave analysis are better predictors of left ventricular mass reduction than cuff pressure. American Journal of Hypertension. 2007;20(4):378–384. doi: 10.1016/j.amjhyper.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 29.van Trijp MJCA, Bos WJW, Uiterwaal CSPM, et al. Determinants of augmentation index in young men: the ARYA study. European Journal of Clinical Investigation. 2004;34(12):825–830. doi: 10.1111/j.1365-2362.2004.01433.x. [DOI] [PubMed] [Google Scholar]