Abstract
The saturated fatty acid palmitate alters normal cell function via disruption of cell signaling, and these effects have been implicated in the end-organ damage associated with dyslipidemia. Neuregulin-1β (NRG-1β) is a growth and survival factor in cardiac myocytes. We tested the hypothesis that palmitate alters NRG-1β signaling and biology in isolated neonatal rat cardiac myocytes. Palmitate treatment inhibited NRG-1β activation of the PI3kinase/Akt pathway in myocytes. We found that the pro-apoptotic activity of palmitate was increased by NRG-1β treatment. The effects of palmitate on NRG-1β signaling and survival were reversed by the mono-unsaturated fatty acid oleate. Under control conditions NRG-1β decreases p53 expression in myocytes. In the presence of palmitate, NRG-1β caused an increase in p53 expression, bax multimer formation, concurrent with degradation of mdm2, a negative regulator of p53. Thus in the presence of palmitate NRG-1β activates pro-apoptotic, rather than pro-survival signaling in cardiac myocytes.
INTRODUCTION
Neuregulins (NRG) are a complex family of growth factors that regulate cell growth, survival, proliferation and differentiation in multiple tissues including epithelium, nerve, skeletal and cardiac muscle (for review see [1; 2]). NRG signaling is mediated through the coupling and activation of the erbB family of type I receptor tyrosine kinases (RTK). Like other receptor tyrosine kinases, the erbB2, erbB3, and erbB4 proteins couple to both MAPK and PI3kinase pathways, and NRG stimulation can activate growth, differentiation, metabolic and survival responses in diverse cells and tissues [1; 3]. NRG-1β as well as the erbB2 and erbB4 receptors are required for cardiac development [4; 5; 6] and alterations in NRG/erbB signaling have been implicated in the pathophysiology of heart failure [7]. Expression of NRG-1β in the adult heart persists in microvascular endothelial cells, and recombinant NRG-1β can activate growth and survival responses in isolated cardiac myocytes via the erbB2 and erbB4 receptors [8; 9]. NRG-1β activation of Akt via PI3K is anti-apoptotic and protects myocytes from anthracycline and hydrogen peroxide induced cell death in vitro [7; 10; 11]. Mice deficient in NRG-1β are more sensitive to cardiac injury [12]. Thus the NRG-1β/erbB system in the heart appears to be a local cardioprotective signaling system.
Saturated fatty acids disrupt normal cell biology and induce cell death by apoptosis in multiple cell types, including cardiac myocytes [13]. The saturated fatty acid palmitate alters signaling of receptor tyrosine kinases such as insulin and IGF-1 with disruption of PI3-K and Akt activation [14; 15]. These effects have been linked to decreasing insulin sensitivity of peripheral tissue [15; 16] as well as changing glucose-dependent insulin secretion in pancreatic beta cells [17]. We hypothesized that palmitate would alter NRG-1β pro-survival signaling and cytoprotection in cardiac myocytes. We found that palmitate causes selective changes in NRG-1β signaling, and that this was associated with augmentation of palmitate-induced apoptosis by NRG-1β. We investigated the possible role of fatty acid intermediates and kinase pathways in this phenomenon, and discuss the potential implications of these findings in the pathophysiology of end-organ damage in the setting of dyslipidemia.
METHODS
Primary Culture of Ventricular Myocytes
Neonatal rat ventricular myocytes (NRVM) were isolated as previously described [18] from 1-2 day old Sprague Dawley rat pups. Following multiple pre-platings to increase myocyte purity, cells were cultured in DMEM (Gibco) with 7% fetal bovine serum (Gibco) for 24 or 48 hours before serum starvation or treatment.
Cell Culture Conditions and Treatments
Palmitate and oleate (sodium salt) were added to DMEM containing 2% w/v fatty acid free BSA (Sigma #A-0281) (for a final molar ratio of 0.6:1, BSA:FA for [FA] 0.5mM) and either 25mM glucose (‘high glucose’) or 5mM glucose + 20mM mannitol (‘low glucose’). All experiments were performed in ‘low glucose’ unless otherwise noted. The fatty acids were added to the media by first dissolving in 90°C water. Experiments were either carried out under BSA:FA or BSA control conditions. All reagents listed above, (±)-threo-1-Phenyl-2-decanoylamino-3-morpholino-1-propanol hydrochloride (PDMP) and insulin were purchased from Sigma. Recombinant human NRG-1-II-β3 (rhGlial Growth Factor 2, refered to as NRG-1β throughout the paper) was from Mark Marchionni.
Flow Cytometry
Apoptosis was quantified as previously described using flow cytometry [18]. Gating was performed on the cells to exclude very small debris with >2 logs weaker staining for PI than G0 cells. The hypodiploid population of cells remaining was considered apoptotic. Fluorescence of 10,000 cells was measured and apoptosis was calculated as the percent of cells in the sub-G1/Go peak.
Western Blot Analysis
Kinase activation was assessed by immunoblot analysis of myocyte lysates. Cells were lysed in a modified RIPA buffer containing 1%NP-40 (Calbiochem), 0.25% deoxycholic acid, 50mM Tris-HCl (pH 7.4), 1mM EDTA, 150 mM NaCl, 1 mM NaF, 1ug/ml Leupeptin, 1mM PMSF and 1mM sodium orthovanadate. Protein concentrations were quantified with Bradford Reagent (Bio-Rad) and normalized protein between 50-100ug was run on a 10%Tris-HCl ready gel (Bio-Rad). Protein was transferred to a PVDF membrane by semi-dry transfer (Bio-Rad) at a constant current of 200mA/gel for 20 min. Western blotting for phospho-akt (Ser473, Cell Signaling #9271), phospho-p38(Thr180/Tyr182, Cell Signaling #9211), phospho-ERK 1/2 (Thr202/Tyr204, Cell Signaling #9101), total akt (Santa Cruz), phospho-tyrosine (Santa Cruz), p53 Ab-1 (Calbiochem, #OP03T), bax Ab-5 (Neomarkers #MS1335), and mdm2 (Santa Cruz, #sc965), was performed using the recommendations of the manufacturer. In order to analyze receptor phosphorylation, anti-erbB2 or anti-erbB4 (Neomarkers, MS-326 and MS-270) was added to 200-400ug of lysate (normalized for concentration and volume) at a dilution of 1:100 and incubated overnight at 4 °C. Protein was then precipitated with protein A/G plus agarose (Santa Cruz) for three hrs at 4 °C. Following washing with RIPA buffer, protein was dissociated from the beads by boiling in Laemmli buffer, separated on 10%Tris-HCl ready gel, and transferred to a PVDF membrane overnight at a constant voltage of 50mA, and then immunoblotted for phospho-tyrosine (Santa Cruz).
Statistical Analysis
Quantified values were analyzed by one-way or two-way ANOVA as appropriate and Bonferonni Post Test analysis, unless otherwise noted. All error bars presented represent standard error of the mean unless otherwise noted.
RESULTS
Recombinant NRG-1β induced erbB2, erbB4, Akt, and ERK phosphorylation in NRVM, as we have previously reported [8; 9]. Palmitate (0.5 mM) treatment for 18 h suppressed NRG-1β activation of Akt, with relatively little effect on phosphorylation of erbB2 or erbB4. Palmitate treatment resulted in tyrosine-phorylated proteins co-immunoprecipitating with erbB2. Palmitate increased ERK phosphorylation, and NRG-1β stimulation further increased levels of ERK phosphorylation. Palmitate also induced phosphorylation of p38 kinase, in a manner that was unaltered by NRG-1β (Fig. 1A). In skeletal myotubes, palmitate alters insulin activation of ERK 1/2 and Akt [15]. However, in cardiac myocytes, palmitate did not change insulin activation of Akt (Fig. 1B) or ERK (data not shown). Thus, in cardiac myocytes, palmitate inhibits NRG-1β stimulated phosphorylation of Akt, with preservation of both NRG-1β activation of ERK, and insulin stimulation of Akt.
Figure 1. Palmitate prevents NRG-1β activation of Akt without inhibiting MAPK phosphorylation or insulin-induced Akt activation.
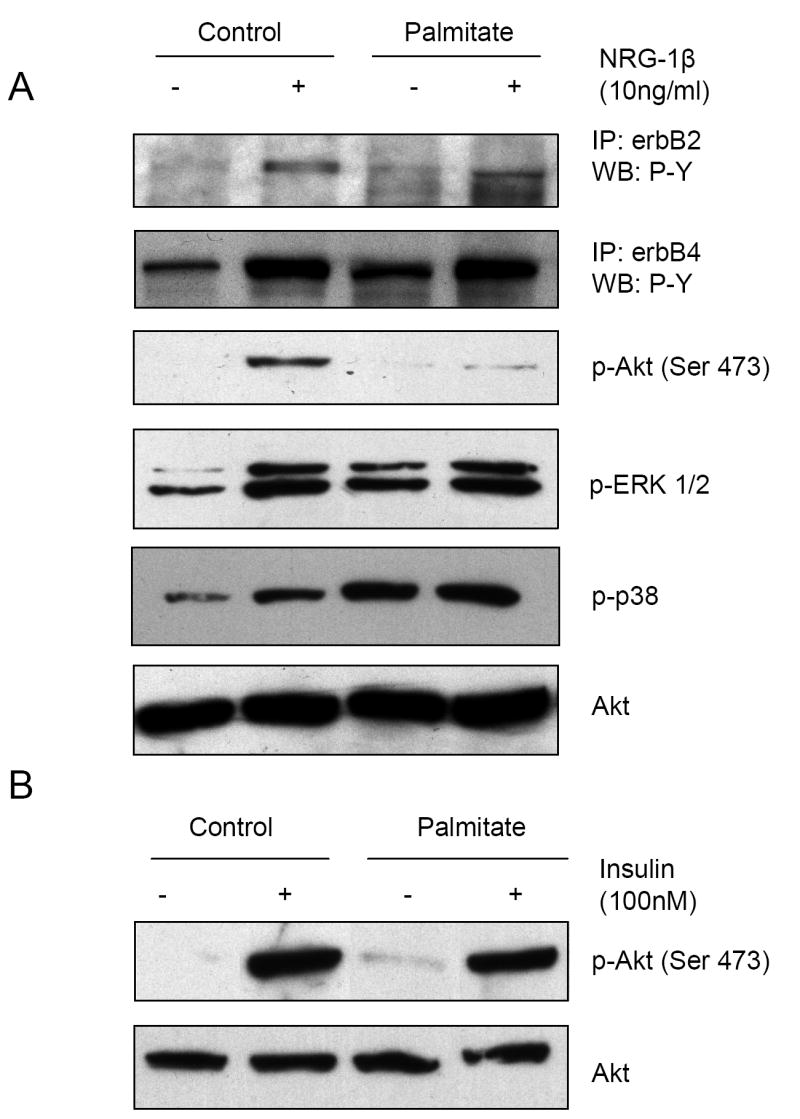
NRVM were pretreated with 0.5mM palmitate for 18 hrs prior to 10ng/ml NRG-1β (A) or 100nM insulin (B) treatment for 10 min. Following lysis, cells were either analyzed by Western blot for p-Akt, p-Erk1/2, p-p38 and Akt or were subjected to immunoprecipitation (IP) with either an erbB2 or erbB4 antibody followed by Western blot for phospho-tyrosine (P-Y) content. Representative blots of 3-6 separate experiments are shown.
NRG-1β protects myocytes from apoptosis induced by serum starvation as well as anthracyclines via activation of PI3-kinase and Akt [8; 11]. We hypothesized that palmitate suppression of Akt activation would prevent NRG-1β from similarly protecting myocytes. To test this hypothesis we examined the effect of NRG-1β on myocyte apoptosis in the presence of palmitate. DNA histograms of PI stained myocytes showed an increase in hypodiploid cells in the presence of palmitate plus NRG-1β. The effect of NRG-1β was visible when cells were examined by phase contrast microscopy (Fig. 2A).
Figure 2. NRG-1β augments palmitate-induced apoptosis.
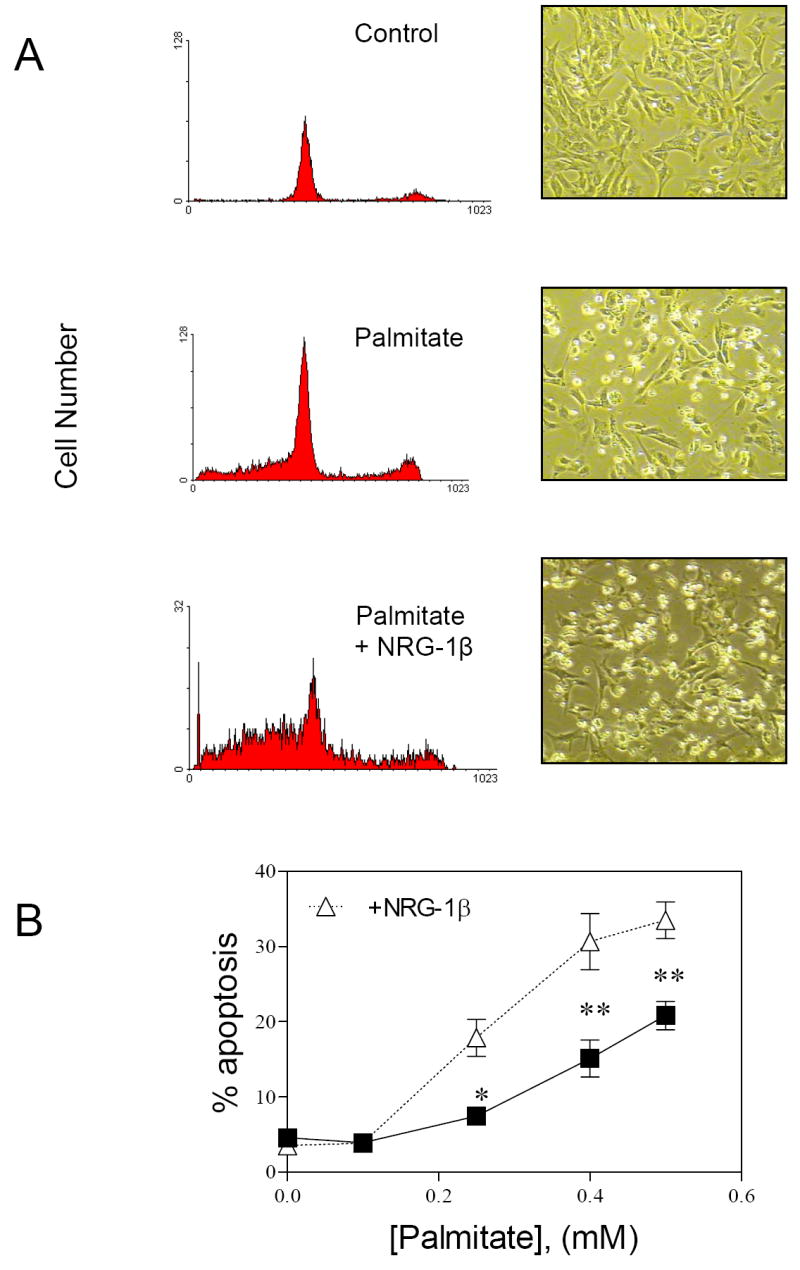
A) Representative flow cytometric analysis of PI stained myocytes and accompanying phase contrast microscopic pictures treated as indicated for 24 hours. B) Quantification of hypodiploid nuclei from flow cytometric analysis of NRVMs treated with varying concentrations of palmitate with (open triangles) and without (closed boxes) 10ng/ml NRG-1β (n=3-15, *p<0.01, **p<0.001 with vs. without NRG-1β) for 24 hrs.
At [palmitate] = 0.1 mM, there was no change in myocyte apoptosis, as quantified by flow cytometry, in the absence or presence of NRG-1β. However, at [palmitate] ≥ 0.25, cotreatment with NRG-1β for 24 hrs caused an augmentation of palmitate-induced apoptosis (Fig. 2B). In contrast, insulin at low and high (data not shown) glucose concentrations did not alter the magnitude of palmitate-induced apoptosis.
Oleate prevents palmitate-induced cytotoxicity [13; 19]. We therefore examined the effect of oleate on palmitate/ NRG-1β interactions. Oleate (0.1mM) prevented palmitate-induced apoptosis in the presence and absence of NRG-1β (Fig. 3A), and restored NRG-1β activation of Akt in the presence of palmitate (Fig. 3B). While palmitate-dependent formation of ceramide [20] has been implicated in apoptosis, as well as alterations in cell signaling [15; 21; 22], overexpression of ceramidase, or inhibition of ceramide synthase with fumonisin B1 had no effecrt on palmitate-suppression of NRG1-β activation of Akt, or the NRG-1β augmentation of palmitate-induced apoptosis (data not shown).
Figure 3. Oleate inhibits palmitate/palmitate + NRG-1β-induced apoptosis and restores NRG-1β activation of Akt.
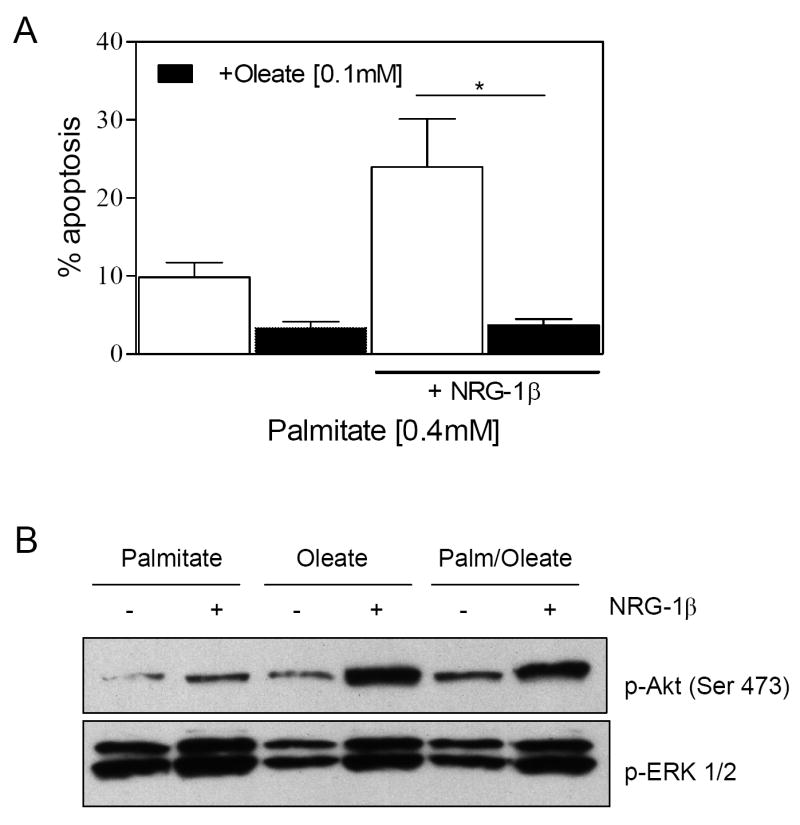
A) NRVM were treated with 0.4mM palmitate with or without 0.1mM oleate and 10ng/ml NRG-1β for 24 hrs before quantifying apoptosis by flow cytometry (n=4, *p<0.01). (B) NRVM is pretreated with either 0.5mM palmitate, 0.5mM oleate or 0.4mM palmitate with 0.1mM oleate for 18 hrs prior to 10 min NRG-1β (10ng/ml) treatment. Following lysis, cells were subjected to Western blot analysis to assess phospho-Akt and phospho-ERK1/2 content. Blots are representative of 3 independent experiments.
We examined the effect of palmitate and NRG-1β on pro-apoptotic pathways including p53 and bax. Palmitate increased p53 expression and NRG-1β augmented the palmitate effect (Fig. 4A,B). Oleate suppressed p53 expression under all conditions. Palmitate alone did not increase bax content compared to the untreated control. However in the presence of palmitate and NRG-1β, total bax content increased, and this was again suppressed by oleate (Figure 4A). This was primarily detected as an increase in oligomers that migrate at approximately 64 kDa, the predicted molecular weight of a toxic, active bax trimer [23]. Palmitate caused cleavage of MDM2 (Figure 4A), a ubiquitin ligase and negative regulator of p53 [24] with known susceptibility to caspase 3-dependent proteolysis.
Figure 4. NRG-1β activates p53/bax in the presence of palmitate.
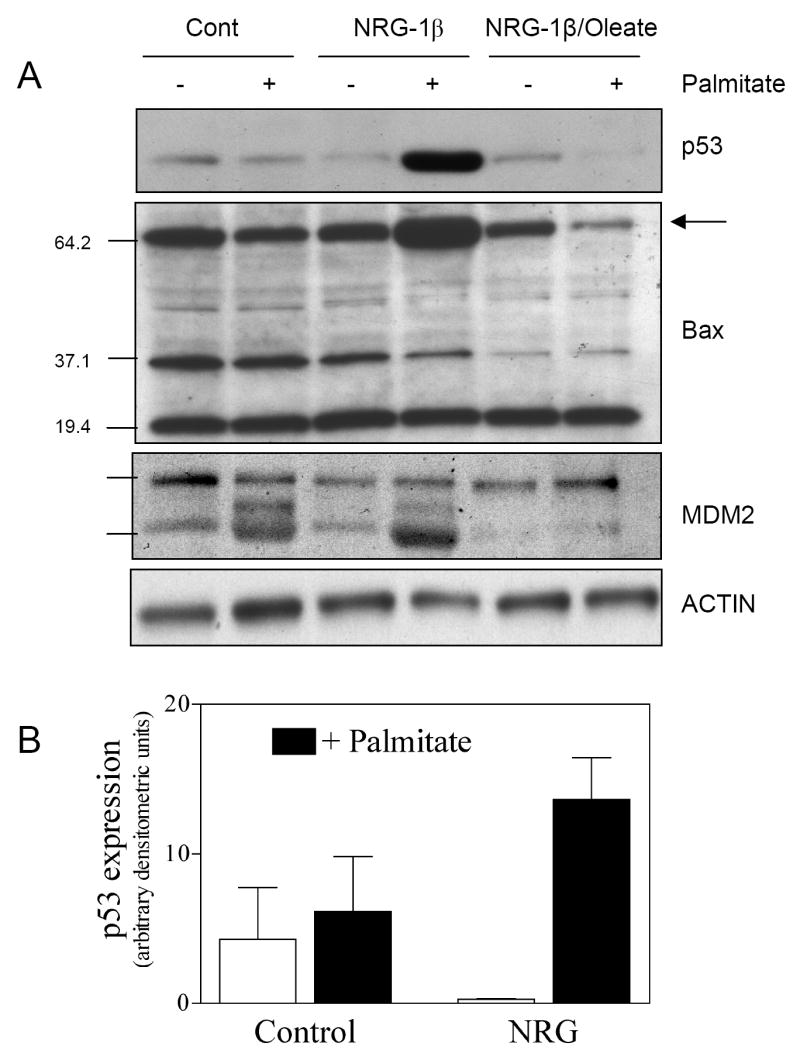
(A) NRVMs were treated with 0.5mM palmitate with or without 0.1mM oleate and 10ng/ml NRG-1β for 24 hrs. Lysates were subjected to Western blot analysis to assess the content of p53, bax and MDM2. Blots are representative of at least three independent experiments. (B) Densitometric analysis was performed for p53 (p=0.03 for interaction between NRG-1β and palmitate, 2-way ANOVA).
DISCUSSION
NRG-1β and its receptors have broad tissue distribution, and regulate tissue architecture during normal growth and development, as well as in the adult organism [1]. While NRG-1β is normally considered a pro-survival growth factor in most tissues, NRG-1β can stimulate both survival as well as apoptotic responses across different malignant cell types [25; 26; 27]. Our finding that palmitate converts NRG-1β from pro-survival to pro-apoptotic in a single cell type suggests that the cellular environment plays an important role in dynamically regulating the response of a given cell to stimulation by NRG-1β. Specifically, saturated fatty acids are known to alter the biology of insulin and contribute to states of cellular insulin resistance [14; 15; 16]. An implication of our findings is that systemic alterations in free fatty acids may alter the biology of paracrine factors such as NRG-1β, and suggest cellular mechanisms for the pathogenesis of end-organ damage in the setting of dyslipidemia where increased myocyte death by apoptosis has been implicated [28; 29].
We do not know the exact mechanism for the effect of palmitate on NRG-1β/erbB signaling and biology. Collectively the data suggest that an interaction between NRG-1β and palmitate occurs at the level of regulating expression and activation of p53 and bax. Alone, NRG-1β stimulation of myocytes lowers levels of p53, likely via Akt-dependent phosphorylation of MDM2 [30], which is known to activate p53 degradation [24]. Palmitate treated cells showed evidence of mdm2 degradation, though this was not associated with increased levels of p53 or bax. MDM2 degradation persists in the presence of NRG-1β, and under these conditions there is a marked increased in p53 and bax expression. We speculate that as palmitate suppresses the Akt/MDM2 signaling via MDM2 degradation, a pro-apoptotic activity of NRG-1β is unmasked. There is a precedent for pro-apoptotic NRG-1β activity. In some cell types NRG-1β activates proteolytic cleavage of erbB4 associated with induction of apoptosis [31]. The released intracellular domain has the potential to regulate genes controlling apoptosis after translocation to the nucleus, or activate mitochondrial membrane permeability transition via a unique BH3 domain [32; 33] that could, in addition to increased bax, promote apoptosis in myocardial cells. Further investigation is needed to determine whether one or more of these mechanisms are playing a role in NRG-1β/palmitate-induced cell death.
Oleate’s rescue of the palmitate-induced phenotype suggests that fatty acid esterification and storage are critical in regulating cell phenotype. Palmitate is known to induce marked increases in diacylglycerol (DAG) in several cell types. In the presence of oleate, however, exogenous lipid is preferentially stored as triacylglycerol (TAG) [19; 34]. In addition to DAG, palmitate is a known precursor for ceramide, ganglioside and sphingolipid synthesis. We would postulate that accumulation of these lipids should also be suppressed when cellular palmitate is incorporated into TAG.
Palmitate inhibits insulin signaling in other cells via mechanisms distal to receptor activation [15], similar to what we observed in the present study for NRG-1β signaling. Palmitate-induced alterations in insulin signaling in other cell types appear to involve ceramide lipids [35; 36; 37]. However we found no effect of ceramidase on palmitate-induced changes in NRG-1β signaling. Moreover, in cardiac myocytes, we were unable to detect any effect of palmitate on insulin signaling, at least under the conditions studied. Collectively, these observations suggest that the effects of palmitate on insulin vs. NRG-1β signaling occur via distinct mechanisms that vary between cell types.
NRG1-β protects cardiac myocytes from stress under a number of conditions [7; 8; 10; 11; 38; 39]. Conditional knockout of erbB2 leads to the development of heart failure [40], and treatment of patients with anti-erbB antibodies promotes the development of cardiac dysfunction [41]. Thus the NRG-1β/erbB signaling axis plays a critical role in the adult heart. This leads to the speculation that palmitate-induced alterations in NRG-1β/erbB biology will have deleterious effects on cardiac structure and function in vivo. Whether such alterations occur in the setting of diabetes and dyslipidemia, and contribute to the associated cardiac dysfunction associated with progressive myocyte apoptosis [28; 29], warrants further investigation. Furthermore it is interesting that NRG-1β and the erbB receptors are expressed and function in multiple tissues that experience end-organ dysfunction in the setting of diabetes: for example, the central and peripheral nervous system [42; 43], musculoskeletal system [44; 45], and vasculature [46]. This raises the intriguing possibility that alterations in NRG-1β/erbB signaling by fatty acids may contribute to the pathogenesis of these other devastating complications.
Acknowledgments
We thank Mark Marchionni and Acorda Therapeutics, Inc. for recombinant neuregulin -1β and Yasuo Ido for adenoviral ceramidase. This work was supported by grants HL068144 from the National Institutes of Health, an Established Investigator Award from the American Heart Association to DBS, and a grant from the Juvenile Diabetes Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res. 2003;284:14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- 2.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 3.Carraway KL, 3rd, Burden SJ. Neuregulins and their receptors. Curr Opin Neurobiol. 1995;5:606–12. doi: 10.1016/0959-4388(95)80065-4. [DOI] [PubMed] [Google Scholar]
- 4.Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- 5.Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature. 1995;378:394–398. doi: 10.1038/378394a0. see comments. [DOI] [PubMed] [Google Scholar]
- 6.Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378:390–394. doi: 10.1038/378390a0. see comments. [DOI] [PubMed] [Google Scholar]
- 7.Rohrbach S, Yan X, Weinberg EO, Hasan F, Bartunek J, Marchionni MA, Lorell BH. Neuregulin in cardiac hypertrophy in rats with aortic stenosis. Differential expression of erbB2 and erbB4 receptors. Circulation. 1999;100:407–412. doi: 10.1161/01.cir.100.4.407. [DOI] [PubMed] [Google Scholar]
- 8.Zhao YY, Sawyer DR, Baliga RR, Opel DJ, Han X, Marchionni MA, Kelly RA. Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem. 1998;273:10261–10269. doi: 10.1074/jbc.273.17.10261. [DOI] [PubMed] [Google Scholar]
- 9.Baliga RR, Pimental DR, Zhao YY, Simmons WW, Marchionni MA, Sawyer DB, Kelly RA. NRG-1-induced cardiomyocyte hypertrophy. Role of PI-3-kinase, p70(S6K), and MEK-MAPK-RSK. Am J Physiol. 1999;277:H2026–37. doi: 10.1152/ajpheart.1999.277.5.H2026. [DOI] [PubMed] [Google Scholar]
- 10.Kuramochi Y, Cote GM, Guo X, LeBrasseur NK, Cui L, Liao R, Sawyer DB. Cardiac endothelial cells regulate ROS-induced cardiomyocyte apoptosis through neuregulin-1beta/erbB4 signaling. J Biol Chem. 2004 doi: 10.1074/jbc.M408662200. [DOI] [PubMed] [Google Scholar]
- 11.Fukazawa R, Miller TA, Kuramochi Y, Frantz S, Kim YD, Marchionni MA, Kelly RA, Sawyer DB. Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt. J Mol Cell Cardiol. 2003;35:1473–9. doi: 10.1016/j.yjmcc.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Liu FF, Stone JR, Schuldt AJ, Okoshi K, Okoshi MP, Nakayama M, Ho KK, Manning WJ, Marchionni MA, Lorell BH, Morgan JP, Yan X. Heterozygous knockout of neuregulin-1 gene in mice exacerbates doxorubicin-induced heart failure. Am J Physiol Heart Circ Physiol. 2005;289:H660–6. doi: 10.1152/ajpheart.00268.2005. [DOI] [PubMed] [Google Scholar]
- 13.Miller TA, Lebrasseur NK, Cote GM, Trucillo MP, Pimentel DR, Ido Y, Ruderman NB, Sawyer DB. Oleate prevents palmitate-induced cytotoxic stress in cardiac myocytes. Biochem Biophys Res Commun. 2005;336:309–15. doi: 10.1016/j.bbrc.2005.08.088. [DOI] [PubMed] [Google Scholar]
- 14.Soltys CL, Buchholz L, Gandhi M, Clanachan AS, Walsh K, Dyck JR. Phosphorylation of cardiac protein kinase B is regulated by palmitate. Am J Physiol Heart Circ Physiol. 2002;283:H1056–64. doi: 10.1152/ajpheart.00275.2002. [DOI] [PubMed] [Google Scholar]
- 15.Schmitz-Peiffer C, Craig DL, Biden TJ. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem. 1999;274:24202–10. doi: 10.1074/jbc.274.34.24202. [DOI] [PubMed] [Google Scholar]
- 16.Olsen GS, Hansen BF. AMP kinase activation ameliorates insulin resistance induced by free fatty acids in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2002;283:E965–70. doi: 10.1152/ajpendo.00118.2002. [DOI] [PubMed] [Google Scholar]
- 17.Carpinelli AR, Picinato MC, Stevanato E, Oliveira HR, Curi R. Insulin secretion induced by palmitate--a process fully dependent on glucose concentration. Diabetes Metab. 2002;28:3S37–44. discussion 3S108-12. [PubMed] [Google Scholar]
- 18.Sawyer DB, Fukazawa R, Arstall MA, Kelly RA. Daunorubicin-induced apoptosis in rat cardiac myocytes is inhibited by dexrazoxane. Circ Res. 1999;84:257–265. doi: 10.1161/01.res.84.3.257. [DOI] [PubMed] [Google Scholar]
- 19.de Vries JE, Vork MM, Roemen TH, de Jong YF, Cleutjens JP, van der Vusse GJ, van Bilsen M. Saturated but not mono-unsaturated fatty acids induce apoptotic cell death in neonatal rat ventricular myocytes. J Lipid Res. 1997;38:1384–94. [PubMed] [Google Scholar]
- 20.Listenberger LL, Ory DS, Schaffer JE. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J Biol Chem. 2001;276:14890–5. doi: 10.1074/jbc.M010286200. [DOI] [PubMed] [Google Scholar]
- 21.Summers SA, Garza LA, Zhou H, Birnbaum MJ. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol Cell Biol. 1998;18:5457–64. doi: 10.1128/mcb.18.9.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chavez JA, Holland WL, Bar J, Sandhoff K, Summers SA. Acid ceramidase overexpression prevents the inhibitory effects of saturated fatty acids on insulin signaling. J Biol Chem. 2005;280:20148–53. doi: 10.1074/jbc.M412769200. [DOI] [PubMed] [Google Scholar]
- 23.Sharpe JC, Arnoult D, Youle RJ. Control of mitochondrial permeability by Bcl-2 family members. Biochim Biophys Acta. 2004;1644:107–13. doi: 10.1016/j.bbamcr.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–45. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 25.Tal-Or P, Di-Segni A, Lupowitz Z, Pinkas-Kramarski R. Neuregulin promotes autophagic cell death of prostate cancer cells. Prostate. 2003;55:147–57. doi: 10.1002/pros.10200. [DOI] [PubMed] [Google Scholar]
- 26.Sartor CI, Zhou H, Kozlowska E, Guttridge K, Kawata E, Caskey L, Harrelson J, Hynes N, Ethier S, Calvo B, Earp HS., 3rd Her4 mediates ligand-dependent antiproliferative and differentiation responses in human breast cancer cells. Mol Cell Biol. 2001;21:4265–75. doi: 10.1128/MCB.21.13.4265-4275.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guerra-Vladusic FK, Vladusic EA, Tsai MS, Lupu R. Signaling molecules implicated in heregulin induction of growth arrest and apoptosis. Oncol Rep. 2001;8:1203–14. doi: 10.3892/or.8.6.1203. [DOI] [PubMed] [Google Scholar]
- 28.Okere IC, Chandler MP, McElfresh TA, Rennison JH, Sharov V, Sabbah HN, Tserng KY, Hoit BD, Ernsberger P, Young ME, Stanley WC. Differential effects of saturated and unsaturated fatty acid diets on cardiomyocyte apoptosis, adipose distribution, and serum leptin. Am J Physiol Heart Circ Physiol. 2006;291:H38–44. doi: 10.1152/ajpheart.01295.2005. [DOI] [PubMed] [Google Scholar]
- 29.Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P. Myocardial cell death in human diabetes. Circ Res. 2000;87:1123–32. doi: 10.1161/01.res.87.12.1123. [DOI] [PubMed] [Google Scholar]
- 30.Zhou BP, Liao Y, Xia W, Zou Y, Spohn B, Hung MC. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol. 2001;3:973–82. doi: 10.1038/ncb1101-973. [DOI] [PubMed] [Google Scholar]
- 31.Carpenter G. ErbB-4: mechanism of action and biology. Exp Cell Res. 2003;284:66–77. doi: 10.1016/s0014-4827(02)00100-3. [DOI] [PubMed] [Google Scholar]
- 32.Williams CC, Allison JG, Vidal GA, Burow ME, Beckman BS, Marrero L, Jones FE. The ERBB4/HER4 receptor tyrosine kinase regulates gene expression by functioning as a STAT5A nuclear chaperone. J Cell Biol. 2004;167:469–78. doi: 10.1083/jcb.200403155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naresh A, Long W, Vidal GA, Wimley WC, Marrero L, Sartor CI, Tovey S, Cooke TG, Bartlett JM, Jones FE. The ERBB4/HER4 intracellular domain 4ICD is a BH3-only protein promoting apoptosis of breast cancer cells. Cancer Res. 2006;66:6412–20. doi: 10.1158/0008-5472.CAN-05-2368. [DOI] [PubMed] [Google Scholar]
- 34.Montell E, Turini M, Marotta M, Roberts M, Noe V, Ciudad CJ, Mace K, Gomez-Foix AM. DAG accumulation from saturated fatty acids desensitizes insulin stimulation of glucose uptake in muscle cells. Am J Physiol Endocrinol Metab. 2001;280:E229–37. doi: 10.1152/ajpendo.2001.280.2.E229. [DOI] [PubMed] [Google Scholar]
- 35.Chavez JA, Summers SA. Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch Biochem Biophys. 2003;419:101–9. doi: 10.1016/j.abb.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 36.Tagami S, Inokuchi Ji J, Kabayama K, Yoshimura H, Kitamura F, Uemura S, Ogawa C, Ishii A, Saito M, Ohtsuka Y, Sakaue S, Igarashi Y. Ganglioside GM3 participates in the pathological conditions of insulin resistance. J Biol Chem. 2002;277:3085–92. doi: 10.1074/jbc.M103705200. [DOI] [PubMed] [Google Scholar]
- 37.Vainio S, Heino S, Mansson JE, Fredman P, Kuismanen E, Vaarala O, Ikonen E. Dynamic association of human insulin receptor with lipid rafts in cells lacking caveolae. EMBO Rep. 2002;3:95–100. doi: 10.1093/embo-reports/kvf010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sawyer DB, Zuppinger C, Miller TA, Eppenberger HM, Suter TM. Modulation of anthracycline-induced myofibrillar disarray in rat ventricular myocytes by neuregulin-1beta and anti-erbB2: potential mechanism for trastuzumab-induced cardiotoxicity. Circulation. 2002;105:1551–4. doi: 10.1161/01.cir.0000013839.41224.1c. [DOI] [PubMed] [Google Scholar]
- 39.Grazette LP, Boecker W, Matsui T, Semigran M, Force TL, Hajjar RJ, Rosenzweig A. Inhibition of ErbB2 causes mitochondrial dysfunction in cardiomyocytes: implications for herceptin-induced cardiomyopathy. J Am Coll Cardiol. 2004;44:2231–8. doi: 10.1016/j.jacc.2004.08.066. [DOI] [PubMed] [Google Scholar]
- 40.Crone SA, Zhao YY, Fan L, Gu Y, Minamisawa S, Liu Y, Peterson KL, Chen J, Kahn R, Condorelli G, Ross J, Jr, Chien KR, Lee KF. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8:459–65. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 41.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 42.Chen D, Xu LG, Chen L, Li L, Zhai Z, Shu HB. NIK is a component of the EGF/heregulin receptor signaling complexes. Oncogene. 2003;22:4348–55. doi: 10.1038/sj.onc.1206532. [DOI] [PubMed] [Google Scholar]
- 43.Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, Role L, Lai C, Schwab MH, Nave KA. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–3. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- 44.LeBrasseur NK, Cote GM, Miller TA, Fielding RA, Sawyer DB. Regulation of neuregulin/ErbB signaling by contractile activity in skeletal muscle. Am J Physiol Cell Physiol. 2003;284:C1149–55. doi: 10.1152/ajpcell.00487.2002. [DOI] [PubMed] [Google Scholar]
- 45.Jacobson C, Duggan D, Fischbach G. Neuregulin induces the expression of transcription factors and myosin heavy chains typical of muscle spindles in cultured human muscle. Proc Natl Acad Sci U S A. 2004;101:12218–23. doi: 10.1073/pnas.0404240101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Russell KS, Stern DF, Polverini PJ, Bender JR. Neuregulin activation of ErbB receptors in vascular endothelium leads to angiogenesis. Am J Physiol. 1999;277:H2205–H2211. doi: 10.1152/ajpheart.1999.277.6.H2205. [DOI] [PubMed] [Google Scholar]


