Abstract
While the importance of cellular and viral kinases in HCMV replication has been demonstrated, relatively little is known about the activity of cellular phosphatases. We conducted a series of experiments designed to investigate the effect of HCMV infection on cellular serine/threonine phosphatase activity. We found that the abundance of two major cellular serine/threonine phosphatases, PP1 and PP2A, increases during HCMV infection. This was associated with an increase in threonine phosphatase activity in HCMV-infected cells. HCMV infection conferred resistance to the effects of the phosphatase inhibitors calyculin A (CA) and okadaic acid with regards to global protein hyperphosphorylation and the shutoff of protein synthesis. The protective effect of HCMV infection could be overcome at a high concentration of CA, suggesting that cellular phosphatase activity is required for critical cellular processes during HCMV infection. Specifically, phosphatase activity was required to limit the accumulation of phospho-eIF2α, but not phospho-PKR, during HCMV infection.
Keywords: cytomegalovirus, phosphatase
Introduction
One process essential to normal cellular homeostasis is the reversible phosphorylation of proteins, a significant proportion of which occurs on serine and threonine residues (Shenolikar, 1994). It has been estimated that the human genome encodes approximately 300 serine/threonine kinases yet only roughly 40 serine/threonine phosphatases (Cohen, 2002). Much of cellular serine/threonine phosphatase activity is provided by protein phosphatase 1 (PP1) isoforms α, δ (or β), γ1, and γ2 and protein phosphatases 2A (PP2A), 2B (calcineurin), and 2C (Bollen, 2001; Cohen, 2002; Gjertsen and Doskeland, 1995; Janssens and Goris, 2001). Since the number of serine/threonine kinases greatly exceeds the number of serine/threonine phosphatases, each phosphatase is responsible for the dephosphorylation of a relatively large number of substrates; therefore, altering the activity of one of these phosphatases is likely to have a dramatic impact on cellular processes. The functional diversity and specificity of the catalytic subunit of each phosphatase is conferred by its ability to interact with a large number of regulatory subunits under varying cellular conditions, which then promote the interaction with a target substrate (Cohen, 2002; Janssens and Goris, 2001).
The phosphorylation of cellular and viral proteins by both cellular and viral kinases contributes to HCMV replication by regulating a wide range of cellular functions, including transcription, cell-cycle control, and translation (Baek et al., 2002; Baek et al., 2004; Bresnahan et al., 1997; Fortunato et al., 2000; Harel and Alwine, 1998; Hertel, Chou, and Mocarski, 2007; Hume et al., 2008; Jault et al., 1995; Prichard et al., 1999; Sanchez et al., 2004). However, little is known about the role(s) played by cellular phosphatases during HCMV infection. The use of serine/threonine phosphatase inhibitors has been shown to affect the sumoylation of the immediate early (IE) 72 protein, the localization of glycoprotein B (gB), and the activity of the US3 region IE enhancer/promoter (Chan, Tseng, and Hayward, 1996; Fish, Soderberg-Naucler, and Nelson, 1998; Spengler et al., 2002). More directly, purified HCMV virions have been found to possess serine/threonine phosphatase activity, resulting in cellular hypophosphorylation within 5 minutes of contact of HCMV with the host cell (Michelson et al., 1996). It was subsequently determined that PP1α and the catalytic subunit of PP2A PP2AC), along with its regulatory subunits PR65 and PR55, are associated with the HCMV virion and are delivered to the host cell upon infection (Michelson et al., 1996; Varnum et al., 2004). Additionally, microarray analysis demonstrated an increase in messenger RNA levels of several phosphatases, including PP1α, at late times during HCMV infection (Hertel and Mocarski, 2004). However, analyses of the abundance and activity of serine/threonine phosphatases over the course of HCMV infection have not been reported.
We conducted a series of experiments designed to further investigate the effect of HCMV infection on the activity of cellular serine/threonine phosphatases. We found detectable increases in both PP1 and PP2AC beginning as early as 1 hour post-infection and continuing throughout the course of infection. This increase in abundance was associated with an increase in phosphatase activity in HCMV-infected cells. HCMV-infected HFs were relatively resistant to global protein hyperphosphorylation and the shutoff of protein synthesis induced by the serine/threonine phosphatase inhibitors calyculin A (CA) and okadaic acid (OA). We then focused specifically on the regulation of the phosphorylation state of the PP1α substrates protein kinase R (PKR) and the alpha subunit of the eukaryotic translation initiation factor 2 (eIF2α) during HCMV infection. HCMV infection protected both PKR and eIF2α from CA-induced hyperphosphorylation, although the mechanisms by which this occurs may differ for the two proteins.
Results
HCMV infection up-regulates serine/threonine phosphatase protein levels and activity
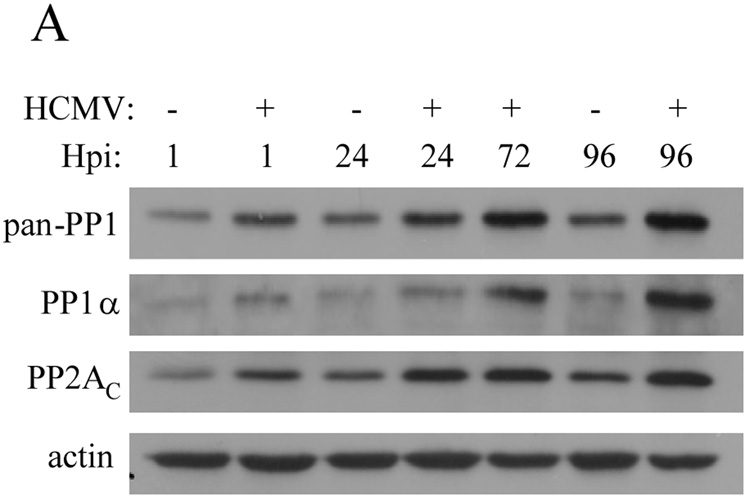
Despite data indicating their delivery to the host cell by the HCMV virion and the results of microarray analyses (Hertel and Mocarski, 2004; Michelson et al., 1996; Varnum et al., 2004), an examination of PP1α and PP2AC at the protein level over the course of HCMV has not been reported. To this end, HFs were mock-infected or infected with HCMV (rTowne-1 strain) and protein was harvested at several timepoints post-infection. Using an antibody that reacts with isoforms α, δ, and γ of the catalytic subunit of PP1 (“pan-PP1”), immunoblot analysis demonstrated that PP1 levels were slightly but noticeably increased as early as 1 hpi and continued to increase compared to mock-infected cells over the course of HCMV infection (Figure 1). When an antibody specific for PP1α was used, a similar pattern of increasing levels during HCMV infection was seen. By comparison, the level of PP2AC was also slightly increased compared to mock-infected HFs beginning at 1 hpi, increased over the first 24 hours of infection, then remained fairly constant through 96 hpi. Thus, detectable increases in both PP1α and PP2AC were observed very early after infection and both increased during infection, albeit with differing patterns of accumulation at later times.
Figure 1.
Analysis of PP1 and PP2AC levels during HCMV infection. Human fibroblasts (HFs) were mock-infected or infected with HCMV and equivalent amounts of protein lysates were analyzed by immunoblot using a “pan”-PP1 antibody that detects all known isoforms of the catalytic subunit of PP1, and antibodies specific for the alpha (α) isoform of the catalytic subunit of PP1, the catalytic subunit of PP2A (PP2AC), and actin.
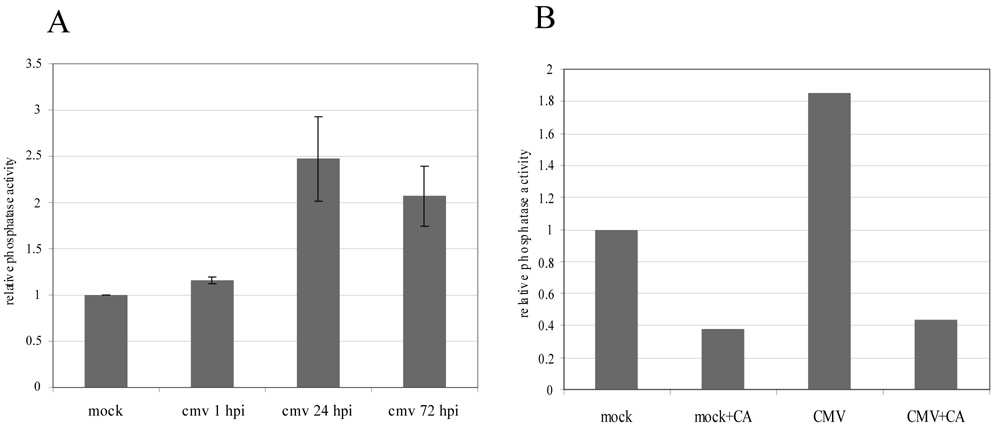
In order to determine whether the increase in PP1 and PP2AC protein levels observed over the course of HCMV infection was accompanied by an increase in cellular phosphatase activity, an in vitro phosphatase activity assay was performed using the phosphopeptide KRpTIRR as a substrate (Guan et al., 2007; Latreille and Larose, 2006) and lysates collected from mock- and HCMV-infected HFs at 1, 24, and 72 hpi. Compared to mock-infected cells, overall phosphatase activity increased slightly at 1 hpi, reached an approximately 2–3 fold induction by 24 hours, and remained elevated at 72 hpi (Figure 2A). Thus, over the course of HCMV infection, cellular threonine phosphatase activity increases along with PP1 and PP2AC protein levels.
Figure 2.
Assessment of phosphatase activity during HCMV infection. (A) HFs were mock-infected or infected with HCMV and at 1, 24, and 72 hpi cell lysates were prepared and equivalent amounts of protein were incubated with the phosphopeptide KRpTIRR for one hour at room temperature. Free phosphate was measured using Malachite Green Phosphate Detection Solution (US Biological) as described in Materials and Methods. Background activity was determined by incubating the phosphopeptide in lysis buffer alone and was subtracted from the values obtained from the mock- and HCMV-infected samples. The results are expressed as fold change compared to mock-infected HFs and represent the mean and standard deviation of one set of lysates tested independently in duplicate. The entire experiment was repeated once and yielded similar results. (B) Phosphatase activity in lysates from mock-infected or HCMV-infected HFs at 72 hpi was measured after one hour of mock-treatment or treatment with [1 µM] calyculin A (CA). Results are expressed as fold change compared to mock-infected, mock-treated HFs after subtraction of background phosphatase activity and are representative of three independent experiments.
As a control for the assay, mock- and HCMV-infected cells (72 hpi) were mock-treated or treated with the serine/threonine phosphatase inhibitor calyculin A (CA) ([1 µM]), a broad and fast-acting serine/threonine phosphatase inhibitor (PP1 [IC50], 0.5 to 10 nM; PP2AC [IC50], 0.1 to 1nM (Brush, Weiser, and Shenolikar, 2003; Favre, Turowski, and Hemmings, 1997; Ishihara et al., 1989)), for one hour prior to protein harvest. Consistent with the results above, lysates from mock-treated, HCMV-infected cells at this timepoint demonstrated an almost two-fold increase in phosphatase activity compared to mock-treated, mock-infected cells (Figure 2B). CA treatment inhibited phosphatase activity in both samples (Figure 2B), thereby confirming the specificity of the assay in measuring phosphatase activity.
HCMV-infected HFs are resistant to the phosphatase inhibitors CA and okadaic acid
In order to investigate what functional consequences the increase in cellular phosphatase levels and activity had during HCMV infection, we assessed whether HCMV infection resulted in resistance to the effects of CA and okadaic acid (OA). Previous reports have demonstrated that in several cell lines, 30 minutes of CA treatment at concentrations of 0.1 µM and 1 µM resulted in cell rounding and detachment from the tissue culture wells, although whether these changes represent apoptosis or necrosis is unknown (Fladmark et al., 1999; Gjertsen et al., 1994). We observed a similar effect of CA in mock-infected HFs by phase contrast microscopy, while HCMV-infected HFs at 72 hpi retained typical viral CPE at [0.1 µM] but not [1 µM] CA (Supplemental Figure 1).
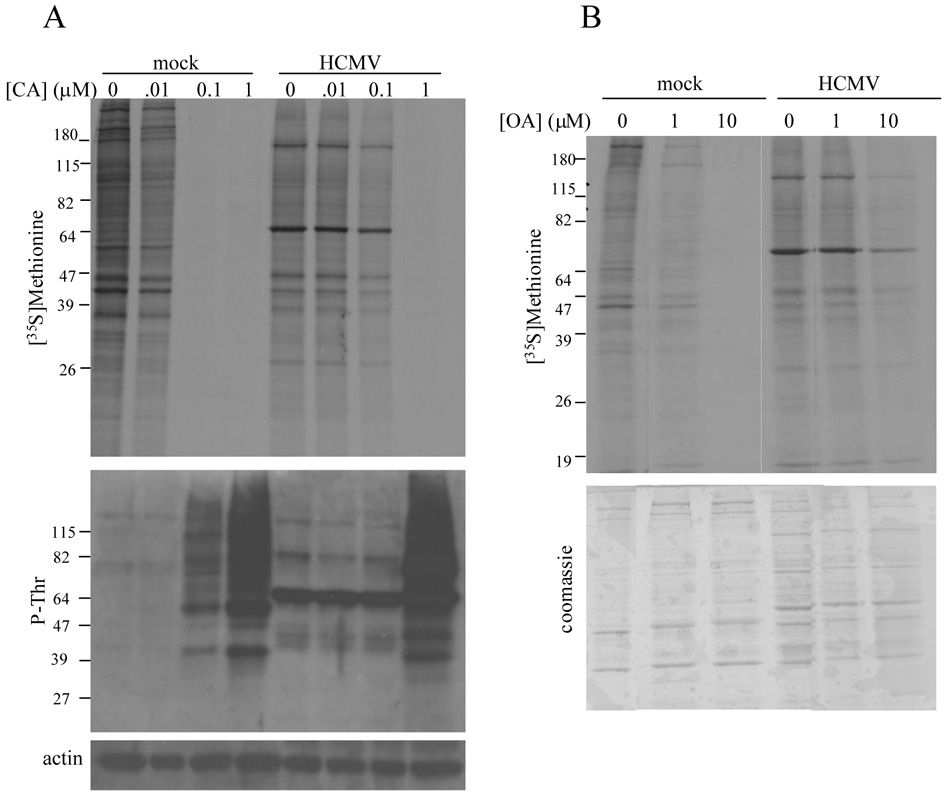
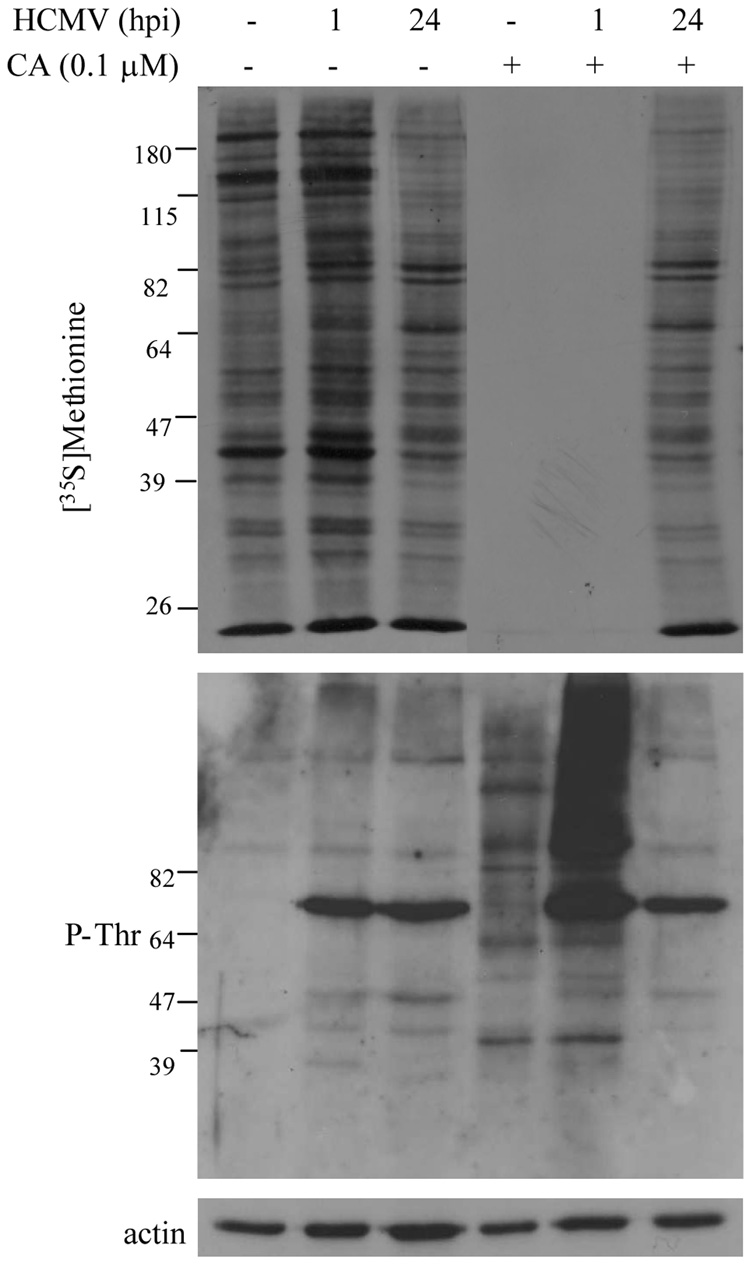
In order to determine whether these CA-induced morphological changes were reflected by changes in cellular protein synthetic activity and protein phosphorylation, and the impact of HCMV infection on the effects of CA, mock-infected or HCMV-infected (72 hpi) HFs were treated for 30 minutes with increasing concentrations of CA, ranging from 0.01µM to 1 µM, followed by [35S]methionine labeling for 30 minutes. Protein synthesis was assessed by SDS-PAGE and autoradiography while global threonine phosphorylation was determined by immunoblot analysis using a phospho-threonine (P-Thr) specific antibody. Consistent with the changes observed by microscopy, [0.1µM] and [1 µM] CA induced the shut-off of protein synthesis and increased cellular threonine phosphorylation in mock-infected cells (Figure 3A). HCMV-infected HFs appeared essentially unaffected by [0.1 µM] CA, but [1 µM] overcame the protective effect of HCMV infection as protein synthesis was shut off and protein hyperphosphorylation was observed (Figure 3A). A prominent immunoreactive band at ~ 64 kD was consistently observed in lysates from HCMV-infected HFs when using the P-Thr antibody and may represent the 65 kD HCMV UL83 gene product (pp65), which is the major tegument protein and is phosphorylated during CMV replication (Pande et al., 1990; Roby and Gibson, 1986).
Figure 3.
Protein synthetic activity and protein phosphorylation in HCMV-infected cells treated with CA and okadaic acid (OA). (A) Mock-infected or HCMV-infected HFs (72 hpi) were treated with increasing concentrations of CA for 30 minutes followed by [35S]methionine ([100 µCi/ml] labeling for 30 minutes. De novo protein synthesis was assessed by SDS-PAGE and autoradiography (top panel) and the pattern of protein phosphorylation was determined by immunoblot analysis using a phospho-threonine (P-Thr) specific antibody (middle panel). Equivalent protein loading was confirmed by immunoblot analysis for actin (bottom panel). (B) Mock-infected HFs or HCMV-infected HFs were treated with the indicated concentration of OA for two hours beginning at 72 hpi. De novo protein synthesis (top panel) was assessed as described above after Coomassie G-250 staining (bottom panel) to document equivalent protein loading.
To determine whether the protective effect of HCMV infection against phosphatase inhibition was unique to CA, we performed similar experiments using OA. Although both CA and OA are serine/threonine phosphatase inhibitors, they are structurally unrelated and display different biological properties (Shenolikar, 1994). OA is slower-acting than CA but, like CA, is a more potent inhibitor of PP2AC ([IC50] 0.1-1nM) than PP1 ([IC50], 0.05 µM to 1 µM) (Favre, Turowski, and Hemmings, 1997; Gupta et al., 1997; Ishihara et al., 1989), and, like CA, may inhibit other serine/threonine protein phosphatases such as PP4, 5, and 7 at the concentrations used in these experiments (Swingle, Ni, and Honkanen, 2007). Mock-infected or HCMV-infected HFs were treated with [1 µM] or [10 µM] OA for two hours beginning at 72 hpi, followed by [35S]methionine labeling for one hour. As shown in Figure 3B, protein synthesis was markedly impaired in mock-infected cells treated with [1 µM] and [10 µM]. HCMV-infected cells remained resistant to the effects of OA treatment on protein synthesis at [1.0 µM], but protein synthesis was impaired by [10 µM] OA.
Taken together, the results of the above experiments indicate that HCMV infection confers partial resistance to the effects of the phosphatase inhibitors CA and OA on protein synthetic activity and global protein hyperphosphorylation, suggesting that the increase in cellular phosphatase levels and activity observed during HCMV infection has functional significance. Additionally, the finding that the protective effect of HCMV could be overcome at higher concentrations of CA and OA indicate that cellular serine/threonine phosphatase activity is indeed required for critical cellular processes during HCMV infection.
Viral functions required to inhibit the effects of CA and OA
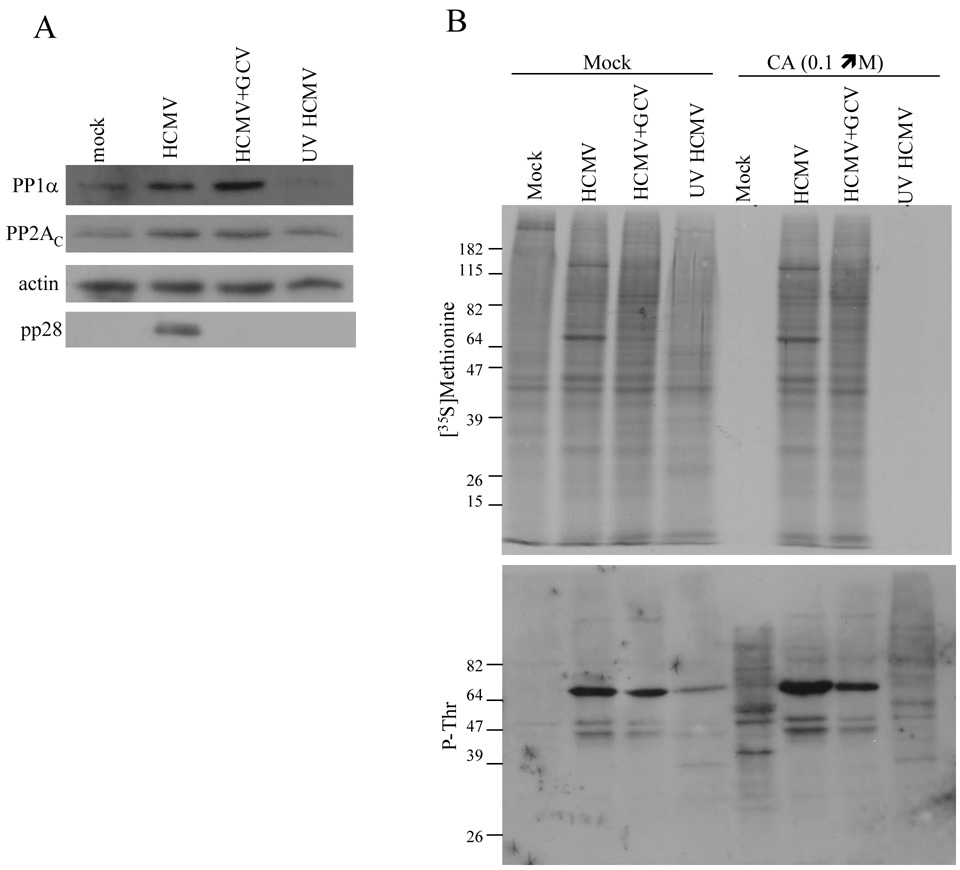
We next sought to determine whether de novo viral gene expression was required to up-regulate cellular phosphatase levels and confer resistance to CA and OA. HFs were mock-infected or infected with HCMV, with UV-inactivated HCMV, or with HCMV in the presence of ganciclovir, an inhibitor of viral replication that results in reduced late gene expression (Chambers et al., 1999). At 72 hpi, the cells were mock-treated or treated with [0.1 µM] CA followed by [35S]methionine labeling. Lysates were harvested and equivalent amounts of protein from the mock-treated samples were subjected to immunoblot analysis for PP1α and PP2AC. An increase in PP1α and PP2AC was observed during HCMV infection, even in the presence of ganciclovir, but not with UV-inactivated HCMV (Figure 4A). Additional immunoblot analyses were performed for actin to control for protein loading and for the late HCMV gene product pp28 (Mocarski, Shenk, and Pass, 2007) to confirm the inhibitory effect of ganciclovir on late gene expression. Consistent with this finding, cells infected with HCMV in the presence of ganciclovir, but not UV-inactivated HCMV, conferred resistance to the CA-induced shutoff of protein synthesis and protein hyperphosphorylation (Figure 4B). Thus, it appears that the expression of immediate-early and/or early HCMV gene products, but not late gene expression, is necessary and sufficient at late times during HCMV infection for the up-regulation of protein phosphatase levels and resistance to the effects of CA.
Figure 4.
Immediate-early and/or early viral gene expression is necessary and sufficient to upregulate cellular phosphatase levels and to inhibit the effects of CA at late times during HCMV infection. (A) Immunoblot analysis of PP1α was performed on lysates obtained at 72 hpi from mock-infected HFs, HCMV-infected HFs, HFs infected with HCMV in the presence of [45 µM] ganciclovir (GCV), or HFs infected with UV-inactivated HCMV. The membrane was stripped followed by immunoblot analysis of PP2AC. Equivalent protein loading was confirmed by immunoblot analysis of actin and the inhibitory effect of ganciclovir on HCMV late gene expression was confirmed by immunoblot analysis of pp28. (B) HFs were mock-infected or infected with HCMV in the presence and absence of GCV or with UV-inactivated HCMV. At 72 hpi, cells were mock-treated or treated with [0.1 µM] CA for 20 minutes, followed by [35S]methionine labeling for 30 minutes. De novo protein synthesis was assessed by SDS-PAGE and autoradiography (top panel) and the pattern of protein phosphorylation was determined by phospho-threonine (P-Thr)-specific immunoblot analysis (bottom panel).
With previous reports having demonstrated that functionally active PP2AC and PP1α are delivered to the cell upon HCMV infection (Michelson et al., 1996; Varnum et al., 2004), we sought to determine whether virion-associated cellular phosphatases contribute to the resistance to CA conferred by HCMV infection. Even though UV-inactivated HCMV failed in this regard at late times during infection (Figure 4B), it is possible that virus-associated phosphatases contribute to resistance at earlier times during infection. To examine this possibility, [0.1 µM] CA was added for 30 minutes at 1 hpi or 24 hpi followed by [35S]methionine labeling for 30 minutes. As shown in Figure 5, protein synthesis was shut off and global protein hyperphosphorylation occurred when CA was added to mock-infected HFs or HCMV-infected HFs at 1 hpi, whereas CA added at 24 hpi had little effect on HCMV-infected HFs. Thus, protection from the effects of CA requires more than the delivery of virus-associated phosphatases and appears to correlate temporally with the de novo expression of PP1 and PP2AC and the increase in cellular phosphatase activity observed by 24 hours (Figure 1 and Figure 2).
Figure 5.
Virion-associated phosphatases are not sufficient to inhibit the effects of calyculin A. Mock-infected HFs or HCMV-infected HFs were treated with [0.1 µM] CA for 30 minutes at the indicated time post-infection. Protein synthesis (top panel), protein phosphorylation (middle panel), and protein loading (bottom panel) were determined as described in figure 3 and figure 4.
Phosphatase activity is required to regulate the accumulation of phosphorylated eIF2α, but not PKR, during HCMV infection
From our observations that HCMV infection partially inhibits global protein hyperphosphorylation and the shutoff of protein synthesis induced by CA, we next sought to determine whether phosphatase activity is required to control the phosphorylation state of specific cellular proteins during HCMV infection, focusing on the regulators of translation protein kinase R (PKR) and the eukaryotic translation initiation factor 2 (eIF2). After binding to double-stranded RNA (dsRNA) that arises during viral infection, PKR undergoes autophosphorylation on several threonine residues, converting it to an active serine/threonine kinase (Dever, Dar, and Sicheri, 2007). Activated PKR then phosphorylates eIF2 on serine 51 of its alpha (α) subunit, resulting in the inhibition of translation initiation and consequently the diminution of de novo protein synthesis (Dever, Dar, and Sicheri, 2007). The phosphorylation state of both proteins in vivo is regulated in part by their PP1α-mediated dephosphorylation (Brush, Weiser, and Shenolikar, 2003; Connor et al., 2001; Jousse et al., 2003; Novoa et al., 2001; Tan et al., 2002) and in fact the inhibition of phosphatase activity by OA has been found to induce the PKR-dependent accumulation of phospho-eIF2α and shutoff of protein synthesis (Morimoto et al., 2004; Morimoto et al., 2005). During HCMV infection, the initial activation of PKR is inhibited by the TRS1 and IRS1 gene products (Child et al., 2004; Child et al., 2002; Hakki et al., 2006). Phospho-eIF2α, however, accumulates at late times during HCMV infection, possibly due to the activation of other eIF2α kinases (Dever, Dar, and Sicheri, 2007; Isler, Skalet, and Alwine, 2005), although global protein synthesis during HCMV infection remains robust (Isler, Skalet, and Alwine, 2005).
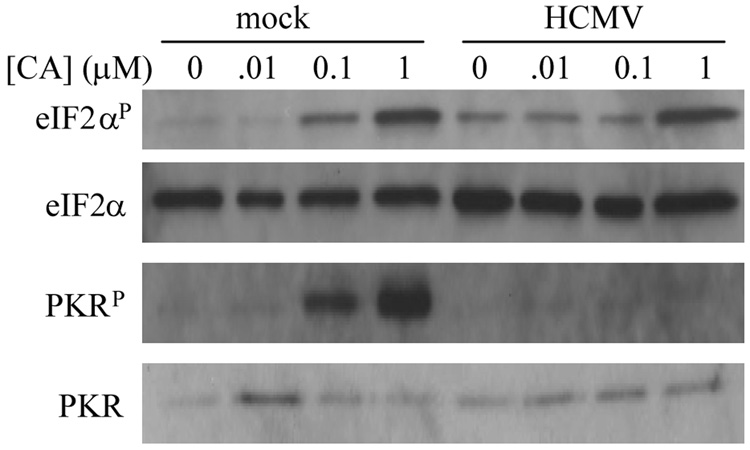
Taking these previous findings into account, we sought to determine what role, if any, phosphatases play in regulating the accumulation of phosphorylated PKR and eIF2α during HCMV infection. In mock-infected HFs, CA treatment at [0.1 µM] and [1 µM] for 30 minutes resulted in the accumulation of phospho-eIF2α and phospho-PKR over basal levels (Figure 6). During HCMV infection, increased phospho-eIF2α was observed by 72 hpi compared to mock-infected HFs in the absence of CA treatment, consistent with previously published work (Isler, Skalet, and Alwine, 2005). However, CA treatment at [0.1 µM] did not cause an increase in phospho-eIF2α relative to no CA or [0.01 µM], whereas at [1 µM] this protective effect was overcome and a marked increase in phospho-eIF2α was observed. Interestingly, HCMV infection inhibited phospho-PKR accumulation at all concentrations of CA, suggesting that the increase in eIF2α phosphorylation observed at [1 µM] CA was not due to activation of PKR. Consistent with this, CA caused the accumulation of phospho-eIF2α independently of PKR and two other major eIF2α kinases, the PKR-like endoplasmic reticulum kinase (PERK) and the general control non-derepressable kinase 2 (GCN2) (Supplemental Figure 2), indicative of its primary mechanism of action being the inhibition of a constitutively active eIF2α phosphatase (Jousse et al., 2003). Taken together, these results indicate that phosphatase activity contributes to, and is indeed required for, regulating the accumulation of phospho-eIF2α, but not phospho-PKR, during HCMV infection.
Figure 6.
Phosphatase activity is required to regulate the accumulation of phospho-eIF2α, but not phospho-PKR, during HCMV infection. Mock-infected or HCMV-infected (72 hpi) HFs were treated with CA at the indicated concentrations for 30 minutes. Cell lysates were subjected to immunoblot analysis for total and phospho-eIF2α and total and phospho-PKR.
Discussion
The phosphorylation state of proteins is determined by the opposing activities of kinases and phosphatases. The importance of cellular and viral kinases in HCMV replication has been demonstrated in many studies (Baek et al., 2004; Bresnahan et al., 1997; Harel and Alwine, 1998; Hertel, Chou, and Mocarski, 2007; Hume et al., 2008; Prichard et al., 1999; Rodems and Spector, 1998; Sanchez et al., 2004). In contrast, little is known about the activity of cellular phosphatases. Two major cellular serine/threonine phosphatases, PP1α and PP2AC, have been found packaged in the HCMV virion (Michelson et al., 1996; Varnum et al., 2004) and the delivery of these phosphatases into the cell upon viral entry results in global protein hypophosphorylation (Michelson et al., 1996). The experiments described in this report were designed to further investigate the regulation of cellular phosphatase levels and activity over the course HCMV infection.
Focusing on PP1α and PP2AC given their known association with HCMV (Michelson et al., 1996), we demonstrated that PP1α, and PP2AC protein levels increased beginning soon after infection, consistent with the delivery of both phosphatases to the host cell by HCMV virions (Michelson et al., 1996; Varnum et al., 2004), although it is also possible that HCMV binding and entry affect the levels of these proteins by alternate mechanisms, such as rapid transcriptional induction. The further increase in PP1α and PP2AC during infection is likely due to regulation at both the transcriptional and post-transcriptional levels (Baharians and Schonthal, 1998; Hertel and Mocarski, 2004; Nakamura et al., 1992) and is dependent upon HCMV immediate early and/or early gene expression but not late gene expression (Figure 4A). HCMV infection may therefore represent a somewhat unusual situation in which alterations in the expression of phosphatase catalytic subunits play an important role in the regulation of their activity. With few exceptions (Nakamura et al., 1992; Nishikawa et al., 1994; Tawara et al., 1993; Wilson et al., 1999), phosphatase catalytic subunit activity is generally determined by their post-translational modification, changes in subcellular localization, and interactions with regulatory subunits, as opposed to alterations in their expression (Brush, Weiser, and Shenolikar, 2003; Bryant, Westphal, and Wadzinski, 1999; Chen, Martin, and Brautigan, 1992; Connor et al., 2001; Damuni, Xiong, and Li, 1994; Favre, Turowski, and Hemmings, 1997; Favre et al., 1994; Flores-Delgado et al., 2007; Helps et al., 2000; Longin et al., 2007; Longin et al., 2008; Tolstykh et al., 2000; Turowski et al., 1995). Therefore, multiple mechanisms may contribute to the regulation of phosphatase activity during HCMV infection.
The increase in the levels of PP1 and PP2AC were indeed associated with an overall increase in threonine phosphatase activity during HCMV infection. Although we cannot state what percentage of the increase in threonine phosphatase activity observed during HCMV infection is attributable to PP1α and PP2AC versus other serine/threonine phosphatases that may also be up-regulated (Hertel and Mocarski, 2004), it is likely, given their relative abundance, that PP1α and PP2AC at least contribute to this effect. While the absolute increase was modest (2–3 fold), the potential opposing effects of serine/threonine kinases that are activated during HCMV infection must be also considered when interpreting the result of an in vitro phosphatase assay using a non-specific phospho-threonine substrate. Additionally, the finding that HCMV-infected HFs were relatively resistant to the effects of the serine/threonine phosphatase inhibitors calyculin A (CA) and okadaic acid (OA) on protein synthesis, and global protein phosphorylation suggests that the increase in phosphatase activity is biologically significant.
The precise pathway(s) regulated by HCMV in order to inhibit the effects of CA and OA are unknown and are complicated by the fact that CA and OA are likely to inhibit other cellular serine/threonine phosphatases in addition to PP1 and PP2AC at the concentrations used in these experiments. Indeed, the shutoff of protein synthesis induced by CA, while associated with eIF2α phosphorylation, is not dependent upon this event, nor is it affected by the pan-caspase inhibitor zVAD.fmk (Supplemental Figure 3), suggesting that the dysregulation of numerous cellular functions contributes to the effects of CA. Regardless, these effects are governed by the actions of these inhibitors on serine/threonine phosphatases and therefore still provide insight into the role of phosphatase activity during HCMV infection. While our finding that HCMV binding and entry alone do not impair the activity of CA (Figure 4B and Figure 5), and although CA and OA possess different structural and functional properties (Shenolikar, 1994), it is nevertheless possible that the protective effect of HCMV infection is due in part to a nonspecific action on the drugs themselves, such impaired entry into the HCMV-infected cell. Additionally, alterations in the levels, localization, and interactions of serine/threonine kinase and phosphatase substrates during HCMV infection may modulate the effects of phosphatase inhibitors. Despite these possibilities, it is likely that the up-regulation of phosphatase levels and activity during HCMV infection contributes to the overall phenotype of CA and OA resistance.
While the increase in phosphatase activity may serve as a general means of compensating for the increased cellular and viral kinase activity that accompanies HCMV replication, it is also likely that HCMV infection affects the phosphatase activity of PP1 and PP2 family members towards individual targets. For example, the increase in phosphatase activity may reflect and promote changes in cell-cycle regulation that occur during HCMV infection. Specifically, HCMV appears to up-regulate the activity of the cdc2-cyclin B phosphatase cdc25C, which functions in controlling the transition to mitosis (Hertel and Mocarski, 2004; Takizawa and Morgan, 2000) and cdc25C is itself activated by a dephosphorylation event mediated by PP1 (Margolis and Kornbluth, 2004; Margolis et al., 2006; Margolis et al., 2003). Additionally, PP1 activity can also regulate alternative splicing events and modulate the function of the NIMA-related kinase 2 (Nek2), which may affect centrosome function during HCMV infection (Helps et al., 2000; Hertel and Mocarski, 2004; Stamm, 2008). Serine/threonine phosphatase activity may also affect viral fusion with cell membranes, transcriptional activity, and protein trafficking during HCMV infection (Barrasa, Harel, and Alwine, 2005; Chan, Tseng, and Hayward, 1996; Fish, Soderberg-Naucler, and Nelson, 1998; Harel and Alwine, 1998; Jarvis et al., 2004; Keay and Baldwin, 1996). Thus, determining how HCMV affects the activity of these phosphatases with regards to specific substrates will be important steps towards understanding the regulation of a diverse array of cellular processes during HCMV replication.
Towards this end, we evaluated the effect of phosphatase inhibition on the PKR-eIF2α pathway during HCMV infection. The dose-dependent effects of CA on these PP1α substrates provide insight into their regulation by HCMV. HCMV blocked the hyperphosphorylation of PKR at [1 µM] CA, a concentration at which HCMV was unable to prevent global protein hyperphosphorylation and the hyperphosphorylation of eIF2α (Figure 3 and Figure 6). Thus, it appears that HCMV exerts greater control over PKR phosphorylation, and consequently activation, than it does over other proteins. Given the importance of PKR to the cellular antiviral response, this is not entirely surprising. Since previous work from our laboratory provided evidence that the HCMV gene products TRS1 and IRS1 inhibit the initial dsRNA-dependent activation of PKR (Child et al., 2004; Hakki et al., 2006), and since we were not able to induce PKR phosphorylation at all with CA during HCMV infection, phosphatase activity does not appear to be required for the regulation of phospho-PKR accumulation during HCMV infection.
The hyperphosphorylation of eIF2α, as opposed to the case with PKR, could be induced by a higher concentration of CA during HCMV infection, indicating that cellular phosphatase activity is in fact required to regulate eIF2α phosphorylation during HCMV infection. CA treatment resulted in the hyperphosphorylation of eIF2α very quickly (within 30 minutes) and independently of three primary eIF2α kinases (Figure 6 and Supplemental Figure 2), suggesting that the CA-induced hyperphosphorylation of eIF2α is due to the inhibition of an eIF2α-phosphatase, most likely PP1α. While PP2AC has been demonstrated to regulate the phosphorylation of eIF2α in reticulocyte lysates (Chen, Kramer, and Hardesty, 1989; Ernst et al., 1982; Petryshyn, Levin, and London, 1982), its role in vivo is less clear. PP1α, on the other hand, dephosphorylates eIF2α in vivo during ER stress and also under basal conditions (Brush, Weiser, and Shenolikar, 2003; Jousse et al., 2003; Novoa et al., 2001).
The net result of HCMV infection is the increased accumulation of phosphorylated eIF2α compared to uninfected HFs (Figure 6 and (Isler, Skalet, and Alwine, 2005)), yet the phosphorylation of eIF2α is still limited such that the shutoff of global protein synthesis and cell death that accompanies the sustained hyperphosphorylation of eIF2α is avoided (DeGracia et al., 1997; Isler, Skalet, and Alwine, 2005; Srivastava, Kumar, and Kaufman, 1998). Based on our results, the activity of an eIF2α phosphatase is required to limit the accumulation of phospho-eIF2α during HCMV infection and may be one mechanism that contributes to maintaining robust levels of protein synthesis during HCMV infection. Indeed, several viruses regulate the eIF2α phosphatase activity of PP1α to their benefit during infection. The herpes simplex virus type 1 (HSV-1) γ134.5 and the African swine fever virus DP71L gene products are homologues of GADD34, a PP1α targeting/regulatory cellular protein, that bind to PP1α and promote the dephosphorylation of eIF2α during viral infection (He, Gross, and Roizman, 1997; Rivera et al., 2007). The human papillomavirus type 18 (HPV-18) E6 oncoprotein interacts with the GADD34/PP1α complex to enhance the dephosphorylation of eIF2α (Kazemi et al., 2004). The importance of regulating phospho-eIF2α-dependent protein synthesis is reflected by the fact that γ134.5, DP71L, and E6 are all important determinants of pathogenesis (Chou et al., 1990; Mantovani and Banks, 2001; Zsak et al., 1996). Therefore, defining the mechanism by which HCMV manipulates cellular phosphatase activity in order to control the accumulation of phospho-eIF2α may provide novel insights into HCMV pathogenesis as well.
In summary, we found that over the course of HCMV infection, cellular phosphatase activity increased along with the levels of two primary cellular serine/threonine phosphatases, PP1 and PP2AC. HCMV-infected HFs were partially resistant to the effects of two different serine/threonine phosphatase inhibitors and specifically, HCMV infection appears to regulate the accumulation of phospho-eIF2α in a manner dependent upon phosphatase activity. To the best of our knowledge, this is the first study to directly examine general serine/threonine phosphatase activity during HCMV infection. Given the importance of protein phosphorylation to HCMV replication, elucidating the mechanisms governing the dephosphorylation of specific cellular and viral proteins will be key for understanding many of the regulatory processes that occur during the HCMV life cycle.
Materials and Methods
Cells and viruses
Human fibroblasts (HFs), PKR wild-type (wt) and PKR-null mouse embryonic fibroblasts (MEFs) (Yang et al., 1995), GCN2-null MEFs (Jiang et al., 2003; Zhang et al., 2002b), and PERK-null MEFs (Jiang et al., 2003; Zhang et al., 2002a) were maintained at 37°C in a 5% CO2 atmosphere in Dulbecco’s modified Eagle’s medium supplemented with 10% NuSerum (Collaborative Biomedical), penicillin-streptomycin (100U/ml), and 2mM L-glutamine. eIF2αA/A and wt eIF2αS/S MEFs (Scheuner et al., 2001) were maintained at 37°C in a 5% CO2 atmosphere in Dulbecco’s modified Eagle’s medium supplemented with 10% NuSerum (Collaborative Biomedical), penicillin-streptomycin (100U/ml), 2mM L-glutamine, and 1X essential and non-essential amino acids (Gibco). All HCMV infections were performed in HFs grown to confluence with rTowne-1 (Alderete, Child, and Geballe, 2001) at a multiplicity of infection (MOI) of 3. Inactivation of HCMV by ultraviolet (UV) irradiation to a total dose of 0.72 J/cm2 was performed as previously described (Child et al., 2002). In experiments utilizing ganciclovir (GCV), drug (final [45 µM]) was added to the media beginning one hour post-infection and was left on for the remainder of the experiment.
Reagents and antibodies
Calyculin A and okadaic acid were purchased from A.G. Scientific. Antibodies used for immunoblot analyses against PP1α, PP2AC, phospho-threonine, eIF2α, phospho-eIF2α (serine 51), and phospho-PKR (threonine 451) were purchased from Cell Signaling Technology. PKR (B-10) and pan-PP1 (E-9) antibody were purchased from Santa Cruz Biotechnology. Anti-actin rabbit polyclonal antibody was purchased from Sigma. Anti-pp28 mouse monoclonal antibody was purchased from Virusys Corporation.
Phosphatase activity assay
Mock- or HCMV-infected HFs were collected in phosphatase lysis buffer (20mM imidazole, 2mM EDTA, 2mM EGTA, 2mM benzamidine, and 1mM PMSF), sonicated for 10 seconds, and subjected to centrifugation at 2500 × g for 5 minutes at 4°C. Protein concentrations in the supernatant were determined by fluoraldehyde o-phthalaldehyde (Pierce) assay (Geballe and Mocarski, 1988) and equivalent amounts of protein or lysis buffer alone (to control for background phosphatase activity) were incubated with the phosphopeptide KRpTIRR (US Biological, Upstate Biotechnology; final [250 µM]) and 8.5% (v/v) pNPP Ser/Thr Assay Buffer (US Biological) for one hour at room temperature. Lysis buffer was added to each sample as needed in order to standardize reaction volumes. Twenty five microliters from each reaction were mixed with 100 microliters in Malachite Green Phosphate Detection Solution AB (US Biological) and after 15 minutes optical density was measured at 630nM.
Immunoblot analysis
Cells were washed twice with ice-cold phosphate-buffered saline (PBS) and lysed in 2% sodium dodecyl sulfate (SDS) pre-heated to 95°C. Protein concentrations were determined by fluoraldehyde o-phthalaldehyde assay and equivalent amounts of protein were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), followed by transfer to polyvinylidene difluoride membranes (PVDF; GE Lifesciences) by electroblotting. Immunoblot analyses were then performed using Western Star chemiluminescent detection system (Tropix, Inc) according to manufacturer’s instructions. Where indicated, membranes were stripped in glycine stripping buffer (0.2 M glycine, 0.1% SDS, 1% Tween-20, pH 2.2) according to instructions (Western Star chemiluminescent detection system (Tropix, Inc))
Radiolabeling and autoradiography
Cells were treated with CA for 30 minutes in cysteine- and methionine-free medium and then labeled with [35S]methionine ([100 µCi/ml]; NEG-772 Easytag Express Protein Labeling Mix, PerkinElmer Life and Analytical Sciences) in cysteine/methione-free medium in the absence of CA for 30 minutes. Cells treated with OA were labeled with [35S]methionine as above for one hour without pre-incubation in cysteine/methionine-free medium. After labeling, all cells were washed twice in cold PBS and lysed in hot 2% SDS. After determination of protein concentration by fluoraldehyde o-phthalaldehyde assay, equivalent amounts of protein were separated by SDS-PAGE, stained with Coomassie G-250 (SimplyBlue SafeStain, Invitrogen) to document equivalent protein loading, and then exposed to film for autoradiography.
Supplementary Material
Acknowledgements
We wish to gratefully acknowledge Dr. Bryan Williams (Cleveland Clinic) for providing PKR null MEFs and their isotype controls, Drs. Randal Kaufman and Donalyn Scheuner (University of Michigan) for providing eIF2αA/A and (wt) eIF2αS/S MEFS, and Dr. Douglas Cavener (Pennsylvania State University) for providing GCN2 null, PERK null, and their isotype control MEFs. This work was supported by NIH grant K08 AI058089 (M.H.) and R01 AI26672 (A.P.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alderete JP, Child SJ, Geballe AP. Abundant early expression of gpUL4 from a human cytomegalovirus mutant lacking a repressive upstream open reading frame. J Virol. 2001;75(15):7188–7192. doi: 10.1128/JVI.75.15.7188-7192.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek MC, Krosky PM, He Z, Coen DM. Specific phosphorylation of exogenous protein and peptide substrates by the human cytomegalovirus UL97 protein kinase. Importance of the P+5 position. J Biol Chem. 2002;277(33):29593–29599. doi: 10.1074/jbc.M202312200. [DOI] [PubMed] [Google Scholar]
- Baek MC, Krosky PM, Pearson A, Coen DM. Phosphorylation of the RNA polymerase II carboxyl-terminal domain in human cytomegalovirus-infected cells and in vitro by the viral UL97 protein kinase. Virology. 2004;324(1):184–193. doi: 10.1016/j.virol.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Baharians Z, Schonthal AH. Autoregulation of protein phosphatase type 2A expression. J Biol Chem. 1998;273(30):19019–19024. doi: 10.1074/jbc.273.30.19019. [DOI] [PubMed] [Google Scholar]
- Barrasa MI, Harel NY, Alwine JC. The phosphorylation status of the serine-rich region of the human cytomegalovirus 86-kilodalton major immediate-early protein IE2/IEP86 affects temporal viral gene expression. J Virol. 2005;79(3):1428–1437. doi: 10.1128/JVI.79.3.1428-1437.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen M. Combinatorial control of protein phosphatase-1. Trends Biochem Sci. 2001;26(7):426–431. doi: 10.1016/s0968-0004(01)01836-9. [DOI] [PubMed] [Google Scholar]
- Bresnahan WA, Boldogh I, Chi P, Thompson EA, Albrecht T. Inhibition of cellular Cdk2 activity blocks human cytomegalovirus replication. Virology. 1997;231(2):239–247. doi: 10.1006/viro.1997.8489. [DOI] [PubMed] [Google Scholar]
- Brush MH, Weiser DC, Shenolikar S. Growth arrest and DNA damage-inducible protein GADD34 targets protein phosphatase 1 alpha to the endoplasmic reticulum and promotes dephosphorylation of the alpha subunit of eukaryotic translation initiation factor 2. Mol Cell Biol. 2003;23(4):1292–1303. doi: 10.1128/MCB.23.4.1292-1303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant JC, Westphal RS, Wadzinski BE. Methylated C-terminal leucine residue of PP2A catalytic subunit is important for binding of regulatory Balpha subunit. Biochem J. 1999;339(Pt 2):241–246. [PMC free article] [PubMed] [Google Scholar]
- Chambers J, Angulo A, Amaratunga D, Guo H, Jiang Y, Wan JS, Bittner A, Frueh K, Jackson MR, Peterson PA, Erlander MG, Ghazal P. DNA microarrays of the complex human cytomegalovirus genome: profiling kinetic class with drug sensitivity of viral gene expression. J Virol. 1999;73(7):5757–5766. doi: 10.1128/jvi.73.7.5757-5766.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YJ, Tseng WP, Hayward GS. Two distinct upstream regulatory domains containing multicopy cellular transcription factor binding sites provide basal repression and inducible enhancer characteristics to the immediate-early IES (US3) promoter from human cytomegalovirus. J Virol. 1996;70(8):5312–5328. doi: 10.1128/jvi.70.8.5312-5328.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Martin BL, Brautigan DL. Regulation of protein serine-threonine phosphatase type-2A by tyrosine phosphorylation. Science. 1992;257(5074):1261–1264. doi: 10.1126/science.1325671. [DOI] [PubMed] [Google Scholar]
- Chen SC, Kramer G, Hardesty B. Isolation and partial characterization of an Mr 60,000 subunit of a type 2A phosphatase from rabbit reticulocytes. J Biol Chem. 1989;264(13):7267–7275. [PubMed] [Google Scholar]
- Child SJ, Hakki M, De Niro KL, Geballe AP. Evasion of cellular antiviral responses by human cytomegalovirus TRS1 and IRS1. J Virol. 2004;78(1):197–205. doi: 10.1128/JVI.78.1.197-205.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child SJ, Jarrahian S, Harper VM, Geballe AP. Complementation of vaccinia virus lacking the double-stranded RNA-binding protein gene E3L by human cytomegalovirus. J Virol. 2002;76(10):4912–4918. doi: 10.1128/JVI.76.10.4912-4918.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J, Kern ER, Whitley RJ, Roizman B. Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science. 1990;250(4985):1262–1266. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- Cohen PT. Protein phosphatase 1--targeted in many directions. J Cell Sci. 2002;115(Pt 2):241–256. doi: 10.1242/jcs.115.2.241. [DOI] [PubMed] [Google Scholar]
- Connor JH, Weiser DC, Li S, Hallenbeck JM, Shenolikar S. Growth arrest and DNA damage-inducible protein GADD34 assembles a novel signaling complex containing protein phosphatase 1 and inhibitor 1. Mol Cell Biol. 2001;21(20):6841–6850. doi: 10.1128/MCB.21.20.6841-6850.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damuni Z, Xiong H, Li M. Autophosphorylation-activated protein kinase inactivates the protein tyrosine phosphatase activity of protein phosphatase 2A. FEBS Lett. 1994;352(3):311–314. doi: 10.1016/0014-5793(94)00981-3. [DOI] [PubMed] [Google Scholar]
- DeGracia DJ, Sullivan JM, Neumar RW, Alousi SS, Hikade KR, Pittman JE, White BC, Rafols JA, Krause GS. Effect of brain ischemia and reperfusion on the localization of phosphorylated eukaryotic initiation factor 2 alpha. J Cereb Blood Flow Metab. 1997;17(12):1291–1302. doi: 10.1097/00004647-199712000-00004. [DOI] [PubMed] [Google Scholar]
- Dever TE, Dar AC, Sicheri F. The eIF2alpha Kinases. In: Mathews MB, Sonenberg N, Hershey JWB, editors. "Translational Control in Biology and Medicine". Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2007. pp. 319–344. [Google Scholar]
- Ernst V, Levin DH, Foulkes JG, London IM. Effects of skeletal muscle protein phosphatase inhibitor-2 on protein synthesis and protein phosphorylation in rabbit reticulocyte lysates. Proc Natl Acad Sci U S A. 1982;79(23):7092–7096. doi: 10.1073/pnas.79.23.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre B, Turowski P, Hemmings BA. Differential inhibition and posttranslational modification of protein phosphatase 1 and 2A in MCF7 cells treated with calyculin-A, okadaic acid, and tautomycin. J Biol Chem. 1997;272(21):13856–13863. doi: 10.1074/jbc.272.21.13856. [DOI] [PubMed] [Google Scholar]
- Favre B, Zolnierowicz S, Turowski P, Hemmings BA. The catalytic subunit of protein phosphatase 2A is carboxyl-methylated in vivo. J Biol Chem. 1994;269(23):16311–16317. [PubMed] [Google Scholar]
- Fish KN, Soderberg-Naucler C, Nelson JA. Steady-state plasma membrane expression of human cytomegalovirus gB is determined by the phosphorylation state of Ser900. J Virol. 1998;72(8):6657–6664. doi: 10.1128/jvi.72.8.6657-6664.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fladmark KE, Brustugun OT, Hovland R, Boe R, Gjertsen BT, Zhivotovsky B, Doskeland SO. Ultrarapid caspase-3 dependent apoptosis induction by serine/threonine phosphatase inhibitors. Cell Death Differ. 1999;6(11):1099–1108. doi: 10.1038/sj.cdd.4400590. [DOI] [PubMed] [Google Scholar]
- Flores-Delgado G, Liu CW, Sposto R, Berndt N. A limited screen for protein interactions reveals new roles for protein phosphatase 1 in cell cycle control and apoptosis. J Proteome Res. 2007;6(3):1165–1175. doi: 10.1021/pr060504h. [DOI] [PubMed] [Google Scholar]
- Fortunato EA, McElroy AK, Sanchez I, Spector DH. Exploitation of cellular signaling and regulatory pathways by human cytomegalovirus. Trends Microbiol. 2000;8(3):111–119. doi: 10.1016/s0966-842x(00)01699-1. [DOI] [PubMed] [Google Scholar]
- Geballe AP, Mocarski ES. Translational control of cytomegalovirus gene expression is mediated by upstream AUG codons. J Virol. 1988;62(9):3334–3340. doi: 10.1128/jvi.62.9.3334-3340.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjertsen BT, Cressey LI, Ruchaud S, Houge G, Lanotte M, Doskeland SO. Multiple apoptotic death types triggered through activation of separate pathways by cAMP and inhibitors of protein phosphatases in one (IPC leukemia) cell line. J Cell Sci. 1994;107(Pt 12):3363–3377. doi: 10.1242/jcs.107.12.3363. [DOI] [PubMed] [Google Scholar]
- Gjertsen BT, Doskeland SO. Protein phosphorylation in apoptosis. Biochim Biophys Acta. 1995;1269(2):187–199. doi: 10.1016/0167-4889(95)00117-b. [DOI] [PubMed] [Google Scholar]
- Guan L, Song K, Pysz MA, Curry KJ, Hizli AA, Danielpour D, Black AR, Black JD. Protein kinase C-mediated down-regulation of cyclin D1 involves activation of the translational repressor 4E-BP1 via a phosphoinositide 3-kinase/Akt-independent, protein phosphatase 2A-dependent mechanism in intestinal epithelial cells. J Biol Chem. 2007;282(19):14213–14225. doi: 10.1074/jbc.M610513200. [DOI] [PubMed] [Google Scholar]
- Gupta V, Ogawa AK, Du X, Houk KN, Armstrong RW. A model for binding of structurally diverse natural product inhibitors of protein phosphatases PP1 and PP2A. J Med Chem. 1997;40(20):3199–3206. doi: 10.1021/jm960873x. [DOI] [PubMed] [Google Scholar]
- Hakki M, Marshall EE, De Niro KL, Geballe AP. Binding and nuclear relocalization of protein kinase R by human cytomegalovirus TRS1. J Virol. 2006;80(23):11817–11826. doi: 10.1128/JVI.00957-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel NY, Alwine JC. Phosphorylation of the human cytomegalovirus 86-kilodalton immediate-early protein IE2. J Virol. 1998;72(7):5481–5492. doi: 10.1128/jvi.72.7.5481-5492.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Gross M, Roizman B. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc Natl Acad Sci U S A. 1997;94(3):843–848. doi: 10.1073/pnas.94.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helps NR, Luo X, Barker HM, Cohen PT. NIMA-related kinase 2 (Nek2), a cell-cycle-regulated protein kinase localized to centrosomes, is complexed to protein phosphatase 1. Biochem J. 2000;349(Pt 2):509–518. doi: 10.1042/0264-6021:3490509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertel L, Chou S, Mocarski ES. Viral and cell cycle-regulated kinases in cytomegalovirus-induced pseudomitosis and replication. PLoS Pathog. 2007;3(1):e6. doi: 10.1371/journal.ppat.0030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertel L, Mocarski ES. Global analysis of host cell gene expression late during cytomegalovirus infection reveals extensive dysregulation of cell cycle gene expression and induction of Pseudomitosis independent of US28 function. J Virol. 2004;78(21):11988–12011. doi: 10.1128/JVI.78.21.11988-12011.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume AJ, Finkel JS, Kamil JP, Coen DM, Culbertson MR, Kalejta RF. Phosphorylation of retinoblastoma protein by viral protein with cyclin-dependent kinase function. Science. 2008;320(5877):797–799. doi: 10.1126/science.1152095. [DOI] [PubMed] [Google Scholar]
- Ishihara H, Martin BL, Brautigan DL, Karaki H, Ozaki H, Kato Y, Fusetani N, Watabe S, Hashimoto K, Uemura D, et al. Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem Biophys Res Commun. 1989;159(3):871–877. doi: 10.1016/0006-291x(89)92189-x. [DOI] [PubMed] [Google Scholar]
- Isler JA, Skalet AH, Alwine JC. Human cytomegalovirus infection activates and regulates the unfolded protein response. J Virol. 2005;79(11):6890–6899. doi: 10.1128/JVI.79.11.6890-6899.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001;353(Pt 3):417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MA, Jones TR, Drummond DD, Smith PP, Britt WJ, Nelson JA, Baldick CJ. Phosphorylation of human cytomegalovirus glycoprotein B (gB) at the acidic cluster casein kinase 2 site (Ser900) is required for localization of gB to the trans-Golgi network and efficient virus replication. J Virol. 2004;78(1):285–293. doi: 10.1128/JVI.78.1.285-293.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jault FM, Jault JM, Ruchti F, Fortunato EA, Clark C, Corbeil J, Richman DD, Spector DH. Cytomegalovirus infection induces high levels of cyclins, phosphorylated Rb, and p53, leading to cell cycle arrest. J Virol. 1995;69(11):6697–6704. doi: 10.1128/jvi.69.11.6697-6704.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HY, Wek SA, McGrath BC, Scheuner D, Kaufman RJ, Cavener DR, Wek RC. Phosphorylation of the alpha subunit of eukaryotic initiation factor 2 is required for activation of NF-kappaB in response to diverse cellular stresses. Mol Cell Biol. 2003;23(16):5651–5663. doi: 10.1128/MCB.23.16.5651-5663.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jousse C, Oyadomari S, Novoa I, Lu P, Zhang Y, Harding HP, Ron D. Inhibition of a constitutive translation initiation factor 2alpha phosphatase, CReP, promotes survival of stressed cells. J Cell Biol. 2003;163(4):767–775. doi: 10.1083/jcb.200308075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemi S, Papadopoulou S, Li S, Su Q, Wang S, Yoshimura A, Matlashewski G, Dever TE, Koromilas AE. Control of alpha subunit of eukaryotic translation initiation factor 2 (eIF2 alpha) phosphorylation by the human papillomavirus type 18 E6 oncoprotein: implications for eIF2 alpha-dependent gene expression and cell death. Mol Cell Biol. 2004;24(8):3415–3429. doi: 10.1128/MCB.24.8.3415-3429.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keay S, Baldwin BR. Evidence for the role of cell protein phosphorylation in human cytomegalovirus/host cell fusion. J Gen Virol. 1996;77(Pt 10):2597–2604. doi: 10.1099/0022-1317-77-10-2597. [DOI] [PubMed] [Google Scholar]
- Latreille M, Larose L. Nck in a complex containing the catalytic subunit of protein phosphatase 1 regulates eukaryotic initiation factor 2alpha signaling and cell survival to endoplasmic reticulum stress. J Biol Chem. 2006;281(36):26633–26644. doi: 10.1074/jbc.M513556200. [DOI] [PubMed] [Google Scholar]
- Longin S, Zwaenepoel K, Louis JV, Dilworth S, Goris J, Janssens V. Selection of protein phosphatase 2A regulatory subunits is mediated by the C terminus of the catalytic Subunit. J Biol Chem. 2007;282(37):26971–26980. doi: 10.1074/jbc.M704059200. [DOI] [PubMed] [Google Scholar]
- Longin S, Zwaenepoel K, Martens E, Louis JV, Rondelez E, Goris J, Janssens V. Spatial control of protein phosphatase 2A (de)methylation. Exp Cell Res. 2008;314(1):68–81. doi: 10.1016/j.yexcr.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Mantovani F, Banks L. The human papillomavirus E6 protein and its contribution to malignant progression. Oncogene. 2001;20(54):7874–7887. doi: 10.1038/sj.onc.1204869. [DOI] [PubMed] [Google Scholar]
- Margolis SS, Kornbluth S. When the checkpoints have gone: insights into Cdc25 functional activation. Cell Cycle. 2004;3(4):425–428. [PubMed] [Google Scholar]
- Margolis SS, Perry JA, Weitzel DH, Freel CD, Yoshida M, Haystead TA, Kornbluth S. A role for PP1 in the Cdc2/Cyclin B-mediated positive feedback activation of Cdc25. Mol Biol Cell. 2006;17(4):1779–1789. doi: 10.1091/mbc.E05-08-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis SS, Walsh S, Weiser DC, Yoshida M, Shenolikar S, Kornbluth S. PP1 control of M phase entry exerted through 14-3-3-regulated Cdc25 dephosphorylation. Embo J. 2003;22(21):5734–5745. doi: 10.1093/emboj/cdg545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson S, Turowski P, Picard L, Goris J, Landini MP, Topilko A, Hemmings B, Bessia C, Garcia A, Virelizier JL. Human cytomegalovirus carries serine/threonine protein phosphatases PP1 and a host-cell derived PP2A. J Virol. 1996;70(3):1415–1423. doi: 10.1128/jvi.70.3.1415-1423.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocarski ES, Shenk T, Pass RF. Cytomegaloviruses. In: Knipe DM, Howley PM, editors. "Fields Virology". 5th ed. Vol. 2. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 2701–2772. 2 vols. [Google Scholar]
- Morimoto H, Okamura H, Yoshida K, Kitamura S, Haneji T. Okadaic acid induces apoptosis through double-stranded RNA-dependent protein kinase/eukaryotic initiation factor-2alpha pathway in human osteoblastic MG63 cells. J Biochem (Tokyo) 2004;136(4):433–438. doi: 10.1093/jb/mvh144. [DOI] [PubMed] [Google Scholar]
- Morimoto H, Ozaki A, Okamura H, Yoshida K, Kitamura S, Haneji T. Okadaic acid induces tyrosine phosphorylation of IkappaBalpha that mediated by PKR pathway in human osteoblastic MG63 cells. Mol Cell Biochem. 2005;276(1–2):211–217. doi: 10.1007/s11010-005-4440-y. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Koda T, Kakinuma M, Matsuzawa S, Kitamura K, Mizuno Y, Kikuchi K. Cell cycle dependent gene expressions and activities of protein phosphatases PP1 and PP2A in mouse NIH3T3 fibroblasts. Biochem Biophys Res Commun. 1992;187(1):507–514. doi: 10.1016/s0006-291x(05)81523-2. [DOI] [PubMed] [Google Scholar]
- Nishikawa M, Omay SB, Toyoda H, Tawara I, Shima H, Nagao M, Hemmings BA, Mumby MC, Deguchi K. Expression of the catalytic and regulatory subunits of protein phosphatase type 2A may be differentially modulated during retinoic acid-induced granulocytic differentiation of HL-60 cells. Cancer Res. 1994;54(18):4879–4884. [PubMed] [Google Scholar]
- Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol. 2001;153(5):1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande H, Lee TD, Churchill MA, Zaia JA. Structural analysis of a 64-kDa major structural protein of human cytomegalovirus (Towne): identification of a phosphorylation site and comparison to pp65 of HCMV (AD169) Virology. 1990;178(1):6–14. doi: 10.1016/0042-6822(90)90374-z. [DOI] [PubMed] [Google Scholar]
- Petryshyn R, Levin DH, London IM. Regulation of double-stranded RNA-activated eukaryotic initiation factor 2 alpha kinase by type 2 protein phosphatase in reticulocyte lysates. Proc Natl Acad Sci U S A. 1982;79(21):6512–6516. doi: 10.1073/pnas.79.21.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard MN, Gao N, Jairath S, Mulamba G, Krosky P, Coen DM, Parker BO, Pari GS. A recombinant human cytomegalovirus with a large deletion in UL97 has a severe replication deficiency. J Virol. 1999;73(7):5663–5670. doi: 10.1128/jvi.73.7.5663-5670.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera J, Abrams C, Hernaez B, Alcazar A, Escribano JM, Dixon L, Alonso C. The MyD116 African swine fever virus homologue interacts with the catalytic subunit of protein phosphatase 1 and activates its phosphatase activity. J Virol. 2007;81(6):2923–2929. doi: 10.1128/JVI.02077-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roby C, Gibson W. Characterization of phosphoproteins and protein kinase activity of virions, noninfectious enveloped particles, and dense bodies of human cytomegalovirus. J Virol. 1986;59(3):714–727. doi: 10.1128/jvi.59.3.714-727.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodems SM, Spector DH. Extracellular signal-regulated kinase activity is sustained early during human cytomegalovirus infection. J Virol. 1998;72(11):9173–9180. doi: 10.1128/jvi.72.11.9173-9180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez V, McElroy AK, Yen J, Tamrakar S, Clark CL, Schwartz RA, Spector DH. Cyclin-dependent kinase activity is required at early times for accurate processing and accumulation of the human cytomegalovirus UL122-123 and UL37 immediate-early transcripts and at later times for virus production. J Virol. 2004;78(20):11219–11232. doi: 10.1128/JVI.78.20.11219-11232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7(6):1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- Shenolikar S. Protein serine/threonine phosphatases--new avenues for cell regulation. Annu Rev Cell Biol. 1994;10:55–86. doi: 10.1146/annurev.cb.10.110194.000415. [DOI] [PubMed] [Google Scholar]
- Spengler ML, Kurapatwinski K, Black AR, Azizkhan-Clifford J. SUMO-1 modification of human cytomegalovirus IE1/IE72. J Virol. 2002;76(6):2990–2996. doi: 10.1128/JVI.76.6.2990-2996.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava SP, Kumar KU, Kaufman RJ. Phosphorylation of eukaryotic translation initiation factor 2 mediates apoptosis in response to activation of the double-stranded RNA-dependent protein kinase. J Biol Chem. 1998;273(4):2416–2423. doi: 10.1074/jbc.273.4.2416. [DOI] [PubMed] [Google Scholar]
- Stamm S. Regulation of alternative splicing by reversible protein phosphorylation. J Biol Chem. 2008;283(3):1223–1227. doi: 10.1074/jbc.R700034200. [DOI] [PubMed] [Google Scholar]
- Swingle M, Ni L, Honkanen RE. Small-molecule inhibitors of ser/thr protein phosphatases. In: Moorhead G, editor. "Protein Phosphatase Protocols". Totowa, NJ: Humana Press; 2007. pp. 23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa CG, Morgan DO. Control of mitosis by changes in the subcellular location of cyclin-B1-Cdk1 and Cdc25C. Curr Opin Cell Biol. 2000;12(6):658–665. doi: 10.1016/s0955-0674(00)00149-6. [DOI] [PubMed] [Google Scholar]
- Tan SL, Tareen SU, Melville MW, Blakely CM, Katze MG. The direct binding of the catalytic subunit of protein phosphatase 1 to the PKR protein kinase is necessary but not sufficient for inactivation and disruption of enzyme dimer formation. J Biol Chem. 2002;277(39):36109–36117. doi: 10.1074/jbc.M205109200. [DOI] [PubMed] [Google Scholar]
- Tawara I, Nishikawa M, Morita K, Kobayashi K, Toyoda H, Omay SB, Shima H, Nagao M, Kuno T, Tanaka C, et al. Down-regulation by retinoic acid of the catalytic subunit of protein phosphatase type 2A during granulocytic differentiation of HL-60 cells. FEBS Lett. 1993;321(2–3):224–228. doi: 10.1016/0014-5793(93)80113-9. [DOI] [PubMed] [Google Scholar]
- Tolstykh T, Lee J, Vafai S, Stock JB. Carboxyl methylation regulates phosphoprotein phosphatase 2A by controlling the association of regulatory B subunits. Embo J. 2000;19(21):5682–5691. doi: 10.1093/emboj/19.21.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turowski P, Fernandez A, Favre B, Lamb NJ, Hemmings BA. Differential methylation and altered conformation of cytoplasmic and nuclear forms of protein phosphatase 2A during cell cycle progression. J Cell Biol. 1995;129(2):397–410. doi: 10.1083/jcb.129.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnum SM, Streblow DN, Monroe ME, Smith P, Auberry KJ, Pasa-Tolic L, Wang D, Camp DG, 2nd, Rodland K, Wiley S, Britt W, Shenk T, Smith RD, Nelson JA. Identification of proteins in human cytomegalovirus (HCMV) particles: the HCMV proteome. J Virol. 2004;78(20):10960–10966. doi: 10.1128/JVI.78.20.10960-10966.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NJ, Moss ST, Csar XF, Ward AC, Hamilton JA. Protein phosphatase 2A is expressed in response to colony-stimulating factor 1 in macrophages and is required for cell cycle progression independently of extracellular signal-regulated protein kinase activity. Biochem J. 1999;339(Pt 3):517–524. [PMC free article] [PubMed] [Google Scholar]
- Yang YL, Reis LF, Pavlovic J, Aguzzi A, Schafer R, Kumar A, Williams BR, Aguet M, Weissmann C. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. Embo J. 1995;14(24):6095–6106. doi: 10.1002/j.1460-2075.1995.tb00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, McGrath B, Li S, Frank A, Zambito F, Reinert J, Gannon M, Ma K, McNaughton K, Cavener DR. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol. 2002a;22(11):3864–3874. doi: 10.1128/MCB.22.11.3864-3874.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, McGrath BC, Reinert J, Olsen DS, Lei L, Gill S, Wek SA, Vattem KM, Wek RC, Kimball SR, Jefferson LS, Cavener DR. The GCN2 eIF2alpha kinase is required for adaptation to amino acid deprivation in mice. Mol Cell Biol. 2002b;22(19):6681–6688. doi: 10.1128/MCB.22.19.6681-6688.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsak L, Lu Z, Kutish GF, Neilan JG, Rock DL. An African swine fever virus virulence-associated gene NL-S with similarity to the herpes simplex virus ICP34.5 gene. J Virol. 1996;70(12):8865–8871. doi: 10.1128/jvi.70.12.8865-8871.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.