Abstract
We present our experience planning and launching a multinational, NIH/NINDS funded study of thymectomy in myasthenia gravis. We highlight the additional steps required for international sites and analyze and contrast the time investment required to bring U.S. and non-U.S. sites into full regulatory compliance. Results show the mean time for non- U.S. centers to achieve regulatory approval was significantly longer (mean 13.4 ± 0.96 months) than for U.S. sites (9.67 ± 0.74 months; p = 0.003, t-test). The delay for non- U.S. sites was mainly attributable to Federalwide Assurance certification and State Department clearance.
Keywords: Myasthenia gravis, thymectomy, regulatory approval
1. BACKGROUND
This manuscript describes our experience in the planning and launching of an international, multicenter, clinical trial funded by the National Institute of Neurological Diseases and Stroke (NINDS) of the National Institutes of Health (NIH) to study the role of thymectomy in myasthenia gravis (MG). By summarizing our experience, we hope to assist other investigators who are organizing clinical trials that will involve international collaboration.
MG is a chronic autoimmune neuromuscular disease that fulfills established criteria as a rare disease. Prevalence rates range from 0.5 to 20.4 per 100,000 (Phillips, 2003). No regions of particularly high or low incidence or prevalence have been identified, but most studies have focused predominantly on Caucasian populations of western European descent. Studies of South African, African American, and Asian populations suggest that there may be differences in racial susceptibility (Chiu et. al., 1987; Phillips et. al., 1992; Wong et. al., 1992; and Heckmann et. al., 2007). Clinical investigation of MG is further complicated by the presence of disease subgroups based on the presence of antibodies against the acetylcholine receptor, muscle-specific kinase or absence of detectable auto-antibodies. Age of onset, presence of thymoma, and the presence of pure ocular versus generalized disease appear to define other patient subgroups (Compston et. al., 1980).
The thymectomy trial for non-thymomatous myasthenia gravis patients receiving prednisone (MGTX) is an NINDS cooperative multinational, multicenter, two-arm trial that is currently enrolling patients. The study aims to determine if extended transsternal thymectomy plus prednisone compared to prednisone alone results in a greater improvement in MG weakness, a lower total dose of prednisone, and an enhanced quality of life by reducing adverse events and symptoms. The study was funded by NINDS in September 2005, approximately five years after planning of the trial began (Wolfe et. al., 2003). An international trial undergoes extra scrutiny at NIH. In order to fund a grant with a foreign Principal Investigator, a special justification has to be made regarding the added value of international sites and leadership to the Advisory Neurological Disorders and Stroke Council meeting and must be approved by the Director of NINDS.
To achieve adequate recruitment for a sufficiently powered study in a reasonable timeframe, the MGTX organizers assembled a large group of investigators from both in and outside the United States. The multinational composition of the study group was deemed necessary given the relatively small number of centers with clinical expertise in MG in an individual country or continent, and the need for a vast referral network of patients in early stages of disease. Further, when confronted with testing an intervention that has been utilized for over 50 years in MG, a small number of centers declined participation.
At the time of study initiation, 70 centers were invited to participate. Nine centers dropped out for a variety of reasons. In the second quarter of 2007, an additional 18 centers were invited to join the study to boost the rate of recruitment. Of the 79 participating sites, 43% are in the U.S. Table 1 lists the 23 countries and distribution of MGTX study sites.
Table 1.
Distribution of Centers by Country
| Country | # of Centers |
|---|---|
| Argentina | 1 |
| Australia | 2 |
| Brazil | 3 |
| Canada | 5 |
| Chile | 1 |
| Germany | 4 |
| Greece | 1 |
| Holland | 1 |
| Ireland | 1 |
| Italy | 4 |
| Japan | 2 |
| Mexico | 1 |
| Poland | 1 |
| Portugal | 1 |
| Romania | 1 |
| Serbia | 1 |
| South Africa | 1 |
| South Korea | 3 |
| Spain | 2 |
| Taiwan | 1 |
| Thailand | 1 |
| United Kingdom | 7 |
| United States | 34 |
Patient recruitment plays a key role to the success of any clinical trial. However, prior to launching a study, months to years are now required to navigate the ever- increasing paperwork, the body of regulatory approvals, building infrastructure and data management solutions, and the training of personnel on the protocol. For an international study involving multiple centers worldwide, the planning phases are even more challenging as a result of additional regulatory requirements, differences in approval policies between institutions and countries, language barriers, and regional customs.
2. CENTER SELECTION AND PRE-AWARD PREPARATION
Formal planning for the study began in October 2000 under the direction of the late John Newsom-Davis. He assembled a group of investigators based on personal assessment of their expertise and ability to participate in a long, demanding, complex study that would require patients to be randomized to surgical plus medical therapy versus medical therapy alone (Newsom-Davis et. al, in print). The initial meeting took place in Boston, MA, during the annual convention of the American Neurological Association. Three more investigator meetings were held in 2001 in Philadelphia, London, and Chicago. Based on feedback from these meetings, the protocol underwent several modifications. Further revisions to the study plan were made in response to peer reviews of grant applications to the Medical Research Council of the United Kingdom and the National Institutes of Health in 2001, and following a March 2002 meeting with the clinical trials section of the NINDS. The University of Alabama at Birmingham was enlisted as the Data Coordinating Center in the February 2004 NIH grant submission, and following a revised proposal in October 2004, the NINDS Council recommended funding for MGTX.
3. REGULATORY APPROVAL
For participating sites, obtaining regulatory approval is the first and a critical step for any clinical trial for several reasons. First, all regulatory documents must be in place before recruitment can begin. Second, most sites will not receive subcontract approval until regulatory paperwork has been approved. In our experience, regulatory approval was the major source of delay. Although project summary and consent form templates are provided by the coordinating center, each site must negotiate with their own institutional review board (IRB) or ethics committee on regulatory language, patient protections, and a variety of local issues and preferences while preserving the overall framework of the protocol. In the U.S. there are local IRBs and centralized IRBs. University-based centers almost exclusively require local IRB approval for federally funded studies. In the United Kingdom both local and Multicentre Research Ethics Committee (MREC) approvals are required before launching recruitment. Ethics boards or committees govern such investigation in other countries.
The duration of IRB approval ranges from 6 to 12 months in the U.S. before another IRB review takes place in order to continue the study. Ethics committee approval outside the U.S. usually lasts for the duration of the study; it is 5 years for MGTX. As a federally funded study supported by the U.S. government, each institution is required to register their ethics committee and apply for Federalwide Assurance (FWA) through the Office for Human Research Protections (OHRP) in the Department of Health and Human Services. Once granted, FWA must be renewed every 3 years. Institutions holding an FWA number must agree to terms required by OHRP found in the following link: http://www.hhs.gov/ohrp/assurances/assurances_index.html. Such agreement provides assurance that conduct of the study at each institution meets the requirements or follows equivalent guidelines mandated by the U.S. Department of Health and Human Services (Protection of human subjects. Code of Federal Regulations Title 45 Public Welfare, Part 46, June 23, 2005) which may be found in the OHRP website under Regulations.
Finally, each institution outside the United States must receive U.S. State Department clearance so that transfer of federal funds and other business with that institution is considered legal. For MGTX, NINDS bore the responsibility for obtaining State Department clearances. State Department Clearance proved to be a significant source of delay for several institutions in South America and Asia. In general, once State Department clearance is granted, it remains valid for the length of the study.
The MGTX Data Coordinating Center (DCC) is responsible for communicating with and assisting centers on regulatory approvals and addressing local questions that arise in relation to the protocol. The NINDS Cooperative Agreement is with the DCC, and the DCC acts as the funding agency (subcontractor) for the sites, keeping track of IRB/ethics committee approvals, and maintaining copies of informed consent forms, FWA certification, and State Department clearance. Via email, the DCC sends out reminders when renewal is due. Although the primary responsibilities of the DCC are usually viewed as data management and statistical analysis, it is not unusual for the DCC to have assumed these administrative functions (Collins et. al., 2003). In some collaborative studies, these administrative tasks may be delegated to a private research coordination firm.
4. TRAINING AND CERTIFICATION
Each participating center has at least two neurologists involved in the trial. The Principal Investigator (PI) is the person responsible for the conduct of the study. The PI screens, randomizes and then follows study subjects for the next three years. The PI is aware whether a patient is randomized to surgery (an extended transsternal thymectomy) versus no-surgery. The second neurologist, the Blinded Evaluator (BE), is unaware of treatment assignment and begins assessing the condition of the patient 4 months after randomization using a single-blinded strategy. Due to the invasive nature of a transsternal incision and ethical concerns, a sham surgical procedure to permit double-blinding was considered not feasible in this trial. By 4 months after randomization, patients assigned to surgery have already recovered to the point that single-blinded assessments are possible. Subjects are reminded throughout the study not to discuss whether or not they underwent surgery with the BE. As a physical precaution, all subjects wear a high collared jersey during their study evaluations. The main reason for enlisting the BE is to minimize evaluator bias in regard to the subject’s clinical status; it is this status that dictates changes in prednisone dosing.
In the last two weeks of March 2006, training sessions for the PIs and BEs were conducted at two venues – San Francisco, CA and Oxford, England -- to better accommodate the wide geographic spread of the participating sites and to limit travel costs. All centers were represented by at least the PI or BE, but in most cases both attended. All PIs and BEs were required to pass a multiple-choice certification test at the end of the two days of training.
As we will describe, hurdles for regulatory approval vary between sites, and our experience suggests 10–20% of sites will never reach this goal. When it came time to add new centers to the study, we developed a more cost-effective approach since it is costly to train sites at large investigator meetings. Thus, training for these sites was provided locally at the institution by the Project Manager, complemented by video training via the internet. Once regulatory approval was obtained, certification tests were administered electronically.
Because one of the treatment arms requires a thymectomy, the surgeons were required to review a printed manual and a video illustrating the extended transsternal thymectomy prescribed by MGTX. In addition, they were required to pass a multiple-choice Certification Test and to sign a Surgical Agreement Form to ensure that they understood the protocol and agreed to follow the prescribed thymectomy technique. Technical training of the surgeons was considered impractical as well as inappropriate.
Finally, each center PI was required to pass the Data Entry System (DES) certification by successfully screening and randomizing a hypothetical patient using the MGTX web-based system. Once completed, the DES registered the date of certification and allowed the center to go “live.”
5. RESULTS: ANALYSES OF TIME TO COMPLETION
MGTX centers are ready to recruit when all regulatory approvals have been obtained, the subcontract is in place, personnel have been trained and certification tests have been passed. As of March 2008, all 34 U.S. sites have obtained IRB approval; 8 of 45 centers outside the U.S. are still completing ethics committee approval. All 8 are among the batch of new sites added to boost recruitment. Of the 61 original centers that remain in the trial, 19 (13 being outside the U.S.) obtained IRB/EC approval before the investigator training meetings were held. Two centers (both new and outside the U.S.) have yet to complete FWA registration, and one new site (Romania) is awaiting State Department clearance. Fifty-nine centers have passed all administrative hurdles and are actively recruiting.
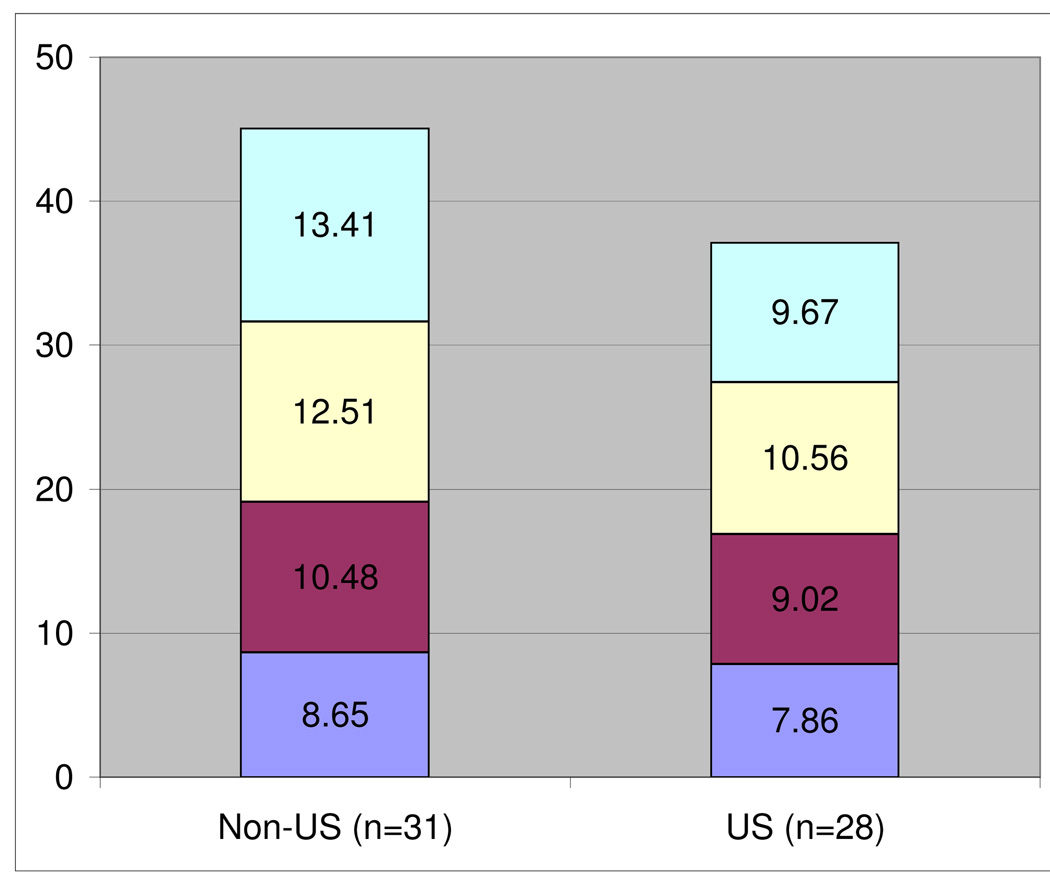
Considering only the 59 centers that have completed all prerequisites, Figure 1 displays the average number of months to complete each stage: (1) regulatory approval, (2) contract, (3) DES certification and (4) surgeon certification. Data is provided for U.S. versus non-U.S. sites. Regulatory approval includes IRB/EC, FWA, and in the case of foreign sites, State Department clearance. In computing the time to completion for each requirement, December 1, 2005 was used as the common start date for original centers, and April 1, 2007 for new centers. Because requirements may be completed in parallel fashion, summation generally exceeds the time it took for a center to be ready to recruit. As expected, U.S. centers had a shorter mean time to complete all requirements than non- U.S. centers. However, the time to complete DES certification, surgeon approval and contracts was not statistically different between the two groups using t-test. In ascending order, contracts, DES certification, and surgeon approval took 8.27 ± 0.67, 9.79 ± 0.60, and 11.58 ± 0.46 months, respectively. Regulatory approval took the longest among the four items considered. The mean time for non-U.S. centers to achieve regulatory approval was significantly longer (mean 13.4 ± 0.96 months) than for U.S. sites (9.67 ± 0.74 months; p = 0.003, t-test). When considering IRB/EC approval alone, there was no significant geographic difference (Table 2). However, the median of 4.4 versus 5.3 months was nearly 1 month longer for the non-U.S. centers. The mean time for IRB/EC approval for all 59 centers was 6.5 ± 0.58 months with a wide range across all sites (less than 1 month to 19 months).
Figure 1.
Average Number of Months until Completion of Study Prerequisites
Table 2.
Summary Statistics on Time until Requirements are Met
| N | Mean | Median | Min | Max | Std Dev | ||
|---|---|---|---|---|---|---|---|
| IRB/EC Approval | Non-US | 31 | 6.37 | 4.40 | 0.20 | 19.27 | 4.62 |
| US | 28 | 6.73 | 5.27 | 0.53 | 18.23 | 4.34 | |
| IRB/EC Approval + FWA and State Dept Clearance | Non-US | 31 | 13.41* | 14.60 | 2.53 | 25.70 | 5.34 |
| US | 28 | 9.67* | 8.13 | 3.90 | 19.10 | 3.90 | |
| Contract | Non-US | 31 | 8.65 | 6.23 | 2.47 | 24.90 | 5.94 |
| US | 28 | 7.86 | 6.60 | 2.53 | 17.20 | 4.25 | |
| Center Ready to Recruit | Non-US | 31 | 14.88# | 14.67 | 7.87 | 25.70 | 4.29 |
| US | 28 | 12.08# | 11.15 | 6.33 | 24.63 | 4.45 | |
p-value=0.003
p-value=0.017
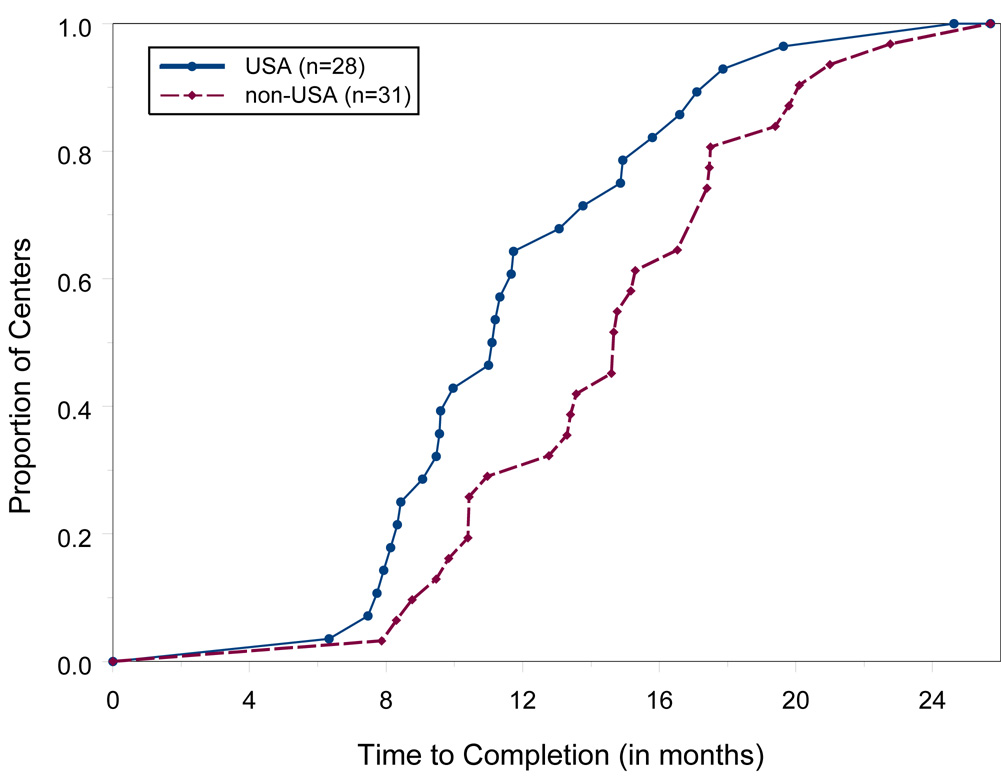
Figure 2 displays the proportion, over time, of the 59 centers that had completed all study prerequisites. Fifty percent of the 28 US centers were ready to recruit within 11.0 months, while 50% of the 31 non-U.S. centers were ready within 14.6 months. The average time for US centers to complete all requirements was 12.1 ± 0.81 months and 14.9 ± 0.80 months for foreign centers. This difference was significant (p = 0.017, t-test). Since the analysis does not include those 20 centers still seeking approvals, mean values are probably underestimated.
Figure 2.
Proportion of Centers Completing Study Prerequisites
6. DISCUSSION
Delay in obtaining regulatory approvals is not unique to the MGTX. Multiple regulatory hurdles and obstacles have been encountered by other multicenter clinical trials (McNay et. al., 2002; Dulley et. al., 2008). Differences in the time for approval of U.S. versus international sites are likely due to several factors. U.S. investigators tend to be more familiar with NIH and federal requirements. Cultural differences (e.g., birth-control policies differ considerably between countries) and language barriers contributed to some of the delays at MGTX sites. Countries including Japan, Taiwan, Netherlands and the United Kingdom would not permit the use of corticosteroids originating in the U.S.. Therefore, pharmacists at these sites required extra training to ensure that the drug dispensing system used to track dosing matched that used in the study protocol.
Most U.S. institutions that participate in clinical research have a preexisting valid FWA which eliminates some of the delay. The FWA requires the signature of a high-level institutional representative. Instructions for this process are available only in English on the Department of Health and Human Services website. Although foreign investigators are usually fluent in English, this is not always the case with administrative officials. In addition, a site’s willingness to sign documents related to FWA may be influenced by differences in local practice in regard to the ethics of human research. The MG center in Taiwan, which in December 2005 was the first to obtain ethics committee approval, did not receive FWA approval until January 2007. A center in Japan received ethics committee approval in February 2006 but did not receive FWA approval until May 2007.
State Department clearance may be delayed by political instability or a tenuous relationship between other countries and the U.S. The Brazilian sites had to wait 17 months for State Department clearance.
Subcontract delays were mainly due to administrative workload issues between the participating sites and the Grants and Contracts Office of the DCC Institution. Some institutional grant management offices have required contracts to be translated into their native language while others insisted they be paid in their local currency instead of the U.S. dollar. For U.S. sites, contract delays were often due to indirect cost accounting issues. Finally, busy work schedules were a source of delay in PIs completing DES certification and surgeons fulfilling their certification obligations.
7. CONCLUSIONS
Research on human subjects appropriately requires rigorously defined regulations. However, the burden placed on investigators in the current regulatory environment is daunting, especially when multinational collaborative studies are involved. Funding agencies, research review committees, grant managers, and investigators must recognize that satisfying the various regulatory hurdles consumes significant resources of time, money and energy. For MGTX, concerns that are unique to a study randomizing patients to an invasive procedure may explain some of the delays encountered with IRB/ethics committee approval. However, FWA certification, State Department clearance, and contractual issues are administrative steps common to all multinational investigations. The impact of these various hurdles on the timely completion of clinical studies deserves further study. As a case in point, the scientific community has begun to organize conferences to address these concerns (Burris, 2008). The time has come for a careful analysis of the cost and effectiveness of the various administrative steps required to enlist sites in a multinational investigative effort, with a streamlining of procedures and paperwork as an ultimate goal.
ACKNOWLEDGEMENTS
This research is supported by a grant from the National Institute of Neurological Disorders and Stroke (5U01NS42685-03 – Thymectomy in Non-Thymomatous MG Patients on Prednisone) and funding from the Myasthenia Gravis Foundation of America. The authors would also like to acknowledge the MGTX Advisory Committee members who have recruited at least 2 patients to date in this study: Dr. Claudio Mazia (Centro de Asistencia Docencia e Investigacion en Miastenia, Buenos Aires, Argentina), Dr. Gabriel Cea (Universidad de Chile, Santiago, Chile), Dr. Amelia Evoli (Catholic University, Rome, Italy), Dr. John King (Royal Melbourne Hospital, Victoria, Australia), Dr. Giovanni Antonini (University of Rome, Rome, Italy), Dr. Jeanine Heckmann (University of Cape Town, Cape Town, South Africa), Dr. Joel Oger (Univeristy of British Columbia, Vancouver, Canada), Dr. Bashar Katirji (Case Western Reserve University, Cleveland, Ohio), Dr. Bryan Lecky (Walton Hospital for Neurology, Liverpool, United Kingdom), Dr. Wilfred Nix (Johannes Guternberg-Universitat, Mainz, Germany), Dr. Mike Pulley (University of Florida, Jacksonville, Florida), Dr. Elza Tosta (Hospital de Base do Distrito Federal, Brasilia, Brazil), and Dr. Hiroaki Yoshikawa (Kanazawa University, Ishikawa, Japan).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors maybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Inmaculada B. Aban, Department of Biostatistics, University of Alabama at Birmingham, 1665 University Boulevard, Birmingham, AL 35294-0022.
Gil I. Wolfe, Department of Neurology, University of Texas Southwestern Medical Center, Dallas, TX 75390-8897.
Gary R. Cutter, Department of Biostatistics, University of Alabama at Birmingham, 1665 University Boulevard, Birmingham, AL 35294-0022.
Henry J. Kaminski, Department of Neurology & Psychiatry, Saint Louis University Medical Center, 1438 South Grand Boulevard, St. Louis, MO 63104.
Alfred Jaretzki, III, Department of Surgery, Columbia University Medical Center, 177 Fort Washington Avenue (MIB 7th Fl), New York, NY, 10032.
Greg Minisman, Department of Biostatistics, University of Alabama at Birmingham, 1665 University Boulevard, Birmingham, AL 35294-0022.
Robin Conwit, Division of Extramural Research, NIH/NINDS, Neuroscience Center, Room 2107, 6001 Executive Blvd MSC 9520, Bethesda, MD 20892-9520.
John Newsom-Davis, formerly from the Department of Clinical Neurology, University of Oxford, West Wing, John Radcliffe Hospital, Oxford OX3 9DU, UK.
REFERENCES
- Burris S. Regulatory innovation in the governance of human subjects research: A cautionary tale and some modest proposals. Regulation & Governance. 2008;2:65–84. [Google Scholar]
- Chiu H-C, Vincent A, Nemsom-Davis J, Hsieh K-H, Hung T-p. Myasthenia gravis: population differences in disease expression and acetylcholine receptor antibody titers between Chinese and Caucasians. Neurology. 1987;37:1854–1857. doi: 10.1212/wnl.37.12.1854. [DOI] [PubMed] [Google Scholar]
- Collins JF, Garg R, Teo KK, Williford WO, Howell CL, on behalf of the DIG Investigators The role of the data coordinating center in the IRB review and approval process: the DIG trial experience. Controlled Clinical Trials. 2003;24:306S–315S. doi: 10.1016/s0197-2456(03)00100-4. [DOI] [PubMed] [Google Scholar]
- Compston DAS, Vincent A, Newsom-Davis J, Batchelor JR. Clinical, pathological, HLA antigen, and immunological evidence for disease heterogeneity in myasthenia gravis. Brain. 1980;103:579–601. doi: 10.1093/brain/103.3.579. [DOI] [PubMed] [Google Scholar]
- Duley L, Antman K, Arena J, Avezum A, Blumenthal M, Bosch J, Chrolavicius S, Li T, Ounpuu S, Perez AC, Sleight P, Svard R, Temple R, Tsouderous Y, Yunis C, Yusuf S. Specific barriers to the conduct of randomized trials. Clin Trials. 2008;5:40–48. doi: 10.1177/1740774507087704. [DOI] [PubMed] [Google Scholar]
- Heckmann JM, Owen EP, Little F. Myasthenia gravis in South Africans: Racial differences in clinical manifestations. Neuromuscul Disord. 2007;17:929–934. doi: 10.1016/j.nmd.2007.07.002. [DOI] [PubMed] [Google Scholar]
- McNay LA, Tavel JA, Oseekey K, McDermott CM, Mollerup D, Bebchuk JD, ESPRIT Group Regulatory approvals in a large multinational clinical trial: the ESPRIT experience. Controlled Clinical Trials. 2002;23:59–66. doi: 10.1016/s0197-2456(01)00183-0. [DOI] [PubMed] [Google Scholar]
- Newsom-Davis J, Cutter G, Wolfe GI, Kaminski HJ, Jaretzki A, III, Minisman G, Aban I, Conwit R. Status of the thymectomy trial for non-thymomatous myasthenia gravis patients receiving prednisone. Annals NY Academy of Science. doi: 10.1196/annals.1405.014. in press. [DOI] [PubMed] [Google Scholar]
- Phillips LH. The epidemiology of myasthenia gravis. Ann N Y Acad Sci. 2003;998:407–412. doi: 10.1196/annals.1254.053. [DOI] [PubMed] [Google Scholar]
- Phillips L, Torner J, Anderson M, Cox G. The epidemiology of myasthenia gravis in central and western Virginia. Neurology. 1992;42:1888–1893. doi: 10.1212/wnl.42.10.1888. [DOI] [PubMed] [Google Scholar]
- Wolfe G, Kaminski HJ, Jaretzki A, Swan A, Newsom-Davis J. Thymectomy trial in non-thymomatous myasthenia gravis patients receiving immunosuppressive therapy. Ann NY Acad Sci. 2003;998:473–480. doi: 10.1196/annals.1254.061. [DOI] [PubMed] [Google Scholar]
- Wong V, Hawkins BR, Yu YL. Myasthenia gravis in Hong Kong Chinese. Acta Neurol Scand. 1992;86:68–72. doi: 10.1111/j.1600-0404.1992.tb08056.x. [DOI] [PubMed] [Google Scholar]