Abstract
Background
Chromogranin A (CHGA) triggers catecholamine secretory granule biogenesis, and its catestatin fragment inhibits catecholamine release. We approached catestatin heritability using twin pairs, coupled with genome-wide linkage, in a series of twin and sibling pairs from 2 continents.
Methods and Results
Hypertensive patients had elevated CHGA coupled with reduction in catestatin, suggesting diminished conversion of precursor to catestatin. Heritability for catestatin in twins was 44% to 60%. Six hundred fifteen nuclear families yielded 870 sib pairs for linkage, with significant logarithm of odds peaks on chromosomes 4p, 4q, and 17q. Because acidification of catecholamine secretory vesicles determines CHGA trafficking and processing to catestatin, we genotyped at positional candidate ATP6N1, bracketed by peak linkage markers on chromosome 17q, encoding a subunit of vesicular H+-translocating ATPase. The minor allele diminished CHGA secretion and processing to catestatin. The ATP6N1 variant also influenced blood pressure in 1178 individuals with the most extreme blood pressure values in the population. In chromaffin cells, inhibition of H+-ATPase diverted CHGA from regulated to constitutive secretory pathways.
Conclusions
We established heritability of catestatin in twins from 2 continents. Linkage identified 3 regions contributing to catestatin, likely novel determinants of sympathochromaffin exocytosis. At 1 such positional candidate (ATP6N1), variation influenced CHGA secretion and processing to catestatin, confirming the mechanism of a novel trans-QTL for sympathochromaffin activity and blood pressure.
Keywords: catecholamines; genes; genetics; hypertension; nervous system, autonomic
Chromogranin A (CHGA) is the major soluble protein found in the core of catecholamine storage vesicles of chromaffin cells and postganglionic sympathetic axons. CHGA plays a necessary role in the formation of secretory granules1 and is cosecreted with catecholamines by exocytosis in both experimental animals and humans.2 The prohormone CHGA is cleaved to a series of biologically active peptides, including the fragment catestatin (human CHGA352–372). This is a potent inhibitor of catecholamine release, acting as an antagonist of the physiological nicotinic cholinergic pathway that triggers secretion from the sympathochromaffin system.3,4 Catestatin circulates in human plasma, and its plasma concentration is predictive of hypertension as well as pressor responses to environmental stimuli.5 Prevention of endogenous catestatin expression by targeted ablation (knockout) of the mouse Chga locus results in severe hypertension that can be “rescued” specifically by replacement of the catestatin peptide.1 Thus, plasma catestatin concentration may be an important “intermediate phenotype” in analysis of genetic risk for cardiovascular disease.6,7
We previously described rare nonsynonymous genetic variants of catestatin with altered nicotinic cholinergic inhibitory potency on chromaffin cells in vitro.8 However, the frequency and significance of more common genetic variants influencing catestatin are not well understood. Many common cardiovascular diseases of later life, such as hypertension, display significant heritability6,7 but are likely to be complex traits9 with multiple determinants, both hereditary and environmental. Grappling with such etiological complexity demands very large sample sizes as well as appropriate kinds of subjects, such as pairs of relatives.9
In the present study, we observed that CHGA is elevated and its processing to catestatin is diminished in hypertension. To approach genetic control of catestatin in the population, we then turned to the human twin pair design.10 Studies of monozygotic (MZ) and dizygotic (DZ) twins allow estimation of the contribution of genetic variation to any trait (as heritability, or h2), and DZ or sibling pairs allow genome-wide approaches such as microsatellite linkage scanning to identify previously unsuspected loci influencing traits of interest. Thus, we measured catestatin in a large series of twin and sibling pairs from both North America and Australia, enabling estimation of heritability, linkage (positional cloning), and association for the trait.
Methods
Subjects
Four groups of subjects, 3 from the University of California at San Diego (UCSD) and 1 from Australia, provided information and blood samples for this project.
UCSD Hypertensive Cases
Initially we studied a series of hypertensive cases and normotensive controls. Characteristics of the subjects were described in previous reports from our group.11 Hypertension (n=452) was defined by a historical diagnosis, current blood pressure (BP) measurement demonstrating diastolic BP (DBP) >90 mm Hg (in triplicate), and/or current antihypertensive drug treatment; at least 2 of these criteria were met in each subject. In controls (n=215), normal BP (DBP <85 mm Hg) was verified by measurement (in triplicate), and none were receiving antihypertensive drugs. Normal renal function was verified in each subject (serum creatinine <1.5 mg/dL). Antecubital blood was drawn for measurement of CHGA116–439 (precursor to active peptides) and CHGA361–372 (catestatin epitope).12
UCSD Twins and Sibling Pairs
San Diego participants were drawn from the UCSD Twin Project,13,14 in which twin pairs were ascertained through a California population-based twin registry15 as well as through newspaper advertising. Twin zygosity was established by self-identification, as well as by extensive microsatellite and single nucleotide polymorphism (SNP) genotyping.13,14 Whenever possible, additional siblings of each twin pair (MZ or DZ) were recruited to facilitate sib-pair linkage. Finally, an additional 69 families with sibship size ≥3 were recruited to provide additional sib pairs. Subjects were recruited during the years 2001–2004. The study was approved by the UCSD Human Research Protection Program.
Australian Twins and Sibling Pairs
Australian participants in this study were described in a previous report.16 They completed a questionnaire in 1989, had a telephone interview in 1993–1994, and provided a blood sample in 1993–1996. Zygosity was determined from responses to questions about physical similarity and the inability of others to tell them apart, supplemented by blood group information and extensive microsatellite or SNP genotyping. Participants gave informed consent to the questionnaire, interview, and blood collection, and the studies were approved by the appropriate ethics review committees. BP was measured on the occasion when blood was collected, with the subjects sitting, with an automated BP recorder (Dinamap 845 Vital Signs Monitor; Critikon Inc). The mean of 2 results taken at 1-minute intervals was calculated.
Population BP Extremes (San Diego)
To test the effect of an ATP6N1A (chromosome 17q21) variant on BP in the population, we studied 1176 individuals of European (white) ancestry, selected from the top and bottom fifth percentiles of BP in a San Diego primary care population of >53 000 individuals.17 Subjects were ascertained by using DBP as the trait because twin and family studies provide evidence that DBP is substantially heritable.18–20 In the higher-BP group (systolic BP [SBP] = 154±0.7/DBP=99±0.3 mm Hg), there were 250 male and 285 female subjects; in the lower-BP group (SBP=108±0.6/DBP=56±0.2 mm Hg), there were 286 male and 355 female subjects. The mean age was 58±0.4 years, and the BP groups did not differ in age.
Laboratory Methods
Biochemical methods for plasma assays and DNA extraction are discussed in Methods in the online-only Data Supplement.
Statistical Methods
Heritability
Estimation of twin-pair correlations, by zygosity and by geographic location, was performed as an initial step. Catestatin results were log-transformed to reduce skewness in the frequency distribution. Models of sources of variation, initially including additive genetic variance (A), shared environmental effects (C), and nonshared environmental effects (E), were fitted to the data with the use of a structural equation modeling by Mx 1.57 (http://www.vcu.edu/mx/), and A and C effects were dropped in turn to test whether they could be omitted without significant effect.
Linkage
Genotyping data were collected from a ≈5-centimorgan (cM) (710 to 780 microsatellite) genome scan done by the Mammalian Genotyping Service of the National Heart, Lung, and Blood Institute, Marshfield, Wis, for the San Diego subjects and from this and other sources described by Cornes et al21 for the Australians. The genotype data were combined and checked for mendelian consistencies,22 and sib-pair linkage analysis was performed with MERLIN variance components, as described by Abecasis et al.23 We applied winsorization to reduce the impact of outliers on the linkage analysis24; this was done by setting values for all residuals >3 times the SD above or below the (log-transformed) mean to values equivalent to 3 times the SD above or below the mean, respectively. Linkage analysis, as well as the results of simulations to determine the genome-wide empirical probability value, is reported for the winsorized dataset. Ninety-six California MZ pairs and 23 Australia MZ pairs were included in MERLIN linkage; because MZ pairs are genetically identical, MERLIN computes a trait mean for the pair and considers that MZ pair to be a single individual within a sibship.
Empirical Genome-Wide Thresholds
Empirical genome-wide suggestive and significant thresholds were calculated in MERLIN through the use of 1000 simulations of marker data under the null hypothesis of no linkage to observed phenotypes, as described by Abecasis et al.25 The empirical genome-wide thresholds for suggestive or significant linkage were defined as the thresholds for which we observed on average 1 or 0.05 peak per simulation with a logarithm of odds (LOD) score greater than or equal to the threshold, respectively.
Allelic associations (SNP or microsatellite marker-on-trait) were performed with generalized estimating equations14 or the transmission disequilibrium test (QTDT) for quantitative traits26 (http://www.sph.umich.edu/csg/abecasis/QTDT/). Such tests account for the nonindependence of observations in relative pairs. Positional candidate loci within/beneath LOD peaks were selected on the basis of the physical positions (in Mb) of linked peak microsatellite markers, as outlined by Duffy (http://orpheus.qimr.edu.au/mapview/index.html). SNP genotyping at a positional candidate locus (ATP6N1, 3′-UTR T3246C, rs938671) was performed by the primer extension-based luminescent Pyrosequencing technique.27
Molecular Mechanism of Candidate Gene Effect on Sympathochromaffin Secretion
Methods to probe the role of vesicular acidification in CHGA and catestatin trafficking and processing, including a CHGA/embryonic alkaline phosphatase chimera, chromaffin cell transfection, H+-ATPase inhibition, and chimera exocytotic secretion, are discussed in Methods in the online-only Data Supplement.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Hypertensive Patients and Controls
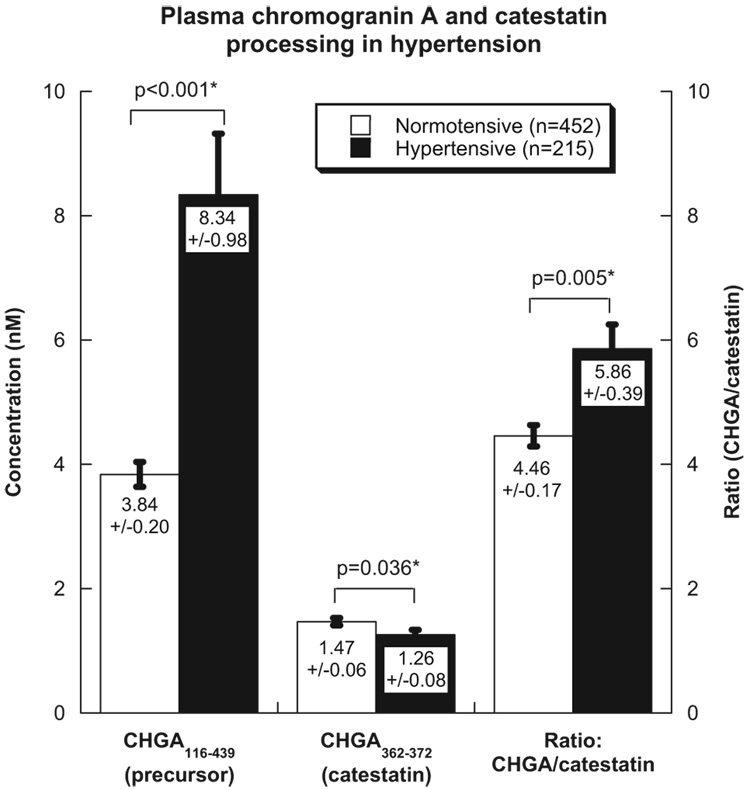
The CHGA precursor (epitope: CHGA116–439) and its catestatin product (epitope: CHGA361–372) were measured in a series of hypertensive cases and normotensive controls, as shown in Figure 1. There was an overall inverse correlation between precursor and product in plasma (Spearman ρ=−0.153, P=0.002), suggesting a spectrum of conversion of the CHGA precursor to its product in plasma (Figure I in the online-only Data Supplement). CHGA116–439 was increased by 117% (P<0.001) in hypertensive patients, whereas the catestatin fragment was reduced by 15% (P=0.036); the ratio of CHGA/catestatin was thus increased by 31% (P=0.005), implying decreased conversion of CHGA to its catecholamine release–inhibitory fragment in hypertension (Figure 1).
Figure 1.
Plasma CHGA and catestatin in hypertension. Each subject had normal renal function. CHGA was assayed by the CHGA116–439 epitope, and catestatin (CHGA352–372) was assayed by the CHGA361–372 epitope. Results in hypertensive patients (n=215) vs normotensive controls (n=452) are shown.
Twin and Sibling Pairs
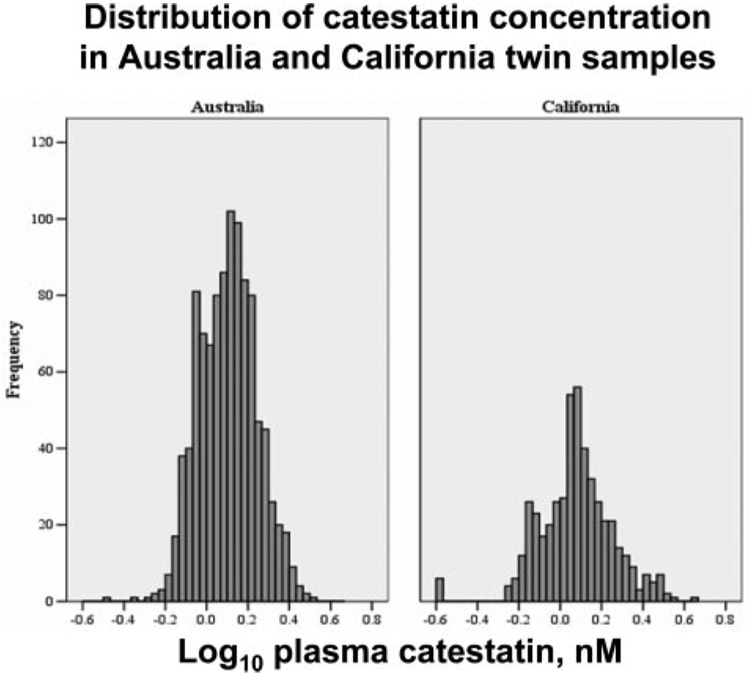
Catestatin results were obtained for 1966 samples from individuals ascertained by twinships, including 306 complete MZ and 639 DZ twin pairs. Demographic characteristics of the US and Australian participants are compared in Table 1. The Australian twins were 6 to 8 years older than the Californians. California subjects were somewhat more ethnically diverse than those in Australia. Both SBP and DBP were higher in male than in female subjects in both California and Australia. Catestatin was 8% to 15% higher in female than in male subjects in both the Australia (P<0.0001) and California (P=0.001) subjects. The plasma catestatin concentrations in the 2 samples are summarized in Table 1; the means and frequency distributions were similar in Australia and California (Figure 2). Catestatin correlations with BP were marginal (SBP r=0.093, DBP r=0.056), perhaps reflecting the limited range of BP values in these predominantly normotensive samples (Australia ≈95%, California ≈90%). Plasma and urine catecholamines were also measured in the California subjects (Table 1): Women exhibited decreased epinephrine in plasma (P<0.01) and urine (P<0.05) and decreased urinary norepinephrine (P<0.05).
Table 1.
Characteristics of the Australia and California Twin and Sibling Study Participants
| California | Australia | |
|---|---|---|
| N total | 519 | 1447 |
| n, men | 125 | 522 |
| n, women | 394 | 925 |
| Nuclear families | 230 | 540 |
| Age, y | ||
| Male | 36.2±14.8 | 44.6±11.4 |
| Female | 39.5±15.0 | 45.9±11.8 |
| Biogeographic ancestry | 74.9% European, 6.9% Hispanic, 4.6% black, 3.9% Asian, 9.3% mixed/other |
98.2% European, 1.8% Asian |
| Body mass index, kg/m2 | ||
| Male | 28.1±6.5 | 25.8±3.5 |
| Female | 26.2±6.2 | 25.2±4.7 |
| BP, mm Hg (systolic/diastolic) | ||
| Male | 135.6±12.8*/74.3±10.5* | 131.3±12.9†/80.7±10.1† |
| Female | 128.7±16.1/70.8±9.3 | 124.6±16.1/75.6±10.5 |
| CHGA361–372, nmol/L (catestatin) |
||
| Male | 1.17±0.47* | 1.26±0.35† |
| Female | 1.35±0.58 | 1.36±0.47 |
| CHGA116–439, nmol/L (precursor) |
||
| Male | 3.46±1.30 | 3.66±1.47† |
| Female | 3.68±1.60 | 3.99±1.82 |
| Plasma norepinephrine, pg/mL | ||
| Male | 355±26.8 (77) | … |
| Female | 330±15.9 (267) | |
| Plasma epinephrine, pg/mL |
||
| Male | 32.1±4.2 (77)‡ | … |
| Female | 18.6±1.3 (267) | |
| Urine norepinephrine, ng/g | ||
| Male | 32 447±3319 (77)§ | … |
| Female | 23 455±1519 (267) | |
| Urine epinephrine, ng/g | ||
| Male | 13 526±1337 (77)§ | … |
| Female | 9879±686 (267) |
Quantitative results are shown as mean±1 SD. Numbers in (parentheses) represent the No. of observations for that parameter, if less than the entire group.
US men<women
P≤0.001
P< 0.01
P< 0.05.
Australia men<women: P< 0.001.
Figure 2.
Distribution of plasma catestatin immunoreactivity in Australia vs California twins. Log10 results are shown to reduce the influence of outliers.
Genetic and Environmental Sources of Variation in Catestatin
Twin pair correlations (maximum likelihood estimates) for plasma catestatin are specified by zygosity type (MZ, DZ), sex, and geographic site in Table I in the online-only Data Supplement. In each case, MZ pair correlations were greater than corresponding DZ pair correlations, suggesting a genetic influence on the catestatin trait. Because there were ≈3 times as many Australia as California twins, sex-specific zygosity correlations are also presented for the Australia subjects.
Twin pair correlations allowed partitioning of trait variance into components (Figure 3) attributable to heredity (A, additive genetic variance) versus environment, further sub-partitioning environmental variance into shared (C) and unique (E, unshared) variance. In both the Australia and California twin samples, the catestatin trait model (Table II in the online-only Data Supplement) with best fit (lowest Akaike’s Information Criterion) to the twin data was the ACE model, and models not containing A were ruled out. The heritability estimate (A/[A+C+E] or h2) for catestatin in Australia twins was 60% compared with California at 44%, whereas E (unique environment) was similar in the 2 geographic sites (28% versus 27%), and C (shared environment) was apparently lower in Australia (11.3%) than California (28.8%) twins. Lower shared environment in Australia twins is consistent with their older age, by 6 to 8 years (Table 1). Although the catestatin mean was higher in female than in male subjects (Table 1), the sex limitation models did not support an effect of sex on heritability (component A) of catestatin.
Figure 3.
Elements of structural equation modeling in twin pairs for decomposition of trait determination. Boxes at the bottom represent measured variables in 2 twins within a twinship. Arrows represent causal pathways. Circles represent latent variables: the components of variance A (additive genetic variance), C (shared environmental variants), and E (unique, unshared environmental variance). Twin pair correlations are specified: genetic correlation (a), shared environmental correlation (c), and unique environmental correlation (e).
Linkage
Because the trait means and distributions were similar (Table 1, Figure 2), the Australia and California sib pairs were combined for sib-pair linkage analysis in 870 sib pairs. MERLIN23 simulations set empirical thresholds for evidence of linkage: Significant linkage (false discovery rate of 0.05, or 1 in 20 simulations per genome scan) was represented by LOD > 3.06, whereas suggestive linkage (false discovery rate of 1 per genome scan) occurred at LOD > 1.29.
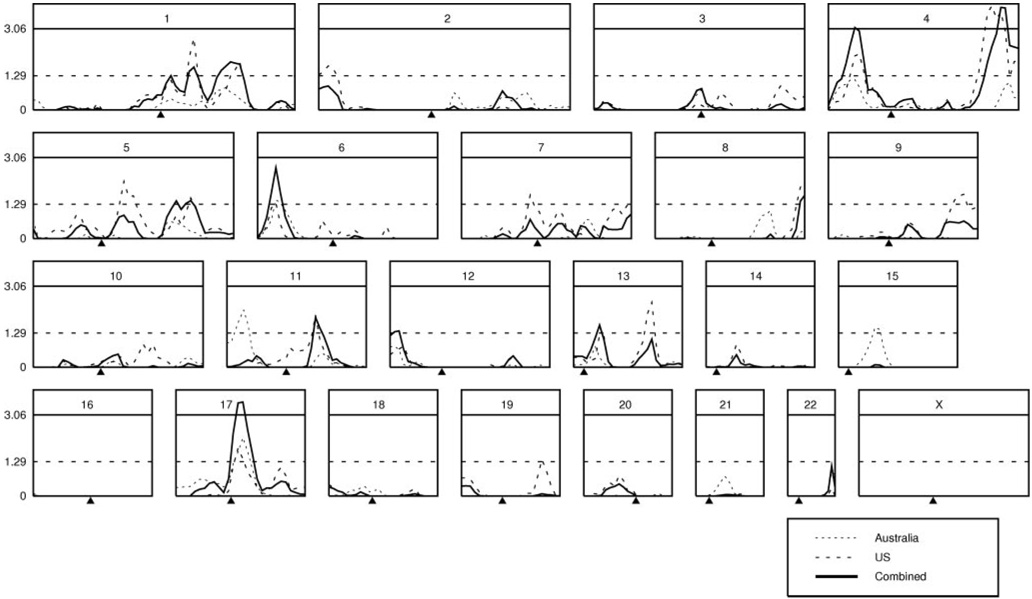
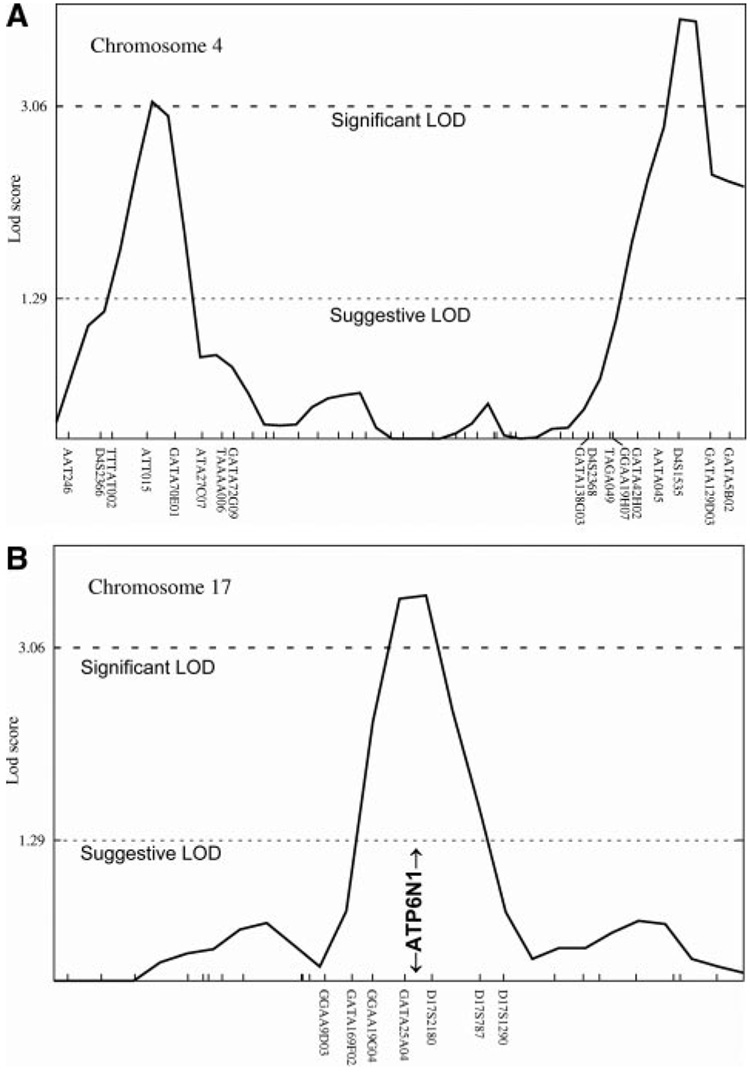
Three regions of the genome (Figure 4 and Figure 5; Table III in the online-only Data Supplement) displayed significant (LOD > 3.06) linkage to plasma catestatin concentration. These were on chromosome 4p (LOD=3.10 at 30 cM, in the region of ATT015), 4q (LOD=3.86 at 195 cM, in the region of D4S1535), and 17q (LOD=3.54 at 70 cM, in the region of D17S2180). Inspection of these LOD peaks (Figure 4) revealed generally additive results for the Australia and California twins, summing to significant LOD peaks (blue lines). Thus, the linkage regions seemed to be consistent across the twin samples. Catestatin-linked regions are shown in detail on chromosome 4 (Figure 5A) and chromosome 17 (Figure 5B).
Figure 4.
Catestatin sib-pair linkage across the genome. Results are plotted across each chromosome. Australia twin results are given in red, California twin results in green, and combined results in blue. Significant (LOD >3.06) and suggestive (LOD >1.29) thresholds are shown by horizontal lines.
Figure 5.
Individual chromosome plots for significant catestatin linkage. Significant (LOD >3.06) and suggestive (LOD >1.29) thresholds are shown by horizontal lines. A, Chromosome 4. The peak LOD scores are 3.10 on 4p at ≈30 cM and 3.86 on 4q at ≈195 cM. B, Chromosome 17. The peak LOD score is 3.54 on 17q at ≈70 cM. Note the location of positional candidate gene ATP6N1 at chromosome 17q21.
Because the most frequent biogeographic ancestry group in each sample (California and Australia) was European, we also performed linkage in European ancestry subjects only. Excluding non-Europeans from both the US and Australian samples did not result in new/significant LOD peaks; this exclusion reduces most linkage peaks but only in proportion to their numbers in the sample, with the obligate loss of power from diminished numbers of sib pairs. We conclude that any ethnic stratification has little effect on our linkage results.
To explore the potential impact of data winsorization (truncation of outliers), we repeated the linkage analysis for the combined sample using the log10-transformed trait catestatin values without winsorization. No new LOD peaks appeared when winsorization was removed. In all other cases, winsorization slightly reduced the linkage peaks or (as for the chromosome 17 peak) had no effect.
We also tested each microsatellite marker for allelic association to the catestatin trait, using the QTDT; there were no significant associations (all LOD scores <3.0).
Positional Candidate Locus (Trans-QTL) on Chromosome 17q: ATP6N1 Allelic Association With CHGA and Catestatin
The peak (LOD=3.54) markers on chromosome 17q (Figure 5B) bracket a candidate locus, ATP6N1 (ATP6N1A, ATP6V0A1, VPP1) (at 37.9 Mb on chromosome 17q21), which encodes a subunit of the vacuolar (V) H+-translocating ATPase, which acidifies the lumen of catecholamine storage vesicles, a process necessary for vesicle formation, as well as secretory protein trafficking into and proteolytic processing within the regulated secretory pathway.28,29
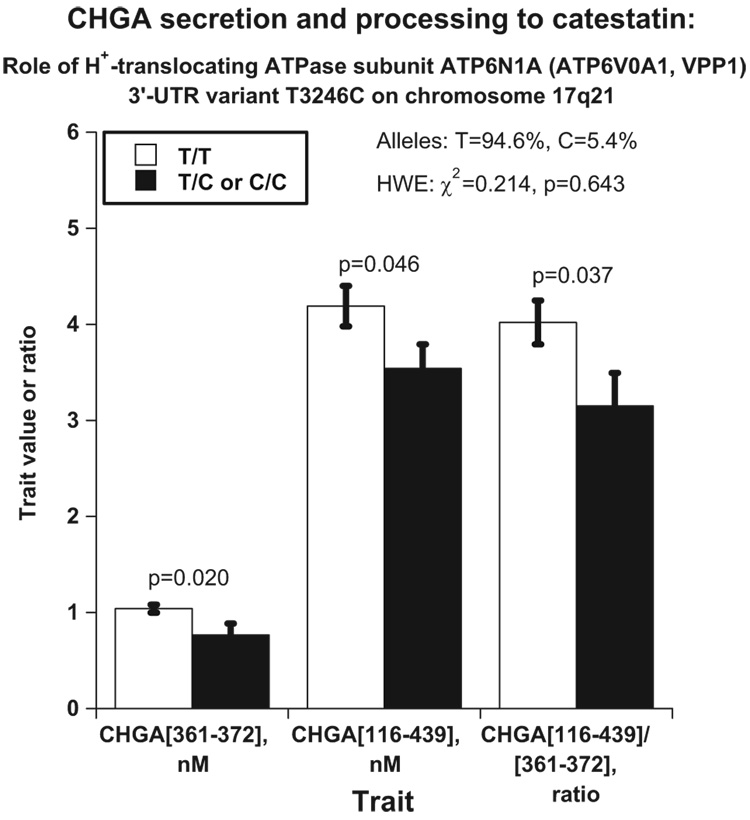
Only 1 common (minor allele frequency >5%) SNP within the transcript is currently known at ATP6N1: 3′-UTR (non-coding) variant T3246C (rs938671). In our population, C is the minor allele (at 5.4% frequency). We scored this SNP in the San Diego twins and sib pairs and tested its effect on sympathochromaffin traits (Figure 6): T3246C associated with the plasma concentration of catestatin (P=0.020), the catestatin precursor (P=0.046; epitope: CHGA116–439), and the processing of CHGA to catestatin, as the ratio CHGA/ catestatin (P=0.037). Presence of the minor (C) allele conferred decreased CHGA as well as decreased processing of CHGA to catestatin. To understand the catestatin processing (CHGA/catestatin ratio) trait, we tested its heritability, finding h2=0.700±0.047 (P=2.14e-18). Then we tested sib-pair linkage of CHGA/catestatin and found suggestive (>1.29) LOD scores, coinciding with the catestatin LOD peak (at 65 to 70 cM; Figure 5B), for CHGA/catestatin at 65 cM on chromosome 17 for the combined sample (LOD=1.11) as well as the San Diego sample (LOD=1.81). T3246C did not associate with BP in the predominantly (≈90%) normotensive San Diego twin sample.
Figure 6.
Effects of a positional candidate locus (trans-QTL) on chromosome 17q21: H+-translocating ATPase (ATP6N1) 3′-UTR variant T3246C (rs938671). CHGA and catestatin formation and release are shown. Results are shown for the plasma concentrations of catestatin, CHGA, or the ratio of CHGA/catestatin, as an index of proteolytic processing. Analyses in twin pairs were performed with the use of generalized estimating equations, establishing an exchangeable correlation matrix to account for intra–twin-pair correlations. HWE indicates Hardy-Weinberg equilibrium.
Combined linkage and association were undertaken to explore the magnitude of effect of the T3246C polymorphism at ATP6N1 on the catestatin trait. At T3246C, the magnitude of the linkage (LOD) peak (in MERLIN variance components) accounted for 27.9% of trait variability. By contrast, allelic association of T3246C with the catestatin trait (in QTDT) accounted for 0.29 nM (or 1.27%) of trait variance.
ATP6N1 Allelic Association With Hypertension
We scored ATP76N1 3′-UTR variant T3246C in 1176 subjects with the most extreme BP values in the population (Table 2). Genotype frequencies differed by the dichotomous trait of BP status: In a 2-by-2 contingency table (BP status by presence or absence of the minor allele), C allele carriers were less likely to have elevated BP than T/T homozygotes (Fisher exact/permutation test, P=0.037). We also conducted permutation tests on 3-by-2 contingency tables (diploid genotype by BP status); the association was significant (P=0.028). Carriers of the minor (C) allele had systolic BP values ≈5 mm Hg lower than major allele homozygotes (T/T; P=0.028). Age (P=0.001) and sex (P=0.002) also influenced SBP, although there was no interaction of genotype with either sex (P=0.636) or age (P=0.960). Genotype did not influence DBP (F=2.15, P=0.141).
Table 2.
ATP6N1 3′-UTR Variant T3246C (rs938671): Effect on BP in the Population
| Diploid Genotype | ||
|---|---|---|
| Group | T/T | T/C or C/C |
| Normotensive | 563 (87.8%) | 78 (12.2%) |
| Hypertensive | 488 (91.2%) | 47 (8.8%) |
Results are shown for 1176 individuals drawn from the top and bottom fifth percentiles of DBP in a primary care population of >53 000 individuals (see Methods). Percentage of each BP group with the indicated genotype is shown in parentheses. The BP groups differed in genotype frequencies (Fisher exact test, P=0.037).
H+-Translocating ATPase and Sympathochromaffin Secretion
To understand how variation at H+-translocating ATPase α subunit ATP6N1 might influence CHGA, we expressed CHGA/reporter chimera in chromaffin cells and then studied secretagogue-regulated versus constitutive secretion during inhibition of the vacuolar ATPase by graded concentrations of bafilomycin A1 (Figure II in the online-only Data Supplement). In the absence of bafilomycin, the exocytosis secretagogue (Ba2+) released far more CHGA than basal values, resulting in a sorting index of ≈1.25; selective ATPase inhibition (1 to 5 nmol/L bafilomycin) progressively reduced the sorting index to ≈0.6, indicating diversion of CHGA out of chromaffin granules and into the constitutive pathway.29
Discussion
Overview
CHGA is known to be important in the physiological control of catecholamine release and BP,1 and the catestatin mechanism is altered in patients with hypertension.5 Because systemic hypertension is a trait with partial determination by heredity,6,7,30 in the present study we probed genetic contributions to the chromogranin traits in humans.
The inverse correlation of plasma catestatin and its CHGA precursor (Figure I in the online-only Data Supplement) confirms previous observations12 and likely reflects processing of the prohormone to its active catestatin fragment in plasma. We further explored the precursor and its fragment in subjects stratified by hypertension (Figure 1) and found evidence for diminished processing in hypertension. To better understand the role of heredity in the formation of catestatin, we then turned to the classic human twin pair design.10
We found that catestatin heritability is significant in both Australia and California twins (Table II in the online-only Data Supplement) and then positioned 3 novel genetic loci (on chromosomes 4p, 4q, and 17q) that contribute significantly to the catestatin trait (Table III in the online-only Data Supplement; Figure 4 and Figure 5). Because CHGA is located on human chromosome 14q32, the new loci represent trans-QTLs.
We then studied a positional candidate gene located centrally beneath the LOD peak on chromosome 17q, ATP6N1 (encoding an ATPase subunit that acidifies secretory vesicles) and found that a conserved 3′-UTR variant associated with not only plasma CHGA but also BP in the population (Table 2).
Linkage, Association, and Positional Candidate Loci: Chromosomes 4p, 4q, and 17q
Having established significant heritability for plasma catestatin concentration and identified chromosomal regions that may affect it, we needed to proceed to identifying genes and ultimately causative alleles. An initial step was to consider candidate genes located close to the significant linkage peaks. Inspection of the linked genomic regions on chromosomes 4 and 17 (Figure 5A and 5B) revealed several loci that could be construed to be in the physiological pathway toward catestatin biosynthesis, release, or actions.
Chromosome 17q: ATP6N1
At the conclusion of linkage and inspection of linked regions for positional candidate loci, we noted a locus directly beneath a LOD peak that encoded a segment of the biochemical/ physiological pathway determining CHGA trafficking and its processing to catestatin. On chromosome 17q21, bracketed by the peak LOD markers (Figure 5B), lies ATP6N1 (otherwise known as ATP6N1A, ATP6V0A1, or VPP1) at 37.9 Mb, an α subunit of the vacuolar (V) H+-translocating ATPase heteromultimeric complex, responsible for acidification of the secretory pathway compartment, including secretory granules (such as chromaffin granules) and the Golgi apparatus.28,31,32
ATP6N1 3′-UTR variant T3246C is associated with plasma catestatin, the catestatin precursor (CHGA), and CHGA-to-catestatin processing (Figure 6). ATP6N1 is an integral membrane protein in the H+-translocating domain of the transporter. Secretory vesicular acidification plays a necessary role in correct trafficking into the regulated pathway and proteolytic processing of secretory proteins such as CHGA29 (Figure 7). Thus, alterations in the amount of ATP6N1 would be expected to change the trafficking, secretion, and cleavage of CHGA to catestatin and indeed the ability of the sympathochromaffin system to form catecholamine storage vesicles29 (Figure 7). Although the local genomic region around ATP6N1 3′-UTR variant T3246C is well conserved in primates (Table IV in the online-only Data Supplement), the variant position is absent by virtue of a local microdeletion in rodents (rats and mice). Mechanistic studies of the functional consequences of this 3′-UTR variant remain to be done; indeed, given the broad confidence interval for linkage (Figure 5B), other (perhaps as yet undiscovered) causative genetic variants within ATP6N1, which spans ≈36 kbp, might be in sufficient linkage disequilibrium with T3246C to account for the marker-on-trait association we observed (Figure 6A).
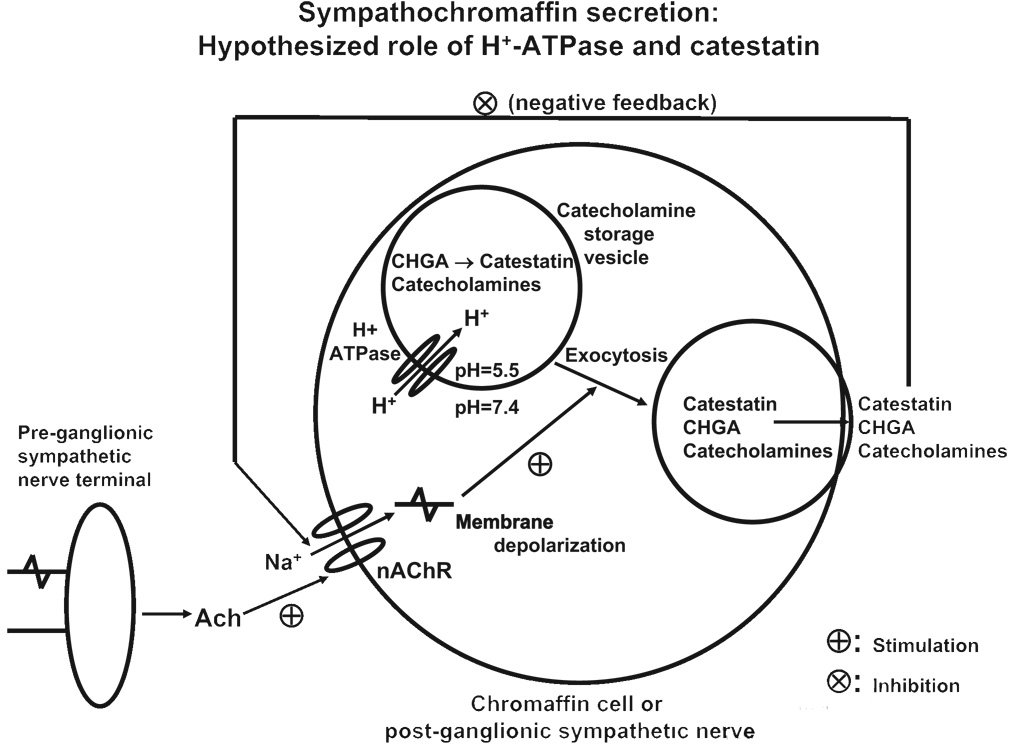
Figure 7.
Hypothetical schema to account for the genetic and secretory observations in this study. The H+-translocating (V) ATPase acidifies the core of the secretory vesicle (chromaffin granule), enabling correct trafficking and proteolytic cleavage of regulated secretory proteins, such as CHGA. CHGA is proteolytically cleaved to catestatin, which is released on nicotinic cholinergic stimulation of the chromaffin cell, after which it may function in autocrine, negative feedback fashion, as an endogenous nicotinic cholinergic antagonist, to limit further secretion. Ach indicates acetylcholine; AChR, nicotinic cholinergic receptor.
Because CHGA and its catestatin fragment are central to BP regulation,1 we then analyzed the effect of the ATP6N1 3′-UTR variant on the most extreme BPs in the population (Table 2) and found that the C (minor) allele carriers were less likely to have hypertension than major allele (T/T) homozygotes (P=0.037). Although this variant influenced mainly SBP (P=0.028), emerging evidence suggests that SBP is an independent and perhaps even more powerful risk factor for hypertensive target-organ damage.33 The T3246C minor (C) allele that reduced CHGA (Figure 6A) also reduced risk of developing hypertension (Table 2); this finding is coordinate with the elevation in CHGA observed in hypertension (Figure 1). Because the C allele reduced CHGA, catestatin, and CHGA/catestatin ratio, the effect of 3246C on BP might be through overall sympathochromaffin exocytosis rather than specifically on CHGA processing to catestatin.
To explore the effect of the H+-ATPase on CHGA secretion, we turned to cultured chromaffin cells and the release of a readily quantified transfectable CHGA chimera (CHGA-EAP) that is ordinarily trafficked to chromaffin granules of the regulated secretory pathway.29 Acute exposure to even nanomolar concentrations of the selective H+-ATPase inhibitor bafilomycin caused a ≈50% decline in sorting index, indicating progressive diversion of CHGA from the secretagogue-regulated pathway (ie, chromaffin granules) into the constitutive (unregulated, continuous) pathway (Figure II in the online-only Data Supplement). Thus, basal/ unregulated secretion of CHGA increases, coupled with unregulated secretion of other transmitters, such as catecholamines. 29 The systemic consequences of a decline in chromaffin granule acidification might be not only unregulated CHGA secretion but also dysregulated storage and release of other transmitters.
Other Linked Chromosomal Regions
On chromosome 4q is SNX25, a sorting nexin that may be involved in vesicular traffic.34 Also on chromosome 4q is the enzyme carboxypeptidase E (CPE), which cleaves carboxy-terminal basic residues from neuropeptides (such as catestatin) after endoproteolytic cleavage of prohormone precursors including CHGA.35 RAB11FIP4 (RAB11 family interacting protein 4) lies on chromosome 17q; it is a lowmolecular-weight GTP-binding protein interacting protein that may be involved in the transport of secretory vesicles.36 Chromosome 17q also harbors RAPGEFL1, a guanine nucleotide exchange factor mediating signal transduction by G protein–coupled receptors. CHGA transcription and exocytotic secretion are controlled by the neuropeptide PACAP and its G protein–coupled receptor PAC1 on chromaffin cells.37,38
Notably, several genes pertinent to the physiological pathway for catestatin biosynthesis and action are not in the catestatin-linked genomic regions on chromosomes 4 and 17 identified by this study. CHGA, the potential cis-QTL, is on chromosome 14q32. Likely proteolytic processing enzymes for CHGA to catestatin include cathepsin L (CTSL)39 on chromosome 9q21, the prohormone convertases PCSK1 (chromosome 5q15) and PCSK2 (chromosome 20p11), and FUR (furin, on chromosome 15q25). Catestatin targets include several neuronal/autonomic nicotinic cholinergic receptor α or β subunits40 encoded by genes outside the catestatin-linked genomic regions on chromosome 4 and 17 (Figure 5A and 5B): the CHRNA3/CHRNA5/CHRNB4 autonomic nicotinic cluster on chromosome 15q24, CHRNA4 on chromosome 20q13, CHRNB2 on chromosome 1q21, and CHRNA7 on chromosome 15q14.
Lack of significant microsatellite allelic associations (microsatellite marker-on-catestatin trait) is perhaps not surprising given the microsatellite linkage (meiotic cosegregation) marker spacing of ≈5 cM (corresponding, on average, to ≈5 Mb); linkage disequilibrium underpinning marker-on-trait association is entirely dependent on the marker-on-marker linkage disequilibrium structure of the local genomic region; because the typical extent of linkage disequilibrium is unlikely to exceed ≈30 to 50 kbp even in populations of predominantly European ancestry, marker-on-trait linkage disequilibrium at ≈5 Mb spacing can be expected to be effective over at best ≈50 kbp/5000 kbp or ≈1% of the genomic region beneath any given linkage LOD peak, and then only in the region of the peak microsatellite marker. To span candidate genes contained within the confidence interval for linkage, additional marker studies will be required beneath the LOD peaks on chromosomes 4 and 17 (Figure 5A and 5B).
Limitations of the Study
Heritability
Heritability can be estimated from several kinds of relative pair correlations,41 including twin or parent/offspring pairs. Twin pair correlations may give rise to higher heritability estimates than parent/offspring pairs; such differences may arise from increased lifestyle sharing in MZ versus DZ twins, inflated estimates of dominance effects, or age-specific genetic effects that are underestimated by the age differences in parent/offspring pairs.42
Causative Genetic Variation
Because the linkage on chromosome 17q (Figure 5B) explained 27.9% of catestatin trait variation, whereas association with ATP6N1 T3246C accounted for 1.27% of trait variance, there must be additional trait-associated variants within ATP6N1 or other loci beneath the confidence interval for catestatin linkage on chromosome 17q21. By selective inhibition of the vesicular H+-ATPase (Figure I in the online-only Data Supplement), we demonstrated that chromaffin vesicular acidification is required for trafficking of CHGA into the regulated secretory pathway. However, even though variant T3246C is located within a conserved (across species) region of the ATP6N1 3′-UTR (Table IV in the online-only Data Supplement), we have not yet provided experimental evidence of the functional significance of this polymorphism.
Conclusions and Perspectives
The complex trait of hypertension has multiple determinants, both hereditary and environmental. The studies reported here establish the heritable determination of an autonomic trait (catestatin) contributing to BP and point to 3 novel genomic regions that may harbor trait loci influencing sympathetic function and, ultimately, BP. The allelic association of a common 3′-untranslated variant T3246C at ATP6N1, a positional candidate locus on chromosome 17q21, with CHGA release (Figure 6A) and, ultimately, systemic BP (Table 2) suggests a mechanism (Figure 7) whereby common genetic variation on chromosome 17 may contribute to interindividual differences in storage and release of CHGA, a central control point in autonomic function. Future studies will explore precisely how genetic variation in this region affects sympathochromaffin exocytosis.
CLINICAL PERSPECTIVE
Chromogranin A (CHGA) plays a necessary role in the formation of catecholamine secretory granules, where it is cleaved to catestatin, an inhibitor of catecholamine release. Catestatin circulates in human plasma and may be an intermediate phenotype in analysis of genetic risk for cardiovascular disease. In the present investigation, we first observed that CHGA is elevated and its processing to catestatin is diminished in hypertension. To approach genetic control of catestatin in the population, we used a human twin pair design. Studies of monozygotic and dizygotic twins allow estimation of the contribution of genetic variation to any trait (as heritability, or h2), and dizygotic or sibling pairs allow genome-wide approaches such as microsatellite linkage scanning to identify previously unsuspected loci influencing traits of interest. Our results indicate that the circulating catestatin concentration has substantial heritability and that novel genetic loci on chromosomes 4p, 4q, and 17q contribute significantly to common interindividual variation in expression, secretion, or enzymatic formation of this biologically active peptide. At the ATP6N1 (H+-ATPase alpha subunit) positional candidate locus on chromosome 17q, a common 3′-UTR variant predicted plasma CHGA, CHGA-to-catestatin processing, and finally, systolic blood pressure in the population. Thus, ATP6N1 represents a novel trans-QTL for sympathochromaffin activity and, ultimately, blood pressure.
Supplementary Material
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/cgi/content/full/CIRCULATIONAHA.107.709105/DC1.
Acknowledgments
Sources of Funding
This work was supported by grants from the National Institutes of Health and the Department of Veterans Affairs. We appreciate the assistance of the National Institutes of Health/National Center for Research Resources–supported General Clinical Research Center (RR00827) and the National Institutes of Health/National Center on Minority Health and Health Disparities EXPORT minority health center (MD000220).
References
- 1.Mahapatra NR, O’Connor DT, Vaingankar SM, Hikim AP, Mahata M, Ray S, Staite E, Wu H, Gu Y, Dalton N, Kennedy BP, Ziegler MG, Ross J, Mahata SK. Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. J Clin Invest. 2005;115:1942–1952. doi: 10.1172/JCI24354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takiyyuddin MA, Cervenka JH, Sullivan PA, Pandian MR, Parmer RJ, Barbosa JA, O’Connor DT. Is physiologic sympathoadrenal catecholamine release exocytotic in humans? Circulation. 1990;81:185–195. doi: 10.1161/01.cir.81.1.185. [DOI] [PubMed] [Google Scholar]
- 3.Mahata SK, O’Connor DT, Mahata M, Yoo SH, Taupenot L, Wu H, Gill BM, Parmer RJ. Novel autocrine feedback control of catecholamine release: a discrete chromogranin a fragment is a noncompetitive nicotinic cholinergic antagonist. J Clin Invest. 1997;100:1623–1633. doi: 10.1172/JCI119686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahapatra NR, Mahata M, Mahata SK, O’Connor DT. The chromogranin A fragment catestatin: specificity, potency and mechanism to inhibit exocytotic secretion of multiple catecholamine storage vesicle co-transmitters. J Hypertens. 2006;24:895–904. doi: 10.1097/01.hjh.0000222760.99852.e0. [DOI] [PubMed] [Google Scholar]
- 5.O’Connor DT, Kailasam MT, Kennedy BP, Ziegler MG, Yanaihara N, Parmer RJ. Early decline in the catecholamine release-inhibitory peptide catestatin in humans at genetic risk of hypertension. J Hypertens. 2002;20:1335–1345. doi: 10.1097/00004872-200207000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Lillie EO, O’Connor DT. Early phenotypic changes in hypertension: a role for the autonomic nervous system and heredity. Hypertension. 2006;47:331–333. doi: 10.1161/01.HYP.0000203980.44717.aa. [DOI] [PubMed] [Google Scholar]
- 7.O’Connor DT, Insel PA, Ziegler MG, Hook VY, Smith DW, Hamilton BA, Taylor PW, Parmer RJ. Heredity and the autonomic nervous system in human hypertension. Curr Hypertens Rep. 2000;2:16–22. doi: 10.1007/s11906-000-0053-8. [DOI] [PubMed] [Google Scholar]
- 8.Mahata SK, Mahata M, Wen G, Wong WB, Mahapatra NR, Hamilton BA, O’Connor DT. The catecholamine release-inhibitory “catestatin” fragment of chromogranin A: naturally occurring human variants with different potencies for multiple chromaffin cell nicotinic cholinergic responses. Mol Pharmacol. 2004;66:1180–1191. doi: 10.1124/mol.104.002139. [DOI] [PubMed] [Google Scholar]
- 9.Lander ES, Schork NJ. Genetic dissection of complex traits. Science. 1994;265:2037–2048. doi: 10.1126/science.8091226. [DOI] [PubMed] [Google Scholar]
- 10.Boomsma D, Busjahn A, Peltonen L. Classical twin studies and beyond. Nat Rev Genet. 2002;3:872–882. doi: 10.1038/nrg932. [DOI] [PubMed] [Google Scholar]
- 11.Brinton TJ, Kailasam MT, Wu RA, Cervenka JH, Chio SS, Parmer RJ, DeMaria AN, O’Connor DT. Arterial compliance by cuff sphygmomanometer: application to hypertension and early changes in subjects at genetic risk. Hypertension. 1996;28:599–603. doi: 10.1161/01.hyp.28.4.599. [DOI] [PubMed] [Google Scholar]
- 12.Stridsberg M, Eriksson B, Oberg K, Janson ET. A panel of 11 region-specific radioimmunoassays for measurements of human chromogranin A. Regul Pept. 2004;117:219–227. doi: 10.1016/j.regpep.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 13.Seasholtz TM, Wessel J, Rao F, Rana BK, Khandrika S, Kennedy BP, Lillie EO, Ziegler MG, Smith DW, Schork NJ, Brown JH, O’Connor DT. Rho kinase polymorphism influences blood pressure and systemic vascular resistance in human twins: role of heredity. Hypertension. 2006;47:937–947. doi: 10.1161/01.HYP.0000217364.45622.f0. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Rao F, Wessel J, Kennedy BP, Rana BK, Taupenot L, Lillie EO, Cockburn M, Schork NJ, Ziegler MG, O’Connor DT. Functional allelic heterogeneity and pleiotropy of a repeat polymorphism in tyrosine hydroxylase: prediction of catecholamines and response to stress in twins. Physiol Genomics. 2004;19:277–291. doi: 10.1152/physiolgenomics.00151.2004. [DOI] [PubMed] [Google Scholar]
- 15.Cockburn M, Hamilton A, Zadnick J, Cozen W, Mack TM. The occurrence of chronic disease and other conditions in a large population-based cohort of native Californian twins. Twin Res. 2002;5:460–467. doi: 10.1375/136905202320906282. [DOI] [PubMed] [Google Scholar]
- 16.Whitfield JB, Fletcher LM, Murphy TL, Powell LW, Halliday J, Heath AC, Martin NG. Smoking, obesity, and hypertension alter the dose-response curve and test sensitivity of carbohydrate-deficient transferrin as a marker of alcohol intake. Clin Chem. 1998;44:2480–2489. [PubMed] [Google Scholar]
- 17.Rana BK, Insel PA, Payne SH, Abel K, Beutler E, Ziegler MG, Schork NJ, O’Connor DT. Population-based sample reveals gene-gender inter-actions in blood pressure in White Americans. Hypertension. 2007;49:96–106. doi: 10.1161/01.HYP.0000252029.35106.67. [DOI] [PubMed] [Google Scholar]
- 18.Evans A, Van Baal GC, McCarron P, DeLange M, Soerensen TI, De Geus EJ, Kyvik K, Pedersen NL, Spector TD, Andrew T, Patterson C, Whitfield JB, Zhu G, Martin NG, Kaprio J, Boomsma DI. The genetics of coronary heart disease: the contribution of twin studies. Twin Res. 2003;6:432–441. doi: 10.1375/136905203770326439. [DOI] [PubMed] [Google Scholar]
- 19.Kupper N, Willemsen G, Riese H, Posthuma D, Boomsma DI, de Geus EJ. Heritability of daytime ambulatory blood pressure in an extended twin design. Hypertension. 2005;45:80–85. doi: 10.1161/01.HYP.0000149952.84391.54. [DOI] [PubMed] [Google Scholar]
- 20.Snieder H, Harshfield GA, Treiber FA. Heritability of blood pressure and hemodynamics in African- and European-American youth. Hypertension. 2003;41:1196–1201. doi: 10.1161/01.HYP.0000072269.19820.0D. [DOI] [PubMed] [Google Scholar]
- 21.Cornes BK, Medland SE, Ferreira MA, Morley KI, Duffy DL, Heijmans BT, Montgomery GW, Martin NG. Sex-limited genome-wide linkage scan for body mass index in an unselected sample of 933 Australian twin families. Twin Res Hum Genet. 2005;8:616–632. [PubMed] [Google Scholar]
- 22.Abecasis GR, Cherny SS, Cookson WO, Cardon LR. GRR: graphical representation of relationship errors. Bioinformatics. 2001;17:742–743. doi: 10.1093/bioinformatics/17.8.742. [DOI] [PubMed] [Google Scholar]
- 23.Abecasis GR, Cherny SS, Cookson WO, Cardon LR. MERLIN: rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet. 2002;30:97–101. doi: 10.1038/ng786. [DOI] [PubMed] [Google Scholar]
- 24.Shete S, Beasley TM, Etzel CJ, Fernandez JR, Chen J, Allison DB, Amos CI. Effect of winsorization on power and type 1 error of variance components and related methods of QTL detection. Behav Genet. 2004;34:153–159. doi: 10.1023/B:BEGE.0000013729.26354.da. [DOI] [PubMed] [Google Scholar]
- 25.Abecasis GR, Burt RA, Hall D, Bochum S, Doheny KF, Lundy SL, Torrington M, Roos JL, Gogos JA, Karayiorgou M. Genomewide scan in families with schizophrenia from the founder population of Afrikaners reveals evidence for linkage and uniparental disomy on chromosome 1. Am J Hum Genet. 2004;74:403–417. doi: 10.1086/381713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abecasis GR, Cardon LR, Cookson WO. A general test of association for quantitative traits in nuclear families. Am J Hum Genet. 2000;66:279–292. doi: 10.1086/302698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmadian A, Ehn M, Hober S. Pyrosequencing: history, biochemistry and future. Clin Chim Acta. 2006;363:83–94. doi: 10.1016/j.cccn.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 28.Morel N. Neurotransmitter release: the dark side of the vacuolar-H + ATPase. Biol Cell. 2003;95:453–457. doi: 10.1016/s0248-4900(03)00075-3. [DOI] [PubMed] [Google Scholar]
- 29.Taupenot L, Harper KL, O’Connor DT. Role of H+-ATPase-mediated acidification in sorting and release of the regulated secretory protein chromogranin A: evidence for a vesiculogenic function. J Biol Chem. 2005;280:3885–3897. doi: 10.1074/jbc.M408197200. [DOI] [PubMed] [Google Scholar]
- 30.Hamilton M, Pickering GW, Roberts JA, Sowry GS. Arterial pressures of relatives of patients with secondary and malignant hypertension. Clin Sci. 1963;24:91–108. [PubMed] [Google Scholar]
- 31.Sun-Wada GH, Wada Y, Futai M. Diverse and essential roles of mammalian vacuolar-type proton pump ATPase: toward the physiological understanding of inside acidic compartments. Biochim Biophys Acta. 2004;1658:106–114. doi: 10.1016/j.bbabio.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 32.Beyenbach KW, Wieczorek H. The V-type H+ ATPase: molecular structure and function, physiological roles and regulation. J Exp Biol. 2006;209:577–589. doi: 10.1242/jeb.02014. [DOI] [PubMed] [Google Scholar]
- 33.Franklin SS, Khan SA, Wong ND, Larson MG, Levy D. Is pulse pressure useful in predicting risk for coronary heart Disease? The Framingham Heart Study. Circulation. 1999;100:354–360. doi: 10.1161/01.cir.100.4.354. [DOI] [PubMed] [Google Scholar]
- 34.Worby CA, Dixon JE. Sorting out the cellular functions of sorting nexins. Nat Rev Mol Cell Biol. 2002;3:919–931. doi: 10.1038/nrm974. [DOI] [PubMed] [Google Scholar]
- 35.Che FY, Yan L, Li H, Mzhavia N, Devi LA, Fricker LD. Identification of peptides from brain and pituitary of Cpe(fat)/Cpe(fat) mice. Proc Natl Acad Sci U S A. 2001;98:9971–9976. doi: 10.1073/pnas.161542198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallace DM, Lindsay AJ, Hendrick AG, McCaffrey MW. Rab11-FIP4 interacts with Rab11 in a GTP-dependent manner and its overexpression condenses the Rab11 positive compartment in HeLa cells. Biochem Biophys Res Commun. 2002;299:770–779. doi: 10.1016/s0006-291x(02)02720-1. [DOI] [PubMed] [Google Scholar]
- 37.Taupenot L, Mahata SK, Wu H, O’Connor DT. Peptidergic activation of transcription and secretion in chromaffin cells: cis and trans signaling determinants of pituitary adenylyl cyclase-activating polypeptide (PACAP) J Clin Invest. 1998;101:863–876. doi: 10.1172/JCI1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taupenot L, Mahata M, Mahata SK, O’Connor DT. Time-dependent effects of the neuropeptide PACAP on catecholamine secretion: stimulation and desensitization. Hypertension. 1999;34:1152–1162. doi: 10.1161/01.hyp.34.5.1152. [DOI] [PubMed] [Google Scholar]
- 39.Lee JC, Taylor CV, Gaucher SP, Toneff T, Taupenot L, Yasothornsrikul S, Mahata SK, Sei C, Parmer RJ, Neveu JM, Lane WS, Gibson BW, O’Connor DT, Hook VY. Primary sequence characterization of catestatin intermediates and peptides defines proteolytic cleavage sites utilized for converting chromogranin A into active catestatin secreted from neuroendocrine chromaffin cells. Biochemistry. 2003;42:6938–6946. doi: 10.1021/bi0300433. [DOI] [PubMed] [Google Scholar]
- 40.Herrero CJ, Ales E, Pintado AJ, Lopez MG, Garcia-Palomero E, Mahata SK, O’Connor DT, Garcia AG, Montiel C. Modulatory mechanism of the endogenous peptide catestatin on neuronal nicotinic acetylcholine receptors and exocytosis. J Neurosci. 2002;22:377–388. doi: 10.1523/JNEUROSCI.22-02-00377.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Falconer DS, Mackay TFC. Introduction to Quantitative Genetics. London, UK: Harlow; 1996. [Google Scholar]
- 42.Tambs K, Eaves LJ, Moum T, Holmen J, Neale MC, Naess S, Lund-Larsen PG. Age-specific genetic effects for blood pressure. Hypertension. 1993;22:789–795. doi: 10.1161/01.hyp.22.5.789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/cgi/content/full/CIRCULATIONAHA.107.709105/DC1.