Abstract
Living donor liver transplantation (LDLT) has been controversial since its inception. Begun in response to deceased donor organ shortage and waiting list mortality, LDLT was initiated in 1989 in children, grew rapidly after its first general application in adults in the US in 1998, and has declined since 2001. There are significant risks to the living donor, including the risk of death and substantial morbidity, and two highly publicized donor deaths are thought to have contributed to decreased enthusiasm for LDLT. Significant improvements in outcomes have been seen over recent years and data, including from the NIH-funded Adult-to-Adult Living Donor Liver Transplantation Cohort Study, A2ALL, has established a survival benefit from pursuing LDLT. Despite this, LDLT still comprises less than 5% of adult liver transplants, significantly less than in kidney transplantation where living donors comprise approximately 40% of all transplant performed. The ethics, optimal utility and application of LDLT remain to be defined. In addition, most studies to date have focused on post-transplant outcomes and not included the effect of the learning curve on outcome or the potential impact of LDLT on waiting list mortality. Further growth of LDLT will depend on defining the optimal recipient and donor characteristics for this procedure as well as broader acceptance and experience in the public and in transplant centers.
Introduction
A central tenet in medicine is primum non nocere, first do no harm. Believed to be part of the Hippocratic Oath, despite being in Latin, it guides policy and beliefs, if not practice, in contemporary medicine. On the surface, adult-to-adult living donor liver transplantation (LDLT) contradicts this principle, because a healthy individual undergoes a major operation for no direct, physical benefit.
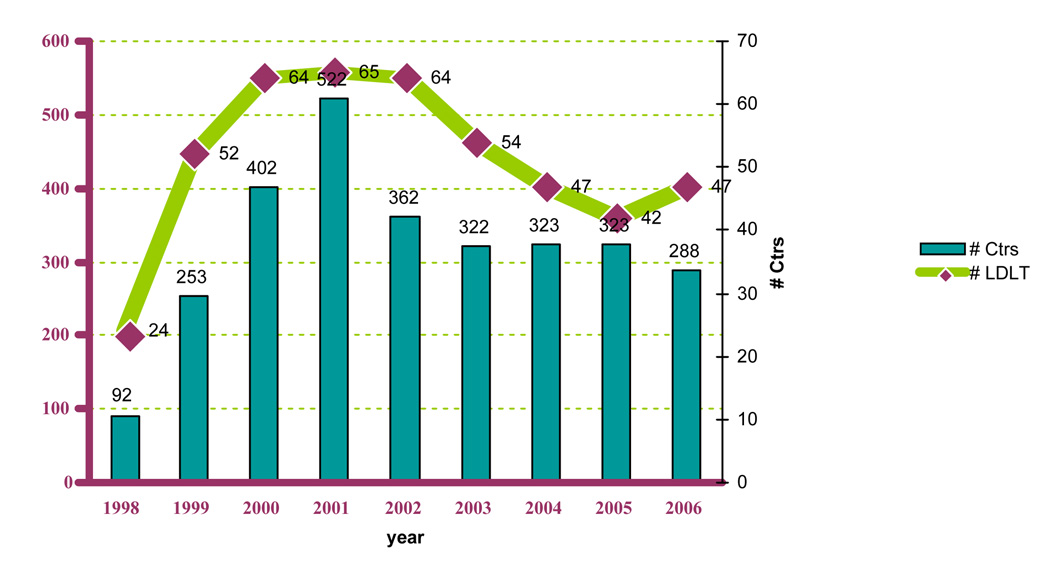
The first adult-to-adult living donor liver transplant (LDLT) was performed in Hong Kong in 1993. Five years later, the first LDLT was performed in the United States, and, today, there are over 90 centers that perform LDLT across the country, though most are done in a smaller number of larger volume centers. The majority of LDLT done in the US are for adults using right lobe grafts. As opposed to a left hepatectomy, this procedure provides the recipient with sufficient hepatic mass to replace the cirrhotic liver while still leaving the donor with enough functioning hepatocytes. In 2000 there was great enthusiasm for adult living donor liver transplantation (LDLT), with forty-nine centers performing at least one LDLT. The enthusiasm was quickly tempered by the death of a donor in January 2002, the second reported death of an adult living transplant donor in the United States. We previously reported that 76% of liver transplant programs that had not performed LDLT planned on starting a program, but since then the climate for living donation had changed 1. From 2001–2006, the number of centers performing LDLT and the number of procedures declined, though it appears to have stabilized at ~250/cases per year, about half of the peak in 20012 (Figure 1). There are several possible reasons why the number of centers performing LDLT and the total number of LDLT’s have declined, including changes in organ allocation and reticence following the donor deaths, but other unrecognized factors may also have played a role. Two factors that may be important were the exhaustion of the initial pool of eligible patient, i.e., all the patients on the waiting list in 1998 (over 17,000 patients), leaving only new additions to the waiting list as potential LDLT candidates. Additionally has been the increased use of extended criteria donor (ECD) livers, which includes those from older donors (over 60 to 70 years old), donation after cardiac death (DCD, formerly called non-heart beating donors), and liver with steatosis or exposure to/infection with Hepatitis B or C.
Figure 1. LDLT Transplant Volumes.
Number of centers and number of adult living donor liver transplants performed from 1998 – 2006 in the United States
In the United States over 1,700 LDLT’s have been performed and two early deaths and two liver transplants have occurred in adult living liver donors. There have been several additional late deaths though these were not clearly related to donation. After the second donor death, a number of position papers, conferences, and review boards have taken place 3–5. New York State created a review committee and document mandating guidelines for transplant centers and physicians who perform LDLT 5. The United Network for Organ Sharing (UNOS) now collects 2-year follow-up data on all donors and is developing standards for evaluating programs as well as resource documents to help standardize the donor consent and evaluation processes. Additionally, a more detailed study of LDLT, A2ALL, is an NIH sponsored multicenter prospective study of LDLT at nine centers in the United States is underway and recently published excellent outcomes including a survival benefit for candidates on the waiting list who pursue LDLT 6.
Selection of the LDLT recipient candidate
At the current time, most experts concur that recipients considered for LDLT should fulfill the same minimal listing criteria established for deceased donor liver transplantation. Some transplant physicians and surgeons believe that LDLT should be extended to patients not felt to be candidates for deceased donor grafts. This is unfortunately potentially coercive and raises an ethical dilemma. The principle of autonomy should allow donors and recipients to make an independent decision even if the risk is prohibitive or a deceased donor transplant is felt contraindicated, e.g. acute alcoholic hepatitis. On the other side is the question of exposing a healthy donor to risk to perform a transplant that wouldn’t be performed with a deceased donor graft. However, much of the seeming contradiction is due to organ scarcity. If deceased donor organs were unlimited, would the outcomes justify the procedure with a deceased donor graft? If the answer is yes, and the transplant physician would proceed with a deceased donor organ at this time, than the candidate is acceptable for LDLT. The ideal candidate for LDLT who derives the maximal benefit is the one that that would benefit from transplant now but is unlikely to receive a deceased donor graft prior to dying or becoming too ill due to waiting list priority, age, or other comorbidities.
Since the major benefit of LDLT is to reduce waiting time mortality, it is possible that patients may receive LDLT too early in their disease course negating that survival benefit. Prior to the implementation of MELD, a substantial proportion (43%) of patients undergoing LDLT were UNOS status 3 (i.e., Child Class B and at home) at the time of transplantation, unlike those undergoing DDLT who were usually Status 2 (i.e. Child Class C or hospitalized with complications of liver disease)7. The LDLT recipient candidate should undergo the same evaluation as the deceased donor recipient. During the proliferation of LDLT, the system for organ prioritization has changed from a waiting time-based system to a severity of illness system based on the Model for End-stage Liver Disease (MELD) score. The optimal MELD score at which patients undergoing LDLT derive a sustained survival benefit by reducing waiting time mortality that is not offset by post-transplant mortality is yet to be determined, but is likely to be the same as for DDLT, which is greater than 15 in most clinical situations 8.
Selection of the LDLT donor candidate
The goal of the donor evaluation is to determine if the donor is medically and psychologically suitable for living donation. Equally important, is to ensure that the donor is well informed of the risks and benefits of the procedure and is making an autonomous and noncoerced decision. Most living donors are in excellent health. Although there is no definitive age cut off, donors are typically between 21 and 55 years old. New York State mandates an upper age limit of 60 years. Donors under 18 are generally felt unacceptable except for an emancipated minor donating to their child. Donors should not have liver disease or significant comorbidities, such as coronary artery disease or cerebrovascular disease. The presence of mild systemic disease, such as well controlled mild hypertension or diet-controlled diabetes, are not necessarily contraindications to donation. Individuals who are significantly obese, with a body mass index over 35 likely are excluded as living donors in many programs due to fear of post-operative complications or the presence of hepatic steatosis. We have shown, however, that selected obese donors with BMI up to 40 can donate safely with good outcomes for both donor and recipient 9. Because the presence of hepatic steatosis may compromise the function of the graft some centers perform liver biopsies on all donor candidates, while other centers rely upon physical exam, risk factors for hepatic steatosis, and imaging studies 10, 11.
Potential donor candidates undergo a similar medical evaluation as the recipient with serologic testing for viral hepatitis, HIV antibody, as well as testing for other chronic liver diseases 11. An independent transplant physician, usually a hepatologist that is not the primary hepatologist of the recipient, should evaluate the LDLT donor candidate. An independent donor advocate who not part of the transplant team has been recommended by UNOS, the Advisory Council on Transplantation (ACOT) and the New York State Commission. We have an independent donor advocate team or IDAT, (Table 1) which evaluates all donors and meets separately from our recipient selection committee. Evaluation of vascular and biliary anatomy can be achieved noninvasively with CT or MR angiography or invasively with conventional angiography and ECRP. The approach varies from to center to center although most centers use noninvasive methods 1. All living donor candidates should undergo a psychosocial evaluation to determine if coercion is present and if they truly understand the risks of the procedure. Between 15% – 45% of donors who present for evaluation may be suitable candidates that eventually proceed with LDLT 1, 11–13. In the multicenter A2ALL consortium the donor acceptance rate overall was 40% but varied markedly between centers and over time 13.
Table 1.
Structure of an independent donor advocate team (IDAT) at New York-Presbyterian. None of the members are directly involved in the care of the recipient.
Those with an asterisk are not members of the liver transplant team
Determining if the donor has adequate hepatic mass to provide both a functional graft and remnant is a key component to LDLT evaluation. A graft to body weight ratio (GRBW) of 0.8% has been recommended as the minimum cut-off for the recipient 10. GRBW of less than 0.8% may be associated with liver failure, or small-for-size syndrome characterized as ascites, jaundice, and hepatic congestion, but is rare with right hepatectomy unless the donor is much smaller than the recipient. Other centers estimate the actual graft volume typically with CT or MRI imaging. The radiologist encircles the right hepatic lobe using the middle hepatic vein as the left border and utilizing computer software calculates the hepatic volume and weight. There are less clear rules for the minimum left lobe size that remains in the donor who can also suffer from small-for-size syndrome if the left lobe remnant is inadequate for immediate post-operative metabolic needs. Small-for-size syndrome usually resolves spontaneously over 10–14 days.
The living donor hepatectomy
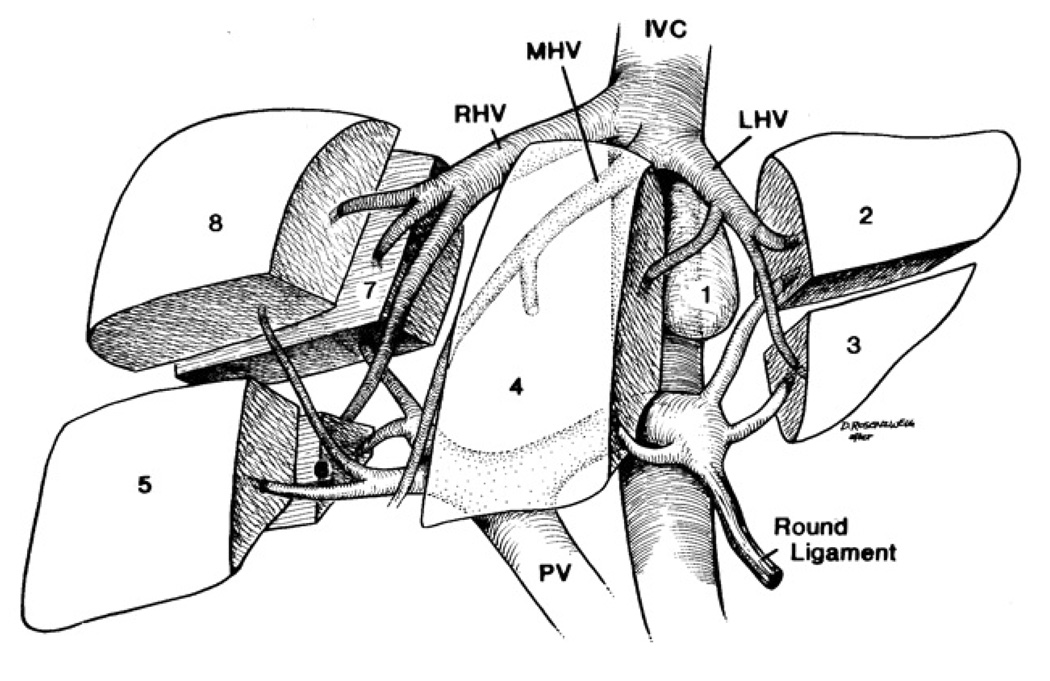
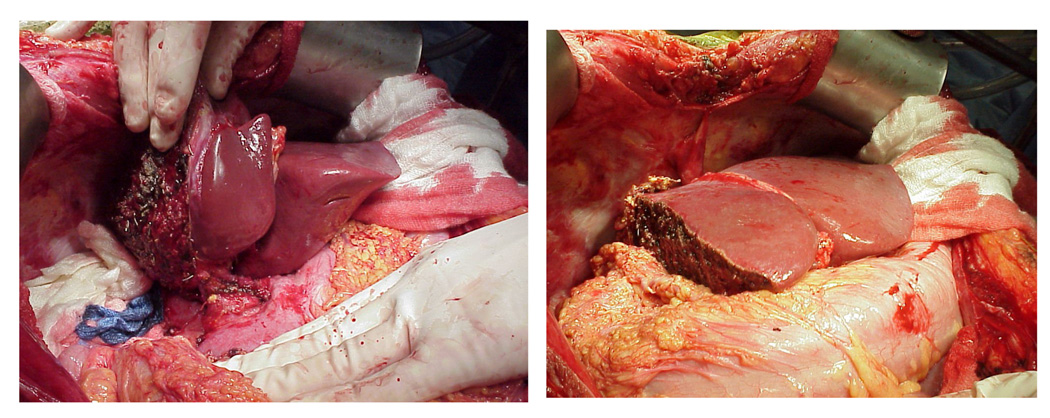
The performance of living donor liver transplantation relies upon an understanding of the vascular and biliary anatomy of the liver. The left lateral lobe consists of Couinaud segments 2 and 3, the median lobe is Couinaud segment 4, and Couinaud segments 5, 6, 7, 8 comprise the right hepatic lobe (See Figure 2). Couinaud segment 1 is the caudate lobe. It is segments 5–8 or the right hepatic lobe, comprising 50–60% of hepatic volume, which are resected from the living donor and transplanted into the recipient in most adult-to-adult LDLT. In some circumstances, usually when the recipient is an adolescent or much smaller than the donor, the left hepatic lobe can be used (Figure 3a). For babies, the left lateral segment, comprising segments 2 and 3 or ~20% of hepatic mass are used. The right hepatectomy is performed through a midline or a right subcostal incision with extension to the upper midline. A cholecystectomy is performed. Intraoperative cholangiogram may be obtained to define biliary anatomy. Intraoperative ultrasound is usually used to isolate the right hepatic artery, right hepatic vein, and right portal vein. In most cases, the liver is divided to the right of the middle hepatic vein with or without inflow occlusion using an ultrasonic aspiration dissector or other device. Some programs in Asia include the middle hepatic vein in the graft to provide improved outflow and hepatic mass to the recipient but the majority of programs in the US feel this is too large a resection for a healthy donor who does not require surgery and increases the risk of small-for-size syndrome in the donor. Whether or not to include the middle hepatic vein in the graft has been the most controversial technical aspect of LDLT. The graft without the middle hepatic vein can be outflow challenged and prone to congestion with cholestasis and portal hypertension early in the recipient, but is safer for the donor. The recipient hepatectomy is usually begun concurrently with the donor operation to minimize cold ischemia time. After complete removal of the recipient’s diseased liver, the right graft is implanted into the recipient. The recipient’s right hepatic vein, hepatic artery, and portal vein are anastomosed to the donor’s right hepatic vein, hepatic artery, portal vein, respectively (Figure 3b). The biliary anastomosis is established through a choledochojejunostomy with a Roux-en-Y anastomosis, to the donor’s right hepatic duct(s) or a duct-to-duct choledochocholedochostomy between the donor’s right hepatic duct and recipient’s common bile duct remnant. Because of variations in biliary anatomy, multiple bile duct anastomoses may need to be performed and this usually requires a Roux-en-Y anastomosis.
Figure 2.
Segmental anatomy of the liver using the Couinaud segments
Figure 3. Figure 3a: Left Lobe Living Donor Graft.
Figure 3b: Right lobe transplant
Left and right lobe liver grafts in situ in the recipient
The Achilles’ heel of LDLT is biliary complications. Anomalous biliary anatomy is present in up to 40% of donors 10. The most common complications reported in donors and recipients specific for LDLT are bile duct leaks or biliary strictures. These complications decrease with time. It is now recognized that a learning curve exists with a lower complication rate and improved graft survival over time. In the A2ALL consortium, the rate of biliary complications in the recipients was 30% in the first 20 cases and decreased to 11% thereafter 14. Vascular complications in the recipient include hepatic artery thrombosis and hepatic venous congestion and are the most common cause of graft loss 14. Vascular complications in the donor are rare.
Recipient outcomes for LDLT
Impact of severity of disease
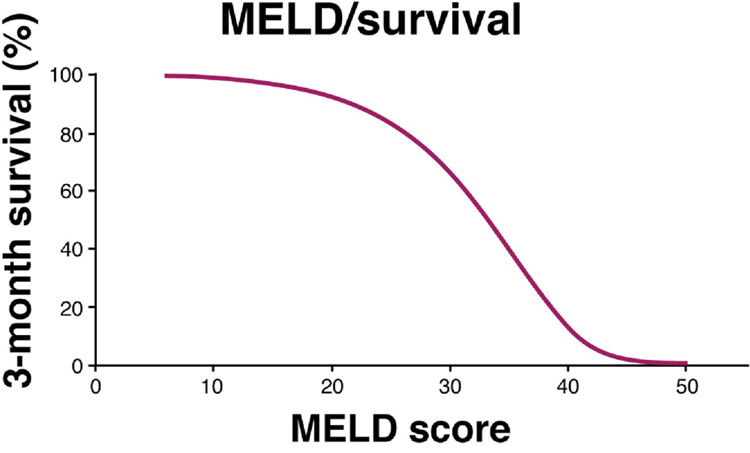
It was initially believed that receiving a whole liver is preferable to receiving a partial liver graft. Recently, however, it has been shown that outcomes from the time transplant are equivalent in similar patient populations between living and deceased donor transplantation in experienced living donor centers 6. Additionally, because of the organ shortage, most transplant centers do not have the luxury of transplanting all of their patients in need of transplantation with MELD allocated deceased donor organs before they die or become too ill. And it is ultimately mortality on the waiting list and overall mortality pre- and post-transplant, not the number of patients waiting for liver transplantation and the number of patients who receive liver transplants, which is of ultimate concern and determines the efficacy of liver replacement therapy. The waiting list mortality increases in patients with advanced liver disease (Figure 4) and patients with a MELD score of 25 have a 20% three-month mortality 15. There is marked regional variability in MELD at transplant across the UNOS regions 16. Thus, depending on the region of the country and the average MELD score at time of transplant within the area served by the organ procurement organization (OPO), LDLT may offer patients a substantially higher likelihood of transplantation than waiting for a deceased donor liver.
Figure 4.
The relationship between MELD scores and likelihood of 3-month survival
When assessing a liver transplant candidate for LDLT, the adequacy of a partial graft for transplantation depends on the candidate’s severity of liver disease. Thus, a balance needs to be struck where the severity of the recipient’s liver disease is sufficient to justify transplantation, but not be so advanced that a partial graft will not provide adequate hepatic mass. Although LDLT has been performed in patients with fulminant liver failure and patients with very advanced liver disease (ICU-bound patients or MELD > 30), post-transplant survival rates are poor in this group of patients 17–19. In one series, patient survival was 57% with an average stay of twenty-three days in the intensive care unit. In comparison, one-year patient survival is 82% in deceased donor transplant recipients who were ICU-bound as UNOS status 2A at the time of transplant 19. This has led most centers in the US to abandon LDLT in the most severely ill patients with high MELD scores, especially now that they are given high priority on the UNOS waiting list. However, since short-term mortality without liver transplantation approaches 100% in these critically ill patients with high MELD scores in areas with low deceased donor organ availability, the decreased post-transplant survival rates with LDLT may be superior to the alternative of the high mortality on the waiting list, especially as outcomes with LDLT improve. This has led to the use of LDLT in this situation in Asia with good outcomes in some studies 20.
We do not have an absolute MELD cut-off for LDLT. Decisions on LDLT are made on a case-by-case basis, but in general it is uncommon to proceed with LDLT in patients with MELD scores above 30. A lower limit of MELD score with LDLT is more controversial and varies from center to center. Since for patients with MELD scores < 15 and certainly < 12, one year survival likelihood of survival is less with transplant than remaining on the waiting list 8, some have advocated not proceeding with LDLT in candidates with MELD scores less than 15. However, in the A2ALL cohort the average MELD at LDLT was 14, though many of these patients had HCC6. Thus decisions need to be made on a case-by-case basis. Particular attention needs to be taken in patients with HCV for whom recurrent HCV could decrease life expectancy if a transplant is performed too early in the absence of pre-transplant viral eradication (see below). We tend to avoid LDLT at low MELD except in patients with hepatocellular carcinoma, a suspicious biliary stricture or dysplasia in the setting of primary sclerosing cholangitis, or significant impairment of quality of life (e.g., refractory pruritus or metabolic bone disease in PBC, or difficult to control ascites and encephalopathy) is present.
Complications in the living donor recipient
Biliary and vascular complications are the major complications that occur in the recipient after LDLT, although wound infection, pneumonia and other typical post-operative complications can occur. Biliary complications, either bile leak or stricture at the anastomotic site or cut edge of the transected liver were reported in 15% – 60% of recipients in early, single center reports 21–24. Stenting the biliary anastomosis has been used to attempt to reduce the rate of bile leaks and strictures, but it is of unproven benefit. Complications are probably underreported and a standardized reporting system has been recommended for LDLT.
Vascular complications include thrombosis of the right hepatic artery at the anastomosis between the recipient and donor artery. Because of the small size of the right hepatic artery, in comparison to the proper hepatic artery in cadaveric liver transplantation, the anastomosis between the recipient’s right hepatic artery and donor’s right hepatic artery may increase the risk of thrombosis. It has been reported that using a Y extension arterial graft with reverse extension bifurcated graft from the gastroduodenal and common hepatic artery may protect arterial inflow 25. It has become increasingly recognized that small tributaries 3– 5 mm in size of the middle hepatic vein that drain segments 5 and 8 should be included in the anastomosis to the recipient hepatic vein or inferior vena cava to prevent hepatic venous congestion of the transplanted right lobe in the recipient.
Post-operatively, regeneration occurs rapidly in the recipient. Initial reports suggested that over 85% of hepatic volume was restored 1 week after transplantation 26. Based on MRI imaging of the abdomen the left lobe increases in mass by 100% in the donor and the right lobe increases by 87% in the recipient. However, subsequent studies suggest regeneration continues over 6 months 27. Liver regeneration is rapid and may be affected by severity of liver disease prior to transplantation and type of reconstruction performed with the middle hepatic vein 27.
Outcomes for Hepatitis C
Hepatitis C remains the most common indication for liver transplant. Early data suggested that patients with HCV that received a LDLT had worse outcomes than did recipients of DDLT 28. These early studies in which LDLT has been associated with increased graft failure have attributed the difference to more rapid HCV progression in the regenerating LDLT graft. One possible explanation for the difference is that recipients of LD receive a smaller grafts that regenerate and, several in-vitro studies suggest that dividing hepatocytes are more vulnerable to HCV infection. This could lead to increased levels of viremia, which is seen in cholestatic HCV, in LDLT recipients. This also may have been due to an increased rate of biliary complications or other problems seen during the learning curve of early LDLT experience. Whether there is an increased risk of cholestatic HCV remains unclear, and warrants further investigation.
More recent data suggests that there is no difference in recurrent HCV between recipients of DDLT and LDLT. These studies were usually based on protocol biopsies and included a later experience with LDLT. In a study of 23 LDLT recipients and 53 DDLT, protocol biopsies at 6 and 12 months were compared for inflammation and fibrosis and there was no difference in mean inflammation scores or fibrosis at any of the time points measured 29. 21% of the recipients of DDLT suffered acute rejection compared to 14% of the LDLT recipients; this difference was not statistically significant. Graft and patient survival rates between the two groups were similar: at 48 months, 82 and 82% for DDLT patients and 76 and 79% for LDLT patients (p=NS). Results from this study, which looked at liver histology do not support the idea that recurrent HCV is more prevalent among recipients of LDLT. Additional studies have also concluded that rates of HCV recurrence are not different among recipients of LDLT 7, 29.
The A2ALL data on HCV was reported recently comparing181 HCV positive LDLT recipients to 94 HCV positive DDLT recipients 30. Although patient survival was similar, 3-year graft survival was lower in LDLT recipients than in DDLT recipients (68% compared to 80% p=0.04). However, center experience was a confounder on the relationship between donor type and outcome. Once the center had performed 20 cases, graft survival was equivalent between DDLT and LDLT. For LDLT < 20 3-year graft survival was only 55% compared to 79% and 80% for LDLT>20 and DDLT recipients, respectively. There was equivalent and excellent patient survival between LDLT > 20 and DDLT as well, 91% and 87%, respectively. Unfortunately, the majority of patients studied in the retrospective arm of the A2ALL group did not have protocol liver biopsies. Of the 63 patients who were biopsied, there was no difference in total necroinflammatory or fibrosis scores between DDLT and LDLT at one-year post transplant. Thus the majority of recent data suggests that outcomes for HCV are similar for LDLT and DDLT at experienced centers and HCV is an acceptable indication for LDLT.
Outcomes for hepatocellular carcinoma
Prior to the implementation of MELD, a large proportion of LDLT were performed for hepatocellular carcinoma 31. Long waiting times and high rates of drop-out on the list as a UNOS Status 2b made LDLT the only viable option for many patients. Currently, despite the increased MELD priority (to 22 points with additional points every 3 months) given to patients who meet the Milan (T2) criteria, i.e., a single lesion <5 cm or 2–3 lesions each less than 3 cm, patients just outside these criteria (e.g. those between the Milan and the more expanded, UCSF criteria) will typically have very long wait list times that make transplant unfeasible. In some regions for some blood types, even patients within Milan criteria may have a 9–12 month wait for DDLT. Thus, LDLT remains an important option for the treatment of HCC particularly in situations where the risk of disease progression on the wait list is substantial.
Although it seems obvious that patients with HCC would benefit from earlier transplant and thus LDLT, to date data has not supported superior outcomes or lower recurrence with LDLT compared to DDLT. Much of this may have to do with differences between LDLT and DDLT recipients and study design. One retrospective study looked at outcomes of transplant in 43 living donor recipients and compared them to the outcomes of 17 deceased donor recipients 32. All of these patients met Milan or UCSF (solitary tumor < 6.5 cm or up to 3 tumor nodules, each < 4.5 cm with a total maximum size of < 8 cm) criteria. The MELD scores, CPT scores, and etiology of liver disease and tumor stage in the explant were comparable in both groups, but there were more patients with Child’s A or MELD < 10 in the LDLT group. 10/40 (25%) of the LDLT group underwent salvage transplant after resection or ablation compared to 1/12 (8%) of the patients who received a DDLT. Tumor recurrence developed in 10/43 (23%) LDLT and 0/17 DDLT patients. Multivariate analysis revealed that salvage transplant (RR 5.2) and tumor outside of UCSF criteria (RR 4.1), but not LDLT, were the only independent predictors of disease recurrence. This study is limited by the small sample size, and the fact that despite the similarities in gross staging, the patients differed in terms of prior therapy and microscopic disease suggesting that more aggressive tumors were disproportionately undergoing LDLT. The authors conclude that the higher recurrence rate seen in LDLT is due to confounding by more advanced disease.
The A2ALL group has also studied LDLT in the setting of HCC. A total of 106 patients were studied retrospectively: 58 LDLT and 34 DDLT recipients. While LDLT recipients enjoyed shorter waiting times compared to DDLT recipients (mean 160 days vs 469 days, p< 0.0001), HCC recurrence was more common in LDLT at 3 years (29% vs 0%, p=0.002) 33. There was no difference in overall mortality between the two groups.
One possible explanation for the increased recurrence of HCC for LDLT may be that the surgical techniques of LDLT make it a less successful cancer operation due to a need to keep vascular margins closer to the liver in LDLT. Another possible explanation for this observed difference is that the groups are not truly comparable. One needs to compare HCC recipients of DDLT and LDLT with caution; LDLT is often used as salvage transplant for patients who have failed to respond to resection or ablation or in patients who are progressing rapidly. This group of patients may represent a particularly aggressive type of tumor that has a high risk of recurrence with any type of transplant. The reason these patients do not recur post-DDLT is that they do not exist; if they do not receive a living donor liver transplant they likely progress rapidly while on the transplant list and drop-out or die prior to receiving DDLT. Thus, the wait list serves as a Darwinian selection mechanism for patients who have favorable tumor biology and a lower recurrence risk. This results in a paradoxical situation in which longer waiting times translate into better outcomes, reflecting more favorable tumor biology rather than an impact of waiting time or type of transplant. Thus, increased recurrence in LDLT recipients may reflect selection of patients with more aggressive disease, not sub-optimal therapy.
The A2ALL results support this theory. Additionally, “fast-tracked” transplants, which were defined as recipients who met the Milan criteria and received additional MELD points through exception or who underwent LDLT, had higher rates of tumor recurrence post-transplant compared to recipients of non-fast-tracked transplants who received transplants on the waiting list prior to being able to receive MELD exception points 34. These results underscore the concept that increased waiting times may provide a filter for patients whose tumor biology is amenable to cure with transplant, not that the operations fundamentally differ in outcomes.
In addition, these studies focus only on post-transplant outcomes. From the patient perspective, only overall (pre- and post-transplant) survival matters. Future studies need to analyze mortality from the time of listing, as well as drop-out due to tumor progression pre-transplant and post-transplant recurrence to adequately assess the impact of LDLT on outcome. If the drop-out pre-transplant with DDLT significantly exceeds the tumor recurrence post-transplant with LDLT, LDLT may offer a substantial overall survival benefit. Additionally, improved methods to risk stratify patients with HCC, and better adjuvant and neoadjuvant treatment regimens are needed. As more is discovered about HCC biology, we will be better able to identify patients with more virulent cancers, who may not benefit from transplant or require more aggressive locoregional or systemic anticancer therapy. LDLT may allow optimization of these therapies and controlled timing of transplantation.
Other centers have reported data more supportive of LDLT for patients with HCC. In a study comparing 36 cases of HCC, 53% outside Milan criteria, that were treated with LDLT to a cohort of 165 recipients of deceased donor organs, there was no significant difference in survival or recurrence rates 35. Furthermore, data suggests that LDLT for patients with HCC not only results in similar disease-free survival rates as DDLT, but that for patients with advanced HCC, outside of Milan criteria, LDLT was shown to provide a 3 year survival rate of 60% 36.
Future studies need to address the role of LDLT in patients with HCC. A true comparison of LDLT and DDLT for HCC should encompass both post-OLT recurrence as well as progression to death or delisting pre-transplant on the waiting list for both groups. For high risk tumors and those not eligible for MELD exceptions, it is likely that tumor progression on the waiting list has a higher risk of mortality than recurrence rates post LDLT.
Donor Outcomes
Donor safety is paramount in LDLT. To date, three donor deaths after right lobe donation have been reported in the United States, two of which occurred within the first post-operative month and were clearly related to the procedure for an overall mortality of 0.15%. One donor died from complications of aspiration pneumonia and one donor died of complications partly related to sepsis 37. One donor died of recreational drug use or suicide 23 months after donation 1, 21. There have also been two liver transplants in living donors for post-operative liver failure. Worldwide other donor deaths have been reported in Europe and Asia, with an overall worldwide estimate of 19 and a donor mortality of 0.15% but the exact number is not known 38.
There has been a wide range of complication rates reported in the literature in donors after LDLT. Overall complications rates have ranged from 0%– 67%, with an overall crude complication rate of 31% 39. Biliary complications have been reported in 0% – 7% of donors, including bile leaks and strictures. Complications related to major abdominal surgery occur in 9% – 19% of donors, including wound infections, small bowel obstruction, pneumonia, and incisional hernia. There are reports of aborted donor hepatectomy at the time of surgery as a result of unexpected findings, including the presence of significant hepatic steatosis, but these figures have not been collected rigorously so the exact number is unknown. Comprehensive data on donor outcomes has been limited due to the lack of a national registry and the majority of data available is generated from single centers with small numbers of patients or self-reported data in national surveys. Additionally it has not been clear whether complication rates reported have included only problems that require intervention or all deviations from standard of care. Earlier studies reported complication rates of 15–32%, likely reflecting differences in the rigor of the donor selection process, the experience of the center, and differences in reporting 40. National data was obtained via voluntary survey of all centers performing LDLT after an early NIH meeting on the topic. Based on this data from 84 different centers, the national overall donor complication rate was estimated to be 14.5%, with a re-hospitalization rate of 8.5%, and a donor mortality rate of 0.2% 1. Currently, overall donor complication rates are estimated at 10%, with mortality rates between 0.2–0.4%, based on a survey of 30 different transplant centers in the country 41. This study also revealed higher complication rates in centers that performed fewer transplants.
A2ALL donor complication data is expected in the near future, but was reported in abstract form to be 38% when all deviations from standard of care were included. Of these 27% were Clavien grade 1 and only 0.8% of these were life-threatening. UNOS now requires follow-up reporting on all living donors for a minimum of 2 years. The combination of detailed data from a smaller number of large volume centers and registry data on all donors will allow a more accurate assessment of donor risk and outcomes.
Because of variation in complication rates and lack of uniform criteria used by centers for defining complications, a standardized system for reporting complications is necessary42, 43. The Clavien system has been modified to include complications that may occur after liver transplantation. This system can be applied to both the living donor and recipient after LDLT and has been adopted by the A2ALL consortium among others 43.
Long-term complications are essentially unknown after right donor hepatectomy because the procedure was not performed on a substantial number of patients until 1999. Therefore, 5 years of data are available on very few patients and 3-year data are just becoming available. The major issue that will evolve is obtaining long-term data from donors because practice patterns for centers following donors after living donation are quite variable as far as the frequency and duration of visits after LDLT44. Furthermore, years after LDLT healthy donors may be lost to follow-up or difficult to contact due to a real or perceived lack of need for medical care, insurance barriers, or access to care.
Donor Quality of Life
Studies assessing donor quality of life after LDLT demonstrate that virtually all donors state that they would donate again, irrespective of recipient outcomes 45, 46. Ninety-six percent of donors were able to return to work after a mean of 10 weeks after surgery. Seventy-one percent of donors reported abdominal symptoms several months after surgery that they attributed to surgery 46. A report on 30 donors at varying time points post-donation reported quality of life at or above US norms on a general quality of life survey 47. In a larger study of 68 Japanese donors at a mean of over 4 years post-donation, there were two donors who indicated that they would not donate again; in both of these cases the recipients had died. The correlation between recipient outcome and donor satisfaction is in contradiction to data in pediatric living donation where few parents express regret regardless of outcome in the child. There was no difference in scores between donors who sustained complications themselves and those who had no complications48. Although overall quality of life data is important, there are specific areas that may be a source of stress and concern to donors, including finances, return to work, and expected recipient outcomes should be addressed both before and after donation 49. A notable limitation to all of these studies is the disproportionately high lack of response from donors whose recipients had serious complications and that the instruments used in these quality of life studies have been general quality of life instruments, e.g., Short Form (SF)-36, which may not capture symptoms or complaints specific to right donor hepatectomy.
Ethical issues
LDLT and performing a right hepatectomy in a healthy individual on the surface challenges the tenet of “first do no harm”. The premise of living donation has to be based on a psychological benefit to the donor from donation. That benefit can be either due to providing a direct benefit to the recipient or satisfaction with the attempt to provide life saving therapy. In order to properly weigh the ethical issues a precise understanding of the risks and benefits to the donor and recipient are needed. Living donor kidney transplantation has been performed for over 4 decades with an estimated mortality risk to the donor of 0.03% 50. Thus, mortality of the donor is 5-fold greater in LDLT compared to living kidney donation. This may be an unfair comparison because there has been over 40 years of experience with living donor kidney transplantation and only 4–5 years of experience with LDLT. There is reason to believe that with experience and improved selection criteria the mortality rate will decrease. However, right donor hepatectomy will always be a more morbid procedure compared to living kidney donation and a real risk of mortality to the donor is unavoidable 50, 51.
The main ethical dilemma is assessing the level of acceptable risk of mortality to the donor and determining whether this is an absolute measure or one that is subject to the clinical situation and the donor preferences. The risk of donor mortality is higher with LDLT than kidney donation. But this is a relative risk. The absolute risk is small and very small compared to the ~20% risk of mortality on the waiting list for the recipient.
The principle of autonomy places the perspective of the donor as the most important. The donor must be informed of the risks associated with the procedure. Coercion of the donor needs to be excluded during an independent, confidential evaluation. But what mortality rate is acceptable when the donor understands the risks and coercion has been excluded? Donors may be willing to accept high rates of mortality if the life of a loved one is in jeopardy, higher than the level acceptable to the transplant physicians and surgeons. In a single study, lay people indicated a willingness to donate with a ~20% mortality risk undergoing hepatectomy for a ~50% anticipated recipient survival 52. There has to be a balance between the risk incurred by the donor and what is acceptable to society, the medical community, and burden to the recipient.
Recipient outcomes are incorporated into decision making about LDLT. From a medical perspective if patient or graft survival rates are markedly lower compared to deceased donor liver transplantation then LDLT may be perceived as a failure. However, from a patient and donor perspective if survival after LDLT is better compared to survival on the waiting list without a liver transplant then LDLT may be acceptable. This issue arises for patients with hepatocellular carcinoma that do not meet the current UNOS criteria for additional MELD priority or those with acute alcoholic hepatitis. With a high risk of death on the list or a current contraindication to transplant and no likelihood of recovery, LDLT may be considered ethical as the potential benefits outweigh the potential risks to both donor and recipient. We currently limit consideration of LDLT to patients we would perform deceased donor liver transplantation if a liver was available. However, dilemmas exist if patients and donors are willing to accept lower post-transplant survival rates if survival to deceased donor transplant is negligible. Similar arguments can be made in the setting of HIV and advanced liver disease or retransplantation for hepatitis C related cirrhosis. Thus far, we have elected to apply the same criteria to LDLT that is applied to deceased donor transplantation, but these standards may be challenged by a society faced with organ shortages.
Costs
There are numerous studies on factors associated with the cost of deceased donor liver transplantation, but there are few studies comparing the cost of LDLT to deceased donor liver transplantation 53–56. DDLT is accepted as a cost-effective therapy for ESLD. The effectiveness of LDLT is established but its cost-effectiveness relative to DDLT has not been well defined.
The first study of the costs of LDLT compared to deceased donor liver transplantation reported costs in arbitrary units and not number of dollars and found that total costs in the deceased donor group were 21% lower compared to the LDLT group, although this difference was not statistically significant 54. On average the cost of LDLT was $25,000–$30,000 higher in the LDLT group. Included in the analysis were costs of donor evaluation (and rejection) and cost of one year of donor follow-up care, including re-transplantation, if applicable. Notably, there were 4 retransplants in the LD group, which markedly increase cost and all of which occurred in the first 10 cases. Thus, if the study was performed further along the program’s learning curve, it is reasonable to assume that costs would be lower with LDLT.
Overall, it is likely that even if LDLT is more costly than DDLT, it will remain cost-effective relative to the alternative of no transplant. Since it adds an additional graft to the pool of available, LDLT should be compared to either no transplant or the costs of waiting with potential DDLT in the future. Future research should look at costs of cases after the first 20 cases in experienced centers, and should make an attempt to include all associated costs, including both donor costs and the costs associated with waiting list morbidity and mortality.
Additionally, donors should be informed that they might be responsible for costs in certain settings. For example, the living donor may be responsible for some costs that occur after initial hospital discharge, including complications that are result of the procedure, such as ventral hernia. These costs may be substantial and a financial counselor to review what the recipient and donor’s health insurance will cover and any potential financial liabilities for the donor.
In one study mean out-of-pocket expenses for the donor were $3,660 46. Complications occur in 15% –30% of donors and donors should be aware that they may be responsible for costs that are not covered by the recipient’s insurance, even if it is related to a complication. After right hepatectomy donors can anticipate not returning to work for at least 2 – 3 months and they should contemplate whether their household can support this period of time off and if their employer will allow it.
Benefits of LDLT
In order to balance the risks and costs outlined above, some quantification of benefit is needed. As indicated above the major benefit to the donor would be increased likelihood of transplant and potential survival and quality of life benefit to the recipient. Studies comparing outcomes in LDLT and deceased donor liver transplant recipients report post-transplant survival rates. One of the main reasons LDLT is offered is to reduce waiting time mortality due to the deceased donor organ shortage 57. Analyses that report post-transplant outcomes fail to capture benefits LDLT may have on waiting time mortality.
Two initial studies of LDLT, one conducted in the United States, reported higher rates of transplantation and lower waiting time mortality rates in the group of patients with living donor volunteers compared to a group without living donor volunteers 58, 59. In a study we performed, waiting time mortality was 10% lower in the group of liver transplant candidates with living donor volunteers compared to the group without volunteers 59. Survival can also be measured from the time of listing to last follow-up, through transplantation, to capture the complete effect of LDLT on survival from listing through transplantation. Using this methodology, a survival benefit to LDLT has recently been demonstrated in the A2ALL consortium. We studied mortality rates in patients who had a donor evaluated for possible LDLT and compared two groups; recipients of LDLT and patients who did not receive a LDLT (including those who received a DDLT, those that remained on the list at study completion, and those that died on the list) 6. LDLT recipients has an adjusted mortality hazard ratio of 0.56 (95% confidence interval 0.42–0.74; p<0.001) relative to patients who were evaluated for but did not receive a living donor graft, controlling for clinical differences at the time of evaluation. This benefit was significantly increased at centers with experience (defined as case number >20), with a hazard ratio of 0.47 (95% CI 0.32–0.69, p< 0.001) associated with LDLT6. This study, which most closely approximates an “intent-to-treat” analysis, quantifies the reduction in waiting list mortality for LDLT compared to remaining on the waiting list as post-transplant survival was the same in DDLT and LDLT at experienced centers (i.e., > 20 cases).
Thus studies from the time of evaluation have all demonstrated substantial benefits of pursuing LDLT on waiting time mortality. Patients are interested in their overall survival, not only if they survive to transplant. It appears that except for patients with high MELD scores, LDLT offers equivalent results to DDLT from the time of transplant at experienced centers, despite an initial belief that for any given severity of illness a whole organ should result in superior or equivalent outcomes compared to a partial organ. Moreover, most centers offer LDLT because transplant candidates die waiting for a whole organ or may become very ill prior to transplantation complicating their post-transplant recovery.
Conclusions
Adult living donor liver transplantation offers improved access to a lifesaving transplant for patients with end stage liver disease in areas where waiting time mortality is high and availability of deceased donor organs falls short of the need of the population. There are significant risks to the living donor, including the risk of death and substantial morbidity, that must be taken into account before patients, physicians, and transplant programs embark in LDLT. Significant improvements in outcomes have been seen over recent years that have now being reported in larger multi-center studies. Despite this, living donor liver transplant remains stagnant. Data support the use of LDLT in patients with ESLD due to HCV as well as HCC, although there remain questions about which patients with HCC are most suitable for LDLT. It is clear that centers with more experience have better outcomes. Future research needs to address optimal donor evaluation, as well as identify the most suitable LDLT donors and recipients. Results of the A2ALL study will help quantify donor risk and recipient outcome, and hopefully allow growth and development of the procedure.
Acknowledgement
Supported in part by National Institute of Digestive Diseases and Kidney grant DK02-010
The author would like to thank Mark Russo, MD, MPH for his review and constructive comments on the manuscript and his years of collaboration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brown RS, Jr, Russo MW, Lai M, Shiffman ML, Richardson MC, Everhart JE, Hoofnagle JH. A survey of liver transplantation from living adult donors in the United States. N Engl J Med. 2003;348:818–825. doi: 10.1056/NEJMsa021345. [DOI] [PubMed] [Google Scholar]
- 2.UNOS. 2007 http://www.unos.org/data/about/viewDataReports.asp.
- 3.American Society of Transplant Surgeons' position paper on adult-to-adult living donor liver transplantation. Liver Transpl. 2000;6:815–817. doi: 10.1053/jlts.2000.18465. [DOI] [PubMed] [Google Scholar]
- 4.Cotler SJ, Cotler S, Gambera M, Benedetti E, Jensen DM, Testa G. Adult living donor liver transplantation: perspectives from 100 liver transplant surgeons. Liver Transpl. 2003;9:637–644. doi: 10.1053/jlts.2003.50109. [DOI] [PubMed] [Google Scholar]
- 5.Donation NYSCoQIiLL. 2002;Volume 2007 http://www.health.state.ny.us/nysdoh/liver_donation/pdf/liver_donor_report_web.pdf.
- 6.Berg CL, Gillespie BW, Merion RM, Brown RS, Jr, Abecassis MM, Trotter JF, Fisher RA, Freise CE, Ghobrial RM, Shaked A, Fair JH, Everhart JE. Improvement in survival associated with adult-to-adult living donor liver transplantation. Gastroenterology. 2007;133:1806–1813. doi: 10.1053/j.gastro.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russo MW, Galanko J, Beavers K, Fried MW, Shrestha R. Patient and graft survival in hepatitis C recipients after adult living donor liver transplantation in the United States. Liver Transpl. 2004;10:340–346. doi: 10.1002/lt.20090. [DOI] [PubMed] [Google Scholar]
- 8.Merion RM, Schaubel DE, Dykstra DM, Freeman RB, Port FK, Wolfe RA. The survival benefit of liver transplantation. Am J Transplant. 2005;5:307–313. doi: 10.1111/j.1600-6143.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- 9.Moss J, Lapointe-Rudow D, Renz JF, Kinkhabwala M, Dove LM, Gaglio PJ, Emond JC, Brown RS., Jr Select utilization of obese donors in living donor liver transplantation: implications for the donor pool. Am J Transplant. 2005;5:2974–2981. doi: 10.1111/j.1600-6143.2005.01124.x. [DOI] [PubMed] [Google Scholar]
- 10.Marcos A. Right lobe living donor liver transplantation: a review. Liver Transpl. 2000;6:3–20. doi: 10.1002/lt.500060117. [DOI] [PubMed] [Google Scholar]
- 11.Trotter JF, Wachs M, Trouillot T, Steinberg T, Bak T, Everson GT, Kam I. Evaluation of 100 patients for living donor liver transplantation. Liver Transpl. 2000;6:290–295. doi: 10.1002/lt.500060323. [DOI] [PubMed] [Google Scholar]
- 12.Trotter JF, Campsen J, Bak T, Wachs M, Forman L, Everson G, Kam I. Outcomes of donor evaluations for adult-to-adult right hepatic lobe living donor liver transplantation. Am J Transplant. 2006;6:1882–1889. doi: 10.1111/j.1600-6143.2006.01322.x. [DOI] [PubMed] [Google Scholar]
- 13.Trotter JF, Wisniewski KA, Terrault NA, Everhart JE, Kinkhabwala M, Weinrieb RM, Fair JH, Fisher RA, Koffron AJ, Saab S, Merion RM. Outcomes of donor evaluation in adult-to-adult living donor liver transplantation. Hepatology. 2007;46:1476–1484. doi: 10.1002/hep.21845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olthoff KM, Merion RM, Ghobrial RM, Abecassis MM, Fair JH, Fisher RA, Freise CE, Kam I, Pruett TL, Everhart JE, Hulbert-Shearon TE, Gillespie BW, Emond JC. Outcomes of 385 adult-to-adult living donor liver transplant recipients: a report from the A2ALL Consortium. Ann Surg. 2005;242:314–323. doi: 10.1097/01.sla.0000179646.37145.ef. discussion 323–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM, Wolfe RA, Krom R. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91–96. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 16.Pomfret EA, Fryer JP, Sima CS, Lake JR, Merion RM. Liver and intestine transplantation in the United States, 1996–2005. Am J Transplant. 2007;7:1376–1389. doi: 10.1111/j.1600-6143.2007.01782.x. [DOI] [PubMed] [Google Scholar]
- 17.Kam I. Adult-adult right hepatic lobe living donor liver transplantation for status 2a patients: too little, too late. Liver Transpl. 2002;8:347–349. doi: 10.1053/jlts.2002.33194. [DOI] [PubMed] [Google Scholar]
- 18.Marcos A, Ham JM, Fisher RA, Olzinski AT, Shiffman ML, Sanyal AJ, Luketic VA, Sterling RK, Posner MP. Emergency adult to adult living donor liver transplantation for fulminant hepatic failure. Transplantation. 2000;69:2202–2205. doi: 10.1097/00007890-200005270-00044. [DOI] [PubMed] [Google Scholar]
- 19.Testa G, Malago M, Nadalin S, Hertl M, Lang H, Frilling A, Broelsch CE. Rightliver living donor transplantation for decompensated end-stage liver disease. Liver Transpl. 2002;8:340–346. doi: 10.1053/jlts.2002.32941. [DOI] [PubMed] [Google Scholar]
- 20.Liu CL, Fan ST, Lo CM, Wong J. Living-donor liver transplantation for high-urgency situations. Transplantation. 2003;75:S33–S36. doi: 10.1097/01.TP.0000047031.65448.47. [DOI] [PubMed] [Google Scholar]
- 21.Ghobrial RM, Saab S, Lassman C, Lu DS, Raman S, Limanond P, Kunder G, Marks K, Amersi F, Anselmo D, Chen P, Farmer D, Han S, Durazo F, Goldstein LI, Busuttil RW. Donor and recipient outcomes in right lobe adult living donor liver transplantation. Liver Transpl. 2002;8:901–909. doi: 10.1053/jlts.2002.35548. [DOI] [PubMed] [Google Scholar]
- 22.Marcos A, Ham JM, Fisher RA, Olzinski AT, Posner MP. Single-center analysis of the first 40 adult-to-adult living donor liver transplants using the right lobe. Liver Transpl. 2000;6:296–301. doi: 10.1053/lv.2000.6354. [DOI] [PubMed] [Google Scholar]
- 23.Miller CM, Gondolesi GE, Florman S, Matsumoto C, Munoz L, Yoshizumi T, Artis T, Fishbein TM, Sheiner PA, Kim-Schluger L, Schiano T, Shneider BL, Emre S, Schwartz ME. One hundred nine living donor liver transplants in adults and children: a single-center experience. Ann Surg. 2001;234:301–311. doi: 10.1097/00000658-200109000-00004. discussion 311–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bak T, Wachs M, Trotter J, Everson G, Trouillot T, Kugelmas M, Steinberg T, Kam I. Adult-to-adult living donor liver transplantation using right-lobe grafts: results and lessons learned from a single-center experience. Liver Transpl. 2001;7:680–686. doi: 10.1053/jlts.2001.26509. [DOI] [PubMed] [Google Scholar]
- 25.Belghiti J, Kianmanesh R. Surgical techniques used in adult living donor liver transplantation. Liver Transpl. 2003;9:S29–S34. doi: 10.1053/jlts.2003.50226. [DOI] [PubMed] [Google Scholar]
- 26.Marcos A, Fisher RA, Ham JM, Shiffman ML, Sanyal AJ, Luketic VA, Sterling RK, Fulcher AS, Posner MP. Liver regeneration and function in donor and recipient after right lobe adult to adult living donor liver transplantation. Transplantation. 2000;69:1375–1379. doi: 10.1097/00007890-200004150-00028. [DOI] [PubMed] [Google Scholar]
- 27.Akamatsu N, Sugawara Y, Kaneko J, Sano K, Imamura H, Kokudo N, Makuuchi M. Effects of middle hepatic vein reconstruction on right liver graft regeneration. Transplantation. 2003;76:832–837. doi: 10.1097/01.TP.0000085080.37235.81. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Retortillo M, Forns X, Llovet JM, Navasa M, Feliu A, Massaguer A, Bruguera M, Fuster J, Garcia-Valdecasas JC, Rimola A. Hepatitis C recurrence is more severe after living donor compared to cadaveric liver transplantation. Hepatology. 2004;40:699–707. doi: 10.1002/hep.20357. [DOI] [PubMed] [Google Scholar]
- 29.Shiffman ML, Stravitz RT, Contos MJ, Mills AS, Sterling RK, Luketic VA, Sanyal AJ, Cotterell A, Maluf D, Posner MP, Fisher RA. Histologic recurrence of chronic hepatitis C virus in patients after living donor and deceased donor liver transplantation. Liver Transpl. 2004;10:1248–1255. doi: 10.1002/lt.20232. [DOI] [PubMed] [Google Scholar]
- 30.Terrault NA, Shiffman ML, Lok AS, Saab S, Tong L, Brown RS, Jr, Everson GT, Reddy KR, Fair JH, Kulik LM, Pruett TL, Seeff LB. Outcomes in hepatitis C virus-infected recipients of living donor vs. deceased donor liver transplantation. Liver Transpl. 2006;13:122–129. doi: 10.1002/lt.20995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rudow DL, Russo MW, Hafliger S, Emond JC, Brown RS., Jr Clinical and ethnic differences in candidates listed for liver transplantation with and without potential living donors. Liver Transpl. 2003;9:254–259. doi: 10.1053/jlts.2003.50037. [DOI] [PubMed] [Google Scholar]
- 32.Lo CM, Fan ST, Liu CL, Chan SC, Ng IO, Wong J. Living donor versus deceased donor liver transplantation for early irresectable hepatocellular carcinoma. Br J Surg. 2007;94:78–86. doi: 10.1002/bjs.5528. [DOI] [PubMed] [Google Scholar]
- 33.Fisher RA, Kulik LM, Freise CE, Lok AS, Shearon TH, Brown RS, Jr, Ghobrial RM, Fair JH, Olthoff KM, Kam I, Berg CL. Hepatocellular carcinoma recurrence and death following living and deceased donor liver transplantation. Am J Transplant. 2007;7:1601–1608. doi: 10.1111/j.1600-6143.2007.01802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kulik L, Abecassis M. Living donor liver transplantation for hepatocellular carcinoma. Gastroenterology. 2004;127:S277–S282. doi: 10.1053/j.gastro.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 35.Gondolesi GE, Roayaie S, Munoz L, Kim-Schluger L, Schiano T, Fishbein TM, Emre S, Miller CM, Schwartz ME. Adult living donor liver transplantation for patients with hepatocellular carcinoma: extending UNOS priority criteria. Ann Surg. 2004;239:142–149. doi: 10.1097/01.sla.0000109022.32391.eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Todo S, Furukawa H. Living donor liver transplantation for adult patients with hepatocellular carcinoma: experience in Japan. Ann Surg. 2004;240:451–459. doi: 10.1097/01.sla.0000137129.98894.42. discussion 459–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller C, Florman S, Kim-Schluger L, Lento P, De La Garza J, Wu J, Xie B, Zhang W, Bottone E, Zhang D, Schwartz M. Fulminant and fatal gas gangrene of the stomach in a healthy live liver donor. Liver Transpl. 2004;10:1315–1319. doi: 10.1002/lt.20227. [DOI] [PubMed] [Google Scholar]
- 38.Trotter JF, Adam R, Lo CM, Kenison J. Documented deaths of hepatic lobe donors for living donor liver transplantation. Liver Transpl. 2006;12:1485–1488. doi: 10.1002/lt.20875. [DOI] [PubMed] [Google Scholar]
- 39.Beavers KL, Sandler RS, Shrestha R. Donor morbidity associated with right lobectomy for living donor liver transplantation to adult recipients: a systematic review. Liver Transpl. 2002;8:110–117. doi: 10.1053/jlts.2002.31315. [DOI] [PubMed] [Google Scholar]
- 40.Trotter JF, Wachs M, Everson GT, Kam I. Adult-to-adult transplantation of the right hepatic lobe from a living donor. N Engl J Med. 2002;346:1074–1082. doi: 10.1056/NEJMra011629. [DOI] [PubMed] [Google Scholar]
- 41.Renz JF, Roberts JP. Long-term complications of living donor liver transplantation. Liver Transpl. 2000;6:S73–S76. doi: 10.1053/jlts.2000.18686. [DOI] [PubMed] [Google Scholar]
- 42.Ghobrial RM, Busuttil RW. Future of adult living donor liver transplantation. Liver Transpl. 2003;9:S73–S79. doi: 10.1053/jlts.2003.50230. [DOI] [PubMed] [Google Scholar]
- 43.Clavien PA, Camargo CA, Jr, Croxford R, Langer B, Levy GA, Greig PD. Definition and classification of negative outcomes in solid organ transplantation. Application in liver transplantation. Ann Surg. 1994;220:109–120. doi: 10.1097/00000658-199408000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beavers KL, Cassara JE, Shrestha R. Practice patterns for long-term follow-up of adult-to-adult right lobectomy donors at US transplantation centers. Liver Transpl. 2003;9:645–648. doi: 10.1053/jlts.2003.50123. [DOI] [PubMed] [Google Scholar]
- 45.Pascher A, Sauer IM, Walter M, Lopez-Haeninnen E, Theruvath T, Spinelli A, Neuhaus R, Settmacher U, Mueller AR, Steinmueller T, Neuhaus P. Donor evaluation, donor risks, donor outcome, and donor quality of life in adult-to-adult living donor liver transplantation. Liver Transpl. 2002;8:829–837. doi: 10.1053/jlts.2002.34896. [DOI] [PubMed] [Google Scholar]
- 46.Trotter JF, Talamantes M, McClure M, Wachs M, Bak T, Trouillot T, Kugelmas M, Everson GT, Kam I. Right hepatic lobe donation for living donor liver transplantation: impact on donor quality of life. Liver Transpl. 2001;7:485–493. doi: 10.1053/jlts.2001.24646. [DOI] [PubMed] [Google Scholar]
- 47.Kim-Schluger L, Florman SS, Schiano T, O'Rourke M, Gagliardi R, Drooker M, Emre S, Fishbein TM, Sheiner PA, Schwartz ME, Miller CM. Quality of life after lobectomy for adult liver transplantation. Transplantation. 2002;73:1593–1597. doi: 10.1097/00007890-200205270-00012. [DOI] [PubMed] [Google Scholar]
- 48.Miyagi S, Kawagishi N, Fujimori K, Sekiguchi S, Fukumori T, Akamatsu Y, Satomi S. Risks of donation and quality of donors' life after living donor liver transplantation. Transpl Int. 2005;18:47–51. doi: 10.1111/j.1432-2277.2004.00028.x. [DOI] [PubMed] [Google Scholar]
- 49.Verbesey JE, Simpson MA, Pomposelli JJ, Richman E, Bracken AM, Garrigan K, Chang H, Jenkins RL, Pomfret EA. Living donor adult liver transplantation: a longitudinal study of the donor's quality of life. Am J Transplant. 2005;5:2770–2777. doi: 10.1111/j.1600-6143.2005.01092.x. [DOI] [PubMed] [Google Scholar]
- 50.Surman OS. The ethics of partial-liver donation. N Engl J Med. 2002;346:1038. doi: 10.1056/NEJM200204043461402. [DOI] [PubMed] [Google Scholar]
- 51.Russo MW, Brown RS. Ethical issues in living donor liver transplantation. Curr Gastroenterol Rep. 2003;5:26–30. doi: 10.1007/s11894-003-0006-x. [DOI] [PubMed] [Google Scholar]
- 52.Cotler SJ, McNutt R, Patil R, Banaad-Omiotek G, Morrissey M, Abrams R, Cotler S, Jensen DM. Adult living donor liver transplantation: Preferences about donation outside the medical community. Liver Transpl. 2001;7:335–340. doi: 10.1053/jlts.2001.22755. [DOI] [PubMed] [Google Scholar]
- 53.Sagmeister M, Mullhaupt B, Kadry Z, Kullak-Ublick GA, Clavien PA, Renner EL. Cost-effectiveness of cadaveric and living-donor liver transplantation. Transplantation. 2002;73:616–622. doi: 10.1097/00007890-200202270-00025. [DOI] [PubMed] [Google Scholar]
- 54.Trotter JF, Mackenzie S, Wachs M, Bak T, Steinberg T, Polsky P, Kam I, Everson GT. Comprehensive cost comparison of adult-adult right hepatic lobe living-donor liver transplantation with cadaveric transplantation. Transplantation. 2003;75:473–476. doi: 10.1097/01.TP.0000047310.04069.ED. [DOI] [PubMed] [Google Scholar]
- 55.Russo MW, Brown RS., Jr Financial impact of adult living donation. Liver Transpl. 2003;9:S12–S15. doi: 10.1053/jlts.2003.50228. [DOI] [PubMed] [Google Scholar]
- 56.Russo MW, Brown RS., Jr Is the cost of adult living donor liver transplantation higher than deceased donor liver transplantation? Liver Transpl. 2004;10:467–468. doi: 10.1002/lt.20102. [DOI] [PubMed] [Google Scholar]
- 57.Everhart JE, Lombardero M, Detre KM, Zetterman RK, Wiesner RH, Lake JR, Hoofnagle JH. Increased waiting time for liver transplantation results in higher mortality. Transplantation. 1997;64:1300–1306. doi: 10.1097/00007890-199711150-00012. [DOI] [PubMed] [Google Scholar]
- 58.Liu CL, Lam B, Lo CM, Fan ST. Impact of right-lobe live donor liver transplantation on patients waiting for liver transplantation. Liver Transpl. 2003;9:863–869. doi: 10.1053/jlts.2003.50163. [DOI] [PubMed] [Google Scholar]
- 59.Russo MW, LaPointe-Rudow D, Kinkhabwala M, Emond J, Brown RS., Jr Impact of adult living donor liver transplantation on waiting time survival in candidates listed for liver transplantation. Am J Transplant. 2004;4:427–431. doi: 10.1111/j.1600-6143.2004.00336.x. [DOI] [PubMed] [Google Scholar]