Abstract
Beyond the elemental role of blood vessels in tumor growth, fluid conducting networks lacking endothelium (termed vasculogenic mimicry) were identified previously in metastatic melanoma and other cancer types. The etiology remains unclear, though it appears to involve dysregulation of the tumor-specific phenotype and transdifferentiation. Instigating the molecular deciphering of this phenomenon, we established a novel technique for microdissecting the spontaneously formed vascular-like networks and the randomly arranged cells (nests) from living 3D cultures of melanoma and performed microgenomics analysis. For the first time we show that despite the shared genotype, transcription was differentially regulated among the phenotypically distinct melanoma structures in vasculogenic mimicry. Several angiogenesis-specific genes were differentially expressed in higher levels in network cells of both uveal and cutaneous melanoma with intriguing representation of the ephrin family of angiogenesis factors, which was confirmed with immunocytochemistry. Moreover, the adjacent nest cells over-expressed ECM-related genes. Expression of angiogenesis-specific genes in melanoma resembled that of normal microvascular cells and was enhanced in melanoma disseminating hematogenously. The findings suggest that melanoma plasticity could enable autopoiesis of vascular-mimicking elements within the tumor infrastructure with significant clinical implications, such as response to anti-angiogenic treatments. Identifying factors regulating tumor plasticity and heterogeneity at the molecular level is essential in designing effective anti-cancer therapies.
Keywords: vasculogenic mimicry, laser capture microdissection (LCM), 3D gels, gene expression profiling, arrays
INTRODUCTION
The elemental role of blood supply in tumor maintenance and growth has been long recognized, studied, and targeted [Carmeliet, 2005; Folkman, 1971]. Apart from obvious contributions from endothelial cells and their progenitors in the development of blood vessels, evidence suggests that tumor perfusion may be also capitalizing on networks lacking endothelial linings [Maniotis et al., 1999], or vessels consisting of both endothelial and tumor cells [Chang et al., 2000]. Vasculogenic mimicry, the formation of fluid conducting networks by non-endothelial cells, has been identified in a plethora of cancer types in vitro and in histological specimens such as aggressive uveal and cutaneous melanomas [Hendrix et al., 2003], sarcomas [van der Schaft et al., 2005], inflammatory and ductal breast carcinomas [Shirakawa et al., 2002], and also ovarian [Sood et al., 2002] and prostatic carcinomas [Sharma et al., 2002].
The etiology of this important vasculogenic phenotype remains unclear but it appears to involve dysregulation of the tumor-specific phenotype and concomitant transdifferentiation of aggressive tumor cells into other cell types [Fang et al., 2005; Hendrix et al., 2003]. Induction and maintenance of plasticity as a set of distinct phenotypes within a generic tumor cell population are uncharted territories in cancer biology. However, the molecular characterization of tumor plasticity is crucial in deciphering the mechanisms underlying pluripotency in tumor cells and its role in tumor progression. It was originally observed that aggressive and genetically dysregulated melanoma cells express endothelia-associated genes and form fluid-conducting vasculogenic-like networks in vitro [Maniotis et al., 1999] and in aggressive tumors of melanoma patients [Seftor et al., 2002]. So far the characterization of vasculogenic mimicry at the molecular level has consisted mostly of the indirect approach of quantifying differences in gene expression of aggressive melanoma cell lines, which are capable of vasculogenic mimicry, compared to non-aggressive counterparts that lack this ability such as VE (vascular endothelial)-cadherin [Hendrix et al., 2001], and tissue factor pathway inhibitor-1 (TFPI-1,-2) [Ruf et al., 2003]. In a more direct approach, specific molecules, such as heparan sulfate proteoglycan [Maniotis et al., 1999], laminin 5 γ2 chain, matrix metalloproteinases (MMP)-1, -2, -9, and MT1-MMP (MMP14) [Seftor et al., 2001], keratin 8,18 [Hendrix et al., 1998], and EphA2 [Hess et al., 2001], were shown to co-localize at vasculogenic mimicry networks.
In long term culture aggressive melanoma cells obtain spontaneously two distinct phenotypes: 3D tubular networks made of multi-cell layers that are interconnected and extend to a total length of several millimeters across the culture; and monolayers of randomly positioned cells (nests) contiguous to the networks (see also [Demou, 2008]). Here we performed the first intra-population microgenomics analysis and quantified differential gene expression amongst these melanoma subpopulations. Synoptically, we have: (i) established a novel and unique application of laser capture microdissection for living 3D cell cultures; (ii) individually microdissected and isolated cells from networks and nests on polymerized collagen type-I gels, and also melanoma and microvascular cells forming networks on Matrigel; and (iii) quantified relative gene expression with two independent transcription profiling assays (gene arrays and real-time PCR arrays) containing genes specific to angiogenesis and extracellular matrix and cell adhesion molecules. The data uniquely delineate the molecular signatures of the heterogeneous cell subpopulations, which evolve spontaneously in 3D culture, and reveal a strong endogenous angiogenic phenotype of the melanoma networks that is common to both uveal and cutaneous melanoma, in parallel with overexpression of ECM-specific genes in nest cells. The angiogenic signature of uveal and cutaneous melanoma was further confirmed to resemble the molecular signature of angiogenesis-associated microvascular endothelial cell networks, the building blocks of blood vessels. Characterization of the transcriptional signatures associated with the phenotypic heterogeneities of aggressive melanoma cells is the first step in deciphering the molecular mechanisms that underlie melanoma plasticity. Our findings suggest that plasticity of aggressive melanoma can enable autopoiesis of critical vascular-mimicking elements within the tumor infrastructure, and may reflect in part the implications of current anti-angiogenic treatments. Understanding the endogenous angiogenic capacities of aggressive melanoma further highlights the significant role of vasculogenic mimicry in tumor perfusion and illuminates new targets for therapeutic intervention.
MATERIALS and METHODS
Cell Culture
The human uveal melanoma liver metastasis cell line MUM2B [Seftor et al., 2002] (recently renamed AUM-2; aggressive uveal melanoma-2), passage number (P) 9-19, and the metastatic cutaneous melanoma C8161 [Welch et al., 1991], P46-56, were cultured in RPMI 1640 media (Invitrogen) supplemented with 10%v/v fetal bovine serum and 0.025 mg/ml gentamicin sulfate (both from Gemini Bioproducts). Normal human microvascular cells (from neonatal dermis) (Cambrex Bio Science) were cultured, up to 5 additional passages from the commercially available stock, in M131 media supplemented with microvascular cell growth supplement (MVGS) in flasks pre-coated with attachment factor (AF) (all from Cascade Biologics). The cells were maintained in a humidified-5% CO2 atmosphere incubator with media renewal every two days and at 80% confluency were passaged, or used for experiments. Melanoma cells were detached with 2mM EDTA (Sigma-Aldrich) and microvascular cells with trypsin/EDTA (Cascade Biologics). The cultures were determined to be free of mycoplasma contamination by using the Gen Probe Mycoplasma Detection Kit III (Fisher Scientific).
3D Cell-Populated Matrices for Laser Capture Microdissection (LCM)
3D gels populated with melanoma or normal microvascular cells were formed in the depressed space of the LCM membrane slides (Molecular Devices Corp.). These are standard size metallic slides 1 mm thick with a rectangular cutout measuring 17 × 45 mm. A porous polyethylene naphthalate (PEN) membrane is glued on one side generating a chamber (17 × 45 × 1 mm) with a membrane base. For the intra-population analysis of networks and nests, the melanoma cells were seeded on top of polymerized rat-tail collagen type I (BD Biosciences) gels (350 μl/slide). Neutralized collagen solution was prepared on ice by diluting the collagen type-I stock to 1 mg/ml in complete culture media and adjusting the pH to 7.4 with sterile NaOH (1N). For the comparison of melanoma to microvascular cell networks, gels (250 μl/slide) were prepared in membrane slides with ice-cold solution of 50% v/v Matrigel (BD Biosciences) in complete RPMI. The constructs were allowed to gel in a humidified - 5% CO2 incubator for 1 hour before cell seeding by overlaying the gels with 1 ml cell suspension. The seeding density was 5×105 melanoma cells/slide on collagen gels, and 2×105 melanoma or microvascular cells/slide on Matrigel. The media were renewed the day after seeding and every two days thereafter for the long-term melanoma cultures. LCM was performed 24 hours after the last media change for all cultures: 14 and 2 days post-seeding for the collagen and Matrigel cultures respectively.
Laser Capture Microdissection of Living 3D Cell Cultures
We established a novel technique for isolating cells from live 3D cultures utilizing the Veritas LCM system (Molecular Devices Corp. former Arcturus). Under RNase free conditions LCM was performed for melanoma cells forming networks and nests (Figure 1) on collagen type-I gels and also for melanoma or microvascular cells forming networks on Matrigel (Figure 2). The slide was wiped with an ethanol moist kimwipe and then placed in the LCM instrument for cell selection and microdissection following standard operational procedures. Briefly the Veritas system combines: a motorized-stage microscope (2x, 10x, 20x objectives) for visualizing and positioning the sample and selecting areas of interest; an ultraviolet (UV) laser to cut around the perimeter of the areas of interest and an infrared (IR) laser to locally attach the microdissected areas onto a collection cap (MacroCapTM, Molecular Devices Corp.).
Figure 1.

Morphology and laser capture microdissection (LCM) of vascular-like networks and nests of melanoma cells. (A) Living AUM-2 melanoma cells spontaneously assembled in vascular-like networks and nests of randomly arranged cells after 14 days in culture on collagen type-I gels (1). The area enclosed in the dashed box is shown magnified in panel (2). (B) Collection cap (appearing as black rim) positioned on field (A) before LCM (1); (2) Holes remaining on the 3D cell culture after LCM of the nests in panel (1); (3) Nests of randomly arranged AUM-2 cells isolated on the collection cap after LCM in field (A). The dashed box provides a topographical reference among the panels. (C) Isolated vascular-like networks of C8161 cells on a collection cap after LCM.
Figure 2.
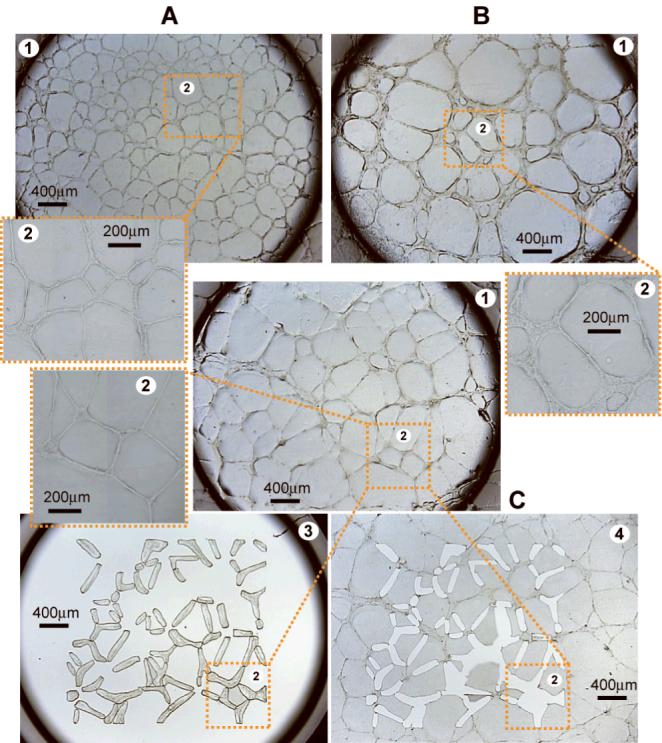
Honeycomb patterning and LCM of AUM-2 and C8161 aggressive melanoma and normal microvascular endothelial cells on Matrigel. Vascular-like network formation on Matrigel targeted for LCM, at low and high magnification panels labeled (1) and (2) respectively, by living normal microvascular cells (A); aggressive melanoma cells AUM-2 (B); and C8161 (C). Microdissected networks from panel (C) isolated on the LCM collection cap (3); and holes remaining on the 3D cell culture after the microdissection of the networks (4).
Total RNA Purification, Quality Testing and Quantification
After LCM, the cell-bearing LCM caps were incubated with 50 μl extraction buffer for 30 min and the extract was stored at -80°C. Prior to gene expression analysis, total RNA was purified from the extracts using the PicoPure kit (Molecular Devices Corp.), a column-based purification method, according to the manufacturer’s instructions. Extract from multiple caps was pooled on the same column to yield up to 200 ng total RNA in 11 μl elution buffer. Good quality of total RNA samples was assured for all downstream applications. The RNA quality and quantity was routinely measured with three independent methods: (i) the sample assessment protocol of the Paradise Reagent System (Molecular Devices Corp.), which is a quantitative real-time PCR method that measures the average β-actin cDNA length by quantification of the PCR product yield ratio of the 3′ end compared to a 5′ sequence target (3′/5′ ratio of 1 corresponds to good quality RNA); (ii) the 2100 Bioanalyzer-Pico Chip (Agilent Technologies); and (iii) the Nanodrop spectrophotometer ND-1000 (Nanodrop Technologies) [details in [Demou, 2008]].
Gene Expression Profiling Assays
(a) Gene Oligo Microarrays
Two types of human gene microarrays were utilized containing angiogenesis- and extracellular matrix (ECM) and cell adhesion-specific genes (SuperArray). Each array contained 113 genes, housekeeping genes, and negative/positive hybridization control spots. The probe was synthesized from 200ng purified total RNA using the Amp2 kit (SuperArray) according to manufacturer’s instructions. The chemiluminescense signal was captured by exposing the arrays to radiographic film for a range of 5 sec to 10 min. The films were scanned generating 8-bit TIFF images that were subsequently analyzed to extract the gray value of each gene spot utilizing a custom made mosaic of regions of interest (ROI) in the NIH ImageJ software (http://rsb.info.nih.gov/ij/). Relative gene expression in each array was quantified by subtracting the gray value of the background (average gray value of negative spots) from the gray value corresponding to each gene spot and then normalizing by dividing with the average gray value of the housekeeping genes on each array.
(b) Real-Time Polymerase Chain Reaction Arrays
Real-time PCR (rtPCR) expression profiling was performed with the RT2 Profiler PCR Array for human angiogenesis-specific and ECM and cell adhesion-specific genes in a 96-well plate format (SuperArray). cDNA synthesis was performed with 150ng purified total RNA using the RT2 PCR Array First Strand Kit according to manufacturer’s instructions (SuperArray). Each 96-well plate contained primers for analyzing 84 function-specific genes, along with housekeeping genes, and reverse transcription control wells. Runs of the reactions based on the SYBR-green chemistry were performed in the AB7500 Fast Real-Time PCR System (Applied Biosystems) utilizing the thermal cycling protocol suggested by the SuperArray kit and they were always followed by a run of the standard dissociation protocol of the AB7500 system. CT values were extracted with the SDS software (Applied Biosystems) and analysis for each 96-well plate was based on the ΔΔCT method, using the average of housekeeping genes as control. Complete gene tables for the above arrays can be found in Supplements 2-5 and at www.superarray.com.
Immunocytochemistry
Cultures of AUM-2 and C8161 melanoma cells were prepared as described above but in Lab-Tek chamber glass slides (Fisher). After fixation with -10°C methanol for 5 min, air drying, and washing (3×) with PBS, the specimens were incubated for 20 min with 10% blocking serum-PBS, washed with PBS, and then incubated for 60 min with one of the following rabbit-derived primary antibodies at 4μg/ml in 1.5% blocking serum-PBS: anti-ephrin-A1 (sc-911), -A3 (sc-1012), -B2 (sc-1010) (Santa Cruz); or 12 μg/ml anti-ephrin-A3 (ZMD.322; Invitrogen); or 12 μg/ml goat-derived anti-ephrin-B2 (AF496; R&D). For blocking, normal donkey serum was used for AF496 and normal goat serum (both from Santa Cruz) for all the other antibodies. Then the specimens were incubated for 45 min with 5 μg/ml Alexa Fluor® 488-conjugated anti-rabbit IgG in 1.5% serum-PBS; or 5 μg/ml donkey anti-goat IgG rhodamin (TRITC)-conjugated secondary antibody (Santa Cruz) for AF496. After washing with PBS the specimens were mounted with UltraCruzTM (Santa Cruz) containing DAPI for counterstaining, kept at 4°C, and subjected to confocal microscopy within 2 days (Zeiss 510 META Confocal Laser Scanning Microscope; excitation at: 488 nm Argon laser for Alexa Fluor®; 543 nm Helium-Neon laser for TRITC; and laser diode 405 nm for DAPI).
RESULTS
Human aggressive uveal AUM-2 and cutaneous C8161 metastatic melanoma cells spontaneously differentiated into networks and nests (Figure 1) when cultured on three-dimensional (3D) gels of polymerized collagen type-I (the main component of skin and tissue stroma). The networks became microscopically visible approximately 7 days post-seeding and reached their peak in about 14 days, marked by distinct cell subpopulations of well-defined 3D networks (reminiscent of vascular formations) and randomly arranged cells (nests). After about 18 days in culture (for the seeding density used in this study) the networks often started to lose cohesion. The end point for the cultures on collagen was set to 14 days, in order to compare gene expression levels in morphologically similar structures of melanoma cells, such as networks of similar width, length, and similar area coverage on the gel (quantified in [Demou, 2008]). Differential gene expression amongst melanoma cells in networks and nests was assayed with microarrays and rtPCR arrays of functionally clustered genes specific to human angiogenesis, and human ECM and cell adhesion molecules. The angiogenic character of the melanoma cells was further confirmed by transcription analysis on Matrigel (tumor derived basement membrane), a commonly utilized assay for in vitro angiogenesis [Nicosia and Ottinetti, 1990], and comparison to normal microvascular endothelial cells on the basis of two parameters: their ability to form tubular structures (honeycomb-like networks) and the expression of angiogenesis-associated genes by the cells forming these structures. Microvascular cells as well as AUM-2 and C8161 cells formed “honeycomb” structures within a couple of hours post-seeding on Matrigel matrices (Figure 2). The length of the network segments and the degree of cell participation in forming them, as opposed to random cell positioning, was dependent on the seeding density for all three cell types. Specifically, at low seeding densities the melanoma and the microvascular cells formed semi-complete “honeycomb” patterns. With increasing density, formations ranged from closed loops to combinations of loops coexisting with randomly arranged cells. For the Matrigel cultures, the endpoint was selected at 2 days post-seeding so that the network pattern would be predominant for all cell types at the time of microdissection. With increasing time in culture, proliferation and migration of melanoma cells on Matrigel gave rise to structures resembling those shown in Figure 1. Similarly, with time in culture the microvascular cells migrated off the “honeycomb” pattern while their networks lost cohesion. Gene expression for the microdissected networks of AUM-2, C8161, and microvascular cells on Matrigel was assayed with microarrays and rtPCR arrays containing angiogenesis-specific genes.
A comprehensive presentation of all gene expression data is summarized in Figure 3A and Supplement 1 (A, B) in the form of normalized relative expression ratios for: angiogenesis-specific genes in AUM-2 and C8161 networks versus nests on collagen-I (Figure 3A); ECM and cell adhesion-specific genes in AUM-2 and C8161 cells in nests versus networks on collagen-I (Supplement 1A); and angiogenesis-specific genes for AUM-2 and C8161 networks compared to microvascular cell networks on Matrigel (Supplement 1B). Each bar represents the mean normalized expression ratio for a specific gene from two biologically independent (independent RNA samples and arrays) experiments. Differences in microarray and rtPCR data may be attributed to the fact that microarrays assay for a larger number of genes (74% of the genes assayed by microarrays were assayed by rtPCR arrays), the sensitivity of the assays and potential false negatives/positives (as expected in multi-gene analyses). Figure 3A and Supplement 1A show that AUM-2 and C8161 genes were differentially expressed in networks compared to nests while both cell lines had strong angiogenic signature in the Matrigel assay compared to microvascular cells (Supplement 1B). For comparative analysis we used cut-off values of 1.5 and 0.6 for the normalized relative gene expression ratio, A/B (Figure 4). Ratio values of at least 1.5 signified expression of a particular gene at a higher levels in condition A compared to B; values equal or smaller than 0.6 signified higher expression levels in condition B compared to A; while values between 0.6 and equal or less than 1.5 corresponded to similar expression levels in conditions A and B.
Figure 3.
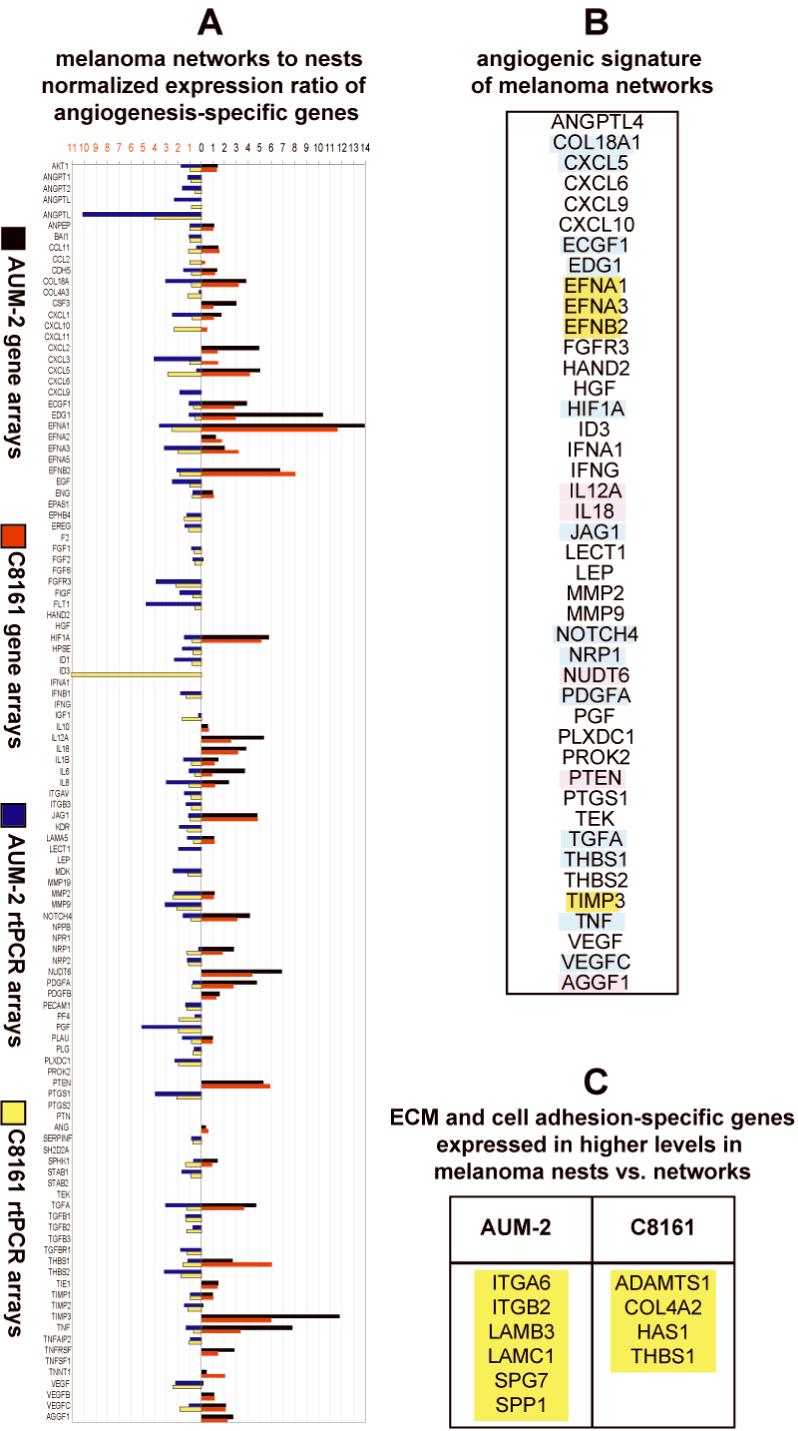
(A) Mean normalized fold expression of angiogenesis-specific genes for AUM-2 and C8161 cells in networks versus nests on collagen type-I gels. Expression was assayed by gene arrays (AUM-2 [black] and C8161 [red]) and by rtPCR arrays (AUM-2 [blue] and C8161 [yellow]); (B) Angiogenesis-specific genes (found collectively by gene and rtPCR arrays to be) expressed in higher levels in networks vs. nests both in AUM-2 and C8161 cells (yellow: detected both by gene and rtPCR arrays; blue: detected only in gene arrays; pink: assayed only by gene arrays); (C) ECM and cell adhesion-specific genes expressed in higher levels in melanoma nests vs. networks of AUM-2 and C8161 cells on collagen type-I gels confirmed both by gene and rtPCR arrays (yellow).
Figure 4.

Sorting of the relative gene expression ratio from gene arrays (GA) and real-time PCR (rtPCR) arrays according to the level of expression with cut-off values 0.6 and 1.5 for the 12 experimental conditions of Figures 4A and Supplement 1 (A,B). (A) Angiogenesis-specific; and (B) ECM- and cell adhesion-specific expression profiling for AUM-2 and C8161 melanoma cells in networks vs. nests on collagen type-I; and (C) Angiogenesis-specific profiling for AUM-2 and C8161 melanoma vs. normal microvascular endothelial cells on Matrigel.
The majority of angiogenesis-specific genes is expressed in higher levels in cells forming networks compared to nests for both uveal and cutaneous melanoma
In Figure 4A, angiogenesis genes were grouped according to the value of the normalized relative expression ratio. In each experimental condition, the total number of expressed genes is the sum of genes expressed in similar (blue), higher (red), and lower levels (green) in networks compared to nests. Taking into consideration the number of genes whose expression was not detectable (gray bars), AUM-2 and C8161 cells expressed about 43% of the angiogenesis genes assayed by microarrays and about 86% of the angiogenesis genes assayed by rtPCR arrays (Figure 4A). The difference reflects the individual sensitivity of the assays. However, microarrays and rtPCR arrays consistently confirmed that a plethora of angiogenesis genes were expressed in higher levels in networks of AUM-2 and C8161 cells compared to nests. Specifically, for all conditions in Figure 4A, over 50% of expressed angiogenesis-specific genes were found at higher levels in networks compared to nests (red vs. green bars, respectively).
Endogenous angiogenic fingerprint of metastatic melanoma networks: Genes expressed in higher levels in uveal and cutaneous melanoma networks versus nests
Relative expression levels of angiogenesis-specific genes in networks compared to nests for AUM-2 and C8161 cells as shown in Figure 3A. Of foremost interest were genes over-expressed in networks compared to nests of both uveal and cutaneous melanoma cells (Figure 3B). A subset of angiogenesis genes, found by both profiling assays (gene and rtPCR arrays) to be expressed over 3-fold higher in networks compared to nests both in AUM-2 and C8161 cells, included three members of the major angiogenesis family of ephrins: EFNA1, EFNA3, EFNB2 and TIMP3. The gene set (highlighted yellow in Figure 3B) delineated the endogenous angiogenic fingerprint of metastatic melanoma networks that was common to uveal and cutaneous melanoma. Higher levels of ephrin-A1, -A3, and -B2 protein in melanoma cells forming networks than nests (as seen in Figure 5) were detected with immunolabeling and confocal microscopy by all five anti-ephrin antibodies used. The immunostaining confirms higher expression of these proteins in the melanoma networks compared to randomly oriented cells as suggested by the gene expression data from the microarrays and real time PCR arrays.
Figure 5.
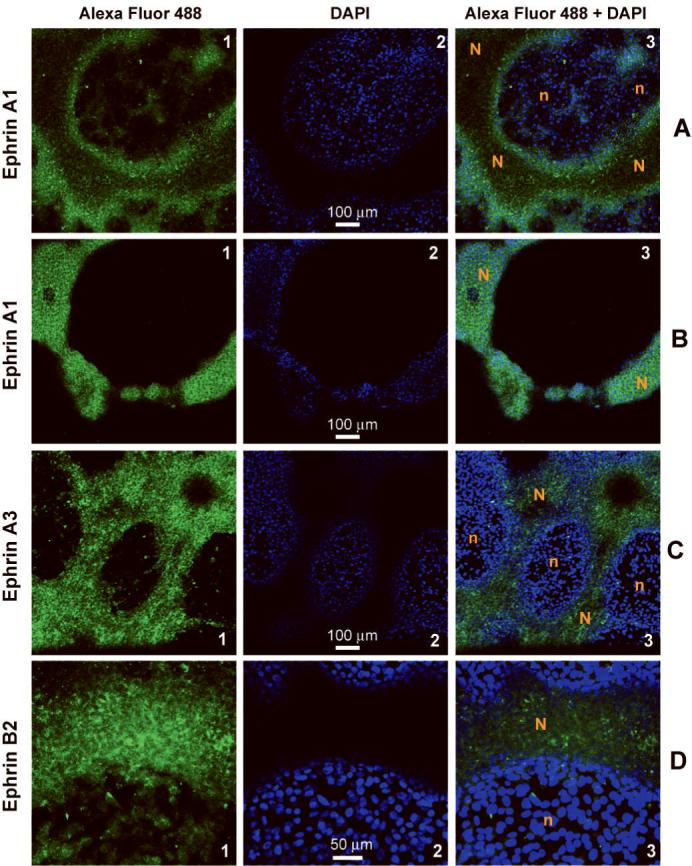
Confocal imaging of melanoma cultures shows increased localization of anti-ephrin immunolabeling on network cells (N) compared to adjacent cells in nests (n). Fluorescence from nest cells and cells forming the base of the network structures is detected in tandem at a single optical slice shown as individual Alexa Fluor® 488 (labeled “1”) and DAPI (labeled “2”) channels and their combination (labeled “3”) for: (A-B) ephrin-A1 (C8161); (C) ephrin-A3 (C8161); and (D) ephrin-B2 (AUM-2). (B) Corresponding signals for an optical slice through the core of the melanoma network and about 30μm above the nest cells of panel (A).
ECM-cell adhesion genes expressed in higher levels by uveal and cutaneous melanoma cells in nests compared to networks
Differential gene expression of ECM and cell adhesion-specific genes by AUM-2 and C8161 cells in networks compared to nests (Supplement 1A) was moderate compared to the abovementioned prominent over-expression of angiogenesis genes in the melanoma networks. Using cut-off values of 0.6 and 1.5 (Figure 4B), over 44% of the ECM-cell adhesion genes were expressed in microarrays for AUM-2 and C8161 cells and over 56% in rtPCR arrays. In contrast to the over-expression of angiogenesis-specific genes by cells in networks, the ECM and cell adhesion-specific genes were mostly expressed in higher levels by cells in nests (green vs. red bars). That was particularly prominent for the AUM-2 cells as data from both microarrays and rtPCR arrays consistently supported this trend. While rtPCR arrays for C8161 showed a definite upregulation of ECM genes in the nests, the upregulation detected by microarrays was not as conclusive. Expression levels of individual ECM and cell adhesion genes obviously depend in part on the cell line and vary for AUM-2 and C8161 cells as shown in Supplement 1A. The genes expressed in higher levels in AUM-2 and C8161 nests versus networks, as extracted by microarrays and rtPCR, are shown in Figure 3C. Contrasting the angiogenesis-specific profiling data, no genes were identified to be over-expressed in nests versus networks in common by AUM-2 and C8161 cells and detectable by both profiling platforms.
Gene expression analysis and angiogenic potential of aggressive melanoma cells compared to normal microvascular cells
Figure 4C shows the cut-off analysis for the expression of angiogenesis-specific genes in melanoma versus microvascular cells on Matrigel (Supplement 1B). Comparing AUM-2 to microvascular cells, 24% of the expressed genes detected by gene arrays (GA) and 19% by rtPCR arrays, were expressed at similar levels. Respectively, 42% (GA) and 36% (rtPCR) of the expressed genes were at higher levels in AUM-2 cells and 33% (GA) and 45% (rtPCR) at higher levels in microvascular cells. Comparing C8161 to microvascular cells, 23% of the expressed genes detected by gene arrays (GA) and 9.7% by rtPCR arrays were expressed at similar levels. 38% (GA) and 37.5% (rtPCR) of the expressed genes were at higher levels in C8161 cells and 38.5% (GA) and 52.8% (rtPCR) at higher levels in microvascular cells. The results indicate that overall more angiogenesis-specific genes are expressed in higher levels by AUM-2 rather than C8161 compared to microvascular cells. This was also confirmed by a detailed relative expression analysis for angiogenesis-specific genes of AUM-2, C8161, and normal microvascular cells on Matrigel summarized in Table 1, specifically columns “AUM-2 vs. MV” and “C8161 vs. MV”. Also more genes are expressed in higher levels by AUM-2 vs. C8161 than in C8161 vs. AUM-2 cells. Finally, more genes were expressed in higher levels by microvascular cells vs. C8161 rather than AUM-2 cells. Two and three-way similarities in gene expression levels are also shown in Table 1.
Table 1.
Relative expression of angiogenesis-specific genes in AUM-2, C8161, and normal microvascular endothelial cells forming networks on Matrigel
| HIGHER EXPRESSION | HIGHER EXPRESSION | HIGHER EXPRESSION | SIMILAR EXPRESSION | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AUM-2 | C8161 | MV | AUM-2 and C8161 |
AUM-2 and MV |
C8161 and MV |
AUM-2 and C8161 and MV |
|||
| vs. C8181 |
vs. MV |
vs. AUM-2 |
vs. MV |
vs. AUM-2 |
vs. C8161 |
||||
| AKT1 | AKT1 | ANGPTL4 | AKT1 | ANGPT2 | ANGPT2 | AKT1 | ANPEP | AKT1 | ANPEP |
| ANGPTL3 | CSF3 | ECGF1 | ANGPTL4 | ANGPTL3 | ANGPTL3 | ANGPT2 | CCL11 | ANPEP | ENG |
| ANGPTL4 | CXCL1 | FIGF | CCL11 | ANGPTL4 | ANGPTL4 | ANPEP | ECGF1 | EFNA3 | MMP2 |
| ANPEP | ECGF1 | HPSE | CCL2 | CDH5 | ANPEP | CXCL1 | EFNA3 | ENG | TIMP1 |
| CCL11 | EFNA2 | LECT1 | CSF3 | COL18A1 | CCL11 | CSF3 | EFNB2 | FIGF | VEGFB |
| CDH5 | EFNA3 | MDK | CXCL1 | CXCL5 | CDH5 | EDG1 | EGF | IL8 | |
| COL18A1 | EPHB4 | NOTCH4 | CXCL10 | EDG1 | COL18A1 | EFNA1 | ENG | MDK | |
| ECGF1 | EREG | PECAM1 | CXCL5 | EFNA1 | CXCL9 | ENG | ID1 | MMP2 | |
| EFNA1 | FGF1 | PLAU | ECGF1 | EFNB2 | ECGF1 | HIF1A | ITGAV | SPHK1 | |
| EFNA2 | FGF2 | PLG | EFNA2 | EPHB4 | EDG1 | IL6 | MMP2 | THBS2 | |
| EFNA3 | HIF1A | PTGS1 | EREG | FGFR3 | EFNA1 | ILIO | NOTCH4 | TIMP1 | |
| EFNB2 | IL1B | STAB1 | FGF1 | FIGF | EFNA3 | ITGB3 | NRP2 | VEGFB | |
| EGF | IL6 | THBS2 | FGF2 | HPSE | EFNB2 | JAG1 | TGFB2 | VEGFC | |
| ENG | IL8 | TGFB2 | FLT1 | IGF1 | EGF | KDR | TIE1 | ||
| EPHB4 | IL10 | HGF | JAG1 | ENG | LAMA5 | TIMP1 | |||
| FGF1 | IL12A | HIF1A | KDR | EPHB4 | MMP2 | VEGFB | |||
| FGF2 | ITGB3 | ID3 | LAMA5 | FGFR3 | PLAU | ||||
| FGFR3 | MMP2 | IFNB1 | LECT1 | HPSE | PLG | ||||
| ID1 | NRP1 | IL10 | MDK | ID1 | PLXDC1 | ||||
| IL1B | PDGFA | IL1B | NOTCH4 | IFNA1 | TGFA | ||||
| IL8 | PDGFB | IL6 | PECAM1 | ITGAV | TIE1 | ||||
| ITGAV | PLAU | ITGB3 | PGF | JAG1 | TIMP1 | ||||
| LAMA5 | PLG | LECT1 | PTGS1 | KDR | TNFAIP2 | ||||
| NOTCH4 | PLXDC1 | LEP | SERPINF1 | LAMA5 | VEGFFB | ||||
| NRP1 | SPHK1 | MMP2 | STAB1 | NOTCH4 | |||||
| NRP2 | TGFA | NRP1 | TGFB1 | NRP1 | |||||
| PDGFA | TGFBR1 | PDGFB | THBS1 | NRP2 | |||||
| PDGFB | TNFAIP2 | PLAU | THBS2 | PDGFA | |||||
| PGF | VEGF | PLG | TIMP2 | PECAM1 | |||||
| SERPINF1 | VEGFC | PLXDC1 | PF4 |
 detected by gene and rtPCR arrays detected by gene and rtPCR arrays |
|||||
| SPHK1 | TGFA | PGF | |||||||
| TGFB1 | TGFB2 | PLG |
 detected by gene arrays detected by gene arrays |
||||||
| TGFBR1 | THBS2 | PTGS1 | |||||||
| THBS1 | TNFAIP2 | SERPINF1 |
 assayed only by gene arrays assayed only by gene arrays |
||||||
| TIMP1 | VEGF | STAB1 | |||||||
| TIMP2 | TEK | ||||||||
| VEGF | TGFB1 | ||||||||
| VEGFC | TGFBR1 | ||||||||
| THBS1 | |||||||||
| TIE1 | |||||||||
| TIMP1 | |||||||||
| TIMP2 | |||||||||
DISCUSSION
Vasculogenic mimicry, a manifestation of melanoma plasticity, has been previously shown to contribute in tumor perfusion while being relatively unresponsive to several angiogenesis inhibitors [van der Schaft et al., 2004]. The vascular phenotype of melanoma cells has been postulated previously [Velazquez and Herlyn, 2003] and endothelia-associated genes, such as EFNA2 [Hess et al., 2001] and VE-cadherin [Hendrix et al., 2001] were shown to be generally over-expressed in aggressive melanoma as opposed to less aggressive counterparts. However, no study has previously addressed the phenotypical heterogeneities of aggressive melanoma cell subpopulations at the molecular level.
Functional dissection of the endogenous angiogenic signature of melanoma networks
As a first step towards deciphering the molecular signature of vasculogenic mimicry, we quantified differential gene expression in spontaneously formed melanoma networks and nests. A novel 3D culture model allowed long-term culture and microdissection of living cell subpopulations. We showed here for the first time that the networks of aggressive melanoma cells on collagen type-I gels have a strong angiogenic phenotype with consistent expression of angiogenesis genes in higher levels by melanoma cells in networks compared to the genotypically identical cells in nests. Of great interest was the high over-expression of the genes EFNA1 (ephrin-A1), EFNA2 (ephrin-A2), EFNB2 (ephrin-B2), both in uveal and cutaneous melanoma cell networks detected here by gene arrays, rtPCR and immunostaining, as they are key members of a single family ephrin/Eph of angiogenesis promoting genes. Ephrin/Eph signaling mechanisms are involved in vasculogenesis, vascular remodeling, angiogenesis, cell migration, metastasis, axon guidance, and synaptic plasticity [Zhang and Hughes, 2006]. EFNA1 promotes formation of capillary-like structures in endothelial cells [Daniel et al., 1996] and plays a key role in TNF-induced angiogenesis [Pandey et al., 1995] by up-regulating various angiogenic factors including VEGF [Kulbe et al., 2007]. Identification of the EFNB2 over-expression by melanoma networks is intriguing due to the well documented role of the ephrinBs in other forms of plasticity, such as hippocampal plasticity [Grunwald et al., 2004]. Moreover, presence of ephrin-B2 receptor is required for synaptic plasticity [Klein, 2004].
Positive regulators of angiogenesis also include: the VEGF family, critical for endothelial cell proliferation, migration, and tube formation; and the angiopoietin/Tie2 family, involved in vessel remodeling, maturation and stabilization. VEGF was over-expressed in the melanoma networks (detected by rtPCR arrays, but not microarrays probably due to small relative over-expression), along with other factors that promote the role of VEGF (key factor in tumor angiogenesis and vascular permeability) [Ferrara et al., 1992]. TGF and epidermal growth factor receptor (EGFR) autocrine signaling can upregulate VEGF [Goldman et al., 1993]. TGFα was also over-expressed in networks. EDG, sphingosine 1-phosphate (SP1), stimulates angiogenesis and activates the endothelial isoform of nitric oxide synthase (eNOS) [Rikitake et al., 2002] (similarly as VEGF). EDG and VEGF independently and possibly synergistically promote angiogenesis [Igarashi et al., 2003]. The IL12 and IL18 interleukins were upregulated in the networks. Increased serum levels of both IL12 and IL18 mark stage IV cancer [Diakowska et al., 2006]. Moreover, IL18 induced production of VEGF in vivo [Cho et al., 2006] and formation of capillary-like tubes in vitro [Park et al., 2001]. Our data highlight an association of VEGF and/or of its signaling partners with the melanoma networks. Moreover, several CXC chemokines were over-expressed in the networks, including CXCL5, which are known for their role in tumor angiogenesis [Strieter et al., 2004]. They are also known to play a role in other forms of plasticity [Klein and Rubin, 2004].
Melanoma networks potentially inclined towards an arterial fate
NOTCH4 and one of its receptors, JAG1, are over-expressed in melanoma networks. The notch signaling pathway had initially been considered key in establishing arterial fate in endothelial cells [Coultas et al., 2005]. Also suggestive of arterial fate is the over-expression of EFNB2 (an arterial marker) and NRP1, the VEGF co-receptor restricted to arteries. Notch and FGF signaling pathways play major roles in self-renewal and carcinogenesis and may be potentially involved in the maintenance of the angiogenic phenotype of melanoma.
Possible maintenance of tumor cell plasticity
NUDT6 through its regulatory effects on the expression of basic FGF may be involved in self-renewal/plasticity of the melanoma networks. NUDT6, nudix (nucleoside diphospahate linked moiety X)-type motif 6 (also known as antisense basic fibroblast growth factor) was over-expressed in the melanoma networks. There is strong evidence of GFG being involved in the regulation of basic FGF (FGF-2), which is one of the first factors associated with hematopoiesis and angiogenesis, tumor cell proliferation and survival [Baguma-Nibasheka et al., 2005]. FGFR3 was also upregulated in the networks and may have a related role.
Endogenous regulators of vessel remodeling in melanoma networks
IL12, IL18, THBS1, and PTEN, previously associated with inhibition of angiogenesis, were shown to be differentially upregulated in melanoma networks. Negative regulation of angiogenesis could be a crucial reaction during remodeling and maturation of the neovascularization networks. Some factors differentially up-regulated in the networks are known for a dual role in both promoting and inhibiting angiogenesis. More specifically, IL18 was shown to enhance THBS1 production and inhibit angiogenesis via the JNK pathway and to promote regression of neovascularization [Qiao et al., 2007]. Furthermore, IL12 and IL18 synergistically inhibited angiogenesis in murine models via IFN-γ production [Coughlin et al., 1998]. Thrombospondin, THBS1, a major inhibitor of angiogenesis is upregulated in the networks. Also PTEN has a negative effect on tumor growth and tumor-induced angiogenesis [Wen et al., 2001]. NUDT6 mRNA, a natural antisense mRNA for FGF-2, post-transcriptionally regulates FGF-2 and its over-expression in tumors reduced the risk of tumor recurrence and increased survival [Barclay et al., 2005]. Proteolytic cleavage (i.e. by MMP-2) of the subunit COL18A1, which is over-expressed in the melanoma networks, produces the fragment endostatin, which is strongly anti-angiogenic [Folkman, 2006]. These genes could play a role in balancing the neovascularization promoting factors for achieving vessel maturation. Another possibility could involve a mechanism to induce a patterned normalization within the melanoma tumor (as they co-localize with the vascular-like networks) in order to allow for organized structures, such as a network of perfusion channels, to emerge within the dysregulated tumor microenvironment and connect ultimately to endothelia-lined vasculature.
Possible endogenous stabilization of the vasculogenic network
The up-regulation of COL18A1 in the networks may also be associated with production of basement membrane. Three variants of the non-fibrillar COL18A1 have been characterized in epithelial and vascular basement membranes and shown to be tissue specific for skin and liver among other tissues [Muragaki et al., 1995]. Moreover, TIMP3 is expressed in higher levels in the melanoma networks both for AUM-2 and C8161 cells. Despite its inhibitory effect on tumor growth, TIMP3 suppression in pericytes was shown to diminish their ability to stabilize endothelial cell lined tubes [Saunders et al., 2006].
Host cell recruiting factors in the melanoma networks may promote their stabilization and mediate co-option
ECGF1, endothelial cell growth factor 1 (platelet derived), also known as thymidine phosphorylase (TP), is chemotactic to endothelial cells and promotes their growth and is known for promoting angiogenesis in vivo [Akiyama et al., 2004]. Upregulation of HIF1A in the networks, a hypoxia induced factor involved in angiogenesis amongst other tumor dissemination mechanisms [Harris, 2002], suggests that the 3D self-assembly of melanoma cells into networks may intrinsically generate hypoxia. This could have an autocrine effect in network formation, or a paracrine one, in attracting blood vessels to achieve co-option with the host circulation. Formation of mosaic vessels, containing both tumor and endothelial cells [Chang et al., 2000], could mediate this process. Obviously, local recruitment of endothelial cells would be essential to achieving perfusion. To this end melanoma networks are also shown here to over-express chemokines CXCL5 and CCL11, which are chemoattractive to endothelial and microvascular cells [Bernardini et al., 2003]. Furthermore, VEGFC, a factor associated with lymphatic metastasis may facilitate co-option of the melanoma with the lymphatic vessels. PDGFA, a tumor secreted factor involved in recruitment of stromal cells that stabilize vascular networks, was also over-expressed in melanoma networks. Collectively, our observations suggest an endogenous capacity of the melanoma cells in networks to potentially co-opt the perfusion system within their host microenvironment, thereby providing a paravascular fluid conducting pathway in aggressive tumors.
Upregulation of ECM genes in the surrounding nests may contribute to the stabilization of the vascular-like melanoma networks
Although several ECM genes were over-expressed in the randomly arranged cells in nests, no genes were identified in our study to be expressed both in uveal and cutaneous melanoma nests. That may reflect the individuality of the tumor stroma specific to each tumor type. However, such patterning of differential gene expression may suggest complementary roles in forming a perfusion network with the angiogenesis gene expressing cells forming vascular-like networks while cells in nests produce the ECM required for their support.
Angiogenic potential of AUM-2 vs. C8161 cells
Differential overexpresion of angiogenesis genes in AUM-2 networks vs. nests had larger values compared to C8161. In addition, a larger number of angiogenesis specific genes were differentially over-expressed in AUM-2 networks than C8161. Moreover, while both AUM-2 and C8161 cells showed similarities with microvascular cells in the Matrigel angiogenesis assay, similarities (in genes and expression level) were more prominent for AUM-2. Specifically, comparison of AUM-2, C8161, and microvascular cells revealed the following patterns: (a) genes expressed in higher levels in melanoma vs. microvascular cells were common to AUM-2 and C8161 cells and include: AKT1, ANGPT1, several CCL and CXC genes, ECGF, several interleukins, NRP1, PLAU, VEGF and others; (b) genes expressed in higher levels in microvascular vs. melanoma cells were in their majority common to AUM-2 and C8161 cells and include: several angiopoietins, CDH5 (VE-cadherin), COL18A1, ephrins, JAG1, LAMA5, NOTCH4, PECAM1, THBS1, and TIMP2; (c) genes expressed in higher levels in AUM-2 vs. C8161 cells had many similarities with the genes over-expressed in microvascular cells vs. melanoma cells, including: angiopoietins, CDH5, COL18A1, ephrins, and NOTCH4. The above collectively highlight a more prominent angiogenic phenotype for the AUM-2 networks compared to the C8161 networks that may relate to the dissemination preferences of these aggressive melanoma lines. It is documented that AUM-2 (or MUM2B) disseminates hematogenously [Hendrix et al., 1998] while C8161 metastasizes hematogenously and/or via the lymphatics [Welch et al., 1991].
In conclusion, we analyzed the gene expression of the phenotypically distinct cell subpopulations of metastatic cutaneous and uveal melanoma, which evolve spontaneously in 3D cultures, and showed that melanoma networks possess a strong endogenous angiogenic potential. Our microgenomics data begin to illuminate the self-sustaining capabilities of tumorigenic melanoma that derive from cell plasticity, and to uncover endogenous survival mechanisms which may contribute to cancer evasion from current therapies.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Elena Pasquale for expert advice on the immunostaining of ephrins.
Grant Support: National Cancer Institute grants CA59702 and CA80318 to M.J.C.H. and Eisenberg Scholar grant to Z.N.D..
Footnotes
Note: The authors declare no competing financial interests.
REFERENCES
- Akiyama S, Furukawa T, Sumizawa T, Takebayashi Y, Nakajima Y, Shimaoka S, Haraguchi M. The role of thymidine phosphorylase, an angiogenic enzyme, in tumor progression. Cancer Science. 2004;95:851–7. doi: 10.1111/j.1349-7006.2004.tb02193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguma-Nibasheka M, Li AW, Osman MS, Geldenhuys L, Casson AG, Too CK, Murphy PR. Coexpression and regulation of the FGF-2 and FGF antisense genes in leukemic cells. Leukemia Research. 2005;29:423–33. doi: 10.1016/j.leukres.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Barclay C, Li AW, Geldenhuys L, Baguma-Nibasheka M, Porter GA, Veugelers PJ, Murphy PR, Casson AG. Basic fibroblast growth factor (FGF-2) overexpression is a risk factor for esophageal cancer recurrence and reduced survival, which is ameliorated by coexpression of the FGF-2 antisense gene. Clinical Cancer Research. 2005;11:7683–91. doi: 10.1158/1078-0432.CCR-05-0771. [DOI] [PubMed] [Google Scholar]
- Bernardini G, Ribatti D, Spinetti G, Morbidelli L, Ziche M, Santoni A, Capogrossi MC, Napolitano M. Analysis of the role of chemokines in angiogenesis. Journal of Immunological Methods. 2003;273:83–101. doi: 10.1016/s0022-1759(02)00420-9. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–6. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- Chang YS, di Tomaso E, McDonald DM, Jones R, Jain RK, Munn LL.Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood.[see comment]. Proceedings of the National Academy of Sciences of the United States of America 20009714608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho ML, Jung YO, Moon YM, Min SY, Yoon CH, Lee SH, Park SH, Cho CS, Jue DM, Kim HY. Interleukin-18 induces the production of vascular endothelial growth factor (VEGF) in rheumatoid arthritis synovial fibroblasts via AP-1-dependent pathways. Immunol Lett. 2006;103:159–66. doi: 10.1016/j.imlet.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Coughlin CM, Salhany KE, Wysocka M, Aruga E, Kurzawa H, Chang AE, Hunter CA, Fox JC, Trinchieri G, Lee WM. Interleukin-12 and interleukin-18 synergistically induce murine tumor regression which involves inhibition of angiogenesis. Journal of Clinical Investigation. 1998;101:1441–52. doi: 10.1172/JCI1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–45. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- Daniel TO, Stein E, Cerretti DP, John PL, Robert B, Abrahamson DR. ELK and LERK-2 in developing kidney and microvascular endothelial assembly. Kidney International - Supplement. 1996;57:S73–81. [PubMed] [Google Scholar]
- Demou ZN. Time-lapse analysis and microdissection of living 3D melanoma cell cultures for genomics and proteomics. Biotechnology and Bioengineering. 2008 doi: 10.1002/bit.21899.:In Press.
- Diakowska D, Markocka-McZka K, Grabowski K, Lewandowski A. Serum interleukin-12 and interleukin-18 levels in patients with oesophageal squamous cell carcinoma. Exp Oncol. 2006;28:319–22. [PubMed] [Google Scholar]
- Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE, Herlyn M. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Research. 2005;65:9328–37. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Houck K, Jakeman L, Leung DW. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocrine Reviews. 1992;13:18–32. doi: 10.1210/edrv-13-1-18. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: therapeutic implications. New England Journal of Medicine. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Folkman J. Antiangiogenesis in cancer therapy--endostatin and its mechanisms of action. Experimental Cell Research. 2006;312:594–607. doi: 10.1016/j.yexcr.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Goldman CK, Kim J, Wong WL, King V, Brock T, Gillespie GY. Epidermal growth factor stimulates vascular endothelial growth factor production by human malignant glioma cells: a model of glioblastoma multiforme pathophysiology. Molecular Biology of the Cell. 1993;4:121–33. doi: 10.1091/mbc.4.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald IC, Korte M, Adelmann G, Plueck A, Kullander K, Adams RH, Frotscher M, Bonhoeffer T, Klein R. Hippocampal plasticity requires postsynaptic ephrinBs. Nature Neuroscience. 2004;7:33–40. doi: 10.1038/nn1164. [DOI] [PubMed] [Google Scholar]
- Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nature Reviews. Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nature Reviews. Cancer. 2003;3:411–21. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- Hendrix MJ, Seftor EA, Meltzer PS, Gardner LM, Hess AR, Kirschmann DA, Schatteman GC, Seftor RE. Expression and functional significance of VE-cadherin in aggressive human melanoma cells: role in vasculogenic mimicry. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:8018–23. doi: 10.1073/pnas.131209798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix MJ, Seftor EA, Seftor RE, Gardner LM, Boldt HC, Meyer M, Pe’er J, Folberg R. Biologic determinants of uveal melanoma metastatic phenotype: role of intermediate filaments as predictive markers. Laboratory Investigation. 1998;78:153–63. [PubMed] [Google Scholar]
- Hendrix MJ, Seftor EA, Seftor RE, Kirschmann DA, Gardner LM, Boldt HC, Meyer M, Pe’er J, Folberg R. Regulation of uveal melanoma interconverted phenotype by hepatocyte growth factor/scatter factor (HGF/SF) American Journal of Pathology. 1998;152:855–63. [PMC free article] [PubMed] [Google Scholar]
- Hess AR, Seftor EA, Gardner LM, Carles-Kinch K, Schneider GB, Seftor RE, Kinch MS, Hendrix MJ. Molecular regulation of tumor cell vasculogenic mimicry by tyrosine phosphorylation: role of epithelial cell kinase (Eck/EphA2) Cancer Research. 2001;61:3250–5. [PubMed] [Google Scholar]
- Igarashi J, Erwin PA, Dantas AP, Chen H, Michel T. VEGF induces S1P1 receptors in endothelial cells: Implications for cross-talk between sphingolipid and growth factor receptors. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10664–9. doi: 10.1073/pnas.1934494100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. Eph/ephrin signaling in morphogenesis, neural development and plasticity. Current Opinion in Cell Biology. 2004;16:580–9. doi: 10.1016/j.ceb.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Klein RS, Rubin JB. Immune and nervous system CXCL12 and CXCR4: parallel roles in patterning and plasticity. Trends in Immunology. 2004;25:306–14. doi: 10.1016/j.it.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Kulbe H, Thompson R, Wilson JL, Robinson S, Hagemann T, Fatah R, Gould D, Ayhan A, Balkwill F. The inflammatory cytokine tumor necrosis factor-alpha generates an autocrine tumor-promoting network in epithelial ovarian cancer cells. Cancer Research. 2007;67:585–92. doi: 10.1158/0008-5472.CAN-06-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, Trent JM, Meltzer PS, Hendrix MJ.Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry.[see comment]. American Journal of Pathology 1999155739–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muragaki Y, Timmons S, Griffith CM, Oh SP, Fadel B, Quertermous T, Olsen BR. Mouse Col18a1 is expressed in a tissue-specific manner as three alternative variants and is localized in basement membrane zones. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:8763–7. doi: 10.1073/pnas.92.19.8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicosia RF, Ottinetti A. Growth of microvessels in serum-free matrix culture of rat aorta. A quantitative assay of angiogenesis in vitro. Laboratory Investigation. 1990;63:115–22. [PubMed] [Google Scholar]
- Pandey A, Shao H, Marks RM, Polverini PJ, Dixit VM. Role of B61, the ligand for the Eck receptor tyrosine kinase, in TNF-alpha-induced angiogenesis. Science. 1995;268:567–9. doi: 10.1126/science.7536959. [DOI] [PubMed] [Google Scholar]
- Park CC, Morel JC, Amin MA, Connors MA, Harlow LA, Koch AE. Evidence of IL-18 as a novel angiogenic mediator. Journal of Immunology. 2001;167:1644–53. doi: 10.4049/jimmunol.167.3.1644. [DOI] [PubMed] [Google Scholar]
- Qiao H, Sonoda KH, Ikeda Y, Yoshimura T, Hijioka K, Jo YJ, Sassa Y, Tsutsumi-Miyahara C, Hata Y, Akira S, Ishibashi T. Interleukin-18 regulates pathological intraocular neovascularization. J Leukoc Biol. 2007;81:1012–21. doi: 10.1189/jlb.0506342. [DOI] [PubMed] [Google Scholar]
- Rikitake Y, Hirata K, Kawashima S, Ozaki M, Takahashi T, Ogawa W, Inoue N, Yokoyama M. Involvement of endothelial nitric oxide in sphingosine-1-phosphate-induced angiogenesis. Arteriosclerosis, Thrombosis & Vascular Biology. 2002;22:108–14. doi: 10.1161/hq0102.101843. [DOI] [PubMed] [Google Scholar]
- Ruf W, Seftor EA, Petrovan RJ, Weiss RM, Gruman LM, Margaryan NV, Seftor RE, Miyagi Y, Hendrix MJ. Differential role of tissue factor pathway inhibitors 1 and 2 in melanoma vasculogenic mimicry. Cancer Research. 2003;63:5381–9. [PubMed] [Google Scholar]
- Saunders WB, Bohnsack BL, Faske JB, Anthis NJ, Bayless KJ, Hirschi KK, Davis GE. Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. Journal of Cell Biology. 2006;175:179–91. doi: 10.1083/jcb.200603176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seftor EA, Meltzer PS, Kirschmann DA, Pe’er J, Maniotis AJ, Trent JM, Folberg R, Hendrix MJ. Molecular determinants of human uveal melanoma invasion and metastasis. Clinical & Experimental Metastasis. 2002;19:233–46. doi: 10.1023/a:1015591624171. [DOI] [PubMed] [Google Scholar]
- Seftor RE, Seftor EA, Koshikawa N, Meltzer PS, Gardner LM, Bilban M, Stetler-Stevenson WG, Quaranta V, Hendrix MJ. Cooperative interactions of laminin 5 gamma2 chain, matrix metalloproteinase-2, and membrane type-1-matrix/metalloproteinase are required for mimicry of embryonic vasculogenesis by aggressive melanoma. Cancer Research. 2001;61:6322–7. [PubMed] [Google Scholar]
- Sharma N, Seftor RE, Seftor EA, Gruman LM, Heidger PM, Jr., Cohen MB, Lubaroff DM, Hendrix MJ. Prostatic tumor cell plasticity involves cooperative interactions of distinct phenotypic subpopulations: role in vasculogenic mimicry. Prostate. 2002;50:189–201. doi: 10.1002/pros.10048. [DOI] [PubMed] [Google Scholar]
- Shirakawa K, Wakasugi H, Heike Y, Watanabe I, Yamada S, Saito K, Konishi F. Vasculogenic mimicry and pseudo-comedo formation in breast cancer. International Journal of Cancer. 2002;99:821–8. doi: 10.1002/ijc.10423. [DOI] [PubMed] [Google Scholar]
- Sood AK, Fletcher MS, Zahn CM, Gruman LM, Coffin JE, Seftor EA, Hendrix MJ. The clinical significance of tumor cell-lined vasculature in ovarian carcinoma: implications for anti-vasculogenic therapy. Cancer Biology & Therapy. 2002;1:661–4. doi: 10.4161/cbt.316. [DOI] [PubMed] [Google Scholar]
- Strieter RM, Belperio JA, Phillips RJ, Keane MP. CXC chemokines in angiogenesis of cancer. Seminars in Cancer Biology. 2004;14:195–200. doi: 10.1016/j.semcancer.2003.10.006. [DOI] [PubMed] [Google Scholar]
- van der Schaft DW, Hillen F, Pauwels P, Kirschmann DA, Castermans K, Egbrink MG, Tran MG, Sciot R, Hauben E, Hogendoorn PC, Delattre O, Maxwell PH, Hendrix MJ, Griffioen AW. Tumor cell plasticity in Ewing sarcoma, an alternative circulatory system stimulated by hypoxia. Cancer Research. 2005;65:11520–8. doi: 10.1158/0008-5472.CAN-05-2468. [DOI] [PubMed] [Google Scholar]
- van der Schaft DW, Seftor RE, Seftor EA, Hess AR, Gruman LM, Kirschmann DA, Yokoyama Y, Griffioen AW, Hendrix MJ. Effects of angiogenesis inhibitors on vascular network formation by human endothelial and melanoma cells. Journal of the National Cancer Institute. 2004;96:1473–7. doi: 10.1093/jnci/djh267. [DOI] [PubMed] [Google Scholar]
- Velazquez OC, Herlyn M. The vascular phenotype of melanoma metastasis. Clinical & Experimental Metastasis. 2003;20:229–35. doi: 10.1023/a:1022987201264. [DOI] [PubMed] [Google Scholar]
- Welch DR, Bisi JE, Miller BE, Conaway D, Seftor EA, Yohem KH, Gilmore LB, Seftor RE, Nakajima M, Hendrix MJ. Characterization of a highly invasive and spontaneously metastatic human malignant melanoma cell line. International Journal of Cancer. 1991;47:227–37. doi: 10.1002/ijc.2910470211. [DOI] [PubMed] [Google Scholar]
- Wen S, Stolarov J, Myers MP, Su JD, Wigler MH, Tonks NK, Durden DL. PTEN controls tumor-induced angiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4622–7. doi: 10.1073/pnas.081063798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Hughes S. Role of the ephrin and Eph receptor tyrosine kinase families in angiogenesis and development of the cardiovascular system. Journal of Pathology. 2006;208:453–61. doi: 10.1002/path.1937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


