Abstract
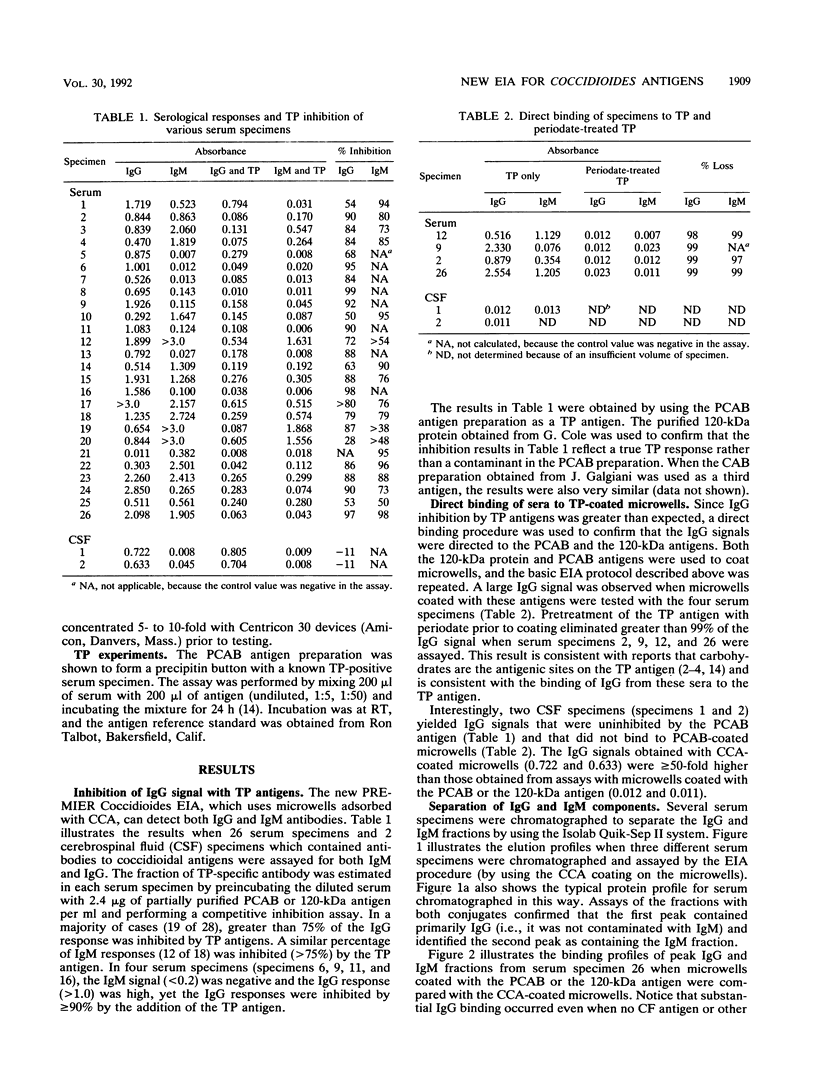
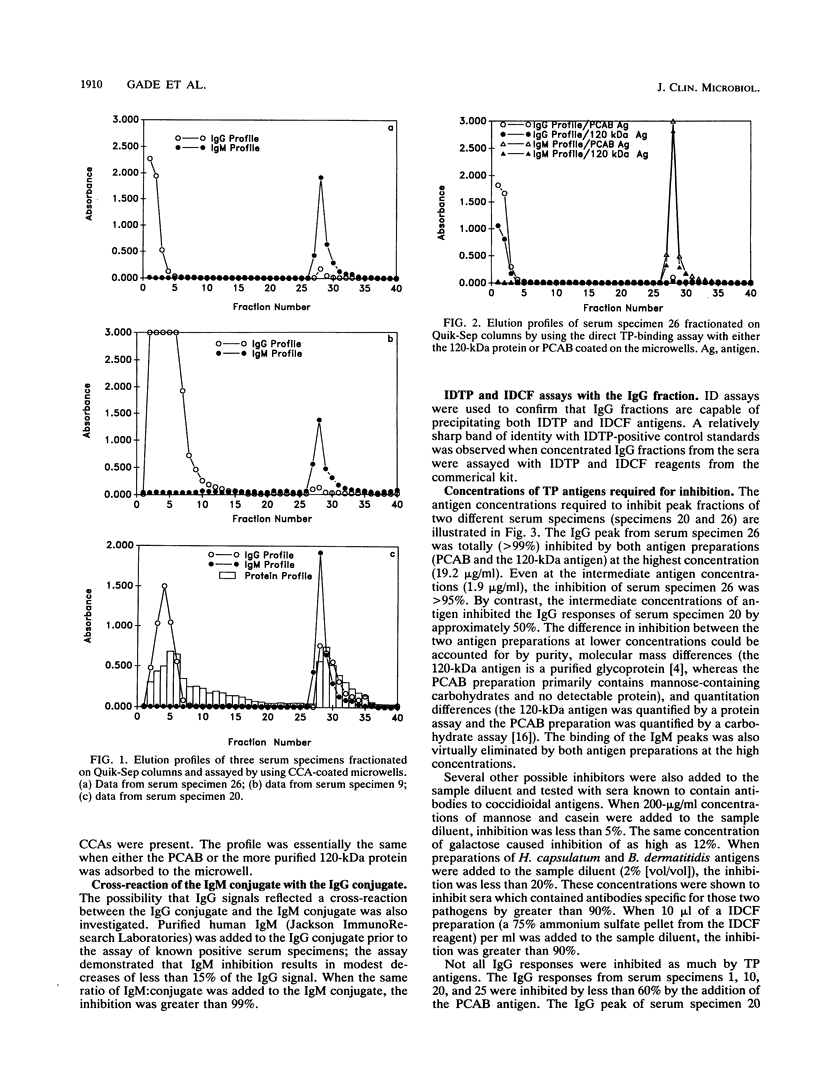
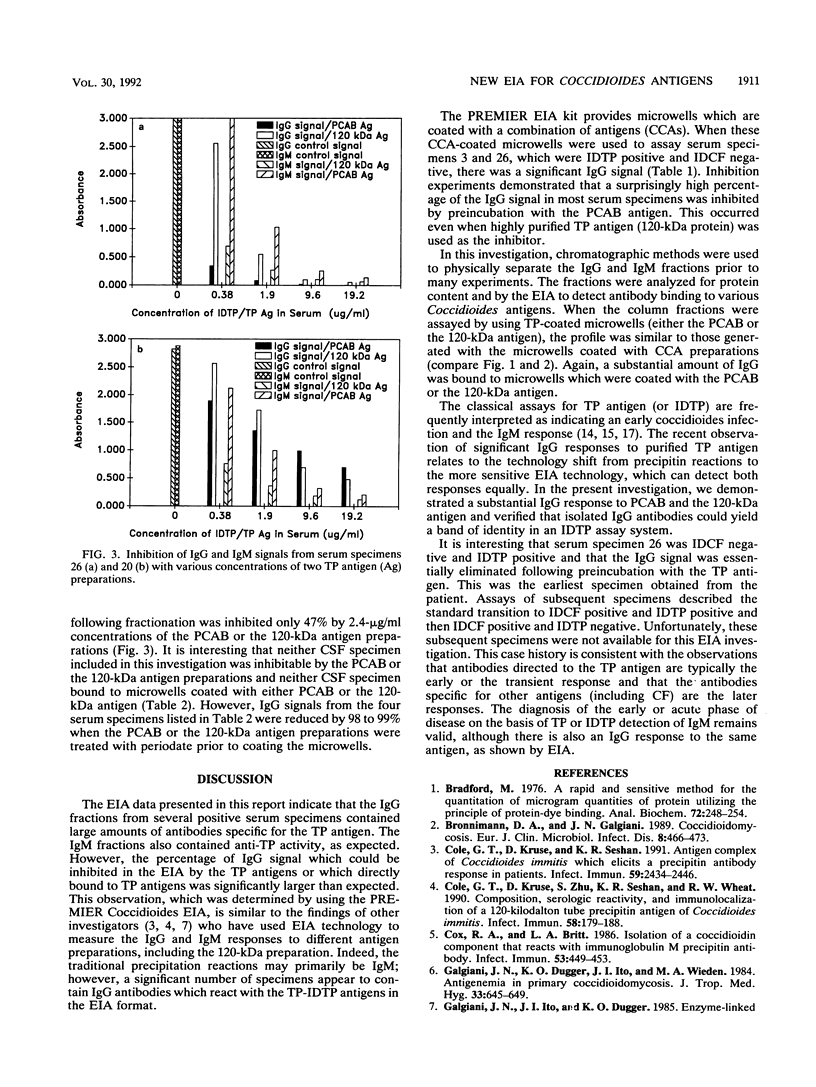
A new enzyme immunoassay (EIA) was used to investigate immunoglobulin M (IgM) and IgG responses to various coccidioidal antigen preparations. Direct binding and inhibition assays both revealed that the IgG responses of many serum specimens were directed largely to the tube precipitin (TP) antigen even when the microwells were coated with a combination of coccidioidal antigens (CCAs). From a panel of 28 serum specimens containing antibodies to CCAs, 4 serum specimens yielded high IgG signals (absorbance, greater than 1.0) and were negative for IgM (absorbance, less than 0.2), yet all four IgG responses were inhibited by at least 90% with partially purified TP antigens (2.4 micrograms/ml). The IgM and IgG fractions of several serum specimens were separated by ion-exchange chromatography and assayed by using different antigen preparations adsorbed to the microwells. The binding of both IgM and IgG peaks to the microwells coated with the CCA preparation was inhibited significantly by preincubation with TP antigens. One serum specimen (specimen 26) yielded a large IgG response (absorbance, greater than 2.0) with the CCA preparation and also bound directly to microwells coated only with TP antigens. The IgG signal (absorbance) of serum specimen 26 was reduced by 98% when it was preincubated with TP antigens prior to the assay. Significant IgG signals from several other serum specimens were observed when microwells were coated with TP antigen preparations, but they were absent when periodate-treated preparations were used. Two cerebrospinal fluid specimens yielded IgG signals with CCA-coated microwells, which were not inhibited by TP antigens, and yielded no signal with microwells coated only with TP antigens. The results are consistent with the concept that the typical serologic response to TP antigens occurs early in disease progression, but they suggest that TP antigens stimulate both IgM and IgG responses.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bronnimann D. A., Galgiani J. N. Coccidioidomycosis. Eur J Clin Microbiol Infect Dis. 1989 May;8(5):466–473. doi: 10.1007/BF01964061. [DOI] [PubMed] [Google Scholar]
- Cole G. T., Kruse D., Seshan K. R. Antigen complex of Coccidioides immitis which elicits a precipitin antibody response in patients. Infect Immun. 1991 Jul;59(7):2434–2446. doi: 10.1128/iai.59.7.2434-2446.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole G. T., Kruse D., Zhu S. W., Seshan K. R., Wheat R. W. Composition, serologic reactivity, and immunolocalization of a 120-kilodalton tube precipitin antigen of Coccidioides immitis. Infect Immun. 1990 Jan;58(1):179–188. doi: 10.1128/iai.58.1.179-188.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Britt L. A. Isolation of a coccidioidin component that reacts with immunoglobulin M precipitin antibody. Infect Immun. 1986 Sep;53(3):449–453. doi: 10.1128/iai.53.3.449-453.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galgiani J. N., Dugger K. O., Ito J. I., Wieden M. A. Antigenemia in primary coccidioidomycosis. Am J Trop Med Hyg. 1984 Jul;33(4):645–649. doi: 10.4269/ajtmh.1984.33.645. [DOI] [PubMed] [Google Scholar]
- Kaufman L., Standard P. G., Huppert M., Pappagianis D. Comparison and diagnostic value of the coccidioidin heat-stable (HS and tube precipitin) antigens in immunodiffusion. J Clin Microbiol. 1985 Oct;22(4):515–518. doi: 10.1128/jcm.22.4.515-518.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman L. Value of immunodiffusion tests in the diagnosis of systemic mycotic diseases. Ann Clin Lab Sci. 1973 Mar-Apr;3(2):141–146. [PubMed] [Google Scholar]
- Pappagianis D., Zimmer B. L. Serology of coccidioidomycosis. Clin Microbiol Rev. 1990 Jul;3(3):247–268. doi: 10.1128/cmr.3.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH C. E., SAITO M. T., SIMONS S. A. Pattern of 39,500 serologic tests in coccidioidomycosis. J Am Med Assoc. 1956 Feb 18;160(7):546–552. doi: 10.1001/jama.1956.02960420026008. [DOI] [PubMed] [Google Scholar]
- Sawaki Y., Huppert M., Bailey J. W., Yagi Y. Patterns of human antibody reactions in coccidioidomycosis. J Bacteriol. 1966 Jan;91(1):422–427. doi: 10.1128/jb.91.1.422-427.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieden M. A., Galgiani J. N., Pappagianis D. Comparison of immunodiffusion techniques with standard complement fixation assay for quantitation of coccidioidal antibodies. J Clin Microbiol. 1983 Sep;18(3):529–534. doi: 10.1128/jcm.18.3.529-534.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer B. L., Pappagianis D. Characterization of a soluble protein of Coccidiodes immitis with activity as an immunodiffusion-complement fixation antigen. J Clin Microbiol. 1988 Nov;26(11):2250–2256. doi: 10.1128/jcm.26.11.2250-2256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


