Abstract
Event-related functional MRI and a version of the Stroop color naming task were used to test two conflicting theories of anterior cingulate cortex (ACC) function during executive processes of cognition. A response-related increase in ACC activity was present when strategic processes were less engaged, and conflict high, but not when strategic processes were engaged and conflict reduced. This is inconsistent with the widely held view that the ACC implements strategic processes to reduce cognitive conflicts, such as response competition. Instead, it suggests that the ACC serves an evaluative function, detecting cognitive states such as response competition, which may lead to poor performance, and representing the knowledge that strategic processes need to be engaged.
With the recent emergence of cognitive neuroscience, no other subject has generated greater interest than understanding the neural substrates of higher cognitive functions. In particular, executive functions, which are necessary for controlled information processing and coordinated actions, are now widely investigated. One region of the brain that has been repeatedly associated with executive functions is the anterior cingulate cortex (ACC), on the medial surface of the frontal lobes (1–4). This region of the brain is active during a wide range of cognitive tasks, which engage executive functions (5), and appears to be particularly vulnerable to disease processes in which executive control is impaired (6, 7).
Two broad theories of ACC function during executive control have been proposed. The first, which is based on a large body of functional neuroimaging data, is that the ACC serves a strategic function, selection for action, defined as “processes which reduce the competition between potential responses to a stimulus” (8). This highly influential theory has been invoked to account for the ACC activity that is observed during a wide range of demanding cognitive tasks. The second, perhaps less well known view, derives from electrophysiological studies of the error-related negativity (ERN), a scalp potential that appears to have its source in the ACC (9–12). The ERN is observed concurrent with subjects generating an incorrect response on a range of speeded response tasks. ERNs are also observed when subjects make “partial errors” on trials in which subjects begin to make an error but then correct themselves. Several aspects of the ERN, including the observation that the largest ERNs are generated by these partial errors and that reaction times (RTs) on subsequent trials are longer when ERN′s are larger (12), suggest that the ACC is part of a circuit involved in error detection and compensation. In other words, this theory proposes that the ACC performs an evaluative function in the service of executive control. Recent functional neuroimaging data support that the ACC does indeed show error-related activity, but that it is likely that rather than detecting errors per se, it detects conflict between incompatible response tendencies (13). This has led to an alternative view regarding the evaluative function of the ACC, that this region of the brain detects processing conflicts and as such is part of an error prevention network (2). It remains unknown whether the ACC also implements strategic processes for conflict reduction.
Previous functional imaging studies of the ACC have not distinguished between the presence of conflict, which might be considered to reflect a demand for strategic processes, and the engagement of these processes to strategically reduce the occurrence of conflict and its impact on cognition. Therefore, in the present study, we used event-related functional MRI (fMRI) and a variant of the Stroop color naming task, which dissociated strategic processes and conflict to clarify the strategic vs. evaluative functions of the ACC. In this classical cognitive paradigm, subjects are required to name the color of a word which is itself the name of a color (14). Subjects are faster to color-name when the color and the word are the same (a congruent stimulus, e.g., RED in red) than when the word and color are different (an incongruent stimulus, e.g., the word RED in blue). This increased response time reflects the fact that word reading interferes with the color naming when the word and color conflict (15, 16), and is referred to as the Stroop effect. In the present experiment, we manipulated subjects' expectancies for congruent and incongruent stimuli. When subjects have a high level of expectancy that a stimulus will be incongruent, they are able to strategically adjust the relative influence of word reading on color naming (16) and reduce the amount of conflict that they experience, which is reflected in a small reaction time Stroop effect. In contrast, when they have a high expectancy for congruent words, they allow word reading to more strongly influence their performance and show a large Stroop effect, reflecting high levels of competition as they process the infrequent incongruent stimuli (16–18). In other words, when expectancy for incongruent stimuli is high, strategic processes implementing a high degree of top down control are engaged, and the prepotent tendency to read the word is overcome, and conflict associated with responding to incongruent stimuli is reduced. When expectancy for congruent stimuli is high, strategic processes implementing a high level of top down control are less engaged, less control is exerted over the prepotent response tendency, and consequently conflict associated with responding to incongruent stimuli is increased. In the present study, this dissociation between conflict and strategic processes implementing top down control allowed us to pit the putative strategic and evaluative functions of the ACC against one another. If the ACC implements strategic processes related to high levels of top down control, then it should show increased activity during trials in which strategic processes are engaged and conflict is reduced. If the ACC is serving an evaluative function, detecting competition between incompatible response tendencies, then it should show activity when strategic processes related to top down control are less engaged and conflict is high.
Methods
Subjects were 12 healthy, young, right-handed adults, who were recruited by advertisement and gave written informed consent prior to participating in the study. Subjects performed the Stroop task during fMRI under conditions of both high expectancy for incongruent stimuli and high expectancy for congruent stimuli. In high expectancy incongruent blocks, 80% of trials were incongruent (I/I) and 20% congruent (I/c). In addition, the first four trials in these blocks were always incongruent and no congruent trial was ever followed by another congruent trial. Similarly, in high expectancy congruent blocks, 80% of trials were congruent (C/c) and 20% incongruent (C/i). The first four trials in these blocks were always congruent and no incongruent trial was ever followed by another incongruent trial. Subjects completed 4 blocks of 16 trials of each condition by using a fixed order (ABBABAAB) design to control for linear and second order polynomial scanner drift during the session.
We used the standard approach in Stroop experiments and required subjects to respond verbally. Our event-related method, in which we acquired multiple scans during the course of a single trial, allowed us to use this approach. The well described 4- to 6-s hemodynamic response lag results in a delay in the BOLD response to cognitive events. Hence, artifactual fMRI signal change introduced by verbal responding will be evident in scans acquired at the time of responding, whereas activation associated with cognitive and motor processes associated with responding to the stimulus will be observed in scans acquired several seconds later.
Subjects' verbal responses were collected by using a nonferromagnetic tubing leading to a microphone placed away from the head coil and recorded for later analysis. Tapes were scored for accuracy then digitized. Response latencies for each trial were obtained from the digitized sound files. In scanner accuracy data were obtained for all 12 subjects. In scanner RT data were not obtained for two subjects because of technical problems. RTs were available for these subjects from testing conducted immediately after the scanning session and showed the expected Stroop effect (RT greater for incongruent than congruent stimuli). This effect was greater during the mostly congruent than during the mostly incongruent blocks.
Functional images were acquired by using a 1.5 T GE Signa whole body scanner with a standard head coil. Sixteen axial slices (3.75 mm3 voxels) were obtained parallel to the AC-PC line. Functional scans were obtained by using a two-shot T2*-weighted spiral-scan pulse sequence (TR 1250 ms, TE 35 ms, flip angle 60 deg FOV 24 cm) (19). Scanning was event-related, with acquisition of images synchronized to stimulus presentation. Structural images were obtained prior to functional scanning by using a standard T1-weighted pulse sequence. Images for all subjects were coregistered to a common reference structural MRI scan by using a 12 parameter automated algorithm (20). They were then smoothed by using an 8-mm full-width half-maximum three-dimensional Gaussian filter, to accommodate individual differences in anatomy. Finally, data were pooled across subjects to increase signal to noise.
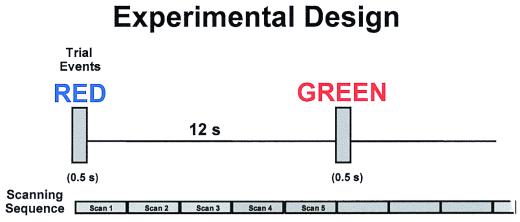
fMRI data were acquired in an event-related manner, with multiple scans within trial providing information about the temporal dynamics of regional brain activity (13). We acquired five 2.5-s scans during a 12.5-s intertrial interval (Fig. 1). We excluded the first scan in each series, during which movement-related artifact would occur. In fact, the amount of additional movement associated with verbal responding was low (<0.05 mm or degrees in any plane), and our results were essentially the same with or without this scan being included.
Figure 1.
Stimuli were presented for 500 ms, followed by an interstimulus interval of 12 s. Image acquisition was synchronized with stimulus onset and five 2.5-s fMRI scans were acquired during the course of a 12.5-s trial.
Results and Discussion
Performance on the Stroop task confirmed that subjects were forming expectancies for incongruent stimuli and engaging strategic processes to reduce the degree of response conflict elicited by incongruent stimuli during the mostly incongruent condition. Error rates were extremely low (0.7% errors, 1% no response or unclear responding). ANOVA conducted on group RT data for correct responses acquired in the scanner with expectancy (mostly congruent, mostly incongruent) and stimulus type (congruent, incongruent) as factors revealed no main effect of expectancy (F[1, 9] = .07, P = .80), and the expected main effect of trial type (F[1, 9] = 21.98, P < .001, with longer latencies observed for incongruent stimuli, [mean 845 ms, SD 152], than for congruent stimuli [mean 747 ms, SD 155]. Most importantly there was also a significant expectancy by trial-type interaction; F[1, 9] = 26.96, P < .001). In the mostly incongruent condition, the mean Stroop effect was 44 ms (I/i 816 ms, SD 156, I/c 772, SD 169), reflecting the engagement of strategic processes and the reduction of conflict. In the mostly congruent condition, the mean Stroop effect was 154 ms (C/i 875 ms, SD 150, C/c 721 ms, SD 144), reflecting that strategic processes were less engaged and conflict was high for incongruent trials in this condition.
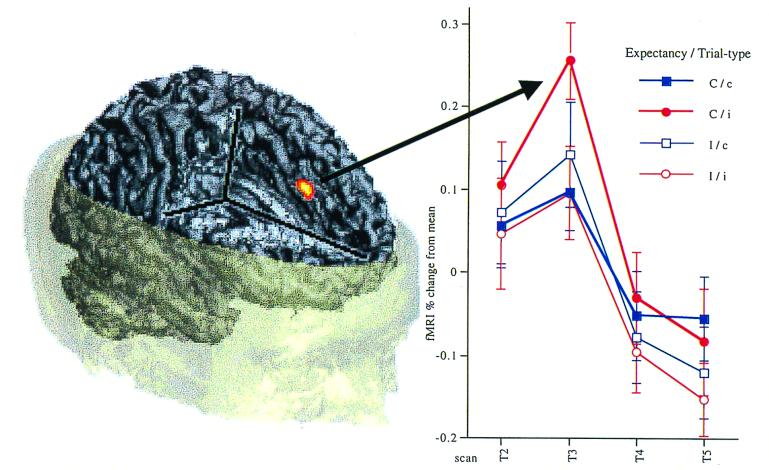
To avoid confounds associated with error-related activity in the ACC, fMRI data were analyzed by using correct trials only. The statistical analysis used voxelwise ANOVAs, with expectancy (mostly incongruent, mostly congruent), stimulus type (congruent, incongruent), and scan-within trial (scans 2–5) as factors. Statistical maps of voxelwise F-ratios were thresholded for significance by using a cluster-size algorithm, which takes account of the spatial extent of activation to correct for multiple comparisons (21). A cluster-size of 8 contiguous voxels and a voxelwise α of 0.01 was chosen, corresponding to an imagewise false positive rate of 0.01. To obtain maximum protection against type I errors that can arise from correlated error in the repeated measurements, we used the minimum possible degrees of freedom for this test, according to the conservative Greenhouse–Geisser adjustment (df = 1, 11). The ACC particle (Talairach coordinates 0, 15, 41) survived this very conservative correction (eight contiguous voxels with F > 9.65). This significant interaction between expectancy, stimulus type, and scan revealed that there was no modulation of ACC activity by trial type in the high control, low conflict incongruent trials in the mostly incongruent blocks. However, ACC activity was robustly increased during the low control, high conflict incongruent trials of the mostly congruent blocks (Fig. 2).
Figure 2.
The time course of the BOLD response in the ACC during the course of a trial for each trial type. Data are displayed as percent change from the mean activity in the region. Transient activity is seen in the ACC, which varies according to expectancy and trial type. In the mostly incongruent condition, ACC activity is not modulated by trial type. In the mostly congruent condition, a robust modulation by trial type is observed, with greater activation in the incongruent, compared with the congruent condition. In addition, when incongruent trials are contrasted directly across the two expectancies, ACC activity is significantly greater in the low control, high conflict, mostly congruent condition, than the high control, low conflict, mostly incongruent condition.
These results are not consistent with a role for the ACC in implementing the strategic processes that reduce response conflict during the mostly incongruent condition. When strategic processes were engaged and conflict reduced, as reflected by the small RT Stroop effect in the mostly incongruent blocks, the ACC was not differentially active during congruent and incongruent trials. In contrast, when strategic processes were relaxed and conflict high, as reflected in the large RT Stroop effect in the mostly congruent condition, the ACC showed robust increases in activity during incongruent trials. Stated in other terms, in this experiment, ACC activity appeared to track the degree of conflict associated with responding to Stroop stimuli, and not the degree to which strategic processes were engaged. Critical in this respect, in the direct contrast of incongruent trials across the two expectancy conditions, activity in the ACC region of interest was significantly higher during low expectancy incongruent trials compared with high expectancy incongruent trials (P < 0.05). When conflict is high and control is low, the ACC is significantly more active than when control is high and conflict is low. These results suggest that the ACC serves an evaluative, rather than strategic, function during executive processes of cognition.
An alternative interpretation of both the reaction time data and the ACC activity would be that rather than reflecting the effects of conflict, driven by expectancy-based variations in top down control, our data reflect the effects of “surprise” effects for the two unexpected stimulus types (unexpected incongruent or C/i, unexpected congruent or I/c). This is a difficult confound to address, because both surprise and strategic effects would result in the same pattern of behavioral effects, slower RTs for C/i compared with I/i (increased interference or surprise), and slower RTs for I/c than C/c (decreased facilitation or surprise). Results of an experiment by Tzelgov et al. (22) do suggest that strategic factors, and not surprise, are driving RT effects in the current study. In that study, expectancies were manipulated by varying the proportions of neutral words in a block while keeping the relative proportions of congruent and incongruent stimuli fixed. Increasing proportions of neutral words, and hence lower expectancies for color words, resulted in increasing Stroop effects (incongruent–congruent, as in the present study). If the expectancy effects were the result of surprise, rather than control, this pattern of results would not have been seen, since congruent and incongruent trials were equally unexpected in the high proportion neutral conditions. A notable neuroimaging finding that is inconsistent with the notion that the ACC activate with surprise is the observation of increased activity in this region when an implicitly learned higher order grammar, which predicted the sequence of button press responses to be generated by the subject, was changed to a novel one unbeknownst to the subject (23). ACC activity associated with conflict between the learned and novel stimulus-response mappings was observed despite the fact that subjects had no explicit awareness of the grammar change.
Also arguing against a “surprise” account of the ACC activation observed in the study is the fact that a direct contrast of the activation in this region of the brain in the I/c and C/c conditions was not significant (P > .8). Finally, we conducted an additional analysis, which speaks to whether “surprise alone” rather than conflict could account for the ACC activation observed in the present study if we assume that surprise is a factor driving this region. We focused on conflict-related activity at the peak of the ACC activity (scan 3) and “subtracted out” activation that may be due to a general effect of surprise (for each subject) by using the formula (C/i − I/i) − [Mean(C/i + I/c) − Mean(I/i + C/c)]. This was significantly greater than zero, (t = 2.62, one-tailed P = .012), indicating that some portion of the difference observed at scan 3 between the expected and unexpected incongruent stimulus is the result of differences in conflict arising from strategic effects.
It is noteworthy that in the present study strategic effects were engaged “tonically,” by allowing subjects to form expectancies as to the likely occurrence of conflict-associated stimuli. In a separate study (24), we have also shown that when strategic processes are modulated on a trial-by-trial basis, ACC activity still reflects the degree of response conflict elicited by a stimulus, and not the degree to which strategic processes are transiently engaged. In that study, we used event-related fMRI and the Eriksen task. It has been shown (25) that when subjects respond to an incompatible stimulus following another incompatible stimulus (I/i trials), they experience less conflict (and show faster RTs) than when they respond to an incompatible stimulus following a compatible one (C/i trials). In that experiment, ACC activity was significantly higher for high conflict, low control C/i trials than for high control, low conflict I/i trials, paralleling the results of the present study and suggesting that the ACC is reflecting response conflict rather than implementing trial-by-trial strategic effects.
One interpretation of our data that might suggest a strategic, rather than evaluative, function for the ACC would be that this region is modulating, in real time, the allocation of attention between color naming and word reading. In this case, when subjects encounter an incongruent trial during the mostly congruent condition, the cingulate would become active as subjects switched from word reading to color naming. If the ACC were subserving such a strategic, real-time “switching” function, we would expect that the activity of this region should be negatively correlated with the disruptive effects of the incongruent stimulus on performance (i.e., the Stroop effect). The more active the ACC, the more efficient the switching, the smaller the influence of the word on color naming. If, on the other hand, the ACC is reflecting the degree of conflict elicited by the task, then the opposite pattern would be expected, with a positive correlation between ACC activity and the Stroop effect. We conducted this analysis on data from the mostly congruent condition and found that the mean size of the Stroop effect for each subject (incongruent RT − congruent RT) was significantly positively correlated with subjects' mean percent change in activation from baseline (scan 1) to scan 3 in the ACC during incongruent trials (r = .58, P < .05). This suggests that ACC activation reflects the degree of conflict elicited by the stimulus, and not the effect of switching attention in response to the stimulus.
In the present experiment one additional region of the brain, left inferior parietal cortex (IPL, BA 40 Talairach coordinates −47, 55, 42), also showed conflict related activity; however, the time course of the BOLD response was quite different to that seen in the ACC. IPL activity was greatest for high conflict trials and, as was also observed in the ACC, this activity peaked in scan 3. Unlike the ACC, where conflict related activity was transient and returned to baseline before the next trial, in IPL this increased conflict-related activity remained high until the onset of the next stimulus. This suggests that one region in the brain that may implement trial-by-trial strategic processes is right IPL.
Two other regions showed significant expectancy by trial type by scan effects, inferior frontal cortex (BA 44/45) bilaterally and extrastriate visual cortex (BA 18/19). All three regions showed transient, response-related increases in activity over the course of a trial. Left and right BA 44/45 (Talairach coordinates −47, −6, 27; 41, −8, 24, respectively) both showed higher activity for c/I than other trial types. Interestingly, in both of these regions, activity peaked fully a scan earlier (scan 2) than was seen in the ACC. The earlier time course of peak response might be consistent with the hypothesis that inferior frontal cortex is engaged according to the selection demands of the task, as recently proposed by Thompson-Schill et al. (26). The extrastriate region (−11, 90, 24) showed lower activity for the c/C stimuli than for the other three types, peaked later than the ACC (scan 4), and was not fully back to baseline by scan 5. The time course of activity was similar in this region to that seen in IPL, suggesting that it, too, might be reflecting trial-by-trial strategic effects.
Under conditions in which their performance indicated that subjects were engaging strategic processes to reduce the effects of response conflict, no increased activity was observed in the ACC. This is inconsistent with the theory that the ACC implements strategic processes to minimize the degree of conflict elicited by the task. In contrast, when subjects' performance indicated that strategic processes were less engaged and conflict high, a transient increase in activity was observed in this region of the brain. This result suggests that the ACC performs an evaluative function, reflecting the degree of response conflict elicited by the task. Because the ERN literature suggests that ACC activity is associated with subsequent “corrective” actions, it is likely that other components of the neural network implementing executive functions are influenced by ACC activity to implement strategic processes. This is consistent with the hypothesized central role for this brain region in the executive control of cognition (27–29). In the present view, the ACC would serve this function by providing an on-line conflict signal, indicating the need to engage brain regions such as dorsolateral prefrontal cortex and IPL to implement strategic processes (2). As such, the ACC would serve as one component of an “error prevention” network. Future functional neuroimaging studies are likely to reveal further details of the modular organization of the neural network subserving executive processes and the mechanisms by which individual components interact to maintain the tightly coordinated yet highly flexible activity that characterizes the normal human cognitive system.
Acknowledgments
This work was supported in part by a National Institute of Mental Health Research Scientist Development Award for Clinicians and by a National Alliance for Research on Schizophrenia and Depression Young Investigators Award (to C.S.C.).
Abbreviations
- ACC
anterior cingulate cortex
- ERN
error-related negativity
- IPL
inferior parietal cortex
- fMRI
functional MRI
- RT
reaction time
References
- 1.Devinsky O, Morrel M J, Vogt B. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 2.Carter C S, Botvinick M M, Cohen J D. Rev Neurosci. 1999;10:49–57. doi: 10.1515/revneuro.1999.10.1.49. [DOI] [PubMed] [Google Scholar]
- 3.Posner M I, Dehaene S. Trends Neurosci. 1994;17:75–79. doi: 10.1016/0166-2236(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 4.D'Esposito M, Detre J A, Alsop D C, Shin R K, Atlas S, Grossman M. Nature (London) 1995;378:279–281. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- 5.Cabeza R, Nyberg L. J Cognit Neurosci. 1997;9:1–26. doi: 10.1162/jocn.1997.9.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Carter C S, Mintun M, Nichols T, Cohen J D. Am J Psychiatry. 1997;154:1670–1675. doi: 10.1176/ajp.154.12.1670. [DOI] [PubMed] [Google Scholar]
- 7.Dolan R J, Bench C J, Brown R E, Scott L C, Frackowiak R J S. Psychol Med. 1994;24:849–854. doi: 10.1017/s0033291700028944. [DOI] [PubMed] [Google Scholar]
- 8.Posner M I, Petersen S E, Fox P T, Raichle M E. Science. 1988;240:1627–1631. doi: 10.1126/science.3289116. [DOI] [PubMed] [Google Scholar]
- 9.Dahaene S, Posner M I, Tucker D M. Psychol Sci. 1994;5:303–305. [Google Scholar]
- 10.Hohnsbein J, Falkenstein M, Hoorman J. J Psychophysiol. 1989;3:32. [Google Scholar]
- 11.Gehring W J, Coles M G H, Meyer D E, Donchin E. Psychophysiology. 1990;27:S34. [Google Scholar]
- 12.Gehring W J, Goss B, Coles M G H, Meyer D E, Donchin E. Psychol Sci. 1993;4:385–390. doi: 10.1177/1745691617715310. [DOI] [PubMed] [Google Scholar]
- 13.Carter C S, Braver T S, Barch D M, Botvinick M M, Noll D, Cohen J D. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 14.Stroop J R. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- 15.MacCleod C M. Psychol Bull. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- 16.Cohen J D, Dunbar K, McClelland J L. Psychol Rev. 1990;97:332–361. doi: 10.1037/0033-295x.97.3.332. [DOI] [PubMed] [Google Scholar]
- 17.Lindsay D S, Jacoby L L. J Exp Psychol Hum Percept Perform. 1994;20:219–234. doi: 10.1037//0096-1523.20.2.219. [DOI] [PubMed] [Google Scholar]
- 18.Logan G D, Zbrodoff N J. Mem Cognit. 1979;7:166–174. [Google Scholar]
- 19.Noll D C, Cohen J D, Meyer C H, Schneider W. J Magn Reson Imaging. 1995;5:49–56. doi: 10.1002/jmri.1880050112. [DOI] [PubMed] [Google Scholar]
- 20.Woods R P, Cherry S R, Mazziotta J C. J Comput Assist Tomogr. 1992;16:620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- 21.Forman S D, Cohen J D, Fitzgerald M, Eddy W F, Mintun M A, Noll D C. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 22.Tzelgov J, Henik A, Berger J Mem Cognit. 1992;20:727–735. doi: 10.3758/bf03202722. [DOI] [PubMed] [Google Scholar]
- 23.Berns G, Cohen J D, Mintun M. Science. 1997;276:1272–1275. doi: 10.1126/science.276.5316.1272. [DOI] [PubMed] [Google Scholar]
- 24.Botvinick, M. M., Nystrom, L., Fissell, K., Carter, C. S & Cohen J. D. (2000) Nature (London), in press. [DOI] [PubMed]
- 25.Gratton G, Coles M G H, Donchin E. J Exp Psychol Gen. 1992;4:480–506. doi: 10.1037//0096-3445.121.4.480. [DOI] [PubMed] [Google Scholar]
- 26.Thompson-Schill S L, D'Esposito M, Kan I P. Neuron. 1999;23:513–522. doi: 10.1016/s0896-6273(00)80804-1. [DOI] [PubMed] [Google Scholar]
- 27.Mesulam M M. Ann Neurol. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- 28.Posner M I, Dehaene S. Trends Neurosci. 1994;17:75–79. doi: 10.1016/0166-2236(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 29.Posner M I, DiGirolamo G J. The Attentive Brain. Cambridge, MA: MIT Press; 1998. pp. 401–423. [Google Scholar]