Abstract
Cortical surface evoked potentials (SEPs) are larger during sleep and characterize a sleep-like state in cortical columns. Since tumor necrosis factor alpha (TNF) may be involved in sleep regulation and is produced as a consequence of waking activity, we tested the hypothesis that direct application of TNF to the cortex will induce a sleep-like state within cortical columns and enhance SEP amplitudes. We found that microinjection of TNF onto the surface of the somatosensory cortex enhanced whisker stimulation-induced SEP amplitude relative to a control heat-inactivated TNF microinjection. We also determined if whisker stimulation enhanced endogenous TNF expression. TNF immunoreactivity (IR) was visualized after 2 h of bilateral deflection of a single whisker bilaterally. The number of TNF-IR cells increased in layers II–IV of the activated somatosensory barrel column. In two separate studies, unilateral deflection of multiple whiskers for 2 h increased the number of TNF-IR cells in layers II–V in columns that also exhibited enhanced Fos-IR. TNF-IR also colocalized with NeuN-IR suggesting that TNF expression was in neurons. Collectively these data are consistent with the hypotheses that TNF is produced in response to neural activity and in turn enhances the probability of a local sleep-like state as determined by increases in SEP amplitudes.
Keywords: barrel field, evoked response potentials, surface evoked potentials, local sleep, cortical columns, cytokine
Theoretical considerations (Krueger and Obál, 1993) and experimental findings (Rector et al., 2005) suggest that sleep is a fundamental property of neural assemblies such as cortical columns. Indeed, by probing neural assemblies with sensory input, e.g. whisker stimulation, the resulting surface evoked potentials (SEPs) of individual cortical columns provide a direct measure of the functional states of those columns. During sleep, average SEP amplitudes are larger than during wakefulness (e.g., Castro-Alamancos et al., 2002; Rector et al., 2005). During whole animal sleep, evoked responses are usually high amplitude and sleep-like, but occasionally drop into a wake-like low amplitude state. During whole animal wakefulness, the evoked responses are usually low amplitude, but sometimes appear sleep-like with high amplitude (Rector et al., 2005). Our earlier studies showed that the neural assembly state properties resemble use-dependent whole animal states to the extent that the probability of occurrence of the sleep-like state is dependent upon the prior duration of the wake-like state (Rector et al., 2005). Enhanced afferent input to a sensory column increases the probability that the column will subsequently be in the sleep-like state. Collectively, such data suggest that individual neural assemblies oscillate between functional sleep and wake states depending upon prior activity. We thus posit that organism sleep/wakefulness states emerge as a statistical property of populations of neural assemblies in various functional sleep/wake states (Krueger and Obál, 1993).
A separate literature dealing with the biochemical regulation of sleep also suggests that sleep is a regional property of brain. Sleep is posited to be initiated locally by activity-dependent production and release of sleep regulatory substances (SRSs) (Krueger and Obál, 1993, 2003). Tumor necrosis factor α (TNF) has met all the criteria for an SRS that we and others have proposed (Borbely and Tobler, 1989; Jouvet, 1984; Obál and Krueger, 2003). For instance, it induces non-rapid eye movement sleep (NREMS) and if inhibited, spontaneous NREMS is reduced (reviewed Obál and Krueger, 2003). TNF levels in brain correlate with sleep propensity while circulating TNF levels correlate with sleep or sleepiness in a variety of pathologies (Krueger et al., 2007). TNF acts on hypothalamic sleep regulatory circuits (Kubota et al., 2002) and the locus coeruleus (De Sarro et al., 1997) to promote NREMS. Unilateral microinjection of TNF onto the surface of the cortex ipsilaterally enhances electroencephalographic (EEG) slow wave activity (SWA) in a state-dependent manner (Yoshida et al., 2004). This observation suggests that TNF can induce local enhancement of sleep intensity and suggests a mechanism for localized sleep homeostasis.
Much additional evidence supports a role for TNF in sleep regulation (reviewed Krueger et al., 2007); although TNF is but one of several substances comprising the sleep homeostat, it remains the most thoroughly documented sleep regulatory substance (SRS). SRSs, such as TNF, produced in response to neural activation, may act locally on cortical columns to alter the functional state of the column (Krueger and Obál, 1993, 2003). To test this hypothesis, we used the whisker to barrel cortex model developed in part by Welker (Welker et al., 1992) in combination with an electrode microarray placed over the barrel cortex to characterize the electrical responses elicited by whisker stimulation during sleep and waking (Rector et al., 2005). We microinjected TNF onto the surface of the somatosensory cortex (Sctx) in an awake rat and then characterized individual cortical column SEPs induced by whisker deflection. We found that TNF enhanced SEP amplitudes in cortical columns thereby suggesting that TNF acts directly on neuronal assemblies to induce functional sleep-like states. To test the idea that TNF is activity-dependent, immunohistochemistry was used to analyze the whisker stimulation-activated cortical columns for Fos, a non-specific activity marker (Filipkowski et al., 2000) and TNF immunoreactivity (IR). We report that neuronal TNF is enhanced in afferent activity-driven columns relative to those with less activity.
EXPERIMENTAL PROCEDURES
ANIMALS
Three groups of male rats were used in these studies. Group A: Eight male Sprague-Dawley rats weighing 250–350 g obtained from Taconic Farms, Inc. (Germantown, NY) were used in the SEP physiology studies. Five of these rats were used for immunohistochemistry. Group B: Seven male rats were used for immunohistochemistry after unilateral manual whisker stimulation of rats contained in a plexiform chamber. Group C: Five additional male rats were used for immunohistochemistry after unilateral manual whisker stimulation of rats placed on top of a centralized, inverted flower pot inside a larger chamber to prevent inadvertent stimulation of contralateral whiskers. The use of rats in these experiments was in accordance with Washington State University guidelines and was approved by the Animal Care and Use Committee. The rats were maintained on a 12:12-h light-dark cycle (lights on at 0500) at 23±2°C ambient temperature inside environmental chambers. Water and food were available ad libitum throughout the experiment.
AGENTS
Rat recombinant TNF was purchased from R&D Systems (Minneapolis, MN) and dissolved in pyrogen-free sterile saline (PFS) at 150 ng/2 µl and stored at −20°C until just prior to the microinjections. The control solution for the physiology experiments was heat-inactivated TNF; boiled for 15 min at 100°C and then centrifuged to remove any precipitated protein.
Group A
BEHAVIORAL ADAPTATION – SEP EXPERIMENTS
In order to stimulate individual whiskers from unanesthetized animals, rats were trained to remain calm for extended periods of time under restraint. Initially rats were subjected to restraint in a simple strait jacket for 15 min and then an additional 15 min of restraint was added each day until the rats reached 2 h/day of restraint. After 8 days, the rats’ whiskers were brushed bilaterally for 2 h/day between 1400–1600 h for 6 days/week until the rats appeared to close their eyes and relax during such treatments. Experiments were performed using the latter half of daylight hours because the endogenous levels of TNF are lower at this time (Floyd and Krueger, 1997). Training typically required 1–2 months. Then the rats were prepared for surgical instrumentation.
SURGICAL INSTRUMENTATION (GROUP A ONLY)
The surgeries were performed during ketamine and xylazine (87 and 13 mg/kg, respectively) anesthesia. The rats were provided two 12-electrode arrays bilaterally; each electrode array (3 mm × 3 mm) had a microinjection guide cannulae glued into the center of the array. The arrays were placed on the primary Sctx as previously described (Rector et al., 2005). Each rat was also provided with stainless steel screws for recording EEG bilaterally over the frontal cortex. EMG was recorded from a pair of stainless steel wires in the neck muscles. A ground reference screw was also placed on the skull 1 mm rostral and 1 mm lateral to the lambda-midline intersection. After the surgery was completed, the dura mater below the microinjection cannulae was broken with a 30 g needle and an obturator was placed in the cannulae to maintain patency. After surgery, the rats were given 7–10 days to recover.
ELECTRICAL MAPPING OF SOMATOSENSORYWHISKER CORTICAL COLUMNS (GROUP A)
SEPs were determined after systematically stimulating a number of individual whiskers, mainly those longer whiskers in the caudal region of the whisker map. During baseline experiments, one electrode from each side was selected that showed the greatest amplitude difference between the first positive peak (P1) and the next negative trough (N1) after whisker stimulation. This electrode was the closest to the cortical column of the stimulated whisker. Whiskers were stimulated by placing them into a hypodermic tube glued to an audio speaker. Tension was placed on the whisker by lifting the tubing so that the whisker was deflected when a random stimulator program moved the speaker. Random stimulation was performed by twitching the whiskers at 75 µm for 0.2 ms at random intervals between 1 and 2 sec. This random stimulation prevented the whisker response from habituating. The rats were stimulated and recorded from for 2 h periods at least 3–5 times per week until sleep during baseline recordings was observed as determined from the EEG recordings. EEG and EMG were continuously sampled during the 2–3 h period at 20 kHz per channel filtered between 0.1 Hz and 3.2 kHz. This typically took another 1–2 months of training. For the 8 rats used in this study, whisker C1 was used for two rats, D1, for four rats and E1 and C0 for the other rats respectively.
MICROINJECTION AND RECORDING (GROUP A)
All microinjections and recordings were performed on a surgical table in the same room (maintained at 23±2°C) as the home cages. All experiments were performed starting at the same time of day as the original baseline adaptation (1400 h). One day prior to the microinjections, the obturators were lifted up within their guide cannulae but not removed, and a baseline was recorded for 2 h to adapt the rat to the microinjection manipulations. Other baselines were recorded between microinjections and after completing microinjections on each side. On the day of the experiment, one obturator was removed and a microinjection needle was placed such that its tip was below the dura 1 min prior to microinjection of heat-inactivated TNF or TNF (150 ng/2 µl). The injection onto the surface of the Sctx was performed with 30 sec pauses between each 0.4 µl injection. The needle was left in the cannulae for an additional 1 min and then removed and the obturator replaced. In another study, after similar injections of a dye, the dye spread 2–3 mm across the surface of the cortex within a few min (Yoshida et al., 2004). On any individual experimental day, only one unilateral microinjection was performed. The opposite side received an injection 7 days later. The heat-inactivated TNF was microinjected first on each side and then 7 days later, TNF microinjections were performed. In two of the rats heat-inactivated TNF microinjection was repeated 7 days after the TNF microinjection. In these rats, the differences in the SEP amplitudes between the TNF and heat-inactivated TNF microinjections were similar to those observed with when the heat-inactivated TNF was microinjected first. After each injection, two whiskers, one on each side of the face that projected to equivalent positions within the somatosensory facial field, were stimulated bilaterally for the next 2–3 h. The recording period varied slightly because whenever the rats removed one of their whiskers from the stimulator tubing, the recording was halted until the whisker could be replaced into the tubing. The final microinjection in 5 of the 8 of the rats was bilateral with one side receiving TNF and the opposite side, heat-inactivated TNF.
SLEEP STATE AND SEP ANALYSIS (GROUP A)
The sleep states were determined using recordings from the frontal EEG and EMG electrodes. The sleep states were evaluated visually in 2 s epochs using a custom computer program that used EEG delta power (0.5–4 Hz) and EMG total power to separate animal state into wake and NREMS as previously described (Rector et al., 2005). In these experiments, 200–500 two-second epochs during wake were averaged, while 75–250 NREM sleep epochs were averaged. We did not observe rapid eye movement sleep (REMS) in our restrained animals. To normalize the data for comparison between different microarrays with different resistances and positions relative to the barrel column, SEP amplitudes were averaged then expressed as a ratio of the averages of the SEP amplitudes obtained from the TNF (or heat-inactivated TNF) injected side relative to average SEP amplitude obtained from the non-injected side before and after TNF (or heat-inactivated TNF) treatments. These normalized SEP amplitude ratios were compared using an unpaired, 2-sided independent t test. Since the resistance and the position of the microarrays relative to the surface of the cortex differed between the two sides, we could not make a direct statistical comparison between the SEP amplitudes on the opposite sides.
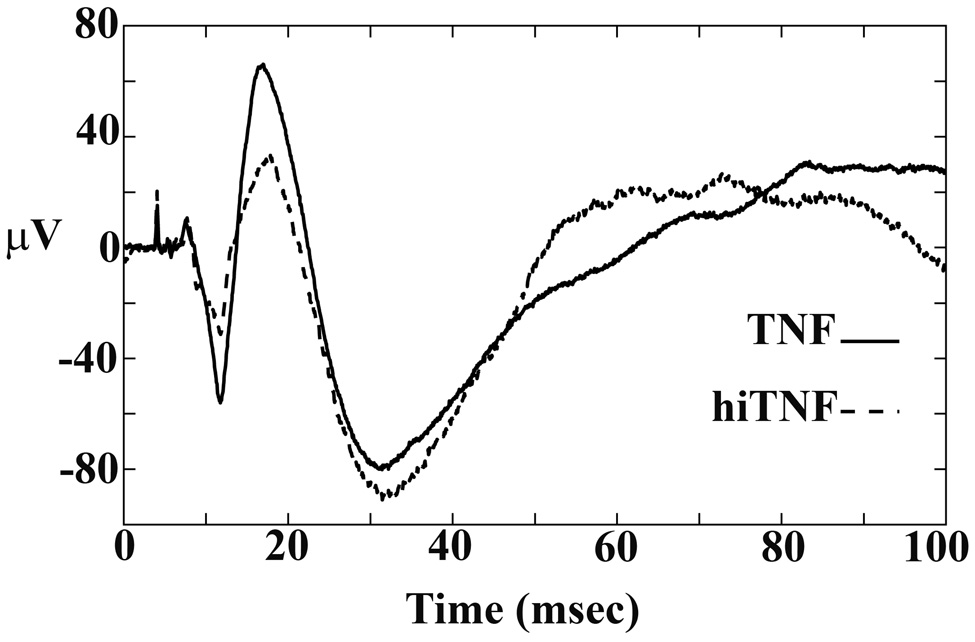
To determine SEP amplitudes, recordings from the electrode arrays were filtered between 10 and 200 Hz. For each SEP, the amplitude difference in microvolts was computed between the height of the first peak, P1 and the first trough, N1. The SEPs for each of 1,000 triggers measured during a typical recording episode was averaged to form a gold standard and used to fit the individual SEPs to extract P1 and N1 amplitude parameters for each individual response. We focused on the P1-N1 amplitude difference (µV units) for this analysis because its origin is from the primary Sctx (Hall and Borbely, 1970; Armstrong-James et al., 1992) and it involves the least amount of data transformation. These values were averaged over the entire recording period. Examples of the SEP amplitudes after each trigger are presented in Figure 2.
Figure 2.
Examples of time-triggered average traces to whisker twitches during NREMS after either TNF or heat-inactivated TNF (hiTNF) microinjection onto the surface of the Sctx. The SEP amplitude is greater after the TNF microinjection than after the heat-inactivated TNF. The increases in SEP amplitudes occurred whether the animal was awake or asleep.
IMMUNOHISTOCHEMISTRY (GROUP A)
In the final experiment with Group A rats, five of the eight rats were injected with heat-inactivated TNF on one side and TNF on the opposite side prior to whisker stimulation for 2 h. Then the rats were anesthetized with isoflurane and cardiac-perfused with warm saline followed by cold 4% paraformaldehyde. Brains were then post-fixed in this fixative for 2 h and sunk in 20% sucrose overnight. The brains were sectioned with the sliding microtome at 30 µm thickness and coronal sections were collected every 360 µm for each of the antigens, using the goat polyclonal antibody to rat recombinant TNF (0.5 µg/ml; R&D Systems, Minneapolis, MN), or rabbit anti-rat Fos antibody (1:10000; Calbiochem, Oncogene Research Products, San Diego, CA), using the immunohistochemical procedures as previously described (Churchill et al., 2005). For double labeling TNF-IR with a neuronal marker, a mouse monoclonal antibody to the neuronal nuclear protein, Neu N (1:1000; Chemicon, Temecula, CA) was used as a primary antibody. For double labeling with TNF & Fos or TNF & NeuN, these antibodies were diluted in 2% chicken and 2% donkey serum. After 3 days of incubation at 4°C with gentle rocking, the sections were incubated for 2 h in secondary antibody (1:500) to goat conjugated with Alexa Fluor-568 (Invitrogen-Molecular Probes, Eugene, OR) and a secondary antibody to rabbit (for Fos) or mouse (for NeuN) conjugated to Alexa Fluor-488 (Invitrogen-Molecular Probes, Eugene, OR) in 2% of the same blocking serum.
SPECIFICITY OF THE TNF ANTIBODY
Western blot analyses for TNF were performed using sleep deprived rat Sctx to demonstrate antibody recognition of the two forms of TNF. Briefly, samples were homogenized in lysis buffer (50 mM Tris-HCl, pH = 7.2, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, 1 mM NaF, 1 mM NaNO3, and containing one tablet of Complete Protease Inhibitor Cocktail (Roche Applied Science, Indianapolis, IN) and incubated on ice for 60 min. The homogenates were centrifuged at 10,000 g for 10 min at 4°C and the supernatants collected. Protein concentration of the samples was determined by the DC Protein Assay Kit (Pierce, Rockford, IL). Twenty micrograms of total protein was separated by electrophoresis through a denaturing 4–15% SDS polyacrylamide gel, and the proteins were transferred to a nitrocellulose membrane. The membranes were blocked in 5% milk and then incubated in a 1:150 dilution of goat anti-rat TNF (R&D systems) in 5% nonfat dry milk/tween tris-buffered saline (TTBS) overnight at 4°C. After three washes in TTBS, the nitrocellulose membranes were incubated in a 1:4,000 dilution of horse anti-goat HRP conjugated secondary antibody (TNF). Immunoreactive protein was detected by the enhanced chemiluminescence detection reagent (ECL kit) according to manufacturer's instructions (GE Healthcare Bio-Sciences Corp, Piscataway, NJ), and bands were visualized by exposure to autoradiographic film Cruz Marker (Santa Cruz Biotechnology, Santa Cruz, CA) molecular weight standards were used to identify the molecular weights of the bands.
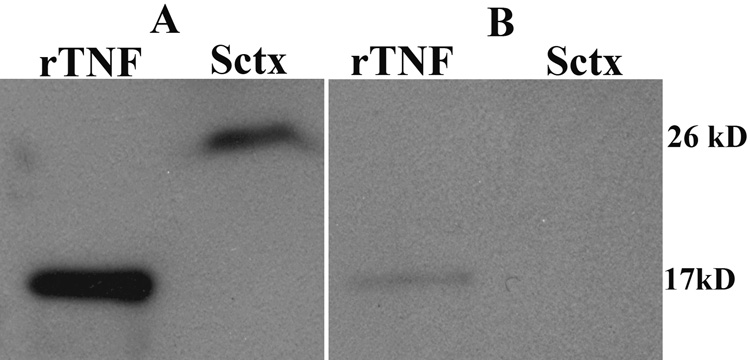
Western blots of rat recombinant TNF indicated the antibody recognized the 17 kD protein and Western blots of Sctx extracts from a sleep-deprived rat indicated that the antibody recognized the membrane bound form of TNF at 26 kD as well (Figure 1). Preabsorption of the antibody with rat recombinant TNF blocked the labeling of both the 17 kD & 26 kD bands in the Western blots. Negative immunohistochemistry controls for TNF were produced by omitting the primary antibody and in separate experiments by pre-incubating the primary antibody (250 ng) with 750 ng recombinant TNF for 24 h prior to the 3-day incubation with the sections. No positive darkly-stained TNF-IR cells were observed in layers I–IV in these control sections (see Figure 3A). A significant reduction in staining was observed in layer V as well. Previously Taishi et al., (2007) showed that decreases in the TNF mRNA levels induced by injection of a TNF siRNA significantly decreased the density of TNF-IR cells in the injected area, demonstrating that knocking down TNF mRNA levels in cortex decreases the specific TNF-protein-IR using this antibody. In another study from our laboratory we showed that this anti-rat TNF antibody also labeled cells in the Sctx of wild type mice, but in TNF knockout mice, there was very little TNF-IR in the cortex. Finally, this anti-TNF antibody was used previously to demonstrate TNF-IR co-localized with NeuN (Figiel and Dzwonek, 2007).
Figure 1.
Goat anti-rat TNF antibody made by R&D Systems showed one band in the Western blot analyses of the rat recombinant TNF (rTNF) at 17 kD (A and B-first lane) and one band in the sleep deprived Sctx at 26 kD (second lane). The 26 kD protein is the membrane bound form of TNF. Preabsorption with rat rTNF (B) blocked the antibody from labeling both recombinant rat TNF (first lane) and the 26 kD band in the Sctx (second lane).
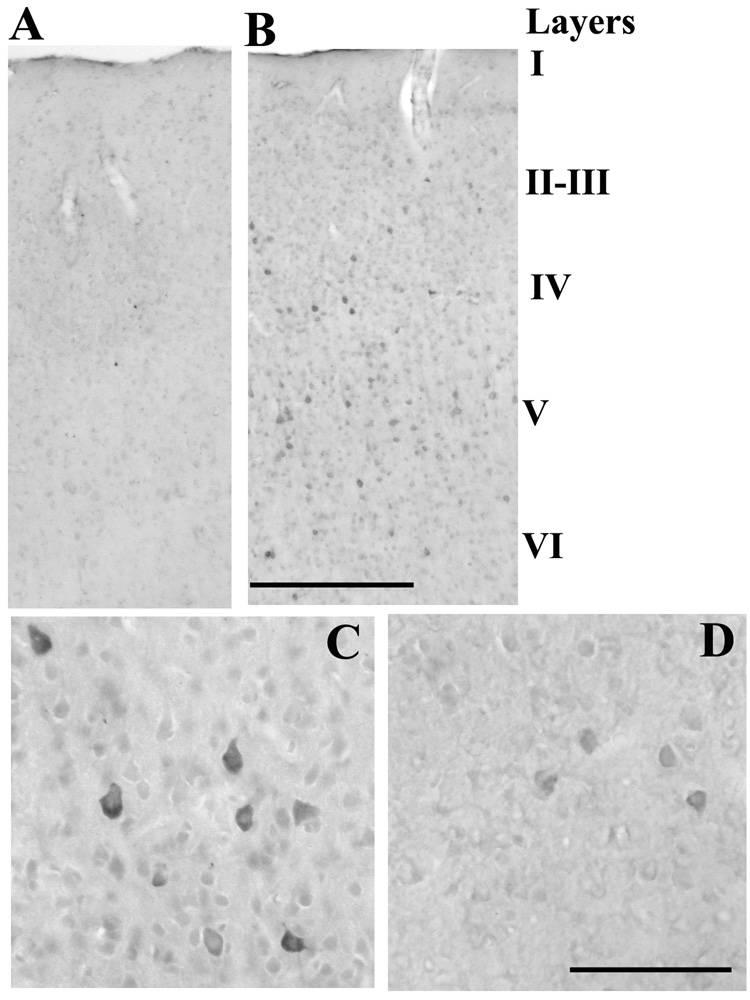
Figure 3.
A. Preabsorption of the primary antibody to rat TNF with rat recombinant TNF (3-fold greater concentration) completely blocked the darkly stained TNF-IR cells evidenced in B. B. Whisker deflection of Group A rats increased the number of TNF-IR cells in layer II–III & V underlying the electrode microarray on the side injected with the control heat-inactivated TNF relative to the adjacent unstimulated column. Bar = 0.7 mm. Higher magnification in the stimulated (C) and unstimulated columns (D) illustrates the neuronal-like shape of the TNF-IR cells with the greatest immunoreactivity surrounding the nuclear region and into the apical dendrite. Bar = 0.05 mm.
Group B
MANUAL WHISKER STIMULATION
A separate group of rats (n=7) were adapted to unilateral manual whisker stimulation of about 30 times per minute by gradually increasing the stimulation time by 15 min intervals until reaching 2 h/day after 8 days. Stimulation began at 1400 h every day. After the initial 8 days of progressive adaptation, the rats were subjected to manual brushing of some of the caudally located mystacial whiskers B0, B1, C0, C1, D0, D1, and E1 for 2 h/day for 2 more days. On these days the start of the whisker stimulation for each rat was spaced at 8 min intervals beginning at 1400 h so that each rat received exactly 2 h of stimulation prior to sacrifice. The direction of the manual brushing was mainly from behind the head toward the snout. Behavioral observations indicated that the rats were accustomed to the whisker brushing on the day of the kill. For instance, the frequency of defecation during the 2 h of stimulation was reduced from the early days of adaptation. Just after 2 h of whisker stimulation on the third day, the rats were anesthetized with isoflurane and killed prior to cardiac perfusion with warm saline (0.9% NaCl) followed by cold 4% paraformaldehyde in phosphate-buffered saline (PBS). The brains were removed, allowed to post-fix for 2 h in 4% paraformaldehyde in PBS, and then sunk in 20% sucrose overnight. The brains were frozen in crushed dry ice and stored at −80°C until sectioning.
Group C
MANUAL WHISKER STIMULATION
A separate group of rats (n=5) were adapted to placement onto the top of an inverted flower pot (5 inch diameter) centrally located above a pool of warm water (37 °C) at least 15 cm from the chamber wall and unilateral manual whisker stimulation was carried out as described for Group B. In Group B, the rats brushed their opposing whiskers against the chamber wall resulting in inadvertent whisker stimulation on the opposite side. The rats were prevented from reaching the chamber wall and stimulating their whiskers on this surface in this experiment. However, some inadvertent whisker stimulation against their own shoulders still occurred when the rats turned their heads to avoid the whisker stimulation. Just after 2 h of whisker stimulation, the rats were anesthetized with isoflurane and killed as described for Group B.
IMAGING
Digital images of the diaminobenzidine (DAB)-stained or fluorescent sections were taken using a light microscope (Leica DMLB) with a Spot camera with a RT slider. Double-stained sections were visualized using a confocal laser scanning microscope LSM510 (Zeiss Imaging, Oberkochen, Germany). The Argon 488nm laser was used for Alexa Flour 488; HeNe 543 nm laser, for Alexa Flour 568. Single plane with full resolution was used for each fluorescent channel. The digital images were adjusted using Adobe Photoshop, only in contrast and brightness. Digital images of the cortical columns in the Sctx that showed Fos activation (primarily in the caudal regions of the whisker map – Bregma −3.0 mm) were prepared and the number of darkly-stained-IR cells counted within a rectangle 0.5 mm wide by 0.225 mm deep for layer II–III, IV and V of the Sctx using a transparent overlay. A stimulated column was defined by having twice or more of the Fos-IR nuclei in layers II–III than in an adjacent unstimulated column. Quantification was done by two individuals blind to the experimental treatment.
STATISTICAL ANALYSES
GROUP A) Amplitude P1-N1
Overall, an unpaired, two sided independent t-test was used to compare the ratio of the averaged SEP responses after TNF to SEP responses on the non-injected side recorded before and after the injection to the ratio of the averaged SEP responses after heat-inactivated TNF to the averaged SEP responses on the non-injected sides before and after the injection. Paired t-tests were used to compare ratios of SEP values obtained during sleep and waking episodes during the initial 25 or 50 minute post-injection periods.
Groups A–C) Activity-dependent changes in the TNF-IR cells
a paired Student’s t-test was used to evaluate the differences in the number of immunoreactive cells between the stimulated (Fos-IR evident) and unstimulated columns (little Fos-IR) on the control side receiving the heat-inactivated TNF (Group A rats) or on the stimulated side opposite to the manual brushing of the whiskers (Group B rats). For Group C rats, a paired Students t-test was used to compare stimulated and unstimulated sides of the cortex.
RESULTS
SEP AMPLITUDES (GROUP A)
The SEP P1-N1 amplitudes were greater after TNF microinjection than after heat-inactivated TNF (for example, see Figure 2). The ratios of SEP amplitudes for the 2–3 hour recording period after TNF treatment (1.14 ± 0.005) were significantly greater than the ratios of SEP amplitudes determined after heat-inactivated TNF treatment (0.96 ± 0.036) (p = 0.009). The averaged latencies for P1 were 29 ± 6 msec, while the averaged latencies for N1 were 48 ± 9 msec; there were no significant differences in latencies after the TNF and heat-inactivated TNF injections (data not shown). Of the animals that slept during the first 50 minute post-injection period (N=7), the P1-N1 amplitudes were larger during sleep than during waking (p = 0.01) as was found in our earlier study (Rector et al., 2005). During the initial 25 minute period, SEP values increased 25 +/− 8% during sleep periods after TNF relative to heat-inactivated TNF treatment (p = 0.05) and 17 +/−5% during waking periods (p = 0.05) in the same animals.
Bilateral Whisker Stimulation Increases TNF-IR Cells (Group A)
TNF-IR in a stimulated column is illustrated relative to an adjacent unstimulated column in Figure 3. The stimulated columns exhibited a significant increase in the number of TNF-IR cells in layer II (Figure 3B, Table 1) (p = 0.035; n = 5) and layer IV (p = 0.016) but not in layer V (p = 0.285). Higher magnification illustrates the neuronal-like shape of the TNF-IR cells (Figure 3C) in the stimulated column and the greater number of cells than in the unstimulated column (Figure 3D).
Table 1.
Number of TNF-IR cells in the stimulated column under the microarray vs. unstimulated columns outside the microarray within layers of the Sctx. The data are expressed as mean ± SEM for 5 rats.
| IMMUNOREACTIVITY IN SCTX LAYERS | TREATMENT |
||
|---|---|---|---|
| STIMULATED | UNSTIMULATED | ||
| TNFα-IR | Primary Sctx-II–III | 12.3 ± 4.1* | 7.9 ± 2.8 |
| Primary Sctx-IV | 22.7 ± 5.9* | 13.6 ± 5.0 | |
| Primary Sctx-V | 28.3 ± 3.2 | 30.5 ± 5.2 | |
p <0.05 comparing stimulated and unstimulated sides, using a paired Students’ t-test.
UNILATERAL WHISKER STIMULATION INCREASES TNF-IR CELLS IN THE EQUIVALENT FOS-ACTIVATED COLUMN RELATIVE TO THE ADJACENT UNSTIMULATED COLUMN (GROUP B)
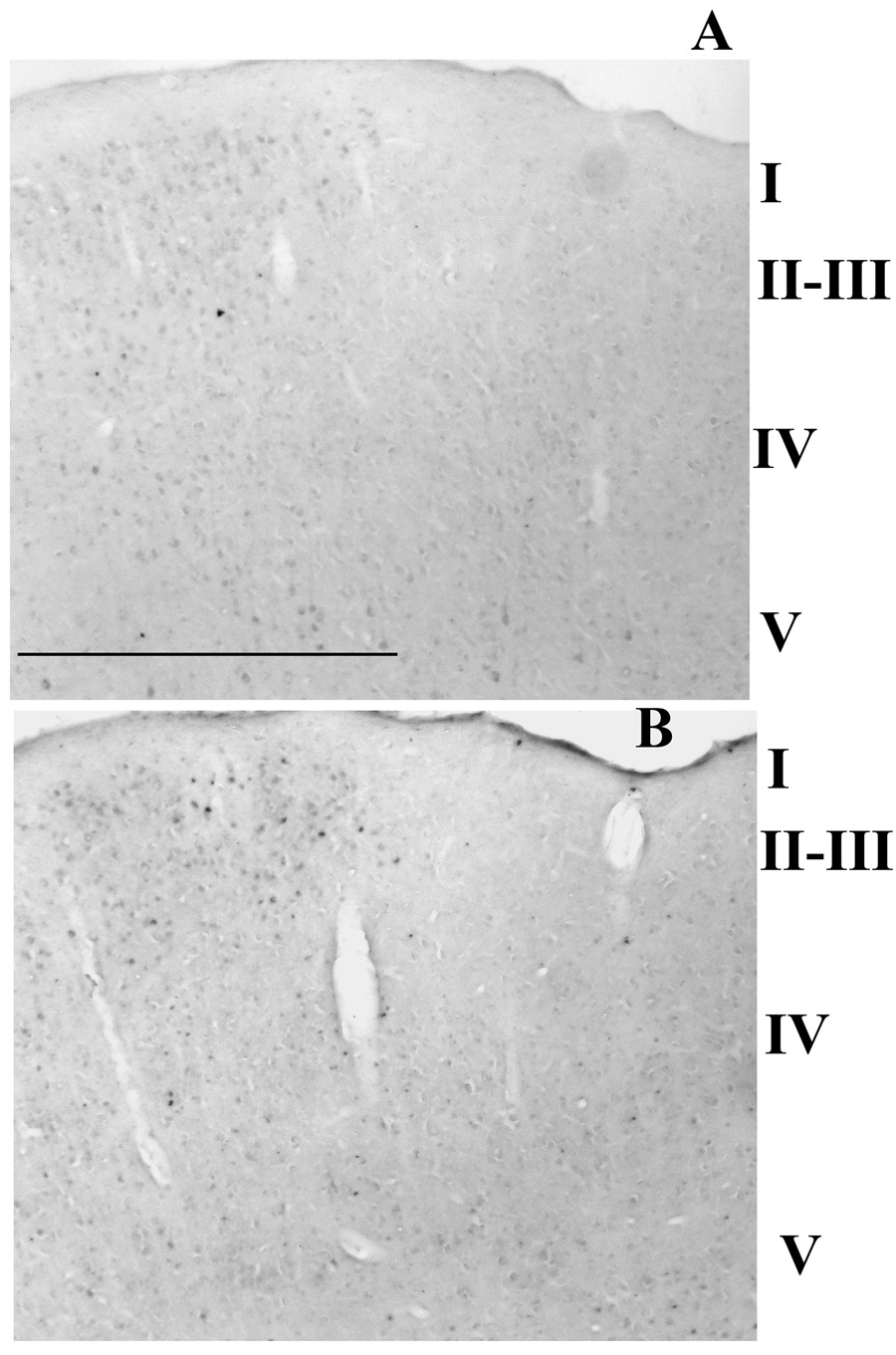
Manual stimulation of multiple whiskers for 2 h increased the number of TNF-IR cells (Figure 4A) in the equivalent Fos-activated columns as marked by the horizontal line (Figure 4B) relative to the adjacent column that did not show Fos activation (to the right of the line). A significantly higher number of TNF-IR cells were observed in layers II–III (p = 0.003) and in layer IV (p = 0.0001) but not in layer V (p = 0.42) when the stimulated column was compared with an adjacent unstimulated column (Table 2). A significant increase in the number of Fos-IR cells was also demonstrated in adjacent sections after 2 h of whisker stimulation in layers II–III [Table 2; p = 0.001; n = 7), layer IV (p = 0.002) and layer V (p = 0.0004)].
Figure 4.
Manual whisker brushing for 2 h in Group B rats increased the number of TNF-IR cells in the Sctx of a stimulated (horizontal bar marks the stimulated column) but not in an adjacent unstimulated cortical columns (A). In this case a large increase in the number of TNF-IR cells was evident in the upper layers of the Sctx. For comparison, whisker stimulation increased the number of Fos-IR cells in an equivalent column of the Sctx in an adjacent section (B). Bar = 0.6 mm.
Table 2.
Fos- and TNF-immunoreactivity in Group B rats after whisker stimulation. Number of Fos- and TNF-IR cells in the stimulated vs. unstimulated columns within layers of the SSctx. The data are expressed as mean ± SEM for 7 rats.
| IMMUNOREACTIVITY IN SCTX LAYERS | TREATMENT | ||
|---|---|---|---|
| STIMULATED | UNSTIMULATED | ||
| Fos | Primary Sctx-II–III | 23.1 ± 4.9* | 8.9 ± 2.06 |
| Primary Sctx-IV | 22.4 ± 5.6* | 10.9 ± 5.6 | |
| Primary Sctx-V | 12.4 ± 3.3* | 8.3 ± 2.8 | |
| TNFα | Primary Sctx-II–III | 17.9 ± 4.2* | 11.3 ± 3.2 |
| Primary Sctx-IV | 11.4 ± 2.2* | 5.9 ± 1.9 | |
| Primary Sctx-V | 15.0 ± 2.8 | 15.1 ± 3.2 | |
p <0.05 comparing stimulated and unstimulated columns, using a paired Students’ t-test.
UNILATERAL WHISKER STIMULATION INCREASES TNF-IR CELLS CONTRALATERALLY RELATIVE TO THE IPSILATERAL SIDE (GROUP C)
Manual unilateral stimulation of multiple whiskers for 2 h also increased significantly the number of TNF-IR cells on the side opposite to the whisker stimulation in layers II–V relative to the opposite cortex receiving input from the unstimulated side (Table 3; II–III (p = 0.009; n = 5), IV (p = 0.0.015) and V (p = 0.016). Fos activation was also increased significantly in the same region of the primary Sctx on the side opposite to whisker stimulation relative to the opposite side (Table 3; layers II (p = 0.045) and IV(p=0.008) but not in layer V (p = 0.118).
Table 3.
Number of Fos- and TNF-IR cells on the stimulated vs unstimulated sides within layers of the Sctx from Group C rats after whisker stimulation for 2h. The data are expressed as mean ± SEM for 5 rats.
| Immunoreactivity in SSctx layers | Treatment |
||
|---|---|---|---|
| Stimulated | Unstimulated | ||
| Fos | Primary Sctx –II–III | 168.4 ± 11.2* | 138.7 ± 4.4 |
| Primary Sctx-IV | 207.3 ± 14.2* | 161.3 ± 20.3 | |
| Primary Sctx-V | 120.1 ± 10.5 | 107.0 ± 7.2 | |
| TNFα | Primary Sctx-II–III | 40.6 ± 5.4* | 25.3 ± 6.0 |
| Primary Sctx-IV | 36.6± 5.5* | 24.9 ± 3.1 | |
| Primary Sctx-V | 46.3 ± 4.1* | 38.5 ± 4.1 | |
p<0.05, comparing stimulated- and unstimulated sides, using a paired Students’ t-test.
COLOCALIZATION OF FOS-IR OR NEUN WITH TNF-IR CELLS (GROUP A)
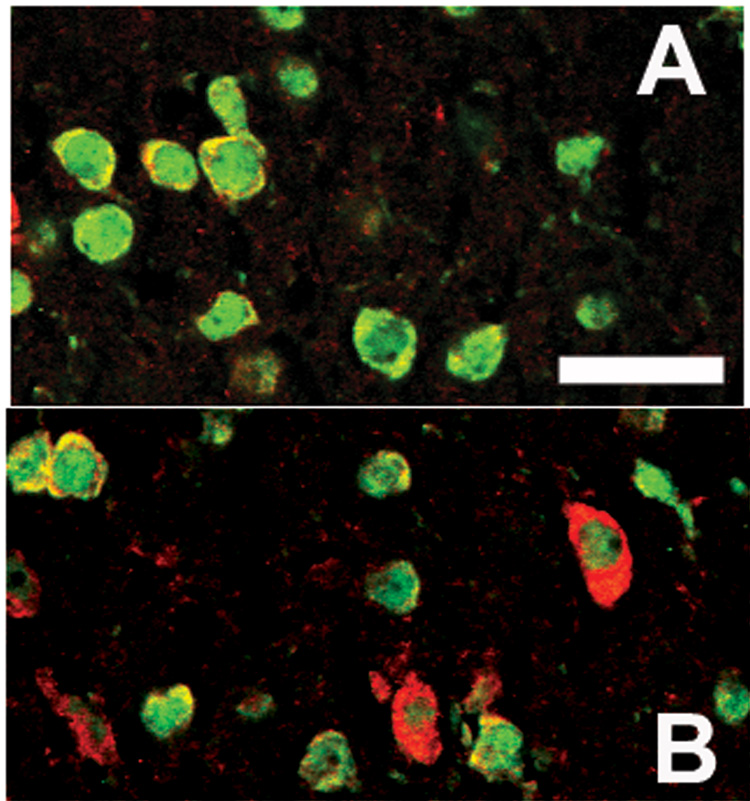
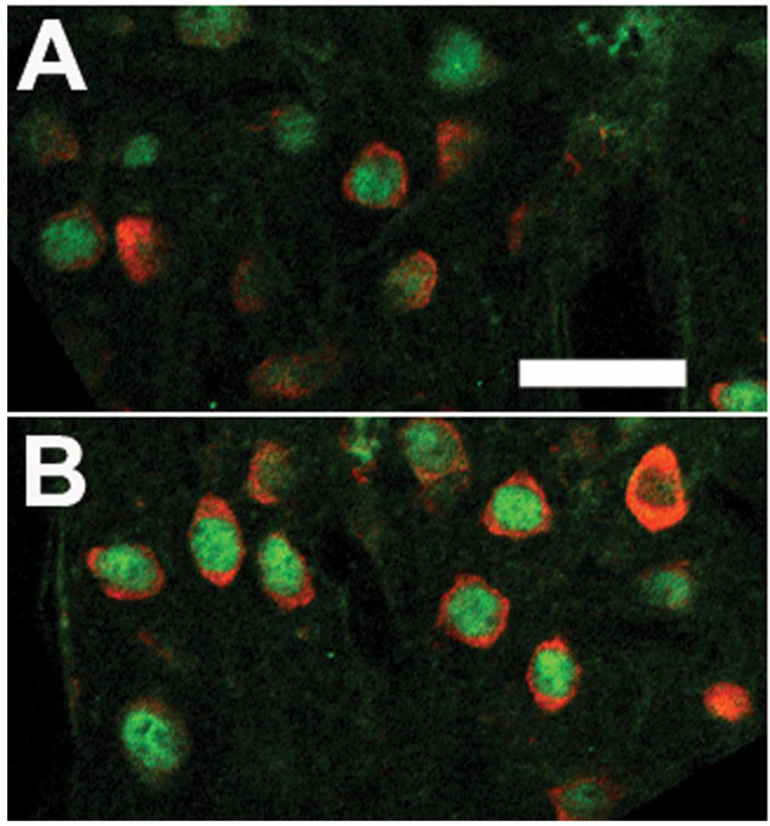
Immunofluorescence using double labeling for the neuronal marker, NeuN and TNF demonstrated double labeled cells (Figure 5). The number of NeuN-IR cells did not differ between the stimulated (Figure 5B) and unstimulated columns (Figure 5A) in layers II–III but the number of double-labeled TNF-IR and NeuN-IR cells were greater in the stimulated column (Figure 5B). Also double labeling immunofluorescence demonstrated Fos-IR in the nuclei of some of the TNF-IR cells in the cytoplasm of cells found in stimulated columns (Figure 6B). The nearby unstimulated column (Figure 6A) showed dramatically less signal for both Fos- and TNF-IR.
Figure 5.
Confocal laser scanning microscopic images of the stimulated (B) and unstimulated (A) columns in the Sctx after double labeling with fluorescent probes for TNF (red)-IR and NeuN (green)-IR. Whisker stimulation increases the number of TNF-IR nuclei (B relative to A), but did not alter the number of NeuN-IR cells in the Sctx. Bar = 0.01 mm.
Figure 6.
Confocal laser scanning microscopic images of the stimulated (B) and unstimulated (A) columns in the Sctx after double labeling with fluorescent probes for TNF (red)-IR and Fos (green)-IR. Whisker stimulation increases the number of TNF- and Fos-IR nuclei (B relative to A). Fos-IR was evident within nuclei surrounded by TNF-IR in the surrounding cytoplasm. Bar = 0.01 mm.
DISCUSSION
Previously, we posited that sleep was a fundamental property of individual cortical columns driven by activity-dependent production of sleep regulatory substances such as TNF (Krueger and Obal 1993, 2003). This hypothesis leads to the prediction that application of exogenous TNF directly to the cortex would increase the probability of a recipient cortical column being in a sleep-like state, high amplitude SEPs, and that the individual cortical column sleep-like state would be semi-autonomous and activity dependent. In the current experiment, we found that TNF applied locally to the surface of the cortex enhances the magnitude of whisker-stimulation-induced SEPs independent of animal sleep-wake state. These results are consistent with our hypothesis. High amplitude SEPs are indicative of a sleep-like state in individual cortical columns and cortical column state oscillations are dependent on prior neuronal activity within the column, prior duration of column state and whole organism state (Rector et al., 2005). Since SEPs are quantified by stimulus onset timed averaging of many individual SEPs of varying amplitudes, during multiple periods within which repeated individual SEPs are evoked to determine average SEPs the columns often oscillate between sleep-like and wake-like states (Rector et al., 2005). Thus, one interpretation of our finding is that locally applied TNF enhances the probability of a column being in the sleep-like state (more high amplitude SEPs averaged together) whether the whole animal is asleep or awake. Such an interpretation is consistent with a model of the coalescing of cortical column states into whole animal state (Roy et al., in press). In the current study, we also show that TNF-IR in cortical neurons is activity-dependent. This finding is consistent with our prior reports of cortical TNF accumulation during waking periods (Floyd and Krueger 1997) and glutamate enhancement of TNF cell content and release in mixed neuron-glia cultures (De et al., 2005). Collectively, these data support the hypothesis that spontaneous activity within neuronal networks enhances TNF and thereby provides an endogenous local source of TNF that in turn induces a sleep-like state within the cortical column producing the TNF.
In other studies, we showed that application of TNF to the surface of the cortex, as was done in the current study, state-dependently enhances EEG delta wave power on the side that received TNF (Yoshida et al., 2004). Those data are consistent with current results to the extent that they suggest that TNF induced a localized intense sleep. However, the relationships between TNF-enhanced SEPs and TNF-enhanced EEG delta power remain unknown. It is possible that enhanced SEPs lead directly to enhanced EEG delta power although this seems unlikely because individual SEPs would have to be exactly synchronized to each other to have such an action. Traditionally the SEPs obtained by averaging single whisker stimulations have been considered distinct from the ongoing EEG background, since the EEG activities independent of the stimulus onset would be filtered out by the averaging process. It seems more likely that TNF is influencing both SEPs and EEG delta power via its ability to promote adenosine A1 receptors via nuclear factor kappa B activation and subsequent enhanced potassium conductance and neuron hyperpolarization (see below and reviewed Krueger et al., 2007).
More recently, there is evidence that the event-related potentials are the result of phase alignments in the ongoing background oscillations (Moratti et al., 2007). However, the background stimulation used to study these alignments did not affect the P1 amplitude. The mechanisms that underly each of the components of the SEP remain unknown. The earliest components of the temporal shape of the evoked response represent input to layer IV and the later components from higher order processing (Knight et al., 1985). Because the P1 component is not as affected by cooling from the surface of the cortex (Kublik et al., 2001), the thalamic input into layer IV was posited to underly the P1 component.
Application of either TNF of interleukin 1β to the surface of the cortex elicited increases in the number of interleukin 1β-IR cells in the layers II–IV of the primary Sctx (Churchill et al., 2005; Yasuda et al., 2007). Also application of either TNF or interleukin 1β to the surface of the Sctx activates the somatic region of the reticular thalamus as indicated by Fos-immunoreactivity (Churchill et al., 2005; Yasuda et al., 2007). These treatments also enhance sleep intensity (EEG delta power) on the side injected. Cortico-reticular thalamic projections likely provide the means by which the thalamus is kept aware of the status of cortical column state. Furthermore, after cortical application of interleukin-1β, the number of Fos-immunoreactive cells increases in the prefrontal cortex, the ventrolateral preoptic nucleus and the median preoptic nucleus thereby suggesting that cortical column state is also communicated to these hypothalamic sleep regulatory circuits (Yasuda et al., 2007). These results along with the known anatomical connections between these regions suggest that cortical column state is likely to be the homeostatic signal that throws the hypothalamic “sleep switch” (Saper et al., 2005). Thus, we posit that neural activity drives production and release of SRSs such as TNF that act locally to alter the input-output response properties of nearby neurons thereby altering the neural assembly state within which they are found. Current results directly demonstrate that TNF does indeed alter cortical column state as determined from SEPs.
SEPs of higher magnitude are observed during NREMS more so than during wake (Rector et al 2005). These sleep-wake differences in SEP amplitudes were observed in the current study. Theoretical reasons indicate that we should find higher amplitude SEPs independent of state after TNF treatment as we found in the current study. There are several possible explanations for the higher P1-N1 differences observed during the sleep-like state: a reduction in inhibitory influences, an increase in excitatory influences, a decrease in random events or a lower initial membrane potential resulting in a larger total change during activation. When animals are awake, the cortical evoked response to thalamocortical stimulation is lower in amplitude (Castro-Alamancos and Oldford, 2002). When animals go to sleep, cortical neurons alternate between hyperpolarized and depolarized states (Steriade, 2001; Steriade et al., 2001; Velluti, 1997); if subsequently stimulated to produce an action potential or EPSP-during the hyperpolarized state, the magnitude of change of the membrane potential might be greater.
Our earlier studies demonstrated a use-dependence of the cortical column sleep-like state since the probability of occurrence of the sleep-like state is higher the longer the particular column is in the wake-like state (Rector et al., 2005). Nevertheless, during whole animal wake or sleep, some columns can be found in the opposite state suggesting a degree of local autonomy in column state regulation. Using a conditioned licking learning paradigm that is dependent on single whisker stimulation, Walker et al. (2005) showed that the error rate was higher if the cortical column receiving the conditioned whisker stimulation was in the sleep-like state than if it was in the wake-like state. This latter finding clearly demonstrates that there are behavioral consequences to cortical column state.
TNF as a proinflammatory cytokine might be expected to increase brain temperature and/or induce inflammation locally. However, preliminary data indicate that microinjection of another proinflammatory cytokine, IL1β (50 ng) did not significantly increase SEP amplitudes as observed with TNF (n=4). Further inflammation induced by systemic sepsis (lipopolysaccaride injection) reduced the SEP amplitudes (Ohnesorge et al., 2003). Finally, microinjection of TNF in smaller doses into the locus coeruleus (LC) in rats initially produced behavioral arousal followed by sedation, while microinjection of IL1β into the LC produced sedation (De Sarro et al., 1997). These results suggest to us that enhanced local temperatures or blood flow (inflammation) are unlikely to be responsible for the TNF-enhanced SEP amplitudes reported here. We think it more likely that TNF as a SRS enhances IL1β, another SRS, in the localized region of the cerebral cortex, but that TNF and IL1β play different roles in promoting SEP amplitude.
Significant increases in TNF-IR were observed in layers II–IV in cortical columns that received enhanced sensory afferent input. Although in sections double labeled with TNF and Fos, Fos-IR nuclei were co-localized with TNF-IR, not all of the Fos-IR nuclei were surrounded by TNF-IR cytoplasm thereby suggesting that TNF is up-regulated in selected neurons whereas Fos is more of a general activity marker. However, the time course of activity-induced TNF expression in neurons is unknown; it, thus, could have peaked before or after Fos expression. Further, different layers may express TNF at different times. For example, in the Group A rats sacrificed after 2 h of whisker stimulation for one whisker where the rats slept during restraint conditions, TNF-IR in layer II–IV was enhanced; layer IV is the input layer. Whereas, in the Group B &C rats sacrificed after 2 h of multiple whisker stimulation where the rats were only restricted to a plexiform chamber or even the top of the centralized inverted flower pot and the rats were not allowed to sleep, more TNF-IR cells were enhanced in layers II –III. Layers II–III are the primary area for corticocortical interactions (Armstrong-Jones et al., 1992) and these differences suggest that whisker stimulation-induced TNF upregulation may have an effect on cross talk between columns. Current data strongly indicate that afferent activity can induce TNF-IR in some neurons.
Current data are also consistent with the previous conclusion that TNF is a SRS (Obál and Krueger, 2003). For instance, a variety of pathologies that are characterized by patient sleepiness also are associated with elevated plasma TNF levels (reviewed Obál and Krueger, 2003). In normal individuals, there also is an association between circulating levels of TNF and EEG delta power (Darko et al., 1995). Sleep apnea patients treated with the soluble TNF receptor have reduced sleepiness (Vgontzas et al., 2004). In fact, the 308A TNF polymorphic variant is associated with the metabolic syndrome (Sookoian et al., 2005) and sleep apnea (Riha et al., 2005). Many animal studies also support a role for TNF in sleep regulation. Thus, injection of TNF promotes NREMS while inhibition of TNF reduces spontaneous sleep. TNF promotes sleep if injected directly into the hypothalamic sleep regulatory circuits (Kubota et al., 2002) and as mentioned above promotes local/regional sleep if applied to the surface of the cortex (Yoshida et al., 2004; this study). Collectively such data strongly implicate TNF in sleep regulation and indicate that TNF acts on every level of the neuroaxis to promote sleep.
There are several contemporary theories of brain organization of sleep and sleep mechanisms that posit that sleep is a regional distributed property of brain and that it is dependent upon prior wakefulness activity (Krueger and Obál, 1993, 2003; Kavanau, 1994; Benington and Heller, 1995; Tononi and Cirelli, 2003). Indeed, there is now a relatively large literature indicating that regional EEG SWA in enhanced if the area was disproportionately activated during prior waking (reviewed Krueger and Obál, 2003). For instance, stimulation of the right hand is associated with enhanced EEG SWA in the left Sctx during the subsequent sleep episode (Kattler et al., 1994). Similarly, activation of Brodman areas 7 and 40 by a learning task is followed by enhanced EEG power in those areas during subsequent sleep (Huber et al., 2004). Many additional studies in both humans and animals demonstrate similar dependence of EEG SWA on prior waking activity (Ferrara et al., 2002; Vyazovskiy et al., 2004; Vyazovskiy and Tobler, 2005; Yasuda et al., 2005). Because activity enhances TNF expression and TNF application to cortical columns enhances the probability of their being in the sleep-like state (this study) and that TNF applied to the cortex enhances EEG delta power (Yoshida et al 2004), these data are consistent with the idea that TNF provides, in part, the signal to enhance local sleep. Regardless, current results clearly indicate that TNF application is associated with cortical column SEPs characteristic of the NREM sleep state and that TNF-IR is activity-dependent.
ACKNOWLEGEMENTS
We would like to acknowledge technical assistance from Jinna A. Navas, Alok De, Krista Ingalsbe, Heather Hallett, Sarah Hall, Susan Schactler, Rachel M. Wood, Alex Carvajal and Kathy Nguyen. We also thank Dr. Ping Taishi for doing the Western Blots and Dr. Bryan Slinker for statistical advice. Research Support was by NIH grants (USA) to J.M. Krueger, NS 25378 and NS 31453, and Murdock Foundation and Beckman Young Investigator Award to DM Rector.
COMPREHENSIVE LIST OF ABBREVIATIONS
- DAB
diaminobenzidine tetrahydrochloride
- EEG
electroencephalography
- H
hour, hours
- IR
immunoreactivity, immunoreactive-like
- msec
milli seconds
- NREMS
non-rapid eye movement sleep
- Sctx
somatosensory cortex
- SRS
sleep regulatory substances
- SWA
slow wave activity
- TNF
Tumor necrosis factor α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Armstrong-James M, Fox K, Das-Gupta A. Flow of excitation within rat barrel cortex on striking a single vibrissa. J Neurophysiol. 1992;68:1345–1358. doi: 10.1152/jn.1992.68.4.1345. [DOI] [PubMed] [Google Scholar]
- Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45:347–360. doi: 10.1016/0301-0082(94)00057-o. [DOI] [PubMed] [Google Scholar]
- Borbely AA, Tobler I. Endogenous sleep-promoting substances and sleep regulation. Physiol Rev. 1989;69:605–670. doi: 10.1152/physrev.1989.69.2.605. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Oldford E. Cortical sensory suppression during arousal is due to the activity-dependent depression of thalamocortical synapses. J Physiol. 2002;541:319–331. doi: 10.1113/jphysiol.2002.016857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill L, Yasuda K, Yasuda T, Blindheim KA, Falter M, Garcia-Garcia F, Krueger JM. Unilateral cortical application of tumor necrosis factor alpha induces asymmetry in Fos-and interleukin-1beta-immunoreactive cells within the corticothalamic projection. Brain Res. 2005;1055:15–24. doi: 10.1016/j.brainres.2005.06.052. [DOI] [PubMed] [Google Scholar]
- De A, Krueger JM, Simasko SM. Glutamate induces the expression and release of tumor necrosis factor-alpha in cultured hypothalamic cells. Brain Res. 2005;1053:54–61. doi: 10.1016/j.brainres.2005.06.044. [DOI] [PubMed] [Google Scholar]
- Darko DF, Miller JC, Gallen C, White J, Koziol J, Brown SJ, Hayduk R, Atkinson JH, Assmus J, Munnel DT, Naitotl P, McCutchen A, Mitler MM. Sleep electroencephalogram delta-frequency amplitude, night plasma levels of tumor necrosis factor alpha, and human immunodeficiency virus infection. Proc Natl Acad Sci USA. 1995;92:12080–12084. doi: 10.1073/pnas.92.26.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sarro G, Gareri P, Sinopoli VA, David E, Rotiroti D. Comparative, behavioural and electrocortical effects of tumor necrosis factor-alpha and interleukin-1 microinjected into the locus coeruleus of rat. Life Sci. 1997;60:555–564. doi: 10.1016/s0024-3205(96)00692-3. [DOI] [PubMed] [Google Scholar]
- Ferrara M, De Gennaro L, Curcio G, Cristiani R, Bertini M. Interhemispheric asymmetry of human sleep EEG in response to selective slow-wave sleep deprivation. Behav Neurosci. 2002;116:976–981. doi: 10.1037//0735-7044.116.6.976. [DOI] [PubMed] [Google Scholar]
- Figiel I, Dzwonek K. TNF alpha and TNF receptor 1 expression in the mixed neuronal-glial cultures of hippocampal dentate gyrus exposed to glutamate or trimethyltin. Brain Res. 2007;1131:17–28. doi: 10.1016/j.brainres.2006.10.095. [DOI] [PubMed] [Google Scholar]
- Filipkowski RK, Rydz M, Berdel B, Morys J, Kaczmarek L. Tactile experience induces c-fos expression in rat barrel cortex. Learn Mem. 2000;7:116–122. doi: 10.1101/lm.7.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd RA, Krueger JM. Diurnal variation of TNF alpha in the rat brain. NeuroReport. 1997;8:915–918. doi: 10.1097/00001756-199703030-00020. [DOI] [PubMed] [Google Scholar]
- Hall RD, Borbely AA. Acoustically evoked potentials in the rat during sleep and waking. Exp Brain Res. 1970;11:93–110. doi: 10.1007/BF00234203. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Jouvet M. Neuromediateurs et facteurs hypnogenes. Rev Neurol (Paris) 1984;140:389–400. [PubMed] [Google Scholar]
- Kattler H, Dijk DJ, Borbely AA. Effect of unilateral somatosensory stimulation prior to sleep on the sleep EEG in humans. J Sleep Res. 1994;3:159–164. doi: 10.1111/j.1365-2869.1994.tb00123.x. [DOI] [PubMed] [Google Scholar]
- Kavanau JL. Sleep and dynamic stabilization of neural circuitry: a review and synthesis. Behav Brain Res. 1994;63:111–126. doi: 10.1016/0166-4328(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Knight RT, Brailowsky S, Scabini D, Simpson GV. Surface auditory evoked potentials in the unrestrained rat: component definition. Electroencephalogr Clin Neurophysiol. 1985;61(5):430–439. doi: 10.1016/0013-4694(85)91035-1. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Obál F., Jr A neuronal group theory of sleep function. J Sleep Res. 1993;2:63–69. doi: 10.1111/j.1365-2869.1993.tb00064.x. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Obál F., Jr Sleep function. Front Biosci. 2003;8:d511–d519. doi: 10.2741/1031. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Rector DM, Churchill L. Sleep and Cytokines. Sleep Medicine Clinics of North America: Sleep, Sleep Disorders and Hormones. 2007;vol. 2(Issue 2) doi: 10.1016/j.jsmc.2007.03.003. pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kublik E, Musiał P, Wróbel A. Identification of principal components in cortical evoked potentials by brief surface cooling. Clin Neurophysiol. 2001;112:1720–1725. doi: 10.1016/s1388-2457(01)00603-4. [DOI] [PubMed] [Google Scholar]
- Kubota T, Li N, Guan Z, Brown RA, Krueger JM. Intrapreoptic microinjection of TNF-alpha enhances non-REM sleep in rats. Brain Res. 2002;932:37–44. doi: 10.1016/s0006-8993(02)02262-x. [DOI] [PubMed] [Google Scholar]
- Moratti S, Clementz BA, Gao Y, Ortiz T, Keil A. Neural mechanisms of evoked oscillations: Stability and interaction with transient events. Hum Brain Mapp. 2007;28:1318–1333. doi: 10.1002/hbm.20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obál F, Jr, Krueger JM. Biochemical regulation of non-rapid-eye-movement sleep. Front Biosci. 2003;8:d520–d550. doi: 10.2741/1033. [DOI] [PubMed] [Google Scholar]
- Ohnesorge H, Bischoff P, Scholz J, Yekebas E, Schulte am Esch J. Somatosensory evoked potentials as predictor of systemic inflammatory response syndrome in pigs? Intensive Care Med. 2003;29:801–807. doi: 10.1007/s00134-003-1657-7. [DOI] [PubMed] [Google Scholar]
- Rector DM, Topchiy IA, Carter KM, Rojas MJ. Local functional state differences between rat cortical columns. Brain Res. 2005;1047:45–55. doi: 10.1016/j.brainres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Riha RL, Brander P, Vennelle M, McArdle N, Kerr SM, Anderson NH, Douglas NJ. Tumour necrosis factor-alpha (−308) gene polymorphism in obstructive sleep apnoea-hypopnoea syndrome. Eur Respir J. 2005;26:673–678. doi: 10.1183/09031936.05.00130804. [DOI] [PubMed] [Google Scholar]
- Roy S, Krueger JM, Rector DM, Wan Y. A network model for activity dependent sleep regulation. J Theor Biol. 2008 doi: 10.1016/j.jtbi.2008.03.033. in press (epub) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Sookoian SC, Gonzalez C, Pirola CJ. Meta-analysis on the G-308A tumor necrosis factor alpha gene variant and phenotypes associated with the metabolic syndrome. Obes Res. 2005;13:2122–2131. doi: 10.1038/oby.2005.263. [DOI] [PubMed] [Google Scholar]
- Steriade M. Impact of network activities on neuronal properties in corticothalamic systems. J Neurophysiol. 2001;86:1–39. doi: 10.1152/jn.2001.86.1.1. [DOI] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- Taishi P, Churchill L, Wang M, Kay D, Davis CJ, Guan X, De A, Yasuda T, Liao F, Krueger JM. TNFα siRNA reduces brain TNF and EEG delta wave activity in rats. Brain Res. 2007;1156:125–132. doi: 10.1016/j.brainres.2007.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62:143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Velluti RA. Interactions between sleep and sensory physiology. J Sleep Res. 1997;6:61–77. doi: 10.1046/j.1365-2869.1997.00031.x. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis E, Lin HM, Bixler EO, Trakada G, Chrousos GP. Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor-α antagonist. J Clin Endocrinol Metab. 2004;89:4409–4413. doi: 10.1210/jc.2003-031929. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Welker E, Fritschy JM, Tobler I. Regional pattern of metabolic activation is reflected in the sleep EEG after sleep deprivation combined with unilateral whisker stimulation in mice. Eur J Neurosci. 2004;20:1363–1370. doi: 10.1111/j.1460-9568.2004.03583.x. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Tobler I. Regional differences in NREM sleep slow-wave activity in mice with congenital callosal dysgenesis. J Sleep Res. 2005;14:299–304. doi: 10.1111/j.1365-2869.2005.00456.x. [DOI] [PubMed] [Google Scholar]
- Walker AJ, Topichiy I, Kouptsov K, Rector DM. ERP differences during conditioned lick response in the rat. Sleep. 2005;28:A15. [Google Scholar]
- Welker E, Rao SB, Dörfl J, Melzer P, van der Loos H. Plasticity in the barrel cortex of the adult mouse: effects of chronic stimulation upon deoxyglucose uptake in the behaving animal. J Neurosci. 1992;12:153–170. doi: 10.1523/JNEUROSCI.12-01-00153.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T, Yasuda K, Brown RA, Krueger JM. State-dependent effects of light-dark cycle on somatosensory and visual cortex EEG in rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1083–R1089. doi: 10.1152/ajpregu.00112.2005. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Churchill L, Yasuda T, Blindheim K, Falter M, Krueger JM. Unilateral cortical application of interleukin-1β (IL1β̣ induces asymmetry in Fos-, IL1 β - and nerve growth factor-immunoreactivity: Implications for sleep regulation. Brain Res. 2007;1131:44–59. doi: 10.1016/j.brainres.2006.11.051. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Peterfi Z, Garcia-Garcia F, Kirkpatrick R, Yasuda T, Krueger JM. State-specific asymmetries in EEG slow wave activity induced by local application of TNF alpha. Brain Res. 2004;1009:129–136. doi: 10.1016/j.brainres.2004.02.055. [DOI] [PubMed] [Google Scholar]