Abstract
Objective
Oxidative stress is thought to play a role in the pathogenesis of fibromyalgia. We examined the hypothesis that oxidative stress was increased in patients with fibromyalgia and related to the severity of symptoms.
Methods
Urinary F2 isoprostane excretion was measured in 48 patients with fibromyalgia and compared to those of 96 control subjects. In patients, we examined the association between oxidative stress and symptoms.
Results
Patients with fibromyalgia were significantly more symptomatic than control subjects, but urinary F2 isoprostane excretion did not differ significantly (2.3±1.9 vs 2.8±2.2 ng/mg creatinine) (p=0.16). In patients with fibromyalgia, F2-isoprostane excretion was associated with fatigue VAS (rho=0.30, p=0.04) but not with pain, quality of life, functional capacity, depression, number of tender points or overall impact of fibromyalgia.
Conclusion
oxidative stress is not increased in patients with fibromyalgia, but as was previously found in patients with systemic lupus erythematosus (SLE), oxidative stress was associated with fatigue.
Keywords: fibromyalgia, oxidative stress, fatigue, F2 isoprostanes
Increased oxidative stress results from an imbalance between products of oxidation and antioxidant defenses. There are several inflammatory clinical conditions associated with increased oxidative stress, but novel data suggest a relationship between oxidative stress and pain perception.1 Furthermore, oxidative stress is increased in patients with chronic fatigue syndrome.2, 3 There is little information about oxidative stress in fibromyalgia but malondialdehyde concentrations are higher and superoxide dismutase lower than in controls.4
Many of the methods used to quantify oxidative stress in vivo are problematic. Recently, the discovery of F2 isoprostanes, prostaglandin-like substances derived from lipid peroxidation, and the development of accurate methods for their quantification have provided a robust marker of oxidative stress in vivo.5-7 We have used this technique to show that oxidative stress is increased in patients with scleroderma,8 and associated with symptoms such as fatigue in patients with systemic lupus erythematosus (SLE).9 Many patients with SLE have associated fibromyalgia, but since we did not formally define fibromyalgia in that study we could not address the possibility that the association between oxidative stress and fatigue in lupus was due to fibromyalgia.
Therefore we examined the hypothesis that oxidative stress, as determined by F2 isoprostane excretion, was increased in patients with fibromyalgia and correlated with severity of symptoms.
PATIENTS AND METHODS
Study subjects
We enrolled 48 eligible patients older than 18 years of age who met the revised American College of Rheumatology criteria for fibromyalgia.10 Patients were recruited from the practices of local rheumatologists, through an email advertisement and using a volunteer database developed by the Vanderbilt General Clinical Research Center (GCRC). Control subjects were selected from the control subjects used in previous studies11, 12 by frequency-matching for age, sex, race, BMI and smoking. Exclusion criteria included the presence of any inflammatory autoimmune disease, diabetes, renal disease, known heart disease, pregnancy, use of corticosteroids, and in the control subjects, a diagnosis of fibromyalgia. The study was approved by the institutional review board of Vanderbilt University Hospital, and all subjects provided written informed consent.
F2 isoprostanes
Urine samples were collected and stored at –70 ºC. F2 isoprostanes were quantified using gas chromatography/mass spectrometry as previously described.13
Patient evaluation
Demographic and clinical data were obtained from patient interview, chart review, physical examination, and patient questionnaires. Subjects completed a modified Health Assessment Questionnaire (MHAQ)14 and visual analogue scales (VAS) for pain and fatigue. Each VAS is a 10 cm scale where 0 represents an absence of the symptom and 10 represents the possible maximum value for the symptom. Fatigue was also evaluated using the Krupp Fatigue Severity Scale (FSS),15 that has 9 questions each scored from 1 (no fatigue) to 7 (severe fatigue) and the score for a subject is the mean of the scores for the 9 questions. Quality of life was assessed using the Spitzer index, a validated index used in patients with chronic illnesses.16 This is a five item questionnaire that evaluates the areas of activity, daily living, health, support, and outlook on life. The score ranges from 0-10, with lower values reflecting worse quality of life. The brief Rheumatology Attitudes Index (RAI) is a five item instrument used to assess the construct of helplessness, defined as a psychological state in which people expect that their efforts will be ineffective. It is scored from 1 to 5 with higher scores indicate more helplessness.17
In patients with fibromyalgia, we measured depression with the use of the Center for Epidemiologic Studies Depression Scale (CES-D),18 the overall impact of fibromyalgia with the Fibromyalgia Impact Questionnaire (FIQ)19 and the number of tender points by digital palpation using 4 kg pressure at 18 standard sites on the body.
STATISTICAL ANALYSIS
Based on previous data showing that the mean F2 isoprostane excretion in control subjects is approximately 2.2 ng/mg creatinine with a standard deviation of 1.4,9 the study required 48 patients with fibromyalgia and 96 control subjects to detect a difference of 0.5 SD (0.7 ng/mg creatinine) in F2 isoprostane excretion with 80% power, a two-sided significance level of 5% and a control: experimental ratio of 1:2. Baseline characteristics and outcome measures were compared between patients and controls using chi squared test for categorical variables and the Wilcoxon rank-sum tests for continuous variables. The relationship between categorical clinical variables and F2 isoprostane excretion in patients with fibromyalgia was evaluated using Spearman’s rank correlation analyses and the results reported as the Spearman’s rank correlation coefficient (rho).
RESULTS
Table 1 shows demographic and clinical characteristics in patient and control groups. The groups were well matched and were of similar age (mean age 47.7±11.9 years and 45.0±11.4 years, respectively), sex (97.9% of female in both groups), race (95.8% white race in both groups), and BMI (27.6±7.3 kg/m2 and 27.5±6.0 kg/m2, respectively). Seven patients with fibromyalgia (14.6%) and 13 control subjects (13.5%) were current smokers. As expected, patients with fibromyalgia had markedly higher levels of fatigue as measured by both the VAS (6.6±2.3 vs. 1.2±1.9 cm, p<0.001) and the FSS (5.6±1.3 vs. 2.5±1.2, p<0.001) than control subjects. Similarly, increased levels of pain (6.3±1.9 vs. 0.9±1.6 cm, p<0.001) and decreased functional capacity (MHAQ 0.6±0.5 vs. 0.1±0.2 p<0.001) were also observed in patients. Patients with fibromyalgia also had poorer attitudes (RAI 3.0±0.9 vs.1.7±0.6 p<0.001) and lower quality of life (QOL 7.6±1.6 vs. 9.5±0.9 p<0.001) than control subjects.
Table 1.
Demographic and Clinical Characteristics of Patients with Fibromyalgia and Control Subjects
| Characteristics | Patients (n=48) | Controls (n=96) | P-value |
|---|---|---|---|
| Age (yrs) | 47.7±11.9 | 45.0±11.4 | 0.14 |
| Female (%) | 97.9 | 97.9 | 1.0 |
| White Race (%) | 95.8 | 95.8 | 1.0 |
| Current Smoking (%) | 14.6 | 13.5 | 0.87 |
| Body Mass Index (kg/m2) | 27.6±7.3 | 27.5±6.0 | 0.87 |
| Pain VAS (cm)* | 6.3±1.9 | 0.9±1.6 | <0.001 |
| Fatigue VAS (cm)* | 6.6±2.3 | 1.2±1.9 | <0.001 |
| MHAQ score † | 0.6±0.5 | 0.1±1.2 | <0.001 |
| Quality of Life score ‡ | 7.6±1.6 | 9.5±0.9 | <0.001 |
Data presented as mean±SD or percentage as appropriate.
VAS - Visual analogue scale
modified health assessment questionnaire
quality of life score (higher score indicates better quality of life)
P values are for Wilcoxon rank sum test or for chi squared test, as appropriate, for comparison among groups
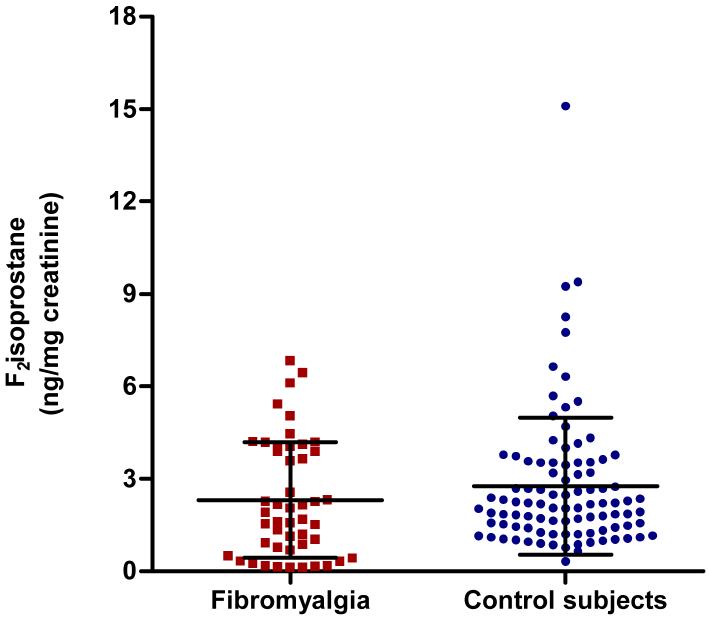
Figure 1 illustrates urinary F2 isoprostane excretion in patients with fibromyalgia and control subjects, showing that they did not differ significantly (2.3±1.9 and 2.8±2.2 ng/mg creatinine respectively, p=0.16).. Table 2 shows the correlations between F2 isoprostane excretion and patient characteristics, showing that F2 isoprostane excretion was correlated significantly with self-reported fatigue (rho=0.30, p=0.04) but not with the fatigue severity score, measures of pain, quality of life, function capacity, depression, number of tender points or overall impact of fibromyalgia.
Figure 1.
F2 Isoprostane excretion in patients with fibromyalgia and control subjects. Error bars represent the mean and SD
Table 2.
Clinical characteristics and relationship between F2 isoprostane excretion
| PATIENT CHARACTERISTICS Mean ± SD |
rho | |
|---|---|---|
| Age (yrs) | 47.7±11.9 | 0.03 |
| Body mass index (kg/m2) | 27.6±7.3 | 0.08 |
| Pack years of smoking | 5.4±11.3 | -0.16 |
| Disease duration (yrs) | 7.5±4.9 | 0.05 |
| Number of tender points | 13.9±2.5 | -0.03 |
| PATIENT SELF-REPORT SCORES | ||
| Pain Visual Analogue Scale | 6.3±1.9 | 0.02 |
| Fatigue Visual Analogue Scale | 6.6±2.3 | 0.30* |
| Fatigue severity score (FSS) | 5.6±1.3 | 0.23 |
| Modified Health Assessment Questionnaire (MHAQ) score |
0.6±0.5 | 0.11 |
| Quality of Life score | 7.6±1.6 | -0.19 |
| Rheumatology Attitudes Index (RAI) score | 3.0±0.9 | 0.03 |
| CES-D Depression Scale | 20.9±11.7 | 0.03 |
| Fibromyalgia Impact Questionnaire (FIQ) | 57.7±14.6 | 0.10 |
Data presented as mean±SD
p=0.04
DISCUSSION
The major finding of this study is that oxidative stress as measured by F2 isoprostane excretion was not different among patients with fibromyalgia and well-matched control subjects. F2 isoprostane excretion was associated with self-reported fatigue in patients with fibromyalgia.
Free radicals generated by oxidative stress result in lipid peroxidation and consequently tissue damage. Recent studies have shown that oxidative stress can also cause peripheral and central sensitization and alter nociception, 20 resulting in hyperalgesia mediated by both local and spinal oxidant mechanisms. Also, isoprostanes, directly enhance the firing of type C-nociceptors.21 Thus, there are mechanisms whereby oxidative stress could contribute to a condition such as fibromyalgia in which tissue damage is not prominent.
We found that oxidative stress was not increased in patients with fibromyalgia. The measurement of malondialdehyde concentrations thiobarbituric acid reactive substances, and the advanced glycation end-product (AGE) pentosidine; less specific markers of oxidative stress than F2 isoprostanes, have yielded results suggesting that fibromyalgia is associated with oxidative stress.4, 22, 23 Thus our study, performed using state of the art measures of oxidative stress in a well characterized group of patients with fibromyalgia, provides valuable information. Our findings in fibromyalgia, also contrast with findings in patients with chronic fatigue syndrome in whom isoprostanes were elevated and correlated with post-exertional malaise.24 This suggests that oxidative stress may be more prominent in patients within the fibromyalgia spectrum of disorders in whom fatigue is more prominent. Indeed, we previously found that oxidative stress is associated with fatigue and not inflammation in patients with SLE,9 and concordant with those findings, there was a weak association between fatigue and oxidative stress in patients with fibromyalgia. Fatigue is one of the most common complains of patients with fibromyalgia. Thus, the finding of an association between oxidative stress and symptoms such as fatigue is of interest, since the mechanisms underlying the symptoms in patients with fibromyalgia remain unknown.
This study has some potential limitations. First, it is possible that medications patients were receiving for fibromyalgia could have affected concentrations of F2 isoprostanes, however, it is noteworthy that patients with fibromyalgia still had significant symptoms as evidenced by their fatigue and quality of life scores. Second, this was a cross sectional study and thus specific studies will be required to define the association between F2 isoprostane excretion, fatigue, and interventions used to treat fibromyalgia.
In conclusion, oxidative stress measured by isoprostane excretion was not different among patients with fibromyalgia and control subjects, but was correlated with self reported fatigue.
Acknowledgments
Supported by grants HL65082, GM15431, P60 AR056116 and 1UL1RR024975 from the National Institutes of Health
Footnotes
Conflict of Interest Cecilia P. Chung: none Dina Titova: none Annette Oeser: none Margaret Randels: none Ingrid Avalos: none Ginger L. Milne: none Jason D. Morrow: none C. Michael Stein: none
Reference List
- 1.Wang ZQ, Porreca F, Cuzzocrea S, et al. A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther. 2004;309(3):869–878. doi: 10.1124/jpet.103.064154. [DOI] [PubMed] [Google Scholar]
- 2.Vecchiet J, Cipollone F, Falasca K, et al. Relationship between musculoskeletal symptoms and blood markers of oxidative stress in patients with chronic fatigue syndrome. Neurosci Lett. 2003;335(3):151–154. doi: 10.1016/s0304-3940(02)01058-3. [DOI] [PubMed] [Google Scholar]
- 3.Keenoy B, Moorkens G, Vertommen J, De L I. Antioxidant status and lipoprotein peroxidation in chronic fatigue syndrome. Life Sci. 2001;68(17):2037–2049. doi: 10.1016/s0024-3205(01)01001-3. [DOI] [PubMed] [Google Scholar]
- 4.Bagis S, Tamer L, Sahin G, et al. Free radicals and antioxidants in primary fibromyalgia: an oxidative stress disorder? Rheumatology International. 2005;25(3):188–190. doi: 10.1007/s00296-003-0427-8. [DOI] [PubMed] [Google Scholar]
- 5.Morrow JD, Chen Y, Brame CJ, et al. The isoprostanes: unique prostaglandin-like products of free-radical-initiated lipid peroxidation. Drug Metab Rev. 1999;31(1):117–139. doi: 10.1081/dmr-100101910. [DOI] [PubMed] [Google Scholar]
- 6.Cracowski JL, Durand T, Bessard G. Isoprostanes as a biomarker of lipid peroxidation in humans: physiology, pharmacology and clinical implications. Trends Pharmacol Sci. 2002;23(8):360–366. doi: 10.1016/s0165-6147(02)02053-9. [DOI] [PubMed] [Google Scholar]
- 7.Lawson JA, Rokach J, FitzGerald GA. Isoprostanes: formation, analysis and use as indices of lipid peroxidation in vivo. J Biol Chem. 1999;274(35):24441–24444. doi: 10.1074/jbc.274.35.24441. [DOI] [PubMed] [Google Scholar]
- 8.Stein CM, Tanner SB, Awad JA, Roberts LJ, Morrow JD. Evidence of free radical-mediated injury (isoprostane overproduction) in scleroderma. Arthritis Rheum. 1996;39(7):1146–1150. doi: 10.1002/art.1780390711. [DOI] [PubMed] [Google Scholar]
- 9.Avalos I, Chung CP, Oeser A, et al. Oxidative stress in systemic lupus erythematosus: relationship to disease activity and symptoms. Lupus. 2007;16(3):195–200. doi: 10.1177/0961203306075802. [DOI] [PubMed] [Google Scholar]
- 10.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33(2):160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 11.Asanuma Y, Oeser A, Shintani AK, et al. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Eng J Med. 2003;349(25):2407–2415. doi: 10.1056/NEJMoa035611. [DOI] [PubMed] [Google Scholar]
- 12.Chung CP, Oeser A, Raggi P, et al. Increased coronary-artery atherosclerosis in rheumatoid arthritis: Relationship to disease duration and cardiovascular risk factors. Arthritis Rheum. 2005;52(10):3045–3053. doi: 10.1002/art.21288. [DOI] [PubMed] [Google Scholar]
- 13.Morrow JD, Roberts LJ. Mass spectrometry of prostanoids: F2-isoprostanes produced by non-cyclooxygenase free radical-catalyzed mechanism. Methods Enzymol. 1994;233:163–174. doi: 10.1016/s0076-6879(94)33019-0. [DOI] [PubMed] [Google Scholar]
- 14.Pincus T, Summey JA, Soraci SA, Jr., Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26(11):1346–1353. doi: 10.1002/art.1780261107. [DOI] [PubMed] [Google Scholar]
- 15.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 16.Spitzer WO, Dobson AJ, Hall J, et al. Measuring the quality of life of cancer patients: a concise QL-index for use by physicians. J Chronic Dis. 1981;34(12):585–597. doi: 10.1016/0021-9681(81)90058-8. [DOI] [PubMed] [Google Scholar]
- 17.Pincus T, Summey JA, Soraci SA, Jr., Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26(11):1346–1353. doi: 10.1002/art.1780261107. [DOI] [PubMed] [Google Scholar]
- 18.Welssman MM, Sholomskas D, Pottenger M, Prusoff B, Locke B. Assessing Depressing symptoms in Five Psychiatric Populations: a Validation Study. Am J Epidemiol. 1977;106(3):203–214. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- 19.Burckhardt CS, Clark SR, Bennett RM. The fibromyalgia impact questionnaire: development and validation. J Rheumatol. 1991;18(5):728–733. [PubMed] [Google Scholar]
- 20.Wang ZQ, Porreca F, Cuzzocrea S, et al. A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther. 2004;309(3):869–878. doi: 10.1124/jpet.103.064154. [DOI] [PubMed] [Google Scholar]
- 21.Evans AR, Junger H, Southall MD, et al. Isoprostanes, novel eicosanoids that produce nociception and sensitize rat sensory neurons. J Pharmacol Exp Ther. 2000;293(3):912–920. [PubMed] [Google Scholar]
- 22.Eisinger J, Zakarian H, Pouly E, Plantamura A, Ayavou T. Protein peroxidation, magnesium deficiency and fibromyalgia. Magnes Res. 1996;9(4):313–316. [PubMed] [Google Scholar]
- 23.Ozgocmen S, Ozyurt H, Sogut S, Akyol O, Ardicoglu O, Yildizhan H. Antioxidant status, lipid peroxidation and nitric oxide in fibromyalgia: etiologic and therapeutic concerns. Rheumatology International. 2006;26(7):598–603. doi: 10.1007/s00296-005-0079-y. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy G, Spence VA, McLaren M, Hill A, Underwood C, Belch JJF. Oxidative stress levels are raised in chronic fatigue syndrome and are associated with clinical symptoms. Free Radical Biology and Medicine. 2005;39(5):584–589. doi: 10.1016/j.freeradbiomed.2005.04.020. [DOI] [PubMed] [Google Scholar]