Abstract
Background & Aims
NK1.1+ TCRαβint CD1-restricted T (NKT) cells are a unique subset of T lymphocytes that are thought to have an immunoregulatory role in a wide range of diseases. Most mouse NKT cells express a T-cell receptor that contains an invariant Vα14Jα18 chain and recognizes antigenic glycolipids presented in association with MHC class Ib (CD1d) molecules. These invariant NKT cells (iNKT) have been implicated in cholestatic liver injury.
Methods
We examined the role of iNKT cells in liver injury associated with biliary obstruction in mice with ligations of the common bile duct.
Results
The number of activated iNKT cells increased markedly in the livers of mice following bile duct-ligation (BDL). Plasma alanine aminotransferase (ALT) levels, an indicator of liver injury, were significantly higher in iNKT cell–deficient (Jα18−/−) mice, compared to wild-type mice, following BDL. Photoimage analysis of histologic sections confirmed that more damage was present in the livers of in Jα18−/−mice; liver damage correlated with increases in keratinocyte-derived chemokine (KC) and macrophage inflammatory protein-2 (MIP-2) production as well as neutrophil sequestration. Liver injury was significantly reduced in Jα18−/−mice treated with anti-KC and anti-MIP-2 or rendered neutrophil-deficient before BDL. Similarly, Jα18−/−mice that were injected with iNKT cells before BDL exhibited significant decreases in neutrophil accumulation and liver damage.
Conclusion
These data document the role of iNKT cells in suppressing the neutrophil proinflammatory response and neutrophil-dependent cholestatic liver damage.
Introduction
NK1.1+ TCRαβint CD1-restricted T (NKT) cells are a unique subset of T lymphocytes thought to play an immuno-regulatory role in a wide range of diseases.1 Indeed, it is widely postulated that NKT cells link innate and adaptive immune responses by responding rapidly and subsequently activating other cell types, e.g., dendritic and NK cells.2 NKT cells comprise approximately 30% of the hepatic lymphoid population in mice (up to 50% in humans) where they reside within the sinusoids adherent to the endothelial cells, crawling rapidly along the vessel walls.3,4 Decidedly fewer reside in the thymus, spleen, lymph nodes and peripheral blood.4,5 Most mouse NKT cells [invariant (i)NKT cells] express a TCR composed of an invariant Vα14Jα18 chain and a β-chain heavily biased toward Vβ8.2, Vβ2 and Vβ7.5 iNKT cells recognize antigenic glycolipids presented in association with non-classical MHC class Ib (CD1d) molecules.
CD1 molecules are cell surface glycoproteins consisting of 43- to 49-kDa heavy chains noncovalently associated with β2-microglobulin. While most mammalian species express multiple CD1 isoforms, mice express only one: CD1d.6 A variety of cell types express CD1d including B cells, dendritic cells and mononuclear phagocytes, as well as epithelial cells, parenchymal cells and vascular smooth muscle cells comprising non-lymphoid tissues including the liver.7 Cell-surface CD1d molecules are internalized and delivered to late endosomes and lysosomes then recycled back to the plasma membrane; thus, they access intracellular compartments that contain both exogenous and endogenous (self) lipid antigens.6 While the origin and identity of these self antigens remain to be delineated fully, a recent study demonstrated the specific recognition of a lysosomal glycosphingolipid, isoglobotrihexosylceramide (iGb3), by iNKT cells in vitro.8 This led the investigators to suggest that iGb3 plays a key role in regulating the development and activity of iNKT cells in vivo. The sparse distribution of iGb3 within mouse and human tissues, however, implies its limited physiologic relevance.9
While CD1-restricted T cells purportedly play a role in a wide range of immune responses, the exact nature of that role is often controversial or unclear.6 NKT cells have been implicated, for example, in immunity to hepatic tumors in patients as well as rodent models. The number of metastatic liver tumors increased significantly in NKT cell-depleted mice inoculated with EL4 tumor cells.10 Conversely, fewer iNKT cells are present in liver biopsies obtained from patients with metastatic disease than from healthy donor organs suggesting that a reduction in iNKT cell number plays a contributing role.11 Likewise, fewer iNKT cells found in the liver and peripheral blood of patients infected with hepatitis C virus suggests the role of iNKT cells in resistance to virus infection and/or protection against disease progression.12 Contrary to these findings, however, NKT cells appear to play a key role in the development of autoimmune hepatitis. The uncontrolled activation of NKT cells, the production of IL-4, IFN-γ and TNF-α, and the expression of Fas ligand contribute to severe liver damage in several animal models.13,14
iNKT cells have also been implicated in the pathogenesis of primary biliary cirrhosis and cholestatic liver injury. Both the expression of CD1d by hepatocytes and small bile duct epithelial cells, and the number of hepatic NKT cells in patients with primary biliary cirrhosis are increased.15 Although the characteristics of these NKT cells and their role in biliary cirrhosis have not been delineated, it has been suggested that NKT cells are a major contributing factor.15 Ligation of the common bile duct in mice provides an excellent experimental model in which to examine the role of iNKT cells in cholestatic liver injury. Here we report that bile duct ligation led to a marked increase in activated, hepatic iNKT cells. In contrast to their alleged role in the pathogenesis of biliary cirrhosis, these iNKT cells suppressed rather than promoted liver injury attending cholestasis. Liver injury that occurred subsequent to ligation was far more severe in iNKT cell-deficient (Vα 14Jα18−/−) mice and dependent upon the increased accumulation of neutrophils in cholestatic livers.
Materials and Methods
Mice
Specific pathogen-free wild-type, C57BL/6J mice were purchased from Jackson Laboratories, Bar Harbor, ME; iNKT cell-deficient, Vα14Jα18−/− mice on a C57BL/6 background were obtained from Dr. M. Taniguchi (Riken Research Center for Allergy) and bred in our animal facility.16 The animals were treated in accordance with NIH publications entitled "Principles for Use of Animals" and "Guide for the Care and Use of Laboratory Animals." All experiments were conducted using female mice between 6 and 16 weeks of age.
Ligation and division of the common bile duct
Surgery was performed on mice under deep anesthesia as we described previously.17 A midline upper abdominal incision was made; the common bile duct was identified, isolated, double-ligated with #4-0 braided silk sutures, and divided between the ligatures; the fascia and skin of the abdominal incision were closed. Control mice underwent sham operations in which the common bile duct was exposed, but not ligated.
Cell preparation, purification and adoptive transfer
Livers were perfused in situ via the portal vein with calcium- and magnesium-free HBSS supplemented with 2% heat-inactivated FBS (wash medium) to remove circulating blood cells. Perfused livers were dissected and teased through 70 µm nylon mesh cell strainers (BD Biosciences, San Jose, CA). The resultant cell suspensions were layered onto a two-step (40/70%) discontinuous Percoll gradient (GE Healthcare Bio-sciences Corp., Piscataway, NJ.) and centrifugation at 900 ×g for 20 min at 25°C. Hepatic leukocyte populations collected at the interface were washed in wash medium. For adoptive transfer experiments, the purified hepatic leukocyte population obtained from wild-type donor mice was stained with PE-conjugated anti-NK1.1 (PK136) and FITC-conjugated anti-TCRβ chain (clone H57–597) purchased from eBioscience Inc., San Diego, CA. Labeled cells were collected using a 3-laser FACSAria and BD FACSDiva Software Version 5.0.2 (BD Biosciences). Jα18−/− recipient mice were inoculated i.v. with 5 ×105 purified NKT cells at approximate 18 hours prior to BDL.
Antibody treatment
CXC chemokine activity was neutralized in mice inoculated i.v. with 50 µg each of rat anti-mouse CXCL1/KC and rat anti-mouse CXCL2/MIP-2 (clones MAB and 40605, respectively, purchased from R&D Systems, Inc., Minneapolis, MN) at 1 hour prior to surgery. Mice were rendered neutrophil deficient by i.p. inoculation twice (on day 2 prior and the day of surgery) with 800 µg of rat IgG2a anti-mouse Ly-6G mAb derived from 1A8 hybridoma cells (Dr. Thomas Malek, University of Miami School of Medicine, Miami, FL). Control animals received an equivalent concentration of normal IgG (Sigma-Aldrich Co., St. Louis, MO). As previously reported,18 the number of circulating peripheral blood neutrophils was reduced more than 10-fold and was <1.0 ×102/µl in mice on day 1 post-inoculation of anti-Ly-6G mAb.
Flow cytometry
Flow cytometry was conducted in accordance with methods previously described.19 Dye-conjugated mAb specific for the following determinants were purchased from eBioscience, Inc. (San Diego, CA) and used: NK1.1 (clone PK136), TCRβ-chain (clone H57–597), CD3 (clone 145-2C11), CD25 (clone PC61.5), CD69 (clone H1.2F3), IL-4 (clone 11B11), and IFN-γ (clone XMG1.2). Conjugated, anti-Ly-6G (clone 1A8) was purchased from BD Biosciences. Invariant NKT cells were stained specifically by incubation with streptavidin-fluorescein-conjugated, PBS-57-loaded mouse CD1d tetramer obtained from the NIH Tetramer Core Facility (NIAID, Emory University Vaccine Center, Atlanta, GA). To enumerate cytokine-producing cells, the freshly isolated hepatic leukocyte population was precultured 6 hours in the presence of brefeldin A to inhibit protein transport, then stained using the BD Biosciences Cytofix/Cytoperm kit according to instructions. All staining was performed in the presence of saturating concentrations of anti-CD16/CD32 (FcγIII/II receptor-block, clone 2.4G2; eBioscience).
RNA extraction, purification, and quantitative real-time RT-PCR
Total cellular RNA was extracted and purified from representative liver samples using TRIzol (Invitrogen Corporation, Carlsbad, CA). Real-time RT-PCR was conducted in accordance with methods we described previously.17 Copies of messenger RNA and 18S ribosomal RNA (the housekeeping standard) were estimated from the PCR cycle number required for the fluorescent signal to reach a fixed intensity, the threshold cycle. The mean number of targeted gene mRNA copies/103 18S rRNA copies ± SE for samples derived from six mice treated comparably is reported. The PCR primers listed in Supplementary Materials Table I online at www.gastrojournal.org were designed from published sequences using Primer3 software (Whitehead Institute, Cambridge, MA) and purchased from Operon Biotechnologies, Inc. (Huntsville, AL).
Cytokine and chemokine analyses
The cytokines and chemokines present in culture supernates, plasma or representative liver samples obtained from individual mice were quantified using the Bio-Plex cytokine array system. In the latter case, tissue samples were homogenized (50–100 mg/ml) in calcium- and magnesium-free HBSS containing 0.5% Triton X-100 and 2x complete protease inhibitor cocktail (Roche Diagnostics Corporation Indianapolis, IN). Homogenates were centrifuged at 13,500g to remove cell debris, and supernatants were stored at −20°C until analyses. Bio-Plex cytokine assay kits were purchased and used with the Bio-Plex 200 system in accordance with the manufacturer’s instructions (Bio-Rad Laboratories GmbH, Munich, Germany). The following panel of potentially relevant cytokines and chemokines were quantified: IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12 (p40), TNF-α, IFN-γ, MIP-2 and KC.
Plasma alanine aminotransferase (ALT)
Plasma samples were collected from mice by cardiac puncture and stored at 4°C until analyzed. ALT activities were quantified spectrophotometrically as we described previously.17
Histochemistry, immunohistochemistry and photo image analysis
Photo image analysis was performed as previously described.17 Representative tissue wedges were fixed in paraformaldehyde and embedded in paraffin; 6-mm sections were cut and stained with trichrome stain (Mr. Paul Monfils, Central Research Laboratory, Rhode Island Hospital). Histologic slides were scanned into Adobe Photoshop (Adobe Systems Inc., San Jose, CA); the blue trichrome stained stroma, a correlate of tissue injury, was quantified using NIH 1.61 Image software and expressed as a percentage of the total section.
To visualize and quantify neutrophils, deparaffinized liver sections were hydrated and incubated with 0.1% hydrogen peroxide to quench endogenous peroxidase activity. Subsequently, the sections were incubated with 3% rat serum to block nonspecific antibody binding, and then with 0.025 mg 1A8 mAb/ml buffer. Washed sections were incubated with biotin-conjugated goat anti-rat IgG (Vector Laboratories, Burlingame, CA), washed, and incubated with VECTASTAIN ABC staining solution (Vector). Washed slides were incubated with 0.05% diaminobenzidine (peroxidase substrate), washed and counterstained with 1% aqueous methyl green.
Myeloperoxidase
The myeloperoxidase activity in liver homogenates was quantified spectrophotometrically by analyzing the H2O2-dependent oxidation of 3,3’5,5’-tetramethylbenzidine (Sigma-Aldrich) as we described previously.20
Statistical analysis
The results were analyzed using the SigmaStat statistics program (Jandel Scientific, San Rafael, CA). Individual means were compared using a non-paired Student's t test or a Mann-Whitney Rank Sum test. Data derived from 3 or more groups were compared by one-way analysis of variance (ANOVA) followed by a Tukey test to identify the groups that differed significantly (P <0.05).
Results
iNKT cell are activated in response to BDL
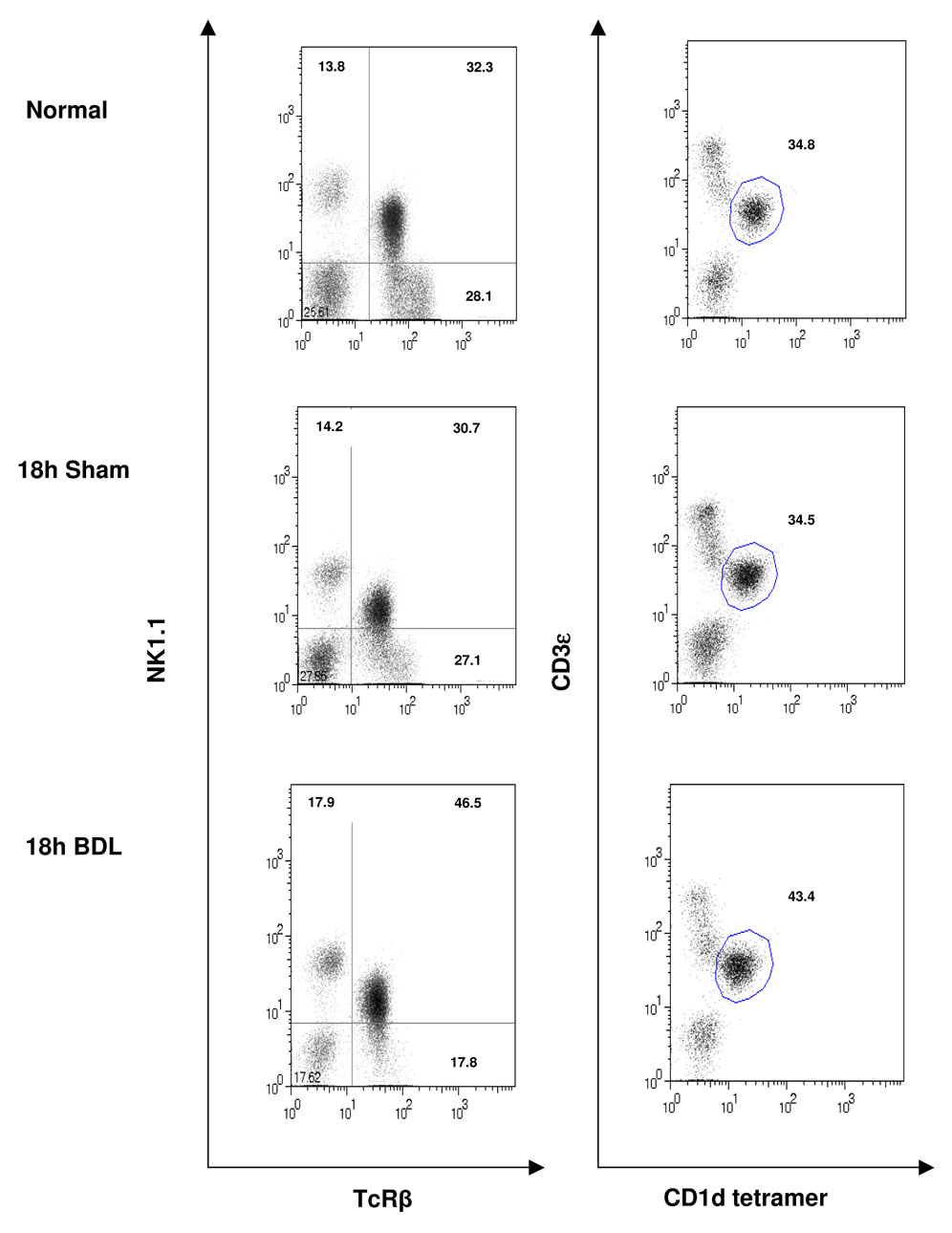
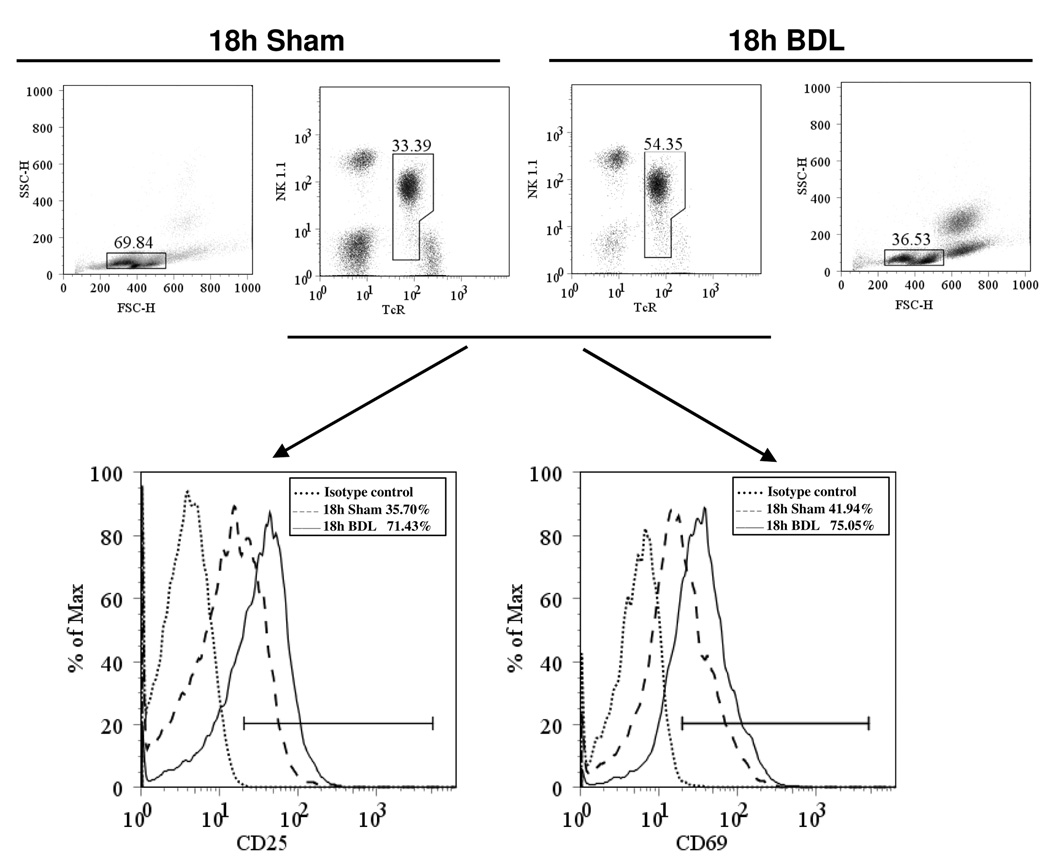
Flow cytometric analyses reveal a significant increase in the percentage of NK1.1+TCR+ cells composing the hepatic lymphoid population derived from BDL, relative to sham-operated, mice assessed at 18 hours post-surgery (Figure 1). A corresponding increase in the percentage of cells stained with fluorescein-conjugated, PBS-57-loaded mouse CD1d tetramer demonstrates directly an increase in hepatic iNKT cells subsequent to biliary obstruction. The elevated expression of CD25 (IL-2Rα) and the early activation marker CD69 further evidences the response of iNKT cells to cholestasis (Figure 2). Two percent or less of these cells produced either IL-4 or IFN-γ detected by intracellular cytokine staining regardless of whether the cells were obtained from sham-operated or bile duct-ligated mice (data not shown). This finding is supported by Bio-Plex bead array analysis of the cytokines produced by cultured nonparenchymal liver cells (NPCs) derived from mice at 2 and 18 hours following surgery. Marked decreases in IL-4 and IFN-γ production by cells derived from BDL, relative to non-operated or sham-operated, animals were observed (Supplementary Table II). In sharp contrast, the NPC population obtained from mice at 2 hours post-administration of α- galactosyl ceramide (α -GalCer) produced elevated quantities of both IL-4 and IFN-γ correlating with 14% and 22% of NKT cells producing IL-4 and IFN-γ, respectively, determined by intracellular cytokine staining (data not shown).
Figure 1. The hepatic iNKT cell population is increased in cholestatic livers.
The hepatic leukocyte populations were obtained from groups of 4 untreated (control) mice, or mice subjected to sham operations or BDL 18 hours previously. NKT cells were stained as indicated and quantified by flow cytometry. Dot plots are the results of a single experiment representative of ≥3 experiments.
Figure 2. CD25- and CD69-expressing iNKT cells accumulate in cholestatic livers.
The hepatic leukocyte populations were obtained from groups of 4 mice at 18 hours following BDL or sham operation. CD25+ (left histogram) and CD69+ (right histogram) iNKT cells were quantified by flow cytometry. The results of a single experiment are depicted; 2 additional experiments yielded similar findings.
iNKT cells suppress cholestatic liver injury
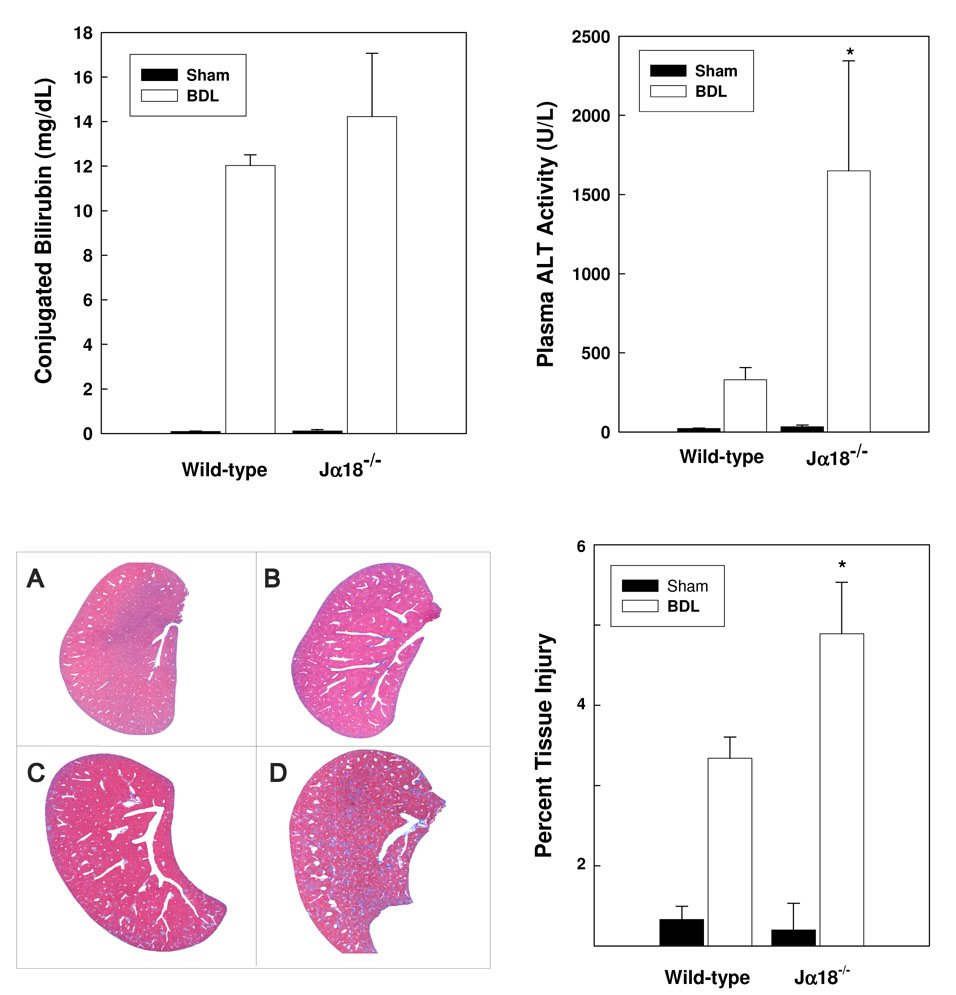
To ascertain the role of iNKT cells in hepatic injury, wild-type and iNKT cell–deficient (J α 18−/−) mice were subjected to BDL or sham operation. Plasma and liver samples were collected on day 3 post-surgery. Conjugated bilirubin and alanine aminotransferase (ALT, an indicator of liver injury) levels were elevated in the plasma of all BDL, relative to sham-operated, animals (Figure 3). ALT activities were significantly higher, however, in ligated J α 18−/− mice evidencing increased liver injury. Photoimage analyses of histologic sections substantiated this finding; a relative increase in the percentage of trichrome blue stained liver stroma (a correlate of tissue damage) was determined in sections of BDL, Jα18−/− mouse livers. Adoptive transfer experiments demonstrate directly the role of iNKT cells in suppressing cholestatic liver injury. The ALT activity in plasma obtained from J α18−/− mice administered NKT cells 18 hours prior to BDL was significantly diminished compared to untreated animals and equivalent to that found in plasma obtained from wild-type mice on day 3 post-surgery (Table I).
Figure 3. iNKT cells suppress cholestatic liver injury.
Sham operations or BDL were performed on groups of 6 wild-type and J α 18−/− mice. Plasma was collected on day 3 post-surgery; conjugated bilirubin (top panel, left) and ALT levels (top panel, right) were quantified [*significantly greater than other groups; P <0.05]. Livers dissected from wild-type (A/C) and Jα18−/− (B/D) mice on day 3 following sham operations (A/B) or BDL (C/D) were sectioned, stained with trichrome stain (bottom panel, left; 100-fold magnification) and subjected to photoimage analysis (bottom panel, right). Percent damaged area stained blue/total area ± SD was calculated. An additional experiment yielded comparable results. *Significantly greater than wild-type mice treated comparably; P=0.037.
Table I.
NKT cells suppress cholestatic liver injury
| Mouse strain | Pre-treatment | BDL | Plasma ALT activity (U/L) |
|---|---|---|---|
| Wild-type | Untreated | − | 30 ± 8 |
| Untreated | sham | 42 ± 11 | |
| Untreated | + | 305 ± 90a | |
| Jα18−/− | Untreated | + | 704 ± 94 |
| Adoptive transfer | + | 391 ± 100b |
NOTE: Groups of mice were untreated or inoculated i.v. with 5 ×105 purified NKT cells (adoptive transfer) at 18 hours prior to surgery. Plasma was collected on day 3 following BDL or sham operations, and ALT levels were quantified. Data are derived from two or more experiments and plasma samples obtained from ≥6 mice.
Significantly greater than non-operated or sham-operated control animals; P<0.001.
Significantly less than Jα18−/ − mice not administered NKT cells: P=0.049.
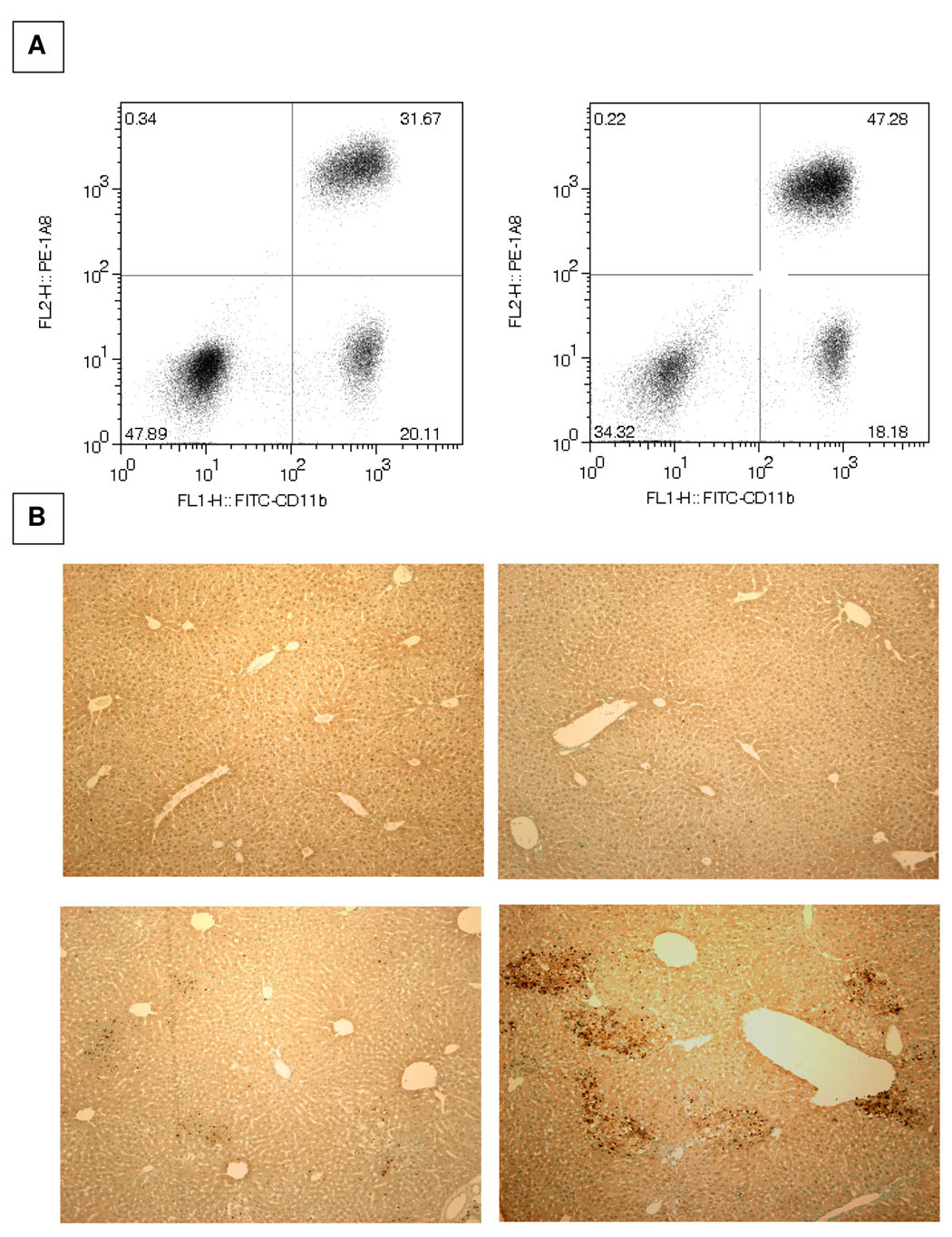
iNKT cells suppress neutrophil sequestration in cholestatic livers
Neutrophils play a significant role in the pathogenesis of cholestatic liver injury; factors that moderate neutrophil accumulation diminish tissue damage.21 It is relevant, therefore, that flow cytometric analysis demonstrated 50% more Ly6-G+ neutrophils in the hepatic leukocyte population obtained from iNKT cell-deficient (Jα18−/−), relative to wild-type, mice at 18 hours post-BDL (Figure 4 top). Neutrophils counted in Wright-Giemsa-stained cytospin smears prepared from these same two populations obtained in a separated experiment yielded similar results. That is, the hepatic populations derived from iNKT cell deficient and wild-type mice contained 32 ± 6% and 24 ± 4% (P=0.018 Student’s t test) neutrophils, respectively. A significant increase in myeloperoxidase activity recovered in representative liver samples obtained from groups of 4 iNKT cell-deficient mice and 4 wild-type mice on day 3 post-BDL, i.e., 0.738 ± 0.289 versus 0.349 ± 0.127 (change OD650/min/100 mg tissue; P=0.049), respectively, demonstrate an actual increase in number of hepatic neutrophils rather than a mere change in percentage of cells reflecting the absence of iNKT cells. This conclusion is supported by the presence of considerably more Ly6-G+ neutrophils in liver sections derived from Jα18−/−, than wild type, mice on day 3 post-BDL (Figure 4 bottom). In this regard, it is pertinent to note that the adoptive transfer of NKT cells prior to surgery resulted in a significant decrease in the percentage of neutrophils constituting hepatic NPC population obtained from Jα18−/− mice on day 3 post-BDL. Thus, in the adoptive transfer experiments illustrated in Table I, neutrophils constituted 51.2 ± 12.6% of the hepatic NPC population obtained from untreated mice versus 37.6 ± 6.7% of the population derived from Jα18−/− mice administered NKT cells prior to surgery (P=0.039).
Figure 4. Increased accumulation of neutrophils in the cholestatic livers of NKT cell-deficient mice.
A. The hepatic leukocyte populations were obtained at 18 hours post-BDL and the percentages of Ly-6G+CD11b+ neutrophils (upper right quadrant) constituting the population derived from wild-type (left panels) and Jα18−/− (right panels) mice were determined by flow cytometric analyses. B. The livers of wild-type (left) and iNKT cell-deficient (right) mice were dissected on day 3 following sham-operation (top) or bile duct ligation (bottom). Fixed and paraffin-embedded tissue samples were sectioned and the presence of neutrophils (distinguished by brown precipitate) was assessed by immunohistochemical staining (original 100x magnification). Two experiments yielded comparable results.
The substantial contribution of neutrophils to cholestatic liver injury observed in Jα18−/− mice is evidenced by an approximate three-fold reduction in plasma ALT levels assessed in BDL mice rendered neutrophil deficient by pretreatment with anti-Ly-6G-specific (1A8) mAb (Table II). Likewise, liver injury was reduced in bile duct-ligated wild-type mice treated with 1A8 mAb prior to surgery. In the latter case, however, plasma ALT levels were reduced reproducibly but not significantly. Consequently, there was no difference in the ALT activity contained in plasma obtained from neutrophil-depleted iNKT cell-deficient and wild-type mice on day 3 following ligation of the common bile duct. These data suggest that the elevated accumulation of neutrophils in the livers of cholestatic Jα18−/− mice is a major factor contributing to increased tissue damage.
Table II.
Neutrophil depletion reduces cholestatic liver injury in iNKT cell-deficient mice
| Mouse strain | Pre-treatment | Plasma ALT (U/L) | Neutrophils (% NPCs) |
|---|---|---|---|
| Jα18−/− | normal IgG | 778 ± 160 | 52.3 ± 15.4 |
| 1A8 mAb | 264 ± 110a | 17.1 ± 11.4b | |
| Wild-type | normal IgG | 332 ± 73 | NDc |
| 1A8 mAb | 204 ± 84 | ND |
NOTE: Groups of 6 mice were pre-treated with the mAb indicated or an equivalent concentration of normal rat IgG; the common bile duct was ligated and divided. Plasma was collected and ALT levels were quantified on day 3 post-surgery. Values are representative of two or more experiments. Significantly less than Jα18−/− mice administered normal rat IgG:
P<0.001;
P=0.012.
ND, not determined
iNKT cells suppress chemokine production
The elevated production of proinflammatory mediators such as cytokine-induced neutrophil chemoattractant (CINC, a rat analog of human IL-8) plays a critical role in promoting neutrophil infiltration and cholestatic liver injury.21,22 Mouse IL-8 analogs, keratinocyte-derived chemokine (KC) and monocyte inhibitory protein (MIP-2), elicit neutrophil immigration and liver injury in a number of experimental mouse models (e.g., trauma and hemorrhage).23 Subsequent experiments demonstrate the ability of iNKT cells to suppress the production of these chemokines following ligation of the common bile duct. KC and MIP-2 transcript levels were increased markedly in the livers of bile duct-ligated wild-type and Jα18−/− mice, relative to sham-operated control animals, at 18 hours post-operation (Figure 5). KC mRNA expression, however, was significantly greater in the livers of ligated, iNKT cell-deficient animals. MIP-2 mRNA expression, on the other hand, was equivalent in wild type and Jα18−/− mice at 18 hours post-BDL and not affected by the absence of iNKT. In contrast to these findings, both IL-4 and IFN-γ transcripts were reduced markedly in the livers of iNKT cell-deficient mice, relative to wild-type animals, following BDL. Moreover, the levels of these two transcripts were comparable in the livers of Jα18−/− mice subjected to either BDL or sham-operation.
Figure 5. iNKT cells suppress chemokine message expression.
Representative liver samples were obtained from groups of wild-type (solid bars) and Jα18−/− (open bars) mice at 18 hours following BDL or sham operation. The RNA was extracted and purified; the transcripts indicated were quantified by real-time RT-PCR. Data are the means ± SE derived from 6 mice treated comparably. A second experiment yielded similar results. Significantly different from that determined in the livers of BDL wild-type animals: *P <0.05; ***P <0.001.
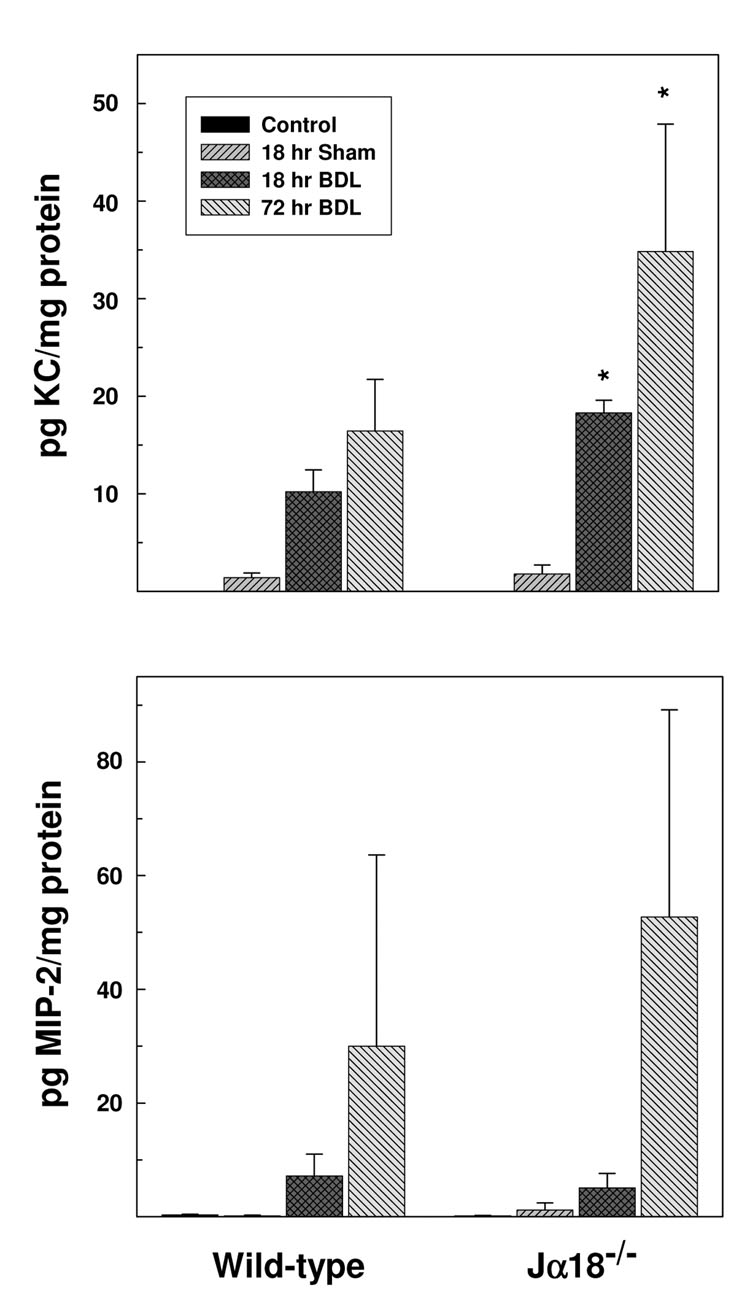
Concordant findings were obtained in experiments that quantified KC and MIP-2 levels in the plasma derived from bile duct-ligated animals. The plasma obtained from wild-type and iNKT cell-deficient mice at 18 hours post-surgery contained 8.8 ± 6.4 pg/ml versus 32.2 ± 16.4 pg/ml KC (P=0.015), respectively; plasma KC levels derived from non-operated control mice were below the limits of detection. Elevated MIP-2 levels were also found in the plasma obtained from wild-type (71.9 ± 27.1 pg/ml) and Jα18−/− (79.630 ± 42.350 pg/ml) mice compared to levels found in control, non-operated animals in each group at 18 hours post-BDL (9.0 ± 1.5pg/ml and 5.6 ± 0.6 pg/ml, respectively). Tissue analyses yielded similar results: significantly more KC in the livers of iNKT cell-deficient mice relative to wild-type mice at both 18 and 72 hours following BDL (Figure 6, top panel). Tissue concentrations of MIP-2 were also elevated in the livers of both wild-type and Jα18−/− mice at 72 hours post-BDL (bottom panel). Despite apparently higher MIP-2 levels in the livers of Jα18−/− mice, however, considerable variation among animals in each group negated statistical significance. These findings suggest that the elevated production of KC and MIP-2 promote neutrophil infiltration and cholestatic liver injury in Jα18−/− mice. Indeed, of the cytokines and chemokines synthesized by iNKT cells and/or widely implicated in cholestatic liver injury, the concentration of just a single additional cytokine, IFN-γ, was elevated in the livers of BDL mice (Supplementary Materials Table III). In sharp contrast to the >100-fold increases in both KC and MIP-2, however, the concentrations of IFN-γ were only two-fold greater than in either non-operated or sham-operated mice at 3 days post-BDL, and were equivalent in wild-type and iNKT-cell deficient animals.
Figure 6. iNKT cells suppress chemokine protein production in the livers of BDL mice.
Representative liver samples were obtained from groups of wild-type and Jα18−/− mice at 0 time (non-operated control), 18 or 72 hours following BDL and/or sham operation. KC (top panel) and MIP-2 (bottom panel) concentrations were determined by Bioplex bead array analysis. Data are the means ± SE derived from 6 mice treated identically. A second experiment yielded comparable results. *Significantly greater than bile duct ligated wild-type mice; P<0.05.
In an effort to demonstrate the combined roles of KC and MIP-2 in promoting neutrophil sequestration and liver injury, Jα18−/− mice were inoculated i.v. with 50 µg each of anti-KC and anti-MIP-2 mAb. Control mice received 100 µg of normal rat IgG. At 1 hour post-treatment, the common bile ducts were ligated; neutrophil accumulation and liver injury were determined on day 3 following surgery. Mice pretreated with anti-KC and anti-MIP-2 exhibited significant decreases in numbers of accumulating neutrophils (40.4 ± 6.0 versus 50.1 ± 6.2 for the controls, P=0.05) and plasma ALT levels (506 ± 145 U/L versus 877 ± 186 U/L for untreated controls, P=0.014).
Discussion
Ostensibly, iNKT cells play an immuno-regulatory role in a wide range of diseases including immune surveillance and tumor immunity, autoimmunity, immune tolerance, and innate host defenses.1 The bulk of iNKT cells express an activated or memory phenotype suggesting that the microenvironment contributes to the size and character of the hepatic iNKT cell population.24 Studies demonstrating the development of equivalent hepatic iNKT cell populations in normal and germ-free animals indicate that the accumulation of activated iNKT cells is not due to microbial antigens presented by CD1d-expressing cells in the liver.24 Rather, most data suggest that the presentation of conserved self-antigens accounts for the size and phenotype of the hepatic iNKT cell population.6 This assertion is supported by in vitro experiments demonstrating the activation of iNKT cells cultured with CD1d-positive antigen-presenting cells in the absence of added foreign antigen.25
The increased replication of a limited number of parasites, bacteria and viruses in the organs of CD1d- or Jα18;deficient mice provides some evidence to support for the role of iNKT cells in innate host resistance to infectious agents.26 In many instances, however, iNKT cells are not protective; wild-type and NKT cell-deficient mice, for example, exhibit comparable susceptibilities to Mycobacterium tuberculosis infections.27 Moreover, in some instances, the response of iNKT cells to bacterial infection [e.g., L. monocytogenes,28 S. cholerasuis29 and E. coli30] is largely detrimental. Notably, the mechanisms that enable iNKT cells to recognize and respond to a wide range of pathogens are not well understood. However, the direct response of iNKT cells to glycolipids associated with a number of microorganisms [e.g., diacylglycerol derived from Borrelia burgdorferi31 or glycosylceramides derived from Sphingomonas32], the indirect response to endogenous self-antigens derived from infected host cells,33 and the nonspecific response to cytokines, i.e., IL-12 and IL-18, produced by antigen presenting cells have been reported.34
Invariant NKT cells recognize and respond to α-GalCer, a glycosphingolipid derived from marine sponges and a potent iNKT cell activator.16 Although α-GalCer serves no known function in normal mammalian physiology, it has been used extensively to quantify and characterize the biological activity of iNKT cells. α-GalCer inoculated i.p. stimulates iNKT cell proliferation and clonal expansion in mice; α-GalCer-activated iNKT cells are characterized by the down-regulated expression of cell-surface NK1.1 and TCR αβ, and the sustained synthesis of cytokines.35,36 α-GalCer stimulates the translation of pre-existing mRNA transcripts and the rapid production of immuno-regulatory cytokines that include IL-4 and IFN-γ.36,37 The uncontrolled activation of iNKT cells, the production of IL-2, IL-4, IL-6, TNF-α and IFN-γ, and the expression of CD178 (Fas ligand) following the administration of α-GalCer result in severe liver damage.14 Similarly, NKT cells play a critical role in concanavalin (Con A)-induced hepatitis dependent upon CD178 expression, and the production of IFN-γ, TNF-α and IL-4.13,38
In sharp contrast to their purported role in the damage induced by α-GalCer or Con A in vivo, experiments reported herein indicate that iNKT cells activated presumably by endogenous factors induced by cholestasis suppressed, rather than promoted, liver injury. iNKT cell-deficient, Jα18−/− mice exhibited significantly greater plasma ALT levels and liver damage than did wild-type animals following bile duct ligation, documenting the role of iNKT cells in suppressing liver injury. The role of iNKT cells in protecting the liver against injury was substantiated directly by adoptive transfer experiments demonstrating a marked reduction in plasma ALT concentrations assessed in Jα18−/− recipient mice administered 5 ×105 NKT cells at 18 hours prior to ligating the common bile duct (Table I).
In further contrast to animals administered α-GalCer or Con A, we found no evidence to demonstrate elevated proinflammatory cytokine production by iNKT cells subsequent to BDL. Rather, IL-2, IL-4, IL-6, TNF-α and IFN-γ production by the hepatic lymphoid population derived from BDL mice was diminished or undetectable relative to the same population derived from non-operated control or α-GalCer-treated mice (flow cytometric analyses, Supplementary Materials Table II, and data not shown). Moreover, while flow cytometric analysis demonstrated an increase in activated, CD25+CD69+ hepatic iNKT cells in mice at 18 hours post-BDL; there was no indication that these cells down-regulated cell-surface NK1.1 or TCR expression in a manner comparable to α-GalCer-activated iNKT cells. Halder et al. reported similar findings in a mouse model of hepatitis, that is, the emergence of an anergic iNKT cell population that suppressed cytokine production and liver injury.39 Furthermore, like the iNKT cells described herein, this anergic iNKT cell population failed to down-regulate the surface expression of NK1.1 and TCR.
Neutrophils play a critical role in pathogenesis of a number of liver injury models, e.g., ischemia-reperfusion,40 endotoxemia,41 Con A-induced hepatitis,42 and bile duct ligation and cholestasis.21 In the latter case, plasma ALT levels and liver necrosis were reduced significantly in mice ligated under conditions in which neutrophil accumulation was diminished, e.g., CD18-deficient mice.21 The experiments reported here document the ability of iNKT cells to suppress neutrophils sequestration in the livers of BDL animals. The marked reduction in plasma ALT levels assessed in wild-type and, particularly, Jα18−/− mice rendered neutrophil-deficient by treatment with anti-mouse neutrophil mAb prior to ligation evidences the contribution of these neutrophils to liver injury. Indeed, liver injury was comparable in wild-type and iNKT cell-deficient mice depleted of neutrophils prior to bile duct ligation. This finding indicates that the ability of iNKT cells to ameliorate cholestatic liver injury is mediated in part by mechanisms that suppress neutrophil sequestration. Although direct cellular interactions between iNKT and neutrophils were not addressed in this study, the iNKT cell-dependent down-regulation of chemokine production appears instrumental. In this regard, it is pertinent to note that hepatocytes serve as a principal source of chemokines synthesized in cholestatic livers and that KC possesses direct, hepatotoxic activity.43,44 Thus, the detrimental effects of KC in the mouse model of cholestatic liver injury presented herein need not depend entirely upon the sequestration of inflammatory neutrophils.
The activated phenotype and preponderance of iNKT cells in the liver relative to lymphoid tissues suggest that hepatic iNKT cells serve a unique function in addition or unrelated to innate host defenses to microbial pathogens proposed by others.26 Rather, we speculate that the primary function of hepatic iNKT cells is to suppress the proinflammatory response of other cell types (e.g., neutrophils) to self-antigens expressed in the liver consequent to the continuous accumulation of toxins, metabolic products, pathogenic microorganisms, debris, etc., and occurring especially during period of exacerbated liver injury. This speculation is supported by experiments demonstrating the rapid expansion of the hepatic iNKT cell population following partial hepatectomy in mice and the purported role of such cells in immune surveillance and hepatocyte regeneration.45 Thus, rather than contribute to the pathogenesis of liver diseases such as viral hepatitis as suggested by others,46 the accumulation of hepatic iNKT cells may represent a beneficial response designed to alleviate tissue damage.
Supplementary Material
Acknowledgments
Supported by National Institutes of Health Research grants DK068097 (to S.H.G.) and AI46709 (to L.B.), Deutsche Forschungsgemeinschaft grant WI2683/1-1 (to P.W.), Deutsche Forschungsgemeinschaft grant GE1193/1-1 (to S.G.), and funds provided by Rhode Island Hospital. Purchase and operation of FACSAria was supported by NCCR SIG grant 1S10RR021051-01A2.
The authors thank Jana M. Hoffmeister and Paul Monfils for their fine technical assistance and Drs. Elizabeth M. Dickson and Johnna D. Wesley for thoughtful discussion.
Abbreviations used
- α-GalCer
α-galactosyl ceramide
- ALT
alanine aminotransferase
- BDL
bile duct ligation
- iGb3
isoglobotrihexosylceramide
- iNKT cell
invariant NKT cell
- Jα18−/−
iNKT cell-deficient
- NPC
nonparenchymal liver cells
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Taniguchi M, Harada M, Kojo S, et al. The regulatory role of Vα14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 2.Fujii S, Shimizu K, Smith C, et al. Activation of natural killer T cells by α-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geissmann F, Cameron TO, Sidobre S, et al. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005;3:e113. doi: 10.1371/journal.pbio.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norris S, Doherty DG, Collins C, et al. Natural T cells in the human liver: cytotoxic lymphocytes with dual T cell and natural killer cell phenotype and function are phenotypically heterogenous and include Vα24-JαQ and γδ T cell receptor bearing cells. Hum Immunol. 1999;60:20–31. doi: 10.1016/s0198-8859(98)00098-6. [DOI] [PubMed] [Google Scholar]
- 5.Hammond KJ, Pellicci DG, Poulton LD, et al. CD1d-restricted NKT cells: an interstrain comparison. J Immunol. 2001;167:1164–1173. doi: 10.4049/jimmunol.167.3.1164. [DOI] [PubMed] [Google Scholar]
- 6.Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 7.Mandal M, Chen XR, Alegre ML, et al. Tissue distribution, regulation and intracellular localization of murine CD1 molecules. Mol Immunol. 1998;35:525–536. doi: 10.1016/s0161-5890(98)00055-8. [DOI] [PubMed] [Google Scholar]
- 8.Zhou D, Mattner J, Cantu C, III, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 9.Speak AO, Salio M, Neville DC, et al. Implications for invariant natural killer T cell ligands due to the restricted presence of isoglobotrihexosylceramide in mammals. Proc Natl Acad Sci U S A. 2007;104:5971–5976. doi: 10.1073/pnas.0607285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seki S, Hashimoto W, Ogasawara K, et al. Antimetastatic effect of NK1+ T cells on experimental haematogenous tumour metastases in the liver and lungs of mice. Immunology. 1997;92:561–566. doi: 10.1046/j.1365-2567.1997.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenna T, Golden-Mason L, Porcelli SA, et al. NKT cells from normal and tumor-bearing human livers are phenotypically and functionally distinct from murine NKT cells. J Immunol. 2003;171:1775–1779. doi: 10.4049/jimmunol.171.4.1775. [DOI] [PubMed] [Google Scholar]
- 12.Lucas M, Gadola S, Meier U, et al. Frequency and phenotype of circulating Vα24/Vβ11 double-positive natural killer T cells during hepatitis C virus infection. J Virol. 2003;77:2251–2257. doi: 10.1128/JVI.77.3.2251-2257.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toyabe S, Seki S, Iiai T, et al. Requirement of IL-4 and liver NK1+ T cells for concanavalin A-induced hepatic injury in mice. J Immunol. 1997;159:1537–1542. [PubMed] [Google Scholar]
- 14.Biburger M, Tiegs G. α-Galactosylceramide-induced liver injury in mice is mediated by TNF-α but independent of Kupffer cells. J Immunol. 2005;175:1540–1550. doi: 10.4049/jimmunol.175.3.1540. [DOI] [PubMed] [Google Scholar]
- 15.Kita H, Naidenko OV, Kronenberg M, et al. Quantitation and phenotypic analysis of natural killer T cells in primary biliary cirrhosis using a human CD1d tetramer. Gastroenterology. 2002;123:1031–1043. doi: 10.1053/gast.2002.36020. [DOI] [PubMed] [Google Scholar]
- 16.Kawano T, Cui J, Koezuka Y, et al. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 17.Gehring S, Dickson EM, San Martin ME, et al. Kupffer cells abrogate cholestatic liver injury in mice. Gastroenterology. 2006;130:810–822. doi: 10.1053/j.gastro.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Daley JM, Thomay AA, Connolly MD, et al. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 19.Wesley JD, Robbins SH, Sidobre S, et al. Cutting edge: IFN-γ signaling to macrophages is required for optimal Vα14i NK T/NK cell cross-talk. J Immunol. 2005;174:3864–3868. doi: 10.4049/jimmunol.174.7.3864. [DOI] [PubMed] [Google Scholar]
- 20.Gregory SH, Cousens LP, van Rooijen N, et al. Complemetary adhesion molecules promote neutrophil-Kupffer cell interaction and the elimination of bacteria taken up by the liver. J Immunol. 2002;168:308–315. doi: 10.4049/jimmunol.168.1.308. [DOI] [PubMed] [Google Scholar]
- 21.Gujral JS, Farhood A, Bajt ML, et al. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology. 2003;38:355–363. doi: 10.1053/jhep.2003.50341. [DOI] [PubMed] [Google Scholar]
- 22.Saito JM, Maher JJ. Bile duct ligation in rats induces biliary expression of cytokine-induced neutrophil chemoattractant. Gastroenterology. 2000;118:1157–1168. doi: 10.1016/s0016-5085(00)70369-6. [DOI] [PubMed] [Google Scholar]
- 23.Frink M, Thobe BM, Hsieh YC, et al. 17beta-Estradiol inhibits keratinocyte-derived chemokine production following trauma-hemorrhage. Am J Physiol Lung Cell Mol Physiol. 2007;292:L585–L591. doi: 10.1152/ajplung.00364.2006. [DOI] [PubMed] [Google Scholar]
- 24.Park SH, Benlagha K, Lee D, et al. Unaltered phenotype, tissue distribution and function of Vα14+ NKT cells in germ-free mice. Eur J Immunol. 2000;30:620–625. doi: 10.1002/1521-4141(200002)30:2<620::AID-IMMU620>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 25.Bendelac A, Lantz O, Quimby ME, et al. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268:863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 26.Kinjo Y, Kronenberg M. Vα14i NKT cells are innate lymphocytes that participate in the immune response to diverse microbes. J Clin Immunol. 2005;25:522–533. doi: 10.1007/s10875-005-8064-5. [DOI] [PubMed] [Google Scholar]
- 27.Dieli F, Taniguchi M, Kronenberg M, et al. An anti-inflammatory role for Vα 14 NK T cells in Mycobacterium bovis bacillus Calmette-Guerin-infected mice. J Immunol. 2003;171:1961–1968. doi: 10.4049/jimmunol.171.4.1961. [DOI] [PubMed] [Google Scholar]
- 28.Szalay G, Ladel CH, Blum C, et al. Cutting edge: anti-CD1 monoclonal antibody treatment reverses the production patterns of TGF-β2 and Th1 cytokines and ameliorates listeriosis in mice. J Immunol. 1999;162:6955–6958. [PubMed] [Google Scholar]
- 29.Ishigami M, Nishimura H, Naiki Y, et al. The roles of intrahepatic Vα14+ NK1.1+ T cells for liver injury induced by Salmonella infection in mice. Hepatology. 1999;29:1799–1808. doi: 10.1002/hep.510290605. [DOI] [PubMed] [Google Scholar]
- 30.Hiromatsu T, Matsuguchi T, Shimizu H, et al. NK T cells stimulated with a ligand for TLR2 at least partly contribute to liver injury caused by Escherichia coli infection in mice. Eur J Immunol. 2003;33:2511–2519. doi: 10.1002/eji.200324077. [DOI] [PubMed] [Google Scholar]
- 31.Kinjo Y, Tupin E, Wu D, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 32.Kinjo Y, Wu D, Kim G, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 33.Brigl M, Bry L, Kent SC, et al. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 34.Nagarajan NA, Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J Immunol. 2007;178:2706–2713. doi: 10.4049/jimmunol.178.5.2706. [DOI] [PubMed] [Google Scholar]
- 35.Wilson MT, Johansson C, Olivares-Villagomez D, et al. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proc Natl Acad Sci U S A. 2003;100:10913–10918. doi: 10.1073/pnas.1833166100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crowe NY, Uldrich AP, Kyparissoudis K, et al. Glycolipid antigen drives rapid expansion and sustained cytokine production by NK T cells. J Immunol. 2003;171:4020–4027. doi: 10.4049/jimmunol.171.8.4020. [DOI] [PubMed] [Google Scholar]
- 37.Stetson DB, Mohrs M, Reinhardt RL, et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198:1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaneko Y, Harada M, Kawano T, et al. Augmentation of Vα14 NKT cell-mediated cytotoxicity by interleukin 4 in an autocrine mechanism resulting in the development of concanavalin A-induced hepatitis. J Exp Med. 2000;191:105–114. doi: 10.1084/jem.191.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halder RC, Aguilera C, Maricic I, et al. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. J Clin Invest. 2007;117:2302–2312. doi: 10.1172/JCI31602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaeschke H. Mechanisms of Liver Injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1083–G1088. doi: 10.1152/ajpgi.00568.2005. [DOI] [PubMed] [Google Scholar]
- 41.Dorman RB, Gujral JS, Bajt ML, et al. Generation and functional significance of CXC chemokines for neutrophil-induced liver injury during endotoxemia. Am J Physiol Gastrointest Liver Physiol. 2005;288:G880–G886. doi: 10.1152/ajpgi.00317.2004. [DOI] [PubMed] [Google Scholar]
- 42.Bonder CS, Ajuebor MN, Zbytnuik LD, et al. Essential role for neutrophil recruitment to the liver in concanavalin A-induced hepatitis. J Immunol. 2004;172:45–53. doi: 10.4049/jimmunol.172.1.45. [DOI] [PubMed] [Google Scholar]
- 43.Kim ND, Moon JO, Slitt AL, et al. Early growth response factor-1 is critical for cholestatic liver injury. Toxicol Sci. 2006;90:586–595. doi: 10.1093/toxsci/kfj111. [DOI] [PubMed] [Google Scholar]
- 44.Stefanovic L, Brenner DA, Stefanovic B. Direct hepatotoxic effect of KC chemokine in the liver without infiltration of neutrophils. Exp Biol Med (Maywood ) 2005;230:573–586. doi: 10.1177/153537020523000809. [DOI] [PubMed] [Google Scholar]
- 45.Minagawa M, Oya H, Yamamoto S, et al. Intensive expansion of natural killer T cells in the early phase of hepatocyte regeneration after partial hepatectomy in mice and its association with sympathetic nerve activation. Hepatology. 2000;31:907–915. doi: 10.1053/he.2000.5850. [DOI] [PubMed] [Google Scholar]
- 46.de Lalla C, Galli G, Aldrighetti L, et al. Production of profibrotic cytokines by invariant NKT cells characterizes cirrhosis progression in chronic viral hepatitis. J Immunol. 2004;173:1417–1425. doi: 10.4049/jimmunol.173.2.1417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.