Summary
The Ras-specific nucleotide exchange factor Son of sevenless (Sos) is inactive without Ras bound to a distal allosteric site. In contrast, the catalytic domain of Ras Guanine Nucleotide Releasing Factor 1 (RasGRF1) is active intrinsically. By substituting residues from RasGRF1 into Sos, we have generated mutants of Sos with basal activity, partially relieved of their dependence on allosteric activation. We have performed molecular dynamics simulations showing how Ras binding to the allosteric site leads to a bias toward the active conformation of Sos. The trajectories show that Sos fluctuates between active and inactive conformations in the absence of Ras and that the activating mutations favor conformations of Sos that are more permissive to Ras binding at the catalytic site. In contrast, unliganded RasGRF1 fluctuates primarily among active conformations. Our results support the premise that the catalytic domain of Sos has evolved an allosteric activation mechanism that extends beyond the simple process of membrane recruitment.
Introduction
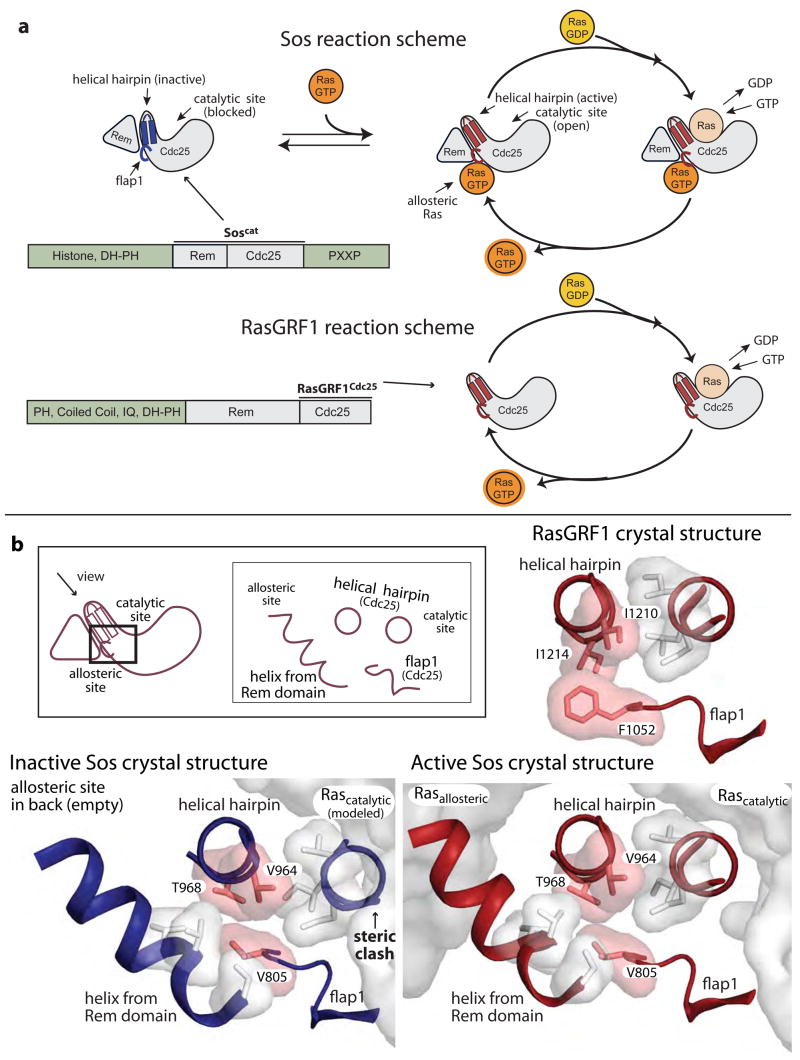
The nucleotide exchange factors Sos and RasGRF1 activate Ras by converting GDP-bound Ras to GTP-bound Ras. A helical hairpin motif in the catalytic domain of Sos binds to and disrupts the nucleotide binding site of Ras, thereby promoting nucleotide release and exchange (Boriack-Sjodin et al., 1998; Vetter and Wittinghofer, 2001) (Fig. 1a). Ras then binds GTP, after which it can activate signaling proteins that control cell growth, differentiation, and survival (Herrmann and Nassar, 1996). Ras is a potent oncogene, and inappropriate activation of Ras has been implicated in the majority of human cancers (Coleman et al., 2004; Hanahan and Weinberg, 2000; Schubbert et al., 2007). The nucleotide exchange activity of Sos and RasGRF1 must therefore be tightly regulated to prevent cellular transformation by constitutively high levels of activated Ras (Egan et al., 1993; Herrmann and Nassar, 1996). Sos itself has been shown to transform cells (Egan et al., 1993), and hyperactivated forms of Sos have been linked to Noonan Syndrome, a developmental disease that stems from dysregulation of the Ras pathway (Roberts et al., 2007; Tartaglia et al., 2007).
Fig. 1.
Comparison of Sos and RasGRF1. (a) Schematic diagram comparing the activation of Ras by Sos and RasGRF1 (b) Interface between Ras and nucleotide exchange factors, and residues mutated in this study. A view down the helical hairpin of Sos highlights the conformational change that occurs upon Ras binding to the allosteric site. The Ras molecules are modeled into the structure of inactive Sos (PDB code: 2II0(Freedman et al., 2006)) from the crystal structure of active Sos (PDB code: 1NVV(Margarit et al., 2003)). When Ras binds to the allosteric site of Sos the Rem domain is pivoted downward to maintain this interaction. The helical hairpin is also pivoted outward to open the catalytic site. Residues V805, V964, and T968, which comprise an interface between the Rem domain, flap1, and the helical hairpin, are highlighted in pink. RasGRF1 (PDB code: 2IJE)(Freedman et al., 2006) assumes an active conformation in the absence of bound Ras.
Two domains of Sos are required for Ras-specific nucleotide exchange activity: a Cdc25 domain named for the activator of Ras in yeast and a Ras exchanger motif (Rem) domain (Fig. 1a). The active site is located entirely within the Cdc25 domain and consists of a hydrophobic pocket for anchoring Ras and the helical hairpin motif that stimulates nucleotide release (Boriack-Sjodin et al., 1998; Freedman et al., 2006). Sos is activated by Ras binding to an allosteric site that bridges the Rem and Cdc25 domains, and we refer to the Ras molecule bound to the allosteric site as “allosteric Ras” (Margarit et al., 2003). In a crystal structure of the isolated Rem and Cdc25 domains of Sos, the helical hairpin is pivoted toward the central core of the Cdc25 domain and occludes the catalytic site (Freedman et al., 2006) (Fig. 1). Allosteric Ras binding pivots the Rem domain outward from the stable core of the Cdc25 domain, repositioning the helical hairpin and opening the catalytic site for Ras binding (Freedman et al., 2006; Margarit et al., 2003).
In a structure of a Sos construct that includes the N-terminal Dbl Homology (DH) and Pleckstrin Homology (PH) domains as well as the Rem and Cdc25 domains (SosDPC), the DH domain blocks the allosteric Ras binding site (Sondermann et al., 2004). As expected, the active site is occluded by the helical hairpin in this structure, and the Rem domain is pivoted away from the conformation in which it interacts with allosteric Ras (Sondermann et al., 2004). The N-terminal histone domain confers an additional level of autoinhibition to Sos (Gureasko et al., 2008) by binding to the PH-Rem domain linker and reinforcing the position of the DH domain (Sondermann et al., 2005 and O. Kuchment, J. Gureasko, and J.K, unpublished data). The histone domain may also prevent a conformational change that opens the allosteric site upon phosphatidylinositol bisphosphate binding to the PH domain (Gureasko et al., 2008). Thus, a significant portion of the regulatory apparatus of Sos is devoted to blocking the allosteric site, and this autoregulation relies on the intrinsic inactivity of the catalytic domain.
In contrast to Sos, the Cdc25 domain of RasGRF1 has intrinsic activity without the Rem domain and without an allosteric effector being bound (Coccetti et al., 1995; Freedman et al., 2006; Lenzen et al., 1995) (Fig. 1a). The crystal structure of the isolated Cdc25 domain of RasGRF1 shows that the helical hairpin is pivoted outward from the core of the Cdc25 domain, leaving the catalytic site open (Freedman et al., 2006). Based on a comparison of crystal structures of active Sos, inactive Sos, and RasGRF1, we have postulated that RasGRF1 is held in an active conformation by bulky residues that comprise an interface between the helical hairpin and an extension of the Cdc25 domain called flap1 (Fig. 1). In addition, we had used Monte Carlo simulations to predict whether swapping residues in the sequences of RasGRF1 and Sos would stabilize the observed backbone conformations (Freedman et al., 2006). In these simulations a cluster of three mutations was predicted to stabilize the active conformation of Sos, but not the inactive conformation. The corresponding positions in RasGRF1 remained unaltered during the Monte Carlo simulations, suggesting that the wild-type sequence of RasGRF1 is superior for stabilizing the active conformation of both RasGRF1 and Sos. These residues (F1052, I1210, and I1214 in RasGRF1 vs. V805, V964, and T968 in Sos) lie in the interface formed by the helical hairpin and flap1 in the Cdc25 domain (Fig. 1b), and we suggested that the bulkier residues from RasGRF1 could be important for maintaining the active conformation (Freedman et al., 2006).
Here we show that substituting one or more residues from flap1 and from the helical hairpin of RasGRF1 into Sos substantially increases the basal activity of Sos in the absence of allosteric Ras binding. We use molecular dynamics simulations to investigate how these substitutions alter the behavior of Sos and show that RasGRF1 and Sos sample different sets of conformations in the absence of Ras binding. In addition, we find that inactive Sos fluctuates between the inactive and active conformations.
Results
Mutations that activate Sos
There are several mechanisms that contribute to Sos activation upon recruitment to the membrane: allosteric activation by membrane-localized Ras, tethering at the membrane through the allosteric site, and release of autoinhibition by lipid binding (Gureasko et al., 2008). To investigate the properties intrinsic to the catalytic domains of Sos that lead to its dependence on allosteric activation, we chose to measure activity in solution rather than at the membrane, and at low concentrations of both Ras and Sos. Thus, we eliminated the effects of crowding at the membrane (Gureasko et al., 2008) and minimized allosteric activation by Ras (Freedman et al., 2006).
We tested the effects of substituting the bulkier residues in the flap1/helical hairpin interface of RasGRF1 into Soscat, a construct of Sos that includes the Rem and Cdc25 domains (Freedman et al., 2006; Margarit et al., 2003) (Fig. 1a). We are unable to express a Rem-Cdc25 construct of RasGRF1 and so restricted our analysis to the Cdc25 construct, which is active. We monitored nucleotide exchange activity as reflected in the decrease in emission intensity of a fluorescent GDP analog, mant-dGDP, upon release from Ras (Freedman et al., 2006; Guo et al., 2005; Lenzen et al., 1998; Lenzen et al., 1995).
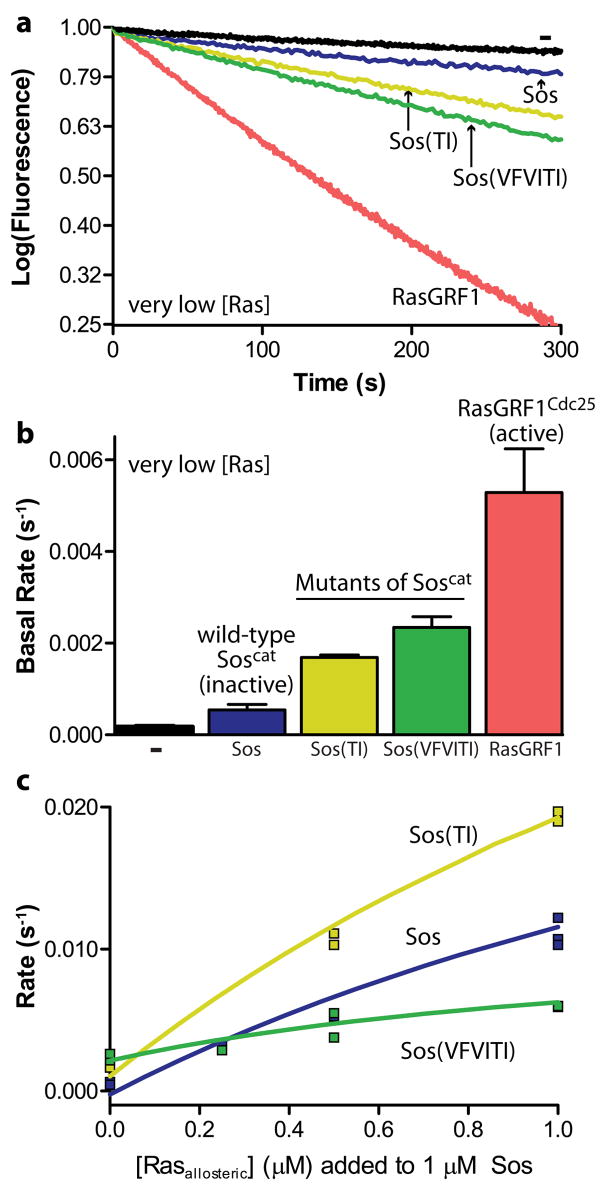
As observed previously, Sos (Soscat, unless otherwise specified) has very little basal activity in the absence of allosteric Ras binding, whereas the rate of nucleotide release stimulated by RasGRF1 (a construct called RasGRF1Cdc25 that includes only the Cdc25 domain) is at least a 10-fold higher than that for Sos under the same conditions (Freedman et al., 2006) (Fig. 2a,b). With a single mutation (T968I) in the helical hairpin of Sos, denoted Sos(TI), ~30% of the activity of RasGRF1 is achieved. A triple mutant with T968I and V964I in the helical hairpin and V805F in flap1, called Sos(VFVITI), has ~45% of the basal activity of RasGRF1, at least a 5-fold increase over wild-type Sos (Fig. 2b). A double mutant (V805F+T968I) and the variant T968L also increase the basal activity of Sos (Supplementary Fig. 1a).
Fig. 2.
Engineering intrinsic activity into Sos. (a,b) Nucleotide release assays show that RasGRF1 (Cdc25 domain) has a high basal activity compared to Sos (Rem and Cdc25 domains). Substituting residues from the flap1/helical hairpin interface of RasGRF1 into Sos increase the basal activity of Sos. Error bars represent the mean +/− the standard deviation of the fit rates at least three independent experiments. (c) Two mutants have different responses to allosteric Ras binding. The single mutant T968I (Sos(TI)) is more active than wild-type Sos at all concentrations of RasY64A, a variant of Ras that binds selectively to the allosteric site. The triple mutant V805F+V964I+T968I (Sos(VFVITI)) has the highest basal activity, but fails to respond to allosteric Ras binding and has a dramatically impaired maximal activity.
To ensure that these measured rate enhancements result from a true increase in basal activity and not, for instance, from increased affinity for allosteric Ras, we also tested these mutations in combination with the mutation W729E in the Rem domain of Sos. The W729E mutation blocks Ras binding to the allosteric site, and would therefore cause a decrease in activity if the original gain was attributable to substrate binding to the allosteric site (Margarit et al., 2003). All of the activated mutants showed similar behavior in backgrounds of wild-type Sos and of Sos with the W729E mutation (Supplementary Fig. 1a), and thus we conclude that substituting residues in the helical hairpin and flap1 of Sos with the corresponding residues from RasGRF1 partially relieves Sos of its dependence on allosteric activation.
Although both Sos(TI) and Sos(VFVITI) are activated relative to wild-type Sos, they differ in their ability to be further stimulated by allosteric Ras binding. We measured the effect of allosteric Ras binding on Sos activity by performing nucleotide exchange reactions with different concentrations of RasY64A, a variant of Ras that binds to the allosteric site but not to the catalytic site of Sos (Hall et al., 2001; Margarit et al., 2003). The RasY64A variant is effective as an allosteric stimulator of Sos, but it does not compete with wild-type Ras for the active site of Sos. At each concentration of RasY64A, Sos(TI) is more active than wild-type Sos, showing that Sos(TI) is sensitive to allosteric activation by Ras. Sos(VFVITI) is, however, almost unresponsive to titration of RasY64A, suggesting that its sensitivity to allosteric activation is impaired (Fig. 2c, Supplementary Fig. 1b,c). These differences in sensitivity to allosteric activation cannot be explained by changes in the affinity of the allosteric site for RasY64A; Sos(VFVITI) actually has increased affinity, whereas the affinity of Sos(TI) for RasY64A is comparable to that of wild-type Sos (Supplementary Fig. 1d). Although both mutants have increased basal activity, the mutation in Sos(TI) does not interfere with its allosteric activation, whereas the mutations in Sos(VFVITI) impair allosteric activation. This implies that these two mutants have different mechanisms for achieving basal activity, as discussed later.
Molecular dynamics simulations of RasGRF1, Sos, and Sos mutants
We generated a series of unbiased molecular dynamics trajectories (Dodson et al., 2008; Karplus and Kuriyan, 2005) for Sos, RasGRF1, and the activated mutants of Sos on the nanosecond timescale (Table 1). The starting structure for each trajectory was generated from crystal structures of RasGRF1, of active Sos with bound allosteric Ras and catalytic-site Ras, and of inactive Sos (Freedman et al., 2006; Margarit et al., 2003). We also modeled the activating mutations into the active or inactive crystal structure of Sos. We used the AMBER software package (Pearlman et al., 1995) to place the protein chain(s) of each modified starting structure into a box of water in solution with 150 mM sodium chloride and counterions to bring the net charge to zero. Independent trajectories of seven nanoseconds each were generated, with at least six trajectories for each starting structure. All of the trajectories were stable throughout, as determined by visual analysis and calculation of the root-mean-square deviation (RMSD) with respect to the starting structures (Supplementary Fig. 2). The trajectories were analyzed extensively using various methods, such as cross-correlation matrices and by calculating normal modes (principal component analysis of the fluctuations). The most informative analysis proved to be simple comparisons of the structures of the proteins as the trajectories evolved, and we therefore restrict our discussion to these structural comparisons. The timescales for the inactive-to-active transitions in Sos and RasGRF1 are not known, but are likely to be in the microsecond to millisecond range. Our simulations do not provide direct information on the nature of the transitions, but rather provide information about the behavior of these proteins when in one state of activation or the other.
Table 1.
Molecular dynamics simulations. The starting structure for RasGRF1 comes from the crystal structure of RasGRF1Cdc25 (2IJE)(Freedman et al., 2006), the starting structure for active Sos comes from the crystal structure of Soscat (1NVV)(Margarit et al., 2003) with two bound Ras molecules, and the starting structure for inactive Sos comes from the crystal structure of apo-Soscat (2II0)(Freedman et al., 2006). Mutations were created in the wild-type crystal structures using Pymol(DeLano, 2002). The abbreviations used are as follows: Rascat= catalytic-site Ras, Rasallo= allosteric-site Ras, Sos= Soscat (Rem+Cdc25 domains), RasGRF1= RasGRF1Cdc25.
| simulation | starting structure | molecules included | mutations | # trajectories (7 ns each) |
|---|---|---|---|---|
| GRF |
|
none | 6 | |
| Ras•SosActive•Ras |
|
none | 1 | |
| Ras•SosActive |

|

|
none | 6 |
| SosActive |

|

|
none | 7 |
| SosInactive |
|
none | 8 | |
| Sos(TI)Inactive |
|
T968I | 6 | |
| Sos(VFVITI)Inactive |
|
V805F+V964I+T968I | 6 | |
| Sos(TI)Active |

|
|
T968I | 6 |
| Sos(VFVITI)Active |

|

|
V805F+V964I+T968I | 6 |
Structures from points along the trajectories were analyzed after alignment on the core of the Cdc25 domain, which does not differ significantly in the active and inactive conformations of Sos (Cα RMSD = 0.3 Å). In all the simulations, these residues in the core of the Cdc25 domain remain close to their starting positions (average Cα RMSD with respect to the starting structures for the residues in the Cdc25-domain core ranges from 0.5 to 0.7 Å for all sets of trajectories; Supplementary Fig. 2). We then compared the conformations of the helical hairpin and the motifs in the Rem domain that interact with allosteric Ras, which differ in crystal structures of active and inactive Sos by Cα RMSD values of 3.3 and 7.8 Å, respectively. Over the course of the trajectories, these residues change position to a greater extent than the residues in the core of the Cdc25 domain. In the following analysis we refer to these residues in the helical hairpin and Rem domain as indicators of conformational change. The average Cα RMSD of the helical hairpin residues with respect to the starting structure (see Methods) varies from 1.6 to 2.9 Å over all sets of trajectories (Supplementary Fig. 3). The average Cα RMSD of the Ras-interacting residues in the Rem domain varies from 2.0 to 7.3 Å over all sets of trajectories (Supplementary Fig. 4).
RasGRF1 and Ras-bound Sos are stable in the active conformation
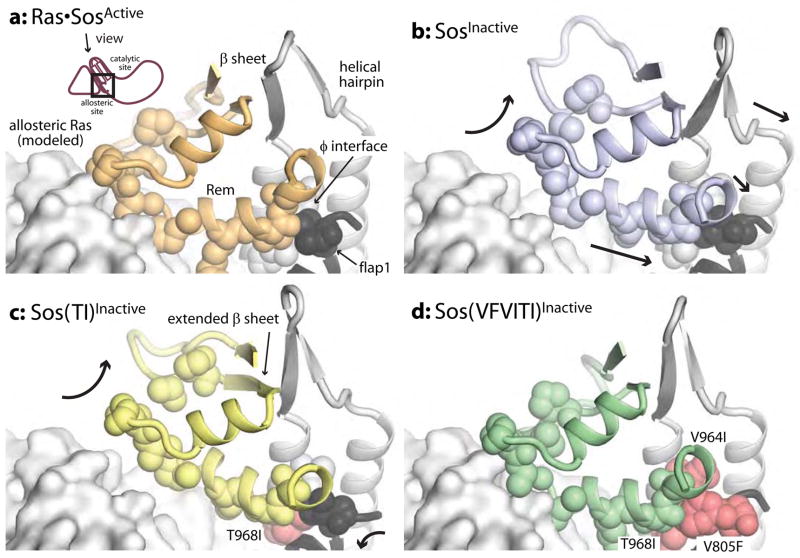
The molecular dynamics trajectories indicate that the isolated Cdc25 domain of RasGRF1 stably maintains the active conformation. The following discussion is based on an analysis of six independent trajectories of 7 ns each (denoted “GRF”, Table 1), but one of these simulations was carried out to 19 ns and yielded consistent results throughout. After alignment on the relatively rigid core of the Cdc25 domain, the mean position of the helical hairpin overall is similar to that in trajectories starting from the crystal structure of RasGRF1 (Fig. 3a, cartoons). The average position of the helical hairpin is also similar in each independent simulation (Fig. 3a, dark surface), but the helical hairpin is flexible compared to the core of the Cdc25 domain, sampling a range of positions around the average conformation (Fig. 3a, light surface). As in the crystal structure of RasGRF1 (Freedman et al., 2006), the average position of the helical hairpin in the GRF trajectories is closer to its position in active Sos than to that in inactive Sos (Fig. 3b, cartoons). The trajectories show that GRF does, on rare occasions, sample conformations similar to the crystal structure of inactive Sos, where the helical hairpin would clash with Ras at the active site (when Ras is modeled into the active site from its position in the crystal structure of Ras-bound Sos, Fig. 3b).
Fig. 3.
Conformation of the helical hairpin in molecular dynamics trajectories of active Sos and RasGRF1. (a) The average conformation over the six GRF trajectories is similar to that observed in the crystal structure of RasGRF1 (pink and purple cartoons, respectively). The light surface reflects the range of sampled conformations, including the average structures for 500 ps windows over all the trajectories and eight instantaneous structures representing the extremes of conformation with respect to active and inactive Sos. (Determined individually by Cα RMSD of the helical hairpin or the Rem domain with respect to comparable regions of active Sos or of inactive Sos. The instantaneous structures with the highest and lowest RMSD values for both regions with respect to both crystal structures represent the diversity of conformations achieved during the trajectories.) The dark surface surrounds the six structures that represent the average conformation of each individual simulation and thus reflects the heterogeneity among different simulations. (b) In the GRF simulations the helical hairpin is in a position more similar to active Sos (red) than inactive Sos (blue). The helical hairpin samples conformations, however, that would clash with Ras bound to the catalytic site. (c) Ras•SosActive simulations are more limited in the range of conformations sampled by the helical hairpin, avoiding clashes with Ras at the catalytic site.
Trajectories of active Sos with Ras molecules bound both at the allosteric and catalytic sites (Ras•SosActive•Ras, Table 1) show, not surprisingly, that the helical hairpin remains close to the active conformation (Supplementary Fig. 5, first panel). Likewise, trajectories for Sos with Ras bound only to allosteric site (Ras•SosActive, Table 1) maintain average helical hairpin conformations similar to that in the crystal structure of active Sos (Fig. 3c, cartoon, dark surface). The helical hairpin in the simulations of Ras•SosActive, however, has considerably less freedom to fluctuate toward the inactive conformation of Sos than seen in the GRF simulations and never approaches a conformation like inactive Sos (Fig. 3c, light surface). This narrowed range of conformations is most likely due to the bound allosteric Ras molecule, which constrains the helical hairpin of Sos in the active conformation.
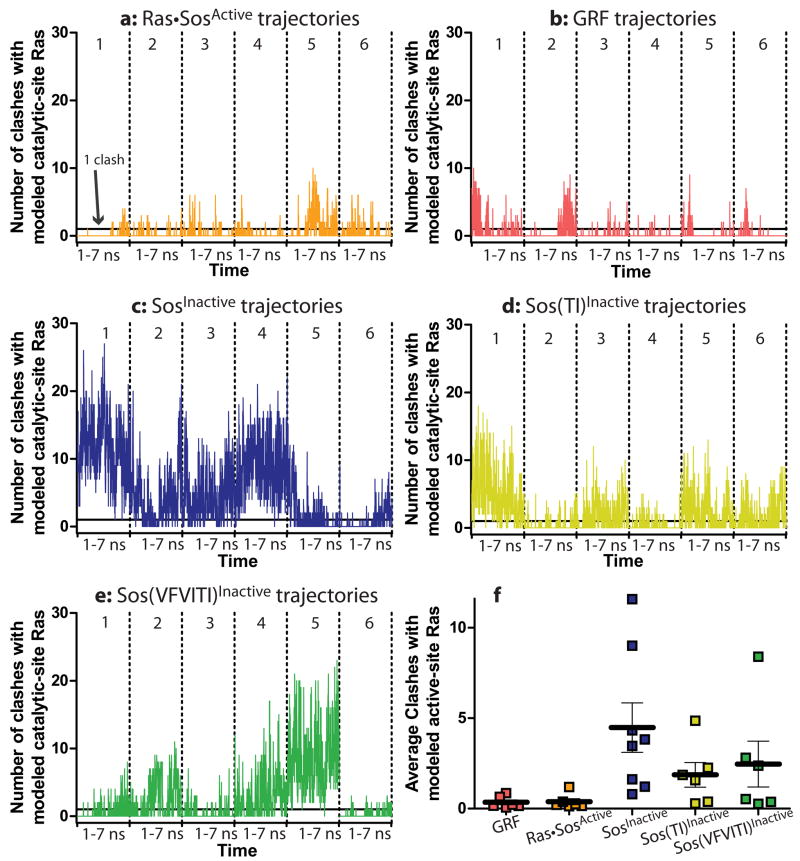
To provide a more quantitative metric for the extent to which the trajectories sample inactive conformations such as the one observed in the crystal structure of isolated Soscat (Freedman et al., 2006), we calculated the number of close contacts between the helical hairpin and a Ras molecule bound at the catalytic site (see Methods for details of this modeling). By this metric, trajectories for Ras•SosActive do occasionally fluctuate into conformations that partially occlude the active site (Fig. 4a). This analysis relies on a rigidly docked Ras molecule at the catalytic site and does not account for complementary motions in Ras and Sos. Thus, for the Ras•SosActive•Ras trajectory, which has Ras bound at the catalytic site of Sos for the duration of the simulation without steric clash, this contact metric also reports occasional clashes (Supplementary Fig. 6, first panel). We therefore consider this low level of clashes to be characteristic of the active conformation. It should be emphasized, however, that because the reference Ras molecule is rigidly docked this metric is only a rough indicator of occlusion of the active site.
Fig. 4.
Active-site occlusion by the helical hairpin during the simulations. (a–e) The number of clashes (Cα -Cα contacts closer than 2.2 Å) between the helical hairpin and a Ras molecule modeled into the active site are counted every 10 ps along the trajectory of the each simulation. The solid line indicates 1 backbone clash between the helical hairpin and catalytic-site Ras. Six simulations are concatenated in each panel, and the dotted lines represent the boundaries between them. (g) The average number of clashes over each trajectory over time is plotted as a square. Points with similar y-axis values are spaced horizontally for clarity. The horizontal bars represent the overall average number of clashes for all simulations. According to an Anova analysis, RasGRF1, Ras•SosActive•Ras (see Supplementary Fig. 6), and Ras•SosActive simulations are not significantly different in their extents of active site occlusion. All other pairs of simulations in this figure have significantly different numbers of clashes between the helical hairpin and active-site Ras (p>.0001).
In this respect, the Ras•SosActive•Ras trajectories and the Ras•SosActive trajectories are not significantly different (averaging 0.2 ± 0.5 clashes for any instantaneous structure over the trajectory of Ras•SosActive•Ras and 0.4 ± 1.0 clashes per instantaneous structure over the six trajectories of Ras•SosActive; Fig. 4f, Supplementary Fig. 6). Therefore, when the average number of these clashes per instantaneous structure over the duration of a simulation is less than 1, we conclude that the active site is not occluded. GRF also fluctuates into conformations that occlude the active site (Fig. 4b), but as with Ras•SosActive, these simulations have, on average, less than 1 clash per instantaneous structure over the all the trajectories (Fig. 4f, 0.3 ± 1.0 clashes per instantaneous structure). This confirms that both RasGRF1 and Sos maintain open active-site conformations.
The Rem domain is important for the coupling of allosteric Ras binding to the activation of Sos (Hall et al., 2001). For analysis of the conformational changes of the Rem domain, we examined the structural motifs that interact with allosteric Ras, which change in a concerted manner upon activation of Sos (Fig. 5a). We define the active conformation of the Rem domain as the conformation observed in the crystal structure of Ras-bound Sos (Margarit et al., 2003), in which these motifs are pivoted so as to interact with allosteric Ras (Fig. 5a, red). These portions of the Rem domain are pivoted into nonproductive positions in the crystal structure of inactive Sos (Fig. 5a, blue) (Freedman et al., 2006). The trajectory for Ras•SosActive•Ras (Supplementary Fig. 7a) shows that, like that of the helical hairpin, the average position of the Rem domain over all the simulations is very close to that seen in the crystal structure of active Sos. Similarly, the Rem domain consistently maintains the active conformation in the Ras•SosActive trajectories (Fig. 5a). Moreover, the average root-mean-square (RMS) fluctuation, which reveals the average degree of motion over each simulation, is low for the Rem domain in the simulations of Ras•SosActive (Fig. 6a). This is consistent with the model that allosteric Ras binding couples the Rem domain and the helical hairpin, holding both in an active conformation.
Fig. 5.
Rem domain and helical hairpin conformations in Sos trajectories. The crystal structures of active and inactive Sos are depicted in red and blue, respectively. In the left column is the result of one trajectory (of the type indicated) with the fewest average number of clashes with Ras modeled into the active site. In the right column is result of one trajectory with the greatest number of clashes. The fraction of related simulations represented by each panel is indicated (for instance, 5/6 means that five simulations of the six performed have a similar degree of active site occlusion to the one shown). The light surface reflects the range of conformations sampled within the simulation, including the conformations with highest and lowest helical-hairpin and Rem-domain RMSD with respect to active and inactive Sos as well as the average structures for every 500 ps of the simulation. Simulations not shown have intermediate degrees of active-site occlusion and are depicted in Supplementary Fig. 7 and Supplementary Fig. 8.
Fig. 6.
Dynamic fluctuations and heterogeneity within simulations. The RMS fluctuation value (related to a crystallographic B factor) of each Cα residue is indicated by color. Individual replicates of each simulation are overlaid after alignment on the rigid core of the Cdc25 domain.
In the absence of Ras, the helical hairpin of Sos fluctuates between active and inactive conformations
We also generated molecular dynamics trajectories for Sos without any bound Ras, starting from the inactive crystal structure (SosInactive, Table 1). Unlike the trajectories for Ras•SosActive and GRF, the independent trajectories for SosInactive vary significantly (Fig. 6b, overlaid structures; Fig. 4f). Thus, the following analysis begins with a discussion of individual trajectories that represent different populations within the set. SosInactive trajectories show dramatic active-site occlusion (Fig. 4c,f; an average of 4.5 clashes per instantaneous structure for SosInactive). Compared to Ras•SosActive, SosInactive trajectories have high RMS fluctuation values, especially in the Rem domain (Fig. 6b, colors). In fact, the average position of the helical hairpin in one SosInactive trajectory closely resembles the one found in active Sos (Fig. 5b, left), whereas in other simulations it more closely resembles the position in inactive Sos or intermediate conformations between the two (Fig. 5b, right; Supplementary Fig. 8a). Although the helical hairpin samples the full range of conformations from inactive to active in the SosInactive simulations, the Rem domain never samples the active conformation (Fig. 5b, surface).
The limited ability of the Rem domain of Sos to switch between the active and inactive conformations is evident also in the trajectories of unliganded Sos starting from the active conformation (SosActive, Table 1). Unlike the Ras•SosActive trajectories, the SosActive trajectories show the helical hairpin fluctuating into conformations that occlude the active site (Supplementary Fig. 6). However, the average conformation of the helical hairpin over the SosActive trajectories remains more like that in active Sos than in the SosInactive trajectories (Supplementary Fig. 5, Supplementary Fig. 7b). This bias toward the starting structure is surprising given the fact that the helical hairpin samples the active and inactive positions multiple times during several of the simulations. The discrepancy suggests that while the helical hairpin may be flexible, the Rem domain has not yet broken free of interactions that bias it toward the active conformation. This conclusion is supported by the SosActive trajectory that displays the most severe active-site occlusion (the greatest average number of close contacts with Ras per instantaneous structure) in which the Rem domain never samples a fully active conformation (Supplementary Fig. 7b, right). In this simulation the Rem domain has overcome some energetic barrier and loses its bias toward the active conformation. Consequently, the helical hairpin fluctuates more often into inactive conformations with an occluded catalytic site. The helical hairpin fluctuates in all the trajectories of SosActive and SosInactive, but only a restricted range of conformations seems to be available to the Rem domain. The correlation of active-site occlusion with the conformational state of the Rem domain pinpoints the Rem domain as the key locking mechanism for the helical hairpin.
Differences in the trajectories of the activated mutants of Sos
As described above, the activated mutant Sos(TI) is responsive to further stimulation by allosteric Ras binding, whereas further allosteric activation of Sos(VFVITI) is impaired. We performed simulations of Sos(TI) and Sos(VFVITI) starting from the active and inactive crystal structures of wild-type Sos (see Methods). In all the simulations the initial steric clash created by inserting the bulkier residues was relieved without any large-scale conformational changes. In simulations of these mutants starting from the inactive structure of Sos (Sos(TI)Inactive and Sos(VFVITI)Inactive, Table 1), there is less severe active-site occlusion than seen in simulations of wild-type SosInactive (Fig. 4d-f).
Although the average position of the helical hairpin is not substantially different in Sos(TI)Inactive than in wild-type SosInactive (Supplementary Fig. 5), the helical hairpin seems to be prevented from sampling conformations in which the active site is completely blocked (Fig 5c, surface). Moreover, the trajectories that do show active-site occlusion have fewer close contacts with Ras modeled in the active site (Fig. 4d,f). Although the average position of the Rem domain in the Sos(TI)Inactive simulations more closely resembles that of inactive Sos than of active Sos (Fig. 7a–c), the β-sheet interactions in the Rem domain are extended and have a shifted register, and there are conformational differences in flap1 as well as a helix in the Rem domain that interacts with flap1, the helical hairpin, and allosteric Ras (Fig. 7c). Furthermore, the Rem domain fluctuates less in the Sos(TI)Inactive trajectories than in those of wild-type SosInactive (Fig. 6c, colors). We therefore speculate that the Rem domain and helical hairpin in Sos(TI) are less flexible than in wild-type Sos. The fact that Sos(TI)Inactive is not already strongly biased toward the active conformation could explain its sensitivity to further stimulation by allosteric Ras binding.
Fig. 7.
Rem-domain position in wild-type and mutant Sos. Average conformation over all simulations. Mutated residues are colored in pink.
Unlike Sos(TI)Inactive, Sos(VFVITI)Inactive is highly flexible. The Rem domains in the Sos(VFVITI)Inactive trajectories have high RMS fluctuation values (Fig. 6d), and Sos(VFVITI)Inactive is highly dynamic even in the simulation with the least active-site occlusion (Fig. 5d, left, surface). The decrease in active-site occlusion in this mutant seems to arise from increased preference for the active conformation in the absence of allosteric Ras binding. In Sos(VFVITI)Inactive the helical hairpin is biased toward the active conformation (Supplementary Fig. 5), and this occurs in conjunction with the Rem domain moving toward the active position (Fig. 5d, left panel). This differs from simulations of SosInactive, where the Rem domain does not move into the active position even when the helical hairpin does (Fig. 5b, left panel). Moreover, when the average position of the helical hairpin is like that in inactive Sos, as it is in a single Sos(VFVITI)Inactive trajectory, the Rem domain is also in the inactive position (Fig. 5d, right panel). Intermediate conformations of the Rem domain accompany intermediate conformations of the helical hairpin (Supplementary Fig. 8b), suggesting that the positions of the Rem domain and helical hairpin are better coupled in the Sos(VFVITI) mutant than in wild-type Sos. The average position of the Rem domain over all the simulations of Sos(VFVITI)Inactive closely resembles that in Ras•SosActive (Fig. 7a,d). Since this mutant no longer requires Ras to achieve the active conformation, its dependence on allosteric activation by Ras could be decreased, explaining the insensitivity of this mutant to further activation by allosteric Ras. It is also possible that the increased flexibility causes a dissipation of the signal generated by allosteric Ras binding, which could result in impaired maximal activity. The idea that flexibility actually impairs the nucleotide exchange reaction is also interesting in light of the observation that the RasGRF1 (flexible) is less active than Ras-bound Sos (rigid) even though both are strongly biased toward the active conformation.
We also performed simulations of Sos mutants starting from the active conformation in the absence of Ras (Sos(TI)Active and Sos(VFVITI)Active, Table 1). As wild-type SosActive is already biased toward an active conformation on the nanosecond timescale, any conformational bias created by the mutations is largely masked. Individual simulations of Sos starting from the active conformation remain trapped in the active conformation and generate very few clashes (Supplementary Fig. 5). Two out of seven SosActive trajectories have this restricted profile, whereas three out of six Sos(TI)Active simulations have this feature. The triple mutant, however, persists in this state in only one of six simulations (Supplementary Fig. 6). This is consistent with the observation of greater flexibility in the Sos(VFVITI)Inactive simulations but not in the Sos(TI) Inactive simulations.
Comparing the average structure over the simulations of wild-type and mutant Sos, we can speculate about the roles of these mutations in coupling and reorienting the Rem domain and helical hairpin. In the simulations of wild-type Sos, these residues are small, and the interaction of the Rem domain with allosteric Ras is necessary for positioning the Rem domain, and with it the helical hairpin, in the active conformation (Fig. 8a,b). The bulky isoleucine residue introduced into the helical hairpin (T968I) distorts flap1 and repositions a helix in the Rem domain that forms part of the binding site for allosteric Ras (Fig. 8c). The addition of the bulky phenylalanine residue in flap1 and the additional isoleucine residue in the helical hairpin (V805F+V964I) seems to further reorient this helix in the Rem domain, and flap1, into the fully active conformation in the simulations of Sos(VFVITI)Inactive (Fig. 8d).
Fig. 8.
Interface created by the Rem domain, helical hairpin, and flap1 in the simulations of Sos. Average conformation over all simulations. Residues participating in the interface of the Rem domain, the helical hairpin, and flap1 are indicated in surface. Mutated residues are colored in pink.
Discussion
From our comparison of molecular dynamics trajectories of RasGRF1 and Sos, we suggest that allosteric Ras binding serves two, membrane-independent functions in Sos: to bias Sos toward the active conformation and to decrease the mobility of the helical hairpin, preventing fluctuations that occlude the active site. We believe that this dynamic block on the helical hairpin is important based on the observation that the catalytic domain of RasGRF1 populates a set of conformations that is biased toward an active position, but is nonetheless a much less efficient exchange factor than activated Sos.
While it seems clear from the simulations that Sos(TI) and Sos(VFVITI) have higher basal activities because they are more receptive to Ras binding at the active site, the manner in which they achieve this is surprising. The Rem domain of Sos(VFVITI) is biased toward the active conformation, but at the expense of high flexibility. Sos(TI), however, samples highly clashing conformations less often, probably because of decreased overall flexibility. The observation from molecular dynamics that the Rem domain of Sos(VFVITI), but not of Sos(TI), is biased toward the active conformation is consistent with the measured affinities of their allosteric sites for Ras. Sos(VFVITI) binds allosteric Ras with higher affinity than does wild-type Sos; increased population of an active-like conformation could lessen the entropic penalty of binding. The affinity of Sos(TI) for allosteric Ras remains unchanged, and in the Sos(TI)Inactive trajectories the Rem domain does not shift toward the active conformation.
We had previously proposed that the bulky residues that pack the helical hairpin/flap1 interface of RasGRF1 contribute to its ability to maintain an active conformation by holding open the active conformation (Freedman et al., 2006). Based on our molecular dynamics simulations, we now refine our analysis of the role of these residues. It seems that the helical hairpin of Sos is intrinsically flexible and samples a variety of conformations along with the Rem domain. We see in our simulations that the active conformation of the helical hairpin is not incompatible with the inactive conformation of the Rem domain. Conformations in which the Rem domain appears to be in an inactive position but the helical hairpin is still pivoted outward have also been observed in a crystal structure. In one of two molecules in the crystallographic asymmetric unit of SosDH-PH-Cdc25, the hydrophobic interface between the helical hairpin and the Rem domain is also broken, suggesting a mechanism for how the position of the helical hairpin may be uncoupled from the Rem domain (Sondermann et al., 2004). However, we never observe the helical hairpin to be in an inactive position if the Rem domain has adopted an active position.
A large component of the regulatory apparatus of Sos relies on the intrinsic inactivity of the catalytic Cdc25 domain. Unlike other regulators of the Ras pathway that have been linked to forms of Noonan Syndrome, Sos does not cause Noonan-Syndrome-associated cancers (Roberts et al., 2007; Swanson et al., 2008; Tartaglia et al., 2007). This could be because the autoregulation of Sos has many checkpoint steps, including release of the histone domain, release of the DH domain, clustering of Ras, and production of Ras•GTP to bind to the allosteric site (Gureasko et al., 2008; Margarit et al., 2003). In addition, Sos is regulated by phosphorylation, adaptor binding, and recruitment to activated receptors. If the catalytic site of Sos were active in the absence of allosteric activation, this regulation could be bypassed, leading to a much more severe hyperactivation of Ras. The intrinsic inactivity of its catalytic domain may explain why Sos has yet to be implicated in human cancers. The helical hairpin of the Rap exchange factor Epac2 undergoes analogous movements to Sos in the switch from active to inactive, but this motion is subtler than the conformational change observed in Sos, and activator-binding domain in this protein directly occludes the active site (Rehmann et al., 2008; Rehmann et al., 2006). Unlike Sos and Epac2, the helical hairpin of RasGRF1 does not seem to collapse inward to block the catalytic site. The levels of RasGRF1 in cells are tightly regulated by cell- and environment-specific expression and by proteolysis (Baouz et al., 1997; Cen et al., 1992; Coccetti et al., 1995; Leaner et al., 2005; Martegani et al., 1992). Thus, it is possible that RasGRF1 may have circumvented the need for the strict control of activity that drove the evolution of a fail-safe mechanism in Sos.
Experimental Procedures
Protein purification
We purified Soscat (human Sos1, residues 566-1049 in pPROEX vector), RasGRF1Cdc25 (mouse RasGRF1, residues 1028-1262 in pGEX-6P-3 vector), and Ras (human H-Ras, residues 1-166 in pPROEX vector) as described previously (Freedman et al., 2006). Briefly, we harvested protein from BL21 DE3* cells (Novagen) after induction with 0.1 mM isopropyl β-D-1-thiogalactopyranoside at 18°C for ~16 h. We performed affinity chromatography (HisTrap for Sos and Ras, GSTrap for RasGRF1, Amersham) followed by ion exchange chromatography (HiTrap Q for Sos and Ras, HiTrap S for RasGRF1, Amersham). We then transferred protein into final buffer (200 mM NaCl and 25 mM Tris, pH 8.0) by gel filtration (Superdex 200, Amersham). SDS PAGE and mass spectrometry confirmed protein homogeneity. We measured protein concentration (30 mg/ml) by absorbance at 280 nm (Gasteiger et al., 2005), which agreed with values obtained in guanidinium chloride (Gill and von Hippel, 1989) and with results of bicinchoninic acid (BCA) colorimetric assays (Sigma). Protein aliquots were frozen in liquid nitrogen and stored at −80°C. The concentration of nucleotide-bound Ras was only measured by the BCA assay. We performed site directed mutagenesis on Sos with the QuikChange system (Invitrogen) and verified the clones by sequencing. We attempted to make the reciprocal RasGRF1 mutants, but even conservative mutants of RasGRF1 were aggregated or insoluble.
Measurement of Ras-specific nucleotide exchange activity and affinity of Ras for the allosteric site of Sos
We measured the decrease in fluorescence as a fluorescent GDP analog was released from Ras to determine the relative activities of RasGRF1 and Sos (Lenzen et al., 1995). Nucleotide exchange reactions were initiated by rapid 1:1 mixing of 2 μM Sos (in a starting mixture of 400 μM GDP, 40 mM Hepes pH 7.5, 10 mM MgCl2, and 1 mM DTT) with 0.2 μM Ras pre-loaded with 3′-O-N-Methyl-anthraniloyl-2′-deoxy-guanosine-5′-diphosphate (Ras•mant-dGDP, Jena Bioscience (Guo et al., 2005), in 40 mM Hepes pH 7.5, 10 mM MgCl2, and 1 mM DTT) on a stopped flow apparatus (Applied Photophysics RX2000) linked to a Jobin Yvon Horiba Fluoromax-3 fluorimeter. After this two-fold dilution, the final concentration of Sos was 1 μM and the final concentration of substrate Ras was 0.1 μM. We premixed RasY64A•GMPPNP with Sos in some reactions to measure allosteric activation of Sos by Ras. To prevent precipitation of RasGRF1 (Freedman et al., 2006; Lenzen et al., 1995) and allow comparison between RasGRF1 and Sos, the samples, the stopped-flow apparatus, and the cuvette were chilled to 15°C before mixing. The progress of each 300 μl reaction was monitored by fluorescence intensity at 430 nm upon excitation at 370 nm. Excitation slits were fixed at 5 nm, and data were recorded every 0.5 s after integration over 0.05 s. Emission slits were set to maximize signal without exceeding the linear range of the instrument, generally between 15 and 25 nm. Whenever possible, reactions were carried out for 20 times the half life of the nucleotide exchange reaction. Data were obtained by averaging three consecutive runs with the same sample, and each reaction was performed in triplicate with independent protein samples.
We used Prism 5.0 (Graphpad) to fit the decay curves to a double exponential function (Y = A0 + A1· e−k1· t + A2 · e−k2 · t), where the higher amplitude phase was the nucleotide exchange rate and the invariant, lower-amplitude phase was attributed to photobleaching. After fitting, we normalized the raw data independently for each reaction with the formula Ynormalized = (Yraw −A0)/(Max − A0), where A0 represents the offset value from the exponential fit and Max is the initial, maximum fluorescence of the sample.
To measure the affinity of Ras for the allosteric site of Sos, the rates of nucleotide release vs. concentration/ratio of added RasY64A, a mutant of Ras that binds only to the allosteric site, were fit to a hyperbolic binding model y = y0 + (Bmax · x)/(Kd + x) where Bmax is the maximum activity when the allosteric site of Sos is saturated with RasY64A. The offset, y0, was used to account for differences in the basal activity of each mutant in the absence of added RasY64A.
Molecular dynamics simulations
Individual molecular dynamics simulations are summarized in Table 1. Two loops that are disordered in the crystal structure of Sos were built using O (Kleywegt and Jones, 1996). To generate the mutants of Sos for simulation, we used Pymol (DeLano, 2002) to model the substitution. Rotamers of the substituted residues were selected based on their appearance in the structure of RasGRF1 (Freedman et al., 2006). Each starting structure, with crystallographic water molecules removed, was placed in a rectangular water box that extended 10 Å beyond the limits of the protein, and Na+ and Cl− ions corresponding to a concentration of ~150 mM. If necessary, extra chloride ions were added to offset the intrinsic charge of the protein. All simulations were performed with the TIP3P explicit water model (Jorgensen et al., 1983). These steps were performed using the LEAP module of AMBER, version 7, with the parm96 force field (Cornell et al., 1995; Pearlman et al., 1995). The charge-charge conflict presented by the proximity of Glu-873 and Asp-792 was removed by protonating Asp-792 (the significance of this ion pair, observed in the crystal structures, is not well understood). The ε nitrogen on His-827 in this cluster of residues was protonated to allow interactions with Glu-792 (a proton on the δ nitrogen of this residue would have no interactions); all other histidine residues were left in the default state of protonation selected by AMBER. After equilibration with positional restraints for the first 50 ps, random velocities were assigned to the atoms (Young et al., 2001). Each starting condition was used to generate 6–8 trajectories, initiated with different random velocities. Each simulation was carried out for at least 7 ns at constant pressure of 1 atm and a constant temperature of 298 K.
The first nanosecond of each simulation was omitted from our analysis to eliminate the effects of initial relaxation. We analyzed the simulations with CHARMM (Brooks et al., 1983) and Pymol (DeLano, 2002). We used CHARMM to calculate average structures from every 10 ps of simulation to approximate instantaneous structures in our analysis. We also used CHARMM to calculate the average structure for each simulation. For this and subsequent analysis, we aligned all instantaneous structures on the rigid core of the Cdc25 domain (residues 1029-1041, 1087-1114, and 1134-1147 for RasGRF1 or 782-794, 839-867, and 888-899 for Sos). CHARMM was also used to calculate RMS fluctuation ((<Δri2>)0.5), which is related to the crystallographic B-value by the formula <Δri2> = 3Bi/8π2. We calculated root-mean-square deviation (RMSD) between the structures from the trajectories and the starting structures with Pymol, using a python script to interatively perform the “rms_cur” command and print the RMSD values for each 10 ns of each trajectory. The residues in the Rem domain that interact with allosteric Ras (residues 683-695 and 615-621) and the helical hairpin (residues 929-943 and 958-976 for Sos, residues 1178-1193 and 1204-1222 for RasGRF1) were compared independently.
To calculate the average structure over multiple simulations, we used a python script developed in the McCammon laboratory http://mccammon.ucsd.edu/~cmura/PyMOL/pymol_mainFrame.html). The average structure does not correspond to a structure actually sampled during the simulations, and has meaningless stereochemistry. For Fig. 3, Fig. 7, Fig. 8, and Supplementary Fig. 5 we used the average structure to select instantaneous structures from the trajectory that have the closest correspondence to the helical hairpin and to the Rem domain. We confirmed these choices by visual inspection.
A python script in Pymol was used to list the number of steric clashes between the backbone atoms of the helical hairpin (residues 1178-1193 of RasGRF1 or 929-976 of Sos) that lie less than 2.2 Å away from a position occupied by Ras in the active site of Soscat in the crystal structure (1NVV, Margarit et al., 2003). For this calculation, we used residues in Ras that surround the anchoring residue Y64. We avoided using residues that belong to the extended region of switch 2 that is opened by binding to the helical hairpin, because it is not clear whether this is the conformation from which Ras would be recognized by Sos, and interactions in the structure of active Sos are very close. In short, we used residues 15-26 and 56-74 of Ras for our calculation. The cutoff for our definition of a steric clash, 2.2 Å, was chosen based on the estimated van der Waal’s radii of the backbone atoms nitrogen, oxygen, and carbon. These have a range of van der Waal’s radii from 1.38 to 1.55 (for carbon and oxygen, respectively, Martz and Sayle, 2000). Steric clash has been defined as occurring when the distance between the two backbone atoms is smaller than 70% of the sum of the van der Waal’s radii (Fernandez-Fuentes et al., 2006a; Fernandez-Fuentes et al., 2006b). For two oxygen atoms, this puts the cutoff at 2.2 Å.
Supplementary Material
Acknowledgments
We thank Doug Lowy for RasGRF1 cDNA, Jodi Gureasko, Nick Levinson, and Xuewu Zhang, for interesting discussions, and David King for mass spectrometry. We thank Susan Marqusee and Dafna Bar-Sagi for guidance and discussions. H.S. was supported by the Leukemia & Lymphoma Society. G.D.F is supported by the NSFGRFP, and T.K. by the Sloan Foundation. J.K. is supported by the NCI (R01 CA096504-02).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baouz S, Jacquet E, Bernardi A, Parmeggiani A. The N-terminal moiety of CDC25(Mm), a GDP/GTP exchange factor of Ras proteins, controls the activity of the catalytic domain. Modulation by calmodulin and calpain. J Biol Chem. 1997;272:6671–6676. doi: 10.1074/jbc.272.10.6671. [DOI] [PubMed] [Google Scholar]
- Boriack-Sjodin PA, Margarit SM, Bar-Sagi D, Kuriyan J. The structural basis of the activation of Ras by Sos. Nature. 1998;394:337–343. doi: 10.1038/28548. [DOI] [PubMed] [Google Scholar]
- Brooks BR, Bruccoleri RE, Olafson BD, States DJ, Swaminathan S, Karplus M. Charmm - a Program for Macromolecular Energy, Minimization, and Dynamics Calculations. J Comput Chem. 1983;4:187–217. [Google Scholar]
- Cen H, Papageorge AG, Zippel R, Lowy DR, Zhang K. Isolation of multiple mouse cDNAs with coding homology to Saccharomyces cerevisiae CDC25: identification of a region related to Bcr, Vav, Dbl and CDC24. Embo J. 1992;11:4007–4015. doi: 10.1002/j.1460-2075.1992.tb05494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccetti P, Mauri I, Alberghina L, Martegani E, Parmeggiani A. The minimal active domain of the mouse ras exchange factor CDC25Mm. Biochem Biophys Res Commun. 1995;206:253–259. doi: 10.1006/bbrc.1995.1035. [DOI] [PubMed] [Google Scholar]
- Coleman ML, Marshall CJ, Olson MF. RAS and RHO GTPases in G1-phase cell-cycle regulation. Nat Rev Mol Cell Biol. 2004;5:355–366. doi: 10.1038/nrm1365. [DOI] [PubMed] [Google Scholar]
- Cornell WD, Cieplak P, Bayly CI, Gould IR, Merz KM, Ferguson DM, Spellmeyer DC, Fox T, Caldwell JW, Kollman PA. A second generation force field for the simulation of proteins, nucleic acids, and organic molecules. J Am Chem Soc. 1995;117:5179–5197. [Google Scholar]
- DeLano WL. The Pymol Molecular Graphics System. 2002 http://www.pymol.org.
- Dodson GG, Lane DP, Verma CS. Molecular simulations of protein dynamics: new windows on mechanisms in biology. EMBO Rep. 2008;9:144–150. doi: 10.1038/sj.embor.7401160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan SE, Giddings BW, Brooks MW, Buday L, Sizeland AM, Weinberg RA. Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature. 1993;363:45–51. doi: 10.1038/363045a0. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fuentes N, Oliva B, Fiser A. A supersecondary structure library and search algorithm for modeling loops in protein structures. Nucl Acids Res. 2006a;34:2085–2097. doi: 10.1093/nar/gkl156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Fuentes N, Zhai J, Fiser A. ArchPRED: a template based loop structure prediction server. Nucleic Acids Res. 2006b;34:W173–176. doi: 10.1093/nar/gkl113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman TS, Sondermann H, Friedland GD, Kortemme T, Bar-Sagi D, Marqusee S, Kuriyan J. A Ras-induced conformational switch in the Ras activator Son of sevenless. Proc Natl Acad Sci U S A. 2006;103:16692–16697. doi: 10.1073/pnas.0608127103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasteiger EHC, Hoogland CAGSDMRWRDA, Bairoch A. Protein Identification and Analysis Tools on the ExPASy Server. In: Walker JM, editor. The Proteomics Protocols Handbook. Humana Press; 2005. [Google Scholar]
- Gill SC, von Hippel PH. Calculation of Protein Extinction Coefficients from Amino-Acid Sequence Data. Analytical Biochemistry. 1989;182:319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- Guo Z, Ahmadian MR, Goody RS. Guanine nucleotide exchange factors operate by a simple allosteric competitive mechanism. Biochemistry. 2005;44:15423–15429. doi: 10.1021/bi0518601. [DOI] [PubMed] [Google Scholar]
- Gureasko J, Galush WJ, Boykevisch S, Sondermann H, Bar-Sagi D, Groves JT, Kuriyan J. Membrane-dependent signal integration by the Ras activator Son of sevenless. Nat Struct Mol Biol. 2008;15:452–461. doi: 10.1038/nsmb.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BE, Yang SS, Boriack-Sjodin PA, Kuriyan J, Bar-Sagi D. Structure-based mutagenesis reveals distinct functions for Ras switch 1 and switch 2 in Sos-catalyzed guanine nucleotide exchange. J Biol Chem. 2001;276:27629–27637. doi: 10.1074/jbc.M101727200. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Herrmann C, Nassar N. Ras and its effectors. Progress in Biophysics and Molecular Biology. 1996;66:1–41. doi: 10.1016/s0079-6107(96)00015-6. [DOI] [PubMed] [Google Scholar]
- Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. Comparison of Simple Potential Functions for Simulating Liquid Water. J Chem Physics. 1983;79:926–935. [Google Scholar]
- Karplus M, Kuriyan J. Molecular dynamics and protein function. Proc Natl Acad Sci U S A. 2005;102:6679–6685. doi: 10.1073/pnas.0408930102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleywegt GJ, Jones TA. Efficient rebuilding of protein structures. Acta Crystallogr D Biol Crystallogr. 1996;52:829–832. doi: 10.1107/S0907444996001783. [DOI] [PubMed] [Google Scholar]
- Leaner VD, Donninger H, Ellis CA, Clark GJ, Birrer MJ. p75-Ras-GRF1 is a c-Jun/AP-1 target protein: its up regulation results in increased Ras activity and is necessary for c-Jun-induced nonadherent growth of Rat1a cells. Mol Cell Biol. 2005;25:3324–3337. doi: 10.1128/MCB.25.8.3324-3337.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzen C, Cool RH, Prinz H, Kuhlmann J, Wittinghofer A. Kinetic analysis by fluorescence of the interaction between Ras and the catalytic domain of the guanine nucleotide exchange factor Cdc25Mm. Biochemistry. 1998;37:7420–7430. doi: 10.1021/bi972621j. [DOI] [PubMed] [Google Scholar]
- Lenzen C, Cool RH, Wittinghofer A. Analysis of intrinsic and CDC25-stimulated guanine nucleotide exchange of p21ras-nucleotide complexes by fluorescence measurements. Methods Enzymol. 1995;255:95–109. doi: 10.1016/s0076-6879(95)55012-7. [DOI] [PubMed] [Google Scholar]
- Margarit SM, Sondermann H, Hall BE, Nagar B, Hoelz A, Pirruccello M, Bar-Sagi D, Kuriyan J. Structural evidence for feedback activation by Ras.GTP of the Ras-specific nucleotide exchange factor SOS. Cell. 2003;112:685–695. doi: 10.1016/s0092-8674(03)00149-1. [DOI] [PubMed] [Google Scholar]
- Martegani E, Vanoni M, Zippel R, Coccetti P, Brambilla R, Ferrari C, Sturani E, Alberghina L. Cloning by functional complementation of a mouse cDNA encoding a homologue of CDC25, a Saccharomyces cerevisiae RAS activator. Embo J. 1992;11:2151–2157. doi: 10.1002/j.1460-2075.1992.tb05274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martz E, Sayle R. What are the values used by RasMol and Chime for van der Waals radii in the spacefill rendering? 2000 [Google Scholar]
- Pearlman DA, Case DA, Caldwell JW, Ross WS, Cheatham TE, Debolt S, Ferguson D, Seibel G, Kollman P. Amber, a Package of Computer-Programs for Applying Molecular Mechanics, Normal-Mode Analysis, Molecular-Dynamics and Free-Energy Calculations to Simulate the Structural and Energetic Properties of Molecules. Comp Phys Commun. 1995;91:1–41. [Google Scholar]
- Rehmann H, Arias-Palomo E, Hadders MA, Schwede F, Llorca O, Bos JL. Structure of Epac2 in complex with a cyclic AMP analogue and RAP1B. Nature. 2008;455:124–127. doi: 10.1038/nature07187. [DOI] [PubMed] [Google Scholar]
- Rehmann H, Das J, Knipscheer P, Wittinghofer A, Bos JL. Structure of the cyclic-AMP-responsive exchange factor Epac2 in its auto-inhibited state. Nature. 2006;439:625–628. doi: 10.1038/nature04468. [DOI] [PubMed] [Google Scholar]
- Roberts AE, Araki T, Swanson KD, Montgomery KT, Schiripo TA, Joshi VA, Li L, Yassin Y, Tamburino AM, Neel BG, Kucherlapati RS. Germline gain-of-function mutations in SOS1 cause Noonan syndrome. Nature Genetics. 2007;39:70–74. doi: 10.1038/ng1926. [DOI] [PubMed] [Google Scholar]
- Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer. 2007;7:295–308. doi: 10.1038/nrc2109. [DOI] [PubMed] [Google Scholar]
- Sondermann H, Nagar B, Bar-Sagi D, Kuriyan J. Computational docking and solution x-ray scattering predict a membrane-interacting role for the histone domain of the Ras activator son of sevenless. Proc Natl Acad Sci U S A. 2005;102:16632–16637. doi: 10.1073/pnas.0508315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondermann H, Soisson SM, Boykevisch S, Yang SS, Bar-Sagi D, Kuriyan J. Structural analysis of autoinhibition in the Ras activator Son of sevenless. Cell. 2004;119:393–405. doi: 10.1016/j.cell.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Swanson KD, Winter JM, Reis M, Bentires-Alj M, Greulich H, Grewal R, Hruban RH, Yeo CJ, Yassin Y, Iartchouk O, et al. SOS1 mutations are rare in human malignancies: implications for Noonan Syndrome patients. Genes Chromosomes Cancer. 2008;47:253–259. doi: 10.1002/gcc.20527. [DOI] [PubMed] [Google Scholar]
- Tartaglia M, Pennacchio LA, Zhao C, Yadav KK, Fodale V, Sarkozy A, Pandit B, Oishi K, Martinelli S, Schackwitz W, et al. Gain-of-function SOS1 mutations cause a distinctive form of Noonan syndrome. Nature Genetics. 2007;39:75–79. doi: 10.1038/ng1939. [DOI] [PubMed] [Google Scholar]
- Vetter IR, Wittinghofer A. The guanine nucleotide-binding switch in three dimensions. Science. 2001;294:1299–1304. doi: 10.1126/science.1062023. [DOI] [PubMed] [Google Scholar]
- Young MA, Gonfloni S, Superti-Furga G, Roux B, Kuriyan J. Dynamic coupling between the SH2 and SH3 domains of c-Src and Hck underlies their inactivation by C-terminal tyrosine phosphorylation. Cell. 2001;105:115–126. doi: 10.1016/s0092-8674(01)00301-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.