1. Introduction
Interest in hypervigilance, a component of some persistent pain conditions, has grown steadily since it was first described, and the concept itself has undergone considerable development during that time [2]. Chapman’s original description of a hypervigilant person was of someone who is unusually alert to “somatic distress signals” including, but not limited to, pain. Chapman regarded hypervigilance as a cognitive tendency, reflecting worry about health concerns. Pennebaker’s experimental research with healthy subjects showed that a hypervigilant attitude can be created by appropriate attentional manipulations, and that it changes the subject’s tendency to detect noxious sensations as well as to report them [18].
This emphasis on perceptual as well as cognitive factors was expanded by McDermid et al., who proposed the Generalized Hypervigilance Hypothesis (GHH), according to which hypervigilance is a “perceptual habit” that involves subjective amplification of a variety of aversive sensations, not just painful ones [15]. They reported that fibromyalgia (FM) patients had higher levels of hypervigilance, and lower pressure-pain threshold and tolerance, than healthy controls. The auditory noise tolerance of the patients was also reduced, suggesting that perceptual amplification is not confined to somesthesis.
The GHH is composed of two elements. The first is that hypervigilance amplifies aversive sensations only. The second is that it does so independent of sensory modality.
The first proposition is widely accepted. FM patients, who on average are markedly hypervigilant, have reduced thresholds and tolerance for experimental pain [6, 8, 10, 11, 12, 15, 17], and direct (i.e. scaling) measures of pain intensity demonstrate amplification in these patients [7, 10, 19]. By documenting reduced pain threshold and tolerance on the forearm of patients with temporomandibular disorders (TMD)--another set of conditions characterized by hypervigilance [20]--Maixner et al. showed that the increased pain sensitivity of hypervigilant clinical populations is at least partly a manifestation of a widespread, and therefore presumably central, disturbance [13].
The second component of the GHH, that perceptual amplification extends to other modalities, is less well established; the case for it relies primarily on auditory tolerance data [15]. We know of no study of hypervigilance in which the perceived intensity of sounds has been measured directly.
In the present study, we evaluated the GHH using several sets of measurements that, in combination, resolve some of the questions left unanswered by earlier work. Perceptual responses of individuals with FM and TMD were examined, because the disparity (confirmed here by questionnaire scores) between the elevated levels of hypervigilance in these clinical groups and the lower amounts in healthy control participants enabled us to make a definitive test of the GHH. We first obtained, in all three groups, direct estimates of sensation intensity using somesthetic and auditory stimuli that spanned wide intensity ranges. To evaluate the idea that hypervigilance selectively amplifies aversive sensations, unpleasantness judgments were also obtained. We also asked on each somesthetic trial whether the stimulus was painful, thus making it possible to compare results between the submodalities of touch and pain. Finally, amount of perceptual amplification and level of hypervigilance were compared across individuals.
2. Methods
2.1 Participants
Twenty-two women with temporomandibular disorders (TMD), 12 with fibromyalgia (FM), and 20 healthy controls (HC) participated in this study. Subjects were recruited by means of flyers posted on campus and other forms of advertisement, and by referral from the Orofacial Pain Clinic in the University of North Carolina at Chapel Hill School of Dentistry, explaining the greater availability of TMD than of FM patients. Potential participants were screened by a phone interview, following which an assessment visit was scheduled to determine their individual case status. During the assessment visit, self-report questionnaires on pain complaints and medical history were administered, and a physical examination was performed by calibrated and validated clinical examiners (PL and MS).
The criteria for classification as an FM subject included self-reported persistent widespread body pain for more than 3 months, and, in most cases, pain on palpation at 11 or more of the 18 body sites (tender points) specified by the American College of Rheumatology [24]. Three of the FM participants had fewer than 11 tender points, but other strong indicators of FM: One had 9/18 tender points but had been diagnosed with primary FM by her physician and had received treatment for it in the Clinic; the other two had 9/18 and 7/18 tender points, respectively, but both reported significant pain and fatigue over the past 3 months, pain in all 4 quadrants, and axial pain lasting more than a year. The criteria for classification as a TMD subject included self-reported orofacial pain for 1 month, and meeting the Research Diagnostic Criteria for TMD following a standardized physical examination [3]. All of the members of the FM group met criteria for TMD as well, a reflection of both the well-documented prevalence of TMD among FM patients [4] and the fact that most of these subjects were recruited through an orofacial pain clinic. As a result, differences in performance between the FM and TMD groups in the present study could reflect either the unique character of fibromyalgia, or the combined impact of both conditions in members of the FM group. HC subjects neither reported any significant pain nor met the criteria for FM or TMD on physical examination.
Individuals were excluded from participation if they had uncontrolled hypertension; heart, lung, or kidney disease; diabetes; hyperthyroidism; epilepsy; drug or alcohol abuse; major psychiatric disorder; were pregnant or nursing; or had other conditions that would have interfered with their carrying out the experimental procedures.
All subjects were female. Average ages of the three groups were: HC, 37.8 (SD=12.1); TMD, 37.7 (11.5); and FM, 35.4 (11.5). A one-way ANOVA showed that these values were not significantly different.
Given the intense chronic pain experienced by many of the patients, and the need to study them in a series of 3 sessions extending, in most cases, over at least two weeks, participants were not asked to discontinue their pain medication. In addition, we felt that limiting recruitment of participants with FM and TMD to those who were willing and able to be medication-free throughout their participation would have resulted in samples biased toward patients with relatively mild signs and symptoms.
After being screened, participants were scheduled for their visits to the laboratory, each lasting 2 hours. The present study concerns measurements made during a single session. Other sessions consisted of unrelated experiments, the results of which will be reported separately. Subjects gave written informed consent for all procedures and were paid for their participation. All aspects of the study were approved by the University’s IRB.
2.2 Apparatus
The pressure stimulator was a weighted vertical rod, with a flat, round Delrin tip 5 mm in diameter, which rested on the dorsal surface of the forearm approximately midway between wrist and elbow. Aluminum scaffolding attached to the top of the rod allowed weights to be added to manipulate downward force, which ranged from 0.8 to 10.8 N, in steps of 1N. A partition blocked the subject’s view of the stimulator and of the stimulated region (and more distal parts) of the arm.
Auditory stimuli were provided by waveforms generated by a PC, amplified by a Grace m902 amplifier, and delivered binaurally through Sennheiser HD265 headphones. Each stimulus was a two-tone combination centered at 1275 Hz. Half the stimuli were a combination of 1200 and 1350 Hz; the other half were a combination of 1020 and 1530 Hz. In all cases the two components were equal in amplitude. We had anticipated that the former pair would sound more discordant than the latter, giving us a way to manipulate unpleasantness independent of loudness. In fact, however, the two combinations yielded closely comparable unpleasantness ratings, and data from them were pooled. Intensity was controlled by adjusting the gain of the amplifier: Sounds ranged in 5 dB steps from 35 to 90 dB SPL (A-weighted).
2.3 Procedure
On their arrival in the laboratory, subjects completed several psychometric instruments including the McGill Pain Questionnaire-Short Form (MPQ-SF) [16], the Pain Catastrophizing Scale (PCS) [22], the State Anxiety Inventory (STAI Form Y-1) [21], the Positive and Negative Affect Scale (PANAS) [23], and the Pennebaker Inventory of Limbic Languidness (PILL) [18], a measure of hypervigilance. Another putative hypervigilance measure, the Kohn Reactivity Scale (KRS) [9], was administered, but some subjects found a number of its items to be remote from their experiences; we therefore rely on the PILL for our psychometric assessment of hypervigilance.
The pressure experiment was conducted next. The subject positioned her right forearm in the apparatus, resting on a small pillow. The experiment consisted of two series of 11 trials, separated by a 5 min break. A series consisted of one presentation of each of the 11 force levels employed (ranging from 0.8 to 10.8 N), in random order. Different random orders were used for the two series, and for each subject. A trial began with the lowering of the weighted rod onto the skin, over the course of about 1 sec. It was left in place for 15 sec, then raised off the skin over the course of an additional second. The subject was then asked to rate the intensity of the sensation caused by the stimulus on a 0–100 scale, with zero representing “no sensation” and 100 indicating “the most intense sensation imaginable.” The participant was then asked to classify the stimulus as “painful, unpleasant but not painful, or neutral.” Finally, the subject gave a second numerical rating, this time of the unpleasantness of the sensation, from 0 (“not at all unpleasant”) to 100 (“the most unpleasant sensation imaginable”). The experimenters next moved the subject’s forearm slightly, to prevent repeated stimulation of the same site, and initiated the next trial. One HC subject did not participate in the pressure experiment because her forearm was too large to fit in the apparatus.
The auditory experiment was conducted later in the session. After receiving instructions, the subject donned headphones and was presented with a series of 24 sounds, in random order. Both combination tones were delivered at the same 12 intensities. After each 2-s stimulus, the participant was asked to rate its loudness on a scale from 0 (“no sound at all”) to 100 (“the loudest sound imaginable”), and to rate its unpleasantness from 0 (“not at all unpleasant”) to 100 (“the most unpleasant sound imaginable”).
Two subjects were excluded from participation in the auditory experiment: one (in the FM group) because she had Menière’s disease, the other (TMD group) because she wore hearing aids. A third subject (also in the TMD group) asked not to participate because she thought the sounds might trigger a migraine. Finally, experimenter error rendered the data of one member of the HC group invalid.
2.4. Statistical analysis
In most cases the data were statistically analyzed using ANOVA. One-way ANOVA was used when individual scores (as on the PILL) were being compared across groups; two-way mixed-model ANOVA was employed when measurements were being compared across intensity levels as well as groups. When a significant group effect was obtained, it was sometimes followed up with post hoc comparisons between specific groups. Alpha was set at 0.05, except in the case of planned comparisons, where it was reduced by Bonferroni correction to 0.025.
3. Results
3.1. Questionnaire measures
Group means and standard deviations for all questionnaires are given in Table 1. There were clear differences among the subjects in terms of their spontaneous pain, as indexed by three scales from the MPQ-SF: the sensory scale (MPQ-S), the affective scale (MPQ-A), and the MPQ-VAS scale on which subjects indicated their current pain level. In each case the scores of the HC participants were the lowest, and those of the FM patients the highest. As indicated in the table, the effect of group was significant (one-way ANOVA) for all three of these measures. For the sensory scale, post hoc comparisons revealed a significant difference between HC and TMD subjects [F(1,53)=7.70, p=.008] as well as between TMD and FM subjects [F(1,53)=5.64, p=.02]. TMD subjects were not statistically different from HC subjects in terms of the affective component of their pain [F(1,53)=1.40, p=.24], while FM subjects reported significantly greater affective pain than the other two groups combined [F(1,53)=11.24, p=.002]. The difference in current pain, as measured by the VAS, between the two clinical groups was not significant [F(1,53)=4.63, p=.04]; however, a highly significant difference between HC subjects and the clinical subjects was observed [F(1,53)=28.00, p<.0001].
Table 1.
Mean (SD) pain and psychosocial subject characteristics for each group
| Group |
|||
|---|---|---|---|
| Questionnaire Measure | HC | TMD | FM |
| MPQ-S** | 0.3 (0.6) | 4.1 (4.2) | 8.0 (7.8) |
| MPQ-A* | 0.1 (0.2) | 0.8 (1.6) | 2.6 (3.6) |
| MPQ-VAS** | 1.4 (3.1) | 18.3 (14.4) | 29.9 (25.1) |
| PILL** | 9.5 (7.5) | 20.5 (8.6) | 28.9 (5.7) |
| KRS | 77.1 (8.6) | 73.2 (15.5) | 83.8 (9.6) |
| PCS* | 8.4 (7.2) | 12.6 (9.2) | 17.8 (7.9) |
| STAI | 29.1 (8.6) | 31.5 (11.3) | 34.8 (11.5) |
| PA* | 34.0 (8.8) | 33.6 (9.5) | 26.3 (8.0) |
| NA | 11.5 (1.7) | 12.5 (4.4) | 12.5 (4.5) |
HC, Healthy Control; TMD, Temporomandibular Disorder; FM, Fibromyalgia; MPQ-S, McGill Pain Questionnaire-Sensory; MPQ-A, McGill Pain Questionnaire-Affective; MPQ-VAS, McGill Pain Questionnaire-Visual Analogue Scale; PILL, Pennebaker Inventory of Limbic Languidness; KRS, Kohn Reactivity Scale; PCS, Pain Catastrophizing Scale; STAI, State Trait Anxiety Inventory Form Y-1; PA, Positive Affect; NA, Negative Affect
p < 0.05
p < 0.001
There was also a highly significant effect of clinical status on hypervigilance, as assessed with the PILL [F(2, 53)=25.81, p<.0001]. As recommended by Pennebaker, a person’s score on this instrument was the number of items (such as “indigestion”, “sweat even in cold weather”, or “twitching of eyelid”) which she reported experiencing at least “every month or so” [18]. Once again, participants with FM had the highest average scores, and healthy controls the lowest. The use of these three groups thus appears to be a reasonable way of comparing individuals with different levels of chronic pain and different levels of hypervigilance. Post hoc comparisons between the individual groups revealed significant differences in PILL scores between HC and TMD subjects [F(1,53)=21.67, p<.0001], and between TMD and FM subjects [F(1,53)=9.50, p=.003]. In addition, scores on our secondary measure of hypervigilance, the KRS, approached a significant difference across groups [F(2,50)=2.93, p=.063]. Also consistent with an association between clinical status and hypervigilance is an effect of group on scores on the PCS [F(2,53)=5.00, p=.010], which contains items “reflecting magnification of the unpleasantness of pain situations and expectancies for negative outcomes” [22, p. 525], such as “I become afraid that the pain may get worse.”
In contrast, measures of state anxiety and negative affect did not significantly distinguish among the groups, although there was a marginally significant effect on positive affect [F(2,53)=3.24, p=.047]. Overall, the questionnaire results support the conclusion that our groups differed selectively in hypervigilance, rather than in some overall aspect of the way they completed psychometric instruments.
3.2. Pressure experiment
Individual estimates of sensation intensity were converted to logarithmic form, as is normally done in ratio scaling experiments as the first step in data analysis [5]. Occasional ratings of 0 were replaced with a small nonzero number (half the smallest nonzero intensity rating given to any of the pressure stimuli by any subject) before the logarithmic conversion. This same procedure was followed in the case of unpleasantness ratings and in the auditory experiment.
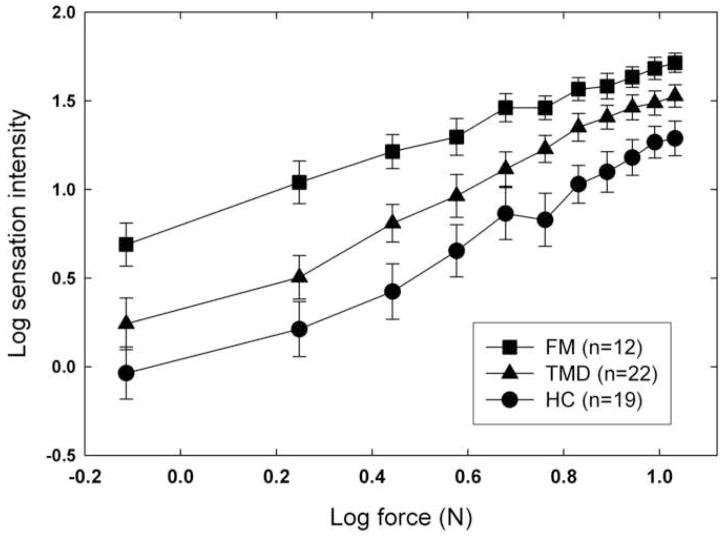
Subjects’ mean log estimates of the sensation intensity caused by the weighted rod are plotted in Figure 1, as a function of the log force exerted by the stimulus. Different symbols show the results for the three groups of subjects. For all subjects, sensation intensity is an increasing function of force. In addition, there is a clear separation between groups, the estimates of the FMs being the highest, and those of the healthy controls, the lowest. An ANOVA confirmed these impressions: both the effect of force [F(10,500)=136.46, p<.0001] and the effect of group [F(2,50)=14.59, p=.001] are highly significant; the interaction between these factors is also significant [F(20,500)=1.72, p=.027], reflecting a tendency for the functions to converge slightly at higher force levels. Post hoc comparisons revealed a difference, achieving Bonferroni-corrected significance, in reported intensity (taking into account ratings at all force levels) between healthy controls and TMD subjects [F(1,52)=5.36, p=.02]; the trend toward a difference between TMD and FM subjects was not significant [F(1,47)=4.03, p=.05].
Fig. 1.
Mean log sensation intensity is plotted as a function of the force exerted on the forearm by the weighted rod. Results for the three groups of subjects are plotted with different symbols, and error bars represent one standard error (S.E.M.) above and below each mean value.
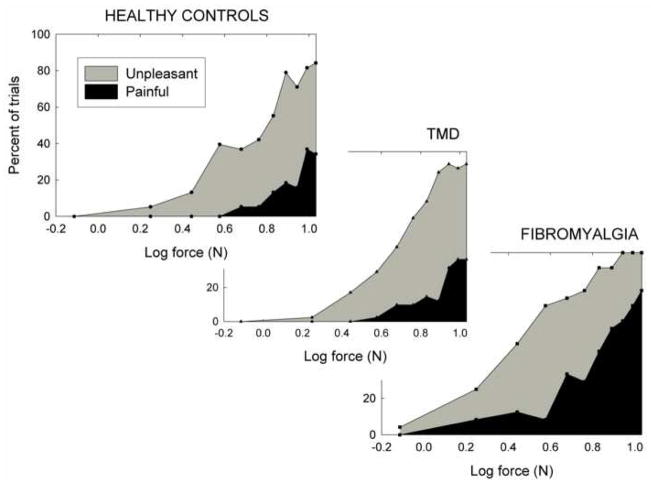
Use of these results to evaluate the Generalized Hypervigilance Hypothesis requires knowledge of how the stimuli were qualitatively perceived. For example, if they were all perceived as painful, their perceptual amplification would be consistent with earlier reports, but would shed no further light on the validity of the GHH. Each subject was therefore asked into which perceptual category (painful, unpleasant but not painful, or affectively neutral) each stimulus fell. These results are shown in Figure 2, where separate panels represent the different groups.
Fig. 2.
Results of the classification task in which each pressure stimulus was classified by the subject as painful, unpleasant but not painful, or neutral. All stimuli were presented twice, in separate random orders, to each participant.
In each group, the trend was for the weakest stimuli to be perceived as neutral; as force increased, responses gradually changed from neutral to unpleasant, and from unpleasant to painful. The figure suggests that these transitions occurred at lighter weights, and the overall frequency of pain responses was greatest, in the FM subjects.
To evaluate this impression, unpleasantness thresholds and pain thresholds were calculated for each subject, by averaging the log values of the lightest weights categorized by the subject as unpleasant and painful, respectively, in each block of trials. In the event that a subject did not report any weight to be unpleasant or painful, the heaviest weight was taken as threshold; if a subject’s ratings switched directly from “neutral” to “painful”, this value was taken as both unpleasantness and pain threshold. An ANOVA revealed a significant effect of group for both unpleasantness threshold [F(2,52)=9.15, p=.0004] and pain threshold [F(2,52)=8.94, p<.0005]. Post hoc comparisons showed that the results for the TMD subjects were similar to those of the healthy controls in terms of both unpleasantness [F(1,52)=.25, p=.62] and pain [F(1,52)=.24, p=.63] threshold. In contrast, FM subjects had significantly lower thresholds than the other two groups combined, for both unpleasantness [F(1,52)=18.18, p<.0001] and pain [F(1,52)=17.77, p=.0001].
For present purposes, it is important to note that the lowest force was almost always judged to produce a neutral (i.e. neither painful nor unpleasant) sensation, the only exception being a single trial for one of the FM subjects. Yet this stimulus evoked significantly different sensation intensity estimates for the three groups of subjects [F(2,52)=5.05, p=.01]. Perceptual amplification is clearly not confined to painful somatosensory stimuli, and these data suggest that it may not even be restricted to unpleasant ones.
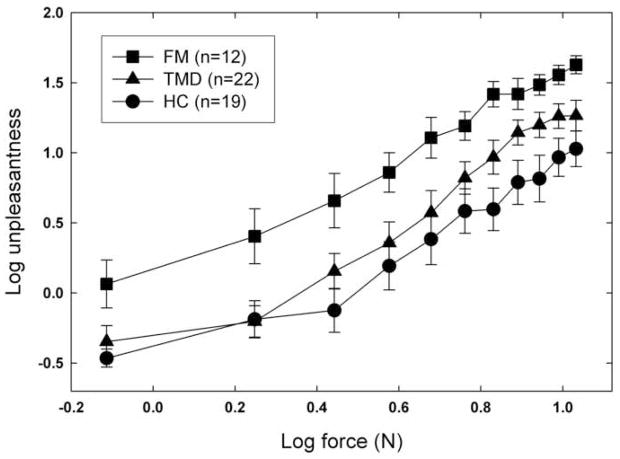
Unpleasantness estimates for the pressure stimuli, using the 0–100 scale, are shown in Figure 3. Despite some overlap of the TMD and HC functions, the data for the different groups are largely separated, and the main effect of this factor is significant [F(2,50)=7.29, p=.002]. The effect of force is also significant [F(10,500)=118.02, p<.0001], but the interaction of group and force is not [F(20,500)=.87, p=.62]. Post hoc comparisons showed no statistical difference between TMD and HC subjects [F(1,52)=2.66, p=.11], but FM subjects reported significantly higher unpleasantness than the other two groups combined [F(1,52)=12.33, p=.001]. A majority of the HC and TMD participants rated the weakest stimulus as 0 (“not at all unpleasant”), while most of the FM subjects gave it small, but nonzero, unpleasantness ratings. Comparing these data with the classification responses, it appears that a sensation may be classified as affectively neutral, yet evoke a nonzero unpleasantness rating when subjects are asked for a numerical estimate. Taken in combination, the data warrant the conclusion that robust perceptual amplification extends to stimuli that are nearly, if not entirely, devoid of unpleasantness, a result not predicted by the GHH.
Fig. 3.
Mean log unpleasantness as a function of the force exerted on the forearm by the weighted rod. Error bars represent one standard error (S.E.M.) above and below each mean value.
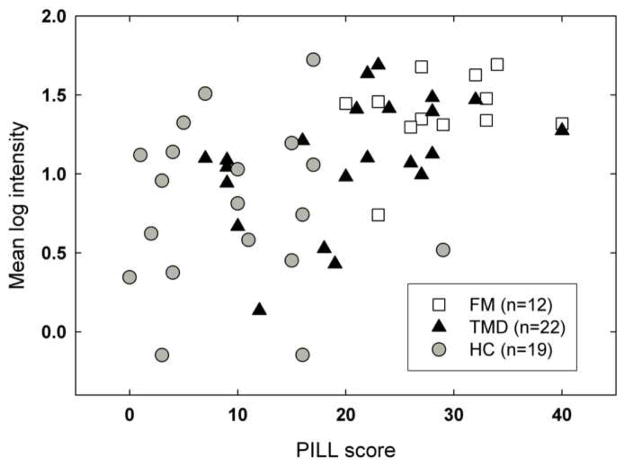
Was the perceptual amplification shown by members of the clinical pain groups a reflection of their elevated levels of hypervigilance? To explore this possibility, the mean log sensation intensity score of each individual was plotted as a function of PILL score (Figure 4). A Pearson correlation showed there was a highly significant positive association between the variables (r=.49, p<.001), and this relationship appears to be present within the largest (i.e., TMD) group as well as across groups. The data are thus consistent with the view that there is a functional relationship between hypervigilance and perceptual amplification.
Fig. 4.
Scatterplot of the relationship between individual subjects’ PILL scores and mean log intensity ratings for the weighted rod. Different symbols are used for subjects in different groups.
Taken as a whole, the results of the weighted rod experiment imply that the GHH is partially valid, but that it may need to be reframed: The present finding that unpleasant but not painful sensations are intensified in two groups of hypervigilant pain patients supports the theory; in contrast, the fact that stimuli that are virtually devoid of unpleasantness show equally robust perceptual amplification is at variance with it.
3.3. Auditory experiment
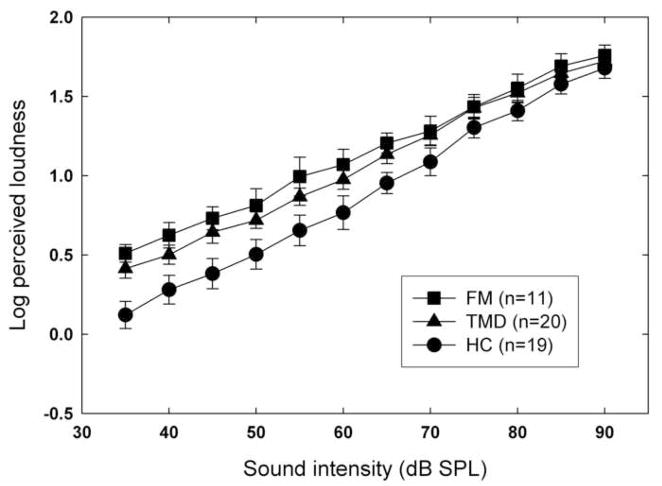
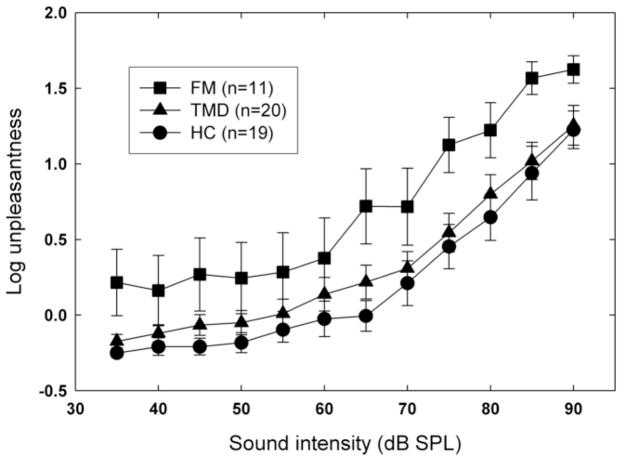
Mean log loudness estimates are shown, as a function of dB level, in Figure 5. The separation of the functions for different groups is less marked than in the weighted rod experiment, but is nevertheless significant [F(2,47)=3.85, p=.028]. Post hoc comparisons revealed a difference approaching Bonferroni-corrected significance between HC and TMD subjects [F(1,47)=4.56, p=.04], whereas no significant difference between the patient groups was observed [F(1,47)=.51, p=.48]. The effect of group on auditory unpleasantness ratings was also significant, F(2,47)=5.86, p=.005 (Figure 6). For this measure, post hoc comparisons revealed a significant difference between the patient groups [F(1,47)=7.09, p=.01] but no significant difference between controls and TMD subjects [F(1,47)=.73, p=.40]. In both cases, the effect of stimulus intensity was highly significant (p<.0001) but the interaction between intensity and group was not (p>.05).
Fig. 5.
Mean log loudness of auditory stimuli (two-tone combinations) as a function of sound level (dB SPL, A-weighted). Error bars show ±1 S.E.M. about each mean log rating.
Fig. 6.
Mean log unpleasantness of the auditory stimuli as a function of sound level (dB SPL, A-weighted). Error bars show ±1 S.E.M. about each mean log rating.
Were the small but significant differences in loudness estimates across groups a reflection of their differing levels of hypervigilance? Apparently so, for a Pearson correlation showed a positive association between the mean log loudness scores of the participants and their PILL scores (r=.36, p=.011).
4. Discussion
The major finding of this study is that perceptual amplification of pressure stimuli, in hypervigilant pain patients, occurs across a wide range of physical intensities, from those that are gentle and innocuous to higher levels that are strongly unpleasant or painful. This result has important implications for the Generalized Hypervigilance Hypothesis, the view that hypervigilance selectively causes amplification of aversive sensations [15]. An additional finding was a small but significant amount of perceptual amplification of auditory sensations in the same subjects; here again, the effect was not confined to unpleasantly intense levels of stimulation.
We used three groups of subjects differing in hypervigilance, as measured with the PILL, a widely recognized instrument for this purpose. They were exposed to a set of pressure stimuli, and to a series of auditory stimuli, and asked to rate the sensory intensity as well as the unpleasantness of each stimulus. To enable comparison of results from the two modalities, we used conditions that were as similar as practicable. In both cases the stimuli were presented in a randomized series of discrete trials, and after each trial the subject was asked to give ratings on 0–100 scales.
In both modalities, unpleasantness ratings confirmed that the weakest stimuli were affectively neutral (or only trivially unpleasant), but that unpleasantness grew steadily as stimulus intensity increased. There was a significant effect of group on unpleasantness ratings, primarily attributable in both modalities to differences between the FM subjects and the other two groups.
Our main interest, however, was in sensation intensity rather than unpleasantness, since the primary aim of the study was to determine the extent to which perceptual amplification—an increase in perceived intensity—occurs. For the pressure stimuli, the effect of group was highly significant, with the FM participants giving the highest ratings, and the HC participants the lowest; in other words, robust perceptual amplification occurred. It was expected that the pain from noxious levels of stimulation would be more intense in the patients, although the role of cognitive factors in this hyperalgesia is unclear. A more open question was whether perceptual amplification would occur for unpleasant stimuli that were not painful; to answer this, subjects were asked to classify every pressure stimulus as painful, unpleasant but not painful, or neutral. For most subjects, the weakest stimuli were described as neutral, and the strongest as painful or nearly so, while intermediate pressures were classified as unpleasant but not painful. The transition from one category to another varied from subject to subject, generally occurring at lower intensities for the FM subjects than for those in the other groups.
Despite these affective and, indeed, qualitative differences in the percepts elicited by the pressure stimuli, perceptual amplification was manifestly present across the full range of physical intensities employed (Figure 1). The fact that sensations of unpleasant but not painful pressure were augmented in the patients is consistent with the Generalized Hypervigilance Hypothesis.
But does this amplification actually reflect hypervigilance, or is it instead merely a result of some other characteristic that differs among participants? For example, individual differences in body-mass index (BMI) might complicate interpretation of the weighted-rod data. To evaluate this possibility, we calculated BMI for the 19 participants (3 FMs, 9 TMDs, and 7 HCs) for whom height and weight data were available. Mean BMIs for the three groups were 31.0, 26.4, and 23.5, respectively. The correlation between BMI and mean log sensation intensity ratings was non-significant [r=.27, p=.28], making it unlikely that BMI had a substantial influence on measured levels of perceptual amplification.
To determine the relevance of hypervigilance per se to the perceptual amplification shown in Figure 1, each individual’s mean log sensation intensity was compared with her PILL (hypervigilance) score. The correlation between these two factors across individuals (and regardless of clinical group) was substantial (r=.49) and highly significant (p<.001). It is thus plausible that perceptual amplification and hypervigilance are aspects of the same process.
It would be interesting to determine whether hypervigilant individuals would show comparable perceptual amplification for other types of cutaneous stimulation, such as warmth and electricity, at non-painful levels. Their use in future studies might give insight into the relative contributions of peripheral and central factors to perceptual amplification [12].
Weaker evidence of perceptual amplification emerged from the auditory experiment. Here there was only a small effect of group on subjective intensity (i.e., loudness), although the most intense sounds were comparable to the highest pressures in unpleasantness (compare Figures 3 and 6). Moreover, the separation among groups in loudness ratings does not grow with dB level (and hence with unpleasantness). These findings pose challenges for the GHH, which predicts that the unpleasantness of sensations (explicitly including auditory ones) is what determines their amplification in hypervigilant individuals [15].
We next examined the overall correlation, across subjects, between mean log loudness estimate and hypervigilance (i.e. PILL) score. The relationship between these measures, although significant (p=.011), was modest (r=.36), perhaps because there was little perceptual amplification for hypervigilance to account for.
It should be noted that McDermid et al.’s case for perceptual amplification in audition was based on auditory tolerance data [15]. These are suggestive of, but do not directly establish, the presence of amplification, since a variety of non-sensory factors may contribute to tolerance measurements. The present study used ratio scaling of sensory intensity, and was therefore able to directly confirm that auditory sensations are somewhat amplified in the presence of hypervigilance.
The disparity between the robust perceptual amplification obtained with pressure stimuli (intensity ratings for FMs were, on average, 292% larger than those of HCs), and the smaller amount (76%) reflected in the auditory data, shows that the phenomenon is not merely a tendency of hypervigilant subjects to give high intensity ratings to all sensory stimuli. The same point was made by Maixner et al., who asked TMD and HC subjects to make continuous VAS recordings of the painfulness of thermal stimuli delivered to the face and forearm, and the brightness of lights [14]. The thermal stimuli were perceived as more painful by the TMD participants than by the controls, but there was no difference between the groups in their ratings of visual stimuli.
In summary, then, we have shown that people with chronic myofascial pain, especially those with high levels of hypervigilance, show robust perceptual amplification for some types of stimuli. However, the present study establishes that the GHH, in its original form, is inadequate to account fully for this process, because it fails to predict the fact that perceptual amplification occurs for low, affectively neutral intensities of pressure and sound. In other words, the unpleasantness of stimuli does not appear to be the factor that determines whether they will be amplified.
But what is the critical factor? Perhaps the answer can be discerned by combining the views of hypervigilance put forward by Chapman [2] and McDermid et al. [15], respectively, into a unified model. Specifically, we propose that hypervigilance begins as a cognitive process, in which an individual is concerned about, and therefore closely monitors, particular types of sensations— especially those that, while not necessarily unpleasant in themselves, accompany or warn of impending pain. We further suggest that sustained direction of this affect-charged attention to a particular form of stimulation produces, over time, an increase in the perceptual gain for all stimuli of that type. For example, in TMD and FM, firm pressure on the skin routinely leads to pain; hence all pressure sensations, even gentle ones that pose no risk of pain, come to be amplified.
This hypothesis is consistent with evidence that transient attention to a visual stimulus increases its vividness [1]; we extend this idea by proposing that if (and to the extent that) attention is habitually focused on sensations of a particular type, their amplification increases and becomes autonomous.
The small amount of perceptual amplification found in the present study for auditory stimuli may be a result of the fact that in some individuals, loud sounds can trigger a headache—a relationship that induced one member of the TMD group to decline participation in the auditory experiment. This explanation of the data is speculative, but consistent with the fact that there is considerable comorbidity between headache and FM and TMD: Among participants in the auditory experiment, for example, headaches occurring at least once a month were reported (question 40 on the PILL) by 91% of FMs and 95% of TMDs, but by only 32% of HCs.
Further tests of the attentional gain control model of hypervigilance proposed here could make use of the fact that some types of pain are associated with sensations in still other modalities—e.g. the fortification illusions of migraine. It would be interesting to determine whether such migraine patients show perceptual amplification for flickering bars of light in the periphery of their visual field. Research on this question would resolve the issue of whether the strong link between somesthesis and hypervigilance is a unique and intrinsic one, or simply the result of experience.
Acknowledgments
We are indebted to Emily Riggan and Jonathan Besas for their help in conducting this research. The study was supported by NINDS grant NS045685. None of the authors have any arrangement or relationship, financial or other, which might represent a conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carrasco M, Ling S, Read S. Attention alters appearance. Nature Neuroscience. 2004;7:308–313. doi: 10.1038/nn1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapman CR. Pain: The perception of noxious events. In: Sternbach RA, editor. The psychology of pain. New York: Raven Press; 1978. pp. 169–202. [Google Scholar]
- 3.Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: Review, criteria, examinations and specifications, critique. J Craniomandib Disord: Facial & Oral Pain. 1992;6:301–355. [PubMed] [Google Scholar]
- 4.Fricton JR. The relationship of temporomandibular disorders and fibromyalgia: Implications for diagnosis and treatment. Current Pain and Headache Reports. 2004;8:355–363. doi: 10.1007/s11916-996-0008-0. [DOI] [PubMed] [Google Scholar]
- 5.Gescheider GA. Psychophysics: The Fundamentals. 3. Mahwah, New Jersey: Lawrence Erlbaum Associates; 1997. [Google Scholar]
- 6.Gibson SJ, Littlejohn GO, Gorman MM, Helme RD, Granges G. Altered heat pain thresholds and cerebral event-related potentials following painful C02 laser stimulation in subjects with fibromyalgia syndrome. Pain. 1994;58:185–193. doi: 10.1016/0304-3959(94)90198-8. [DOI] [PubMed] [Google Scholar]
- 7.Gracely RH, Grant MAB, Giesecke T. Evoked pain measures in fibromyalgia. Best Practice & Res Clin Rheumatol. 2003;17:593–609. doi: 10.1016/s1521-6942(03)00036-6. [DOI] [PubMed] [Google Scholar]
- 8.Granges G, Littlejohn G. Pressure pain threshold in pain-free subjects, in patients with chronic regional pain syndromes, and in patients with fibromyalgia syndrome. Arthritis and Rheum. 1993;36:642–646. doi: 10.1002/art.1780360510. [DOI] [PubMed] [Google Scholar]
- 9.Kohn PM. Sensation-seeking, augmenting-reducing, and strength of the nervous system. In: Spence JT, Izard CE, editors. Motivation, emotion, and personality. Amsterdam: Elsevier; 1985. pp. 167–173. [Google Scholar]
- 10.Kosek E, Ekholm J, Hansson P. Sensory dysfunction in fibromyalgia patients with implications for pathogenic mechanisms. Pain. 1996;68:375–383. doi: 10.1016/s0304-3959(96)03188-0. [DOI] [PubMed] [Google Scholar]
- 11.Lautenbacher S, Rollman GB. Possible deficiencies of pain modulation in fibromyalgia. Clin J Pain. 1997;13:189–196. doi: 10.1097/00002508-199709000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Lautenbacher S, Rollman GB, McCain GA. Multi-method assessment of experimental and clinical pain in patients with fibromyalgia. Pain. 1994;59:45–53. doi: 10.1016/0304-3959(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 13.Maixner W, Fillingim R, Booker D, Sigurdsson A. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain. Pain. 1995;63:341–351. doi: 10.1016/0304-3959(95)00068-2. [DOI] [PubMed] [Google Scholar]
- 14.Maixner W, Fillingim R, Sigurdsson A, Kincaid S, Silva S. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain: evidence for altered temporal summation of pain. Pain. 1998;76:71–81. doi: 10.1016/s0304-3959(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 15.McDermid AJ, Rollman GB, McCain GA. Generalized hypervigilance in fibromyalgia: evidence of perceptual amplification. Pain. 1996;66:133–144. doi: 10.1016/0304-3959(96)03059-x. [DOI] [PubMed] [Google Scholar]
- 16.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–198. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 17.Mikkelsson M, Latikka P, Kautiainen H, Isomeri R, Isomäki H. Muscle and bone pressure pain threshold and pain tolerance in fibromyalgia patients and controls. Arch Phys Med Rehab. 1992;73:814–818. [PubMed] [Google Scholar]
- 18.Pennebaker JW. The Psychology of Physical Symptoms. New York: Springer-Verlag; 1982. [Google Scholar]
- 19.Petzke F, Gracely RH, Park KM, Ambrose K, Clauw DJ. What do tender points measure? Influence of distress on 4 measures of tenderness. J Rheumatol. 2003;30:567–574. [PubMed] [Google Scholar]
- 20.Rollman GB, Gillespie JM. Disturbances of pain perception in temporomandibular pain syndrome. In: Lautenbacher S, Fillingim RB, editors. Pathophysiology of pain perception. New York: Plenum; 2004. pp. 107–118. [Google Scholar]
- 21.Spielberger CD. Manual for the State-Trait Anxiety Inventory (STAI) Palo Alto, California: Consulting Psychologists Press; 1983. [Google Scholar]
- 22.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychol Assessment. 1995;7:524–532. [Google Scholar]
- 23.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Personal Social Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 24.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, Fam AG, Farber SJ, Fiechtner JJ, Franklin CM, Gatter RA, Hamaty D, Lessard J, Lichtbroun AS, Masi AT, McCain GA, Reynolds WJ, Romano TJ, Russell IJ, Sheoin RP. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]