Abstract
In this letter, we report a facile method to prepare robust phospholipid vesicles using commonly available phospholipids that are stabilized via formation of an interpenetrating, acid-labile, cross-linked polymer network, that imparts a site for controlled polymer destabilization and subsequent vesicle degradation. The polymer network was formed in the inner lamella of the phospholipid bilayer using 2-2-Di(methacryloyloxy-1-ethoxy)propane (DMOEP) and butyl methacrylate (BMA). Upon exposure to acidic conditions the highly crosslinked polymer network was partially converted to smaller linear polymers, resulting in substantially reduced vesicle stability upon exposure to chemical and physical insults. Isolated polymers showed a pH-dependent solubility in THF. Transmission electron microscopy, and dynamic light scattering showed time dependent enhanced vesicle stability in high concentrations of surfactant and vacuum conditions at elevated pH, whereas exposure to acidic pH rapidly decreased the vesicle stability, with complete destabilization observed in less than 24 hours. The resultant transiently stabilized vesicles may prove useful for enhanced drug delivery and chemical sensing applications and allow for improved physiological clearance.
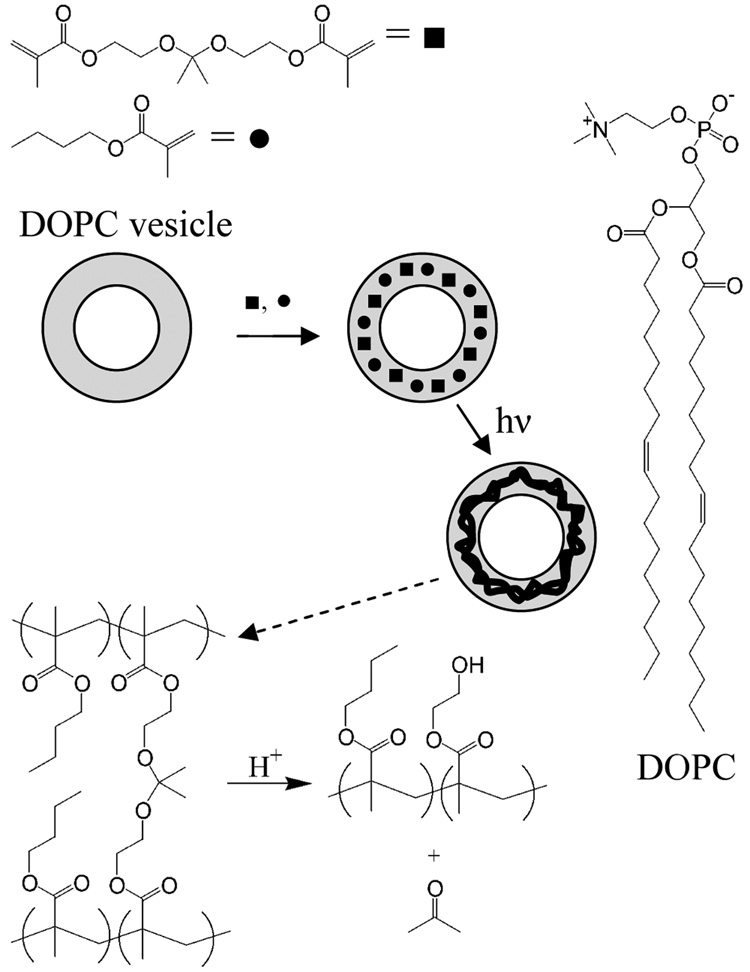
Phospholipid vesicles are routinely used for intracellular delivery, via fusion with the cell membrane and subsequent release of encapsulated cargo. While useful, vesicle delivery systems suffer from a series of physical limitations, including long-term stability and fusion with other substrates, resulting in inefficient cellular delivery of cargo. Transient stabilization of the phospholipid vesicle membrane may provide for better spatial and temporal control of cargo delivery and release. Whereas, vesicles prepared from naturally-occurring phospholipids offer limited stability in harsh chemical and biological environments, stabilization of the vesicle architecture allows many key advantages to be realized.1–6 Vesicle stabilization has been achieved via several approaches, including: a) utilizing synthetic, polymerizable phospholipids, and b) polymer scaffolding - partitioning hydrophobic monomers into the vesicle lamella with subsequent polymerization.1;4;6;7 The resulting stabilized vesicles can be loaded across cellular membranes intact, however, the irreversible stability severely limits cargo release, as well as physiological clearance. Chemically and mechanically robust vesicles that can be controllably destabilized under biologically relevant conditions are more desirable. In this letter, we report a facile method to prepare robust phospholipid vesicles using commonly available phospholipids that are stabilized via formation of an interpenetrating, acid-labile, cross-linked polymer network, that can be controllably destabilized and subsequently degraded.
Stabilized 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) vesicles were prepared by fabricating an acid-cleavable, highly cross-linked copolymer within the hydrophobic lamella of the pre-organized vesicle assembly using dimethacrylate monomer (DMOEP) and butylmethacrylate (BMA) (Scheme I). The inclusion of acid-cleavable acetal moieties within these highly cross-linked networks enables degradation into smaller linear polymers in acidic environments that may be encountered in biological systems, e.g. lysosomes, stomach, or tumors, in a manner analogous to acid-degradable acrylamide and methacrylate-based polymers.8–15 Similar to these previous reports, the lifetime and pH-sensitivity of the cross-linked polymer should be readily tailored to specific biological and chemical environments via modulation of the functionalities on the acetal carbon.11
SCHEME I. Formation and Degradation of Stabilized Vesicles.
Hydrophobic monomers partition into the pre-assembled vesicle bilayer and are polymerized via UV photoirradiation. Upon exposure to acidic media, the highly cross-linked polymer backbone is degraded, allowing chemical or physical disassembly of the vesicle structure.
Vesicle preparation is described in detail in the Supporting Information. Briefly, 2-2-Di(methacryloyloxy-1-ethoxy)propane (DMOEP) was synthesized in a modified one step procedure, and utilized as the acid-cleavable cross-linking agent (Supporting Information).10;11;13 Phospholipid vesicles (nominal diameter = 200 nm) were prepared using freeze-thaw/extrusion methods with DOPC (Supporting Information). The photoinitiator Irgacure 907 (1 µmol), DMOEP (1 µmol), and BMA (1.2 µmol) were added to 1 mL vesicle suspension (10 mg/mL DOPC) then allowed to partition into the hydrophobic lamella of the vesicles overnight, and polymerized via UV irradiation for 30 min (Scheme I). In all cases, vesicles were prepared physiological buffer mimics containing 10 mM phosphate or citrate buffer with 137 mM NaCl and 2.7 mM KCl.
To qualitatively determine the pH-dependent stabilization of the DOPC/BMA/DMOEP polymer-stabilized vesicles, as well as ascertain a rough estimate of polymer molecular weight, a series of solubility experiments were performed. Figure 1 presents representative data obtained from three experiments. For these experiments, polymer-stabilized vesicles were prepared and stored at either pH 2.0 or 7.4 overnight. The polymer was then separated from the lipid via methanol/THF washes and stored in THF overnight as indicated. When DOPC/BMA/DMOEP polymer-stabilized vesicles were stored at neutral pH and the isolated polymer was suspended in acidified THF, the polymer was solubilized (OD < 0.01) (Figure 1A and 1E). Conversely, when the isolated polymer was suspended in neutral THF after overnight storage at neutral pH, the polymer was insoluble (OD = 0.94) (Figure 1B and 1E). When DOPC/BMA/DMOEP polymer-stabilized vesicles were stored overnight at pH 2.0, followed by extraction and resuspension of the polymer in neutral THF, partial solubilization of the polymer is observed (OD = 0.72) (Figure 1C and 1E). To ensure that THF was not primarily responsible for the partial polymer solubilization seen in Figure 1C, polymer was isolated from DOPC/BMA/DMOEP polymer-stabilized vesicles stored at pH 7.4, lyophilized and resuspended in pH 2.0 aqueous buffer overnight (Figure 1D). The resulting solubilization was similar to that seen in acidified THF. The increased solubility after exposure to acidic conditions is likely due to the degradation of the cross-links within the cross-linked polymer, generating smaller, linear polymers that are highly soluble in THF (e.g., see data for 14 kDa poly(BMA) in Supporting Information). Combined, these data suggest that the phospholipid membrane shields the polymer from acid degradation to some degree, allowing only partial conversion to linear polymers. It is unclear though whether this low conversion of the polymer network to render the vesicle more sensitive to degradation. Thus, a key question is whether the low conversion of the polymer network observed is sufficient to affect the overall stability of DOPC/BMA/DMOEP polymer-stabilized vesicles thereby rendering the architecture more prone to vesicle disassembly in the presence of chemical or physical challenges.
Figure 1. pH-dependent solubility of isolated BMA/DMOEP copolymer.
A) BMA/DMOEP copolymer isolated from DOPC liposomes stored at pH 7.4, followed by overnight suspension in acidified THF. B) BMA/DMOEP copolymer isolated from DOPC liposomes stored at pH 7.4, followed by overnight suspension in neutral THF. C) BMA/DMOEP copolymer isolated from DOPC liposomes stored at pH 2.0, followed by overnight suspension in neutral THF. D) BMA/DMOEP copolymer isolated from DOPC liposomes, resuspended in pH 2.0 aqueous buffer overnight. E) Optical density measurements for samples collected following overnight incubation in THF as described in A), B) and C). Measurements were made at 330 nm.
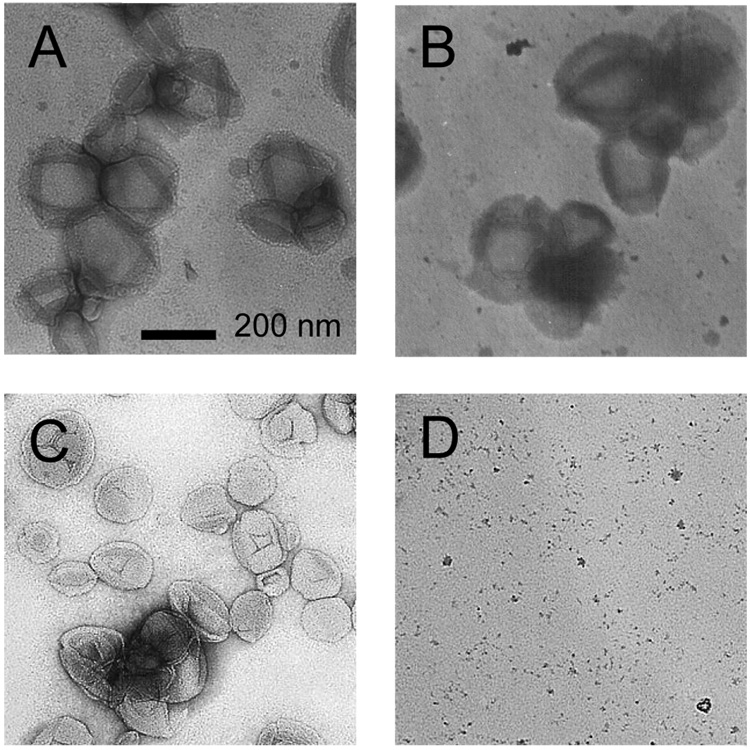
Biological environments are typically rich in phospholipid membrane area, wherein phospholipid vesicles may fuse upon contact. To address whether the polymer stabilized vesicles presented herein are potentially suitable for utilization in harsh biological and chemical environments, we have probed the pH-dependent vesicle stability using TEM of vesicles stored under a variety of conditions (Figure 2). Most notably, we have used a high concentration of surfactant in that stability in this harsh chemical environment strongly suggests the ability to remain intact in membrane rich biological environments. When DOPC/BMA/DMOEP polymer-stabilized vesicles were stored at pH 7.0 in both the absence and presence of excess surfactant (10:1 Triton X-100:DOPC mole ratio), intact vesicle structures were (Figure 2A and 2B). Conversely, when DOPC/BMA/DMOEP polymer-stabilized vesicles were stored at pH 4.0, intact vesicles were only observed when no surfactant had been added (Figure 2C and 2D). Furthermore, DOPC/BMA/DMOEP polymer-stabilized vesicles stored at pH 2.0 yielded no intact structures upon TEM imaging, indicative of the instability of the vesicle structure in vacuum conditions (Supporting Information). A complete absence of intact structures was observed when DOPC vesicles lacking a polymer network were prepared (Supporting Information). These results support the hypothesis that a BMA/DMOEP copolymer is intact at pH 7.0 and stabilizes the vesicle under conditions utilized for TEM, that this stabilization is strongly pH dependent, and storage at pH 2.0 causes sufficient conversion of the cross-linked polymer network to smaller, linear polymers to complete destabilize the vesicle architecture. Interestingly, vesicles stored at pH 4.0 are sufficiently stable to survive the drying process in the absence of surfactant, suggesting partial retention of the cross-linked polymer network (Fig. 2C and 2D).
Figure 2.
TEM images of polymer stabilized phospholipid vesicles stored at A) pH 7.4, B) pH 7.4 and spiked with a 10:1 mole ratio of Triton X-100:DOPC, C) pH 4.0, and D) pH 4.0 and spiked with a 10:1 mole ratio of Triton X-100:DOPC.
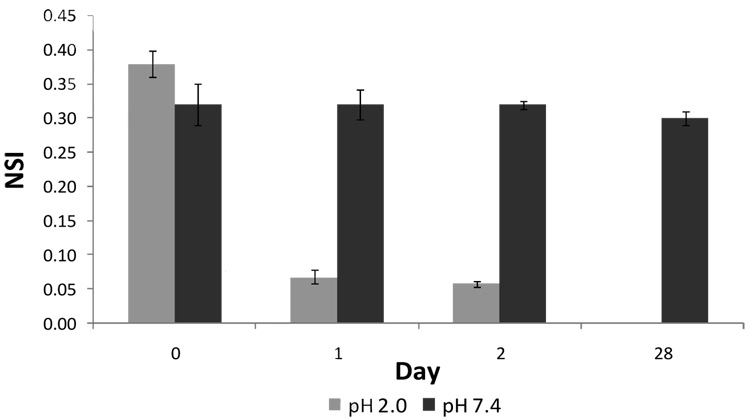
Dynamic Light Scattering (DLS) analysis performed on the same samples provided complementary results. DOPC/BMA/DMOEP polymer-stabilized vesicles were prepared with a nominal mean diameter of 200 nm as described above. The sample was split and stored at either pH 2.0 or pH 7.4. The mean scatter intensity, a measure of the effective concentration of the scattering species, 16 was then recorded after addition of 10:1 surfactant (Figure 3). On the day of preparation, the normalized scatter intensity of all samples was within experimental error. However, within 24 hours, vesicles stored at pH 2.0 were completely reduced to mixed micelles upon addition of 10:1 surfactant, as indicated by the normalized scatter intensity, whereas vesicles stored at pH 7.4 remained as stable as when originally prepared in excess of one month storage. These results strongly suggest rapid, complete destabilization at pH 2.0, allowing vesicle dissolution upon addition of surfactant. Together, these data support the formation of a BMA/DMOEP copolymer that stabilizes the vesicles for an extended time, yet provides for pH-dependent destabilization of the vesicle architecture.
Figure 3. pH-dependent temporal profile of polymer vesicle destabilization.
Normalized scatter intensity for vesicles stored at pH 2.0 and pH 7.4 following addition of 10:1 mole ratio of Triton X-100:DOPC.
The chemical nature of the destabilization was investigated to determine if decreased vesicle stability resulted from: a) hydrolysis of esters in the copolymer to yield poly(methacrylic acid), which is likely insoluble in the bilayer lamella; b) cleavage of acetal cross-links yielding a linear polymer network; or c) a combination of the two. GC-MS analysis showed no byproducts of cleaved esters, butanol or 1,2-dihydroxyethane, ruling out ester cleavage. Acetone released during acetal cleavage was detected in all samples at a concentration that varied with the pH of the storage medium. Acetone observed at pH 7.4 likely results from DMOEP that hydrolyzed before partitioning or did not partition into the bilayer lamella (this quantity was normalized to 100). At pH 4.0, the normalized acetone increased to 124 after seven days of storage. At pH 2.0, a normalized acetone level of 213 was measured after seven days of storage, further supporting the pH dependence of vesicle destabilization.
In conclusion, we have prepared acid-labile, polymer stabilized phospholipid vesicles utilizing an acetal-functionalized methacrylate monomer. The resulting vesicles are stable at physiological pH for extended periods of time, while exhibiting a pH-dependent and time-dependent destabilization. The resulting stabilized vesicle architecture can be envisioned to enter a biological environment wherein a change in pH (e.g. as found in lysosome, stomach, tumor, etc.) partially degrades the polymer network that has to this point imparted the enhanced stability required to cross the cell membrane and survive in membrane rich biological environments. Upon partial destabilization of the polymer network, the vesicle is now susceptible to vesicle fusion and other chemical and biological destabilization events, thus allowing cargo to be delivered and enhancing clearance from the biological environment. Thus, the transiently stabilized vesicle concept presented herein may prove useful for enhanced drug delivery and chemical sensing applications with improved physiological degradation and clearance properties as better control of the temporal and pH-dependent degradation is realized.
Supplementary Material
Detailed procedures and data referenced but not shown in this article. This material is available free of charge via the internet at http://pubs.acs.org/
Acknowledgement
This work was supported by grants from the NSF (CHE-0548167 and CHE-0518702), the Arizona Biomedical Research Commission (ABRC 0701), and the NIH (GM074522) and (EB007047).
REFERENCES
- 1.(a) Hotz J, Meier W. Langmuir. 1998;14:1031–1036. [Google Scholar]; (b) Cheng ZL, Aspinwall CA. Analyst. 2006;131:263–243. doi: 10.1039/b511083a. [DOI] [PubMed] [Google Scholar]
- 2.Meier W. Chimia. 1999;53:214–215. [Google Scholar]
- 3.Rakhmatullina E, Meier W. Langmuir. 2008;24:6254–6261. doi: 10.1021/la8003068. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien DF, Armitage BA, Benedicto A, Bennett DE, Lamparski HG, Lee YS, Srisiri W, Sisson TM. Acc. Chem. Res. 1998;31:861–868. [Google Scholar]
- 5.Mueller A, O’Brien DF. Chem. Rev. 2002;102:727–757. doi: 10.1021/cr000071g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graff A, Winterhalter M, Meier W. Langmuir. 2001;17:919–923. [Google Scholar]
- 7.(a) Cheng ZL, D'Ambruoso GD, Aspinwall CA. Langmuir. 2006;22:9507–9511. doi: 10.1021/la061542i. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Liu SC, O'Brien DF. Macromol. 1999;32:5519–5524. [Google Scholar]
- 8.Declercq RR, Goethals EJ. Macromol. 1992;25:1109–1113. [Google Scholar]
- 9.Gillies ER, Goodwin AP, Frechet JM. J. Bioconj. Chem. 2004;15:1254–1263. doi: 10.1021/bc049853x. [DOI] [PubMed] [Google Scholar]
- 10.Heffernan MJ, Murthy N. Bioconj. Chem. 2005;16:1340–1342. doi: 10.1021/bc050176w. [DOI] [PubMed] [Google Scholar]
- 11.Jain R, Standley SM, Frechet JMJ. Macromol. 2007;40:452–457. [Google Scholar]
- 12.Kafouris D, Themistou E, Patrickios CS. Chem. Mat. 2006;18:85–93. [Google Scholar]
- 13.(a) Murthy N, Thng YX, Schuck S, Xu MC, Frechet JMJ. J. Am. Chem. Soc. 2002;124:12398–12399. doi: 10.1021/ja026925r. [DOI] [PubMed] [Google Scholar]; (b) Themistou E, Patrickios CS. Macromol. 2006;39:73–80. [Google Scholar]
- 14.Themistou E, Patrickios CS. Macromol. 2007;40:5231–5234. [Google Scholar]
- 15.Walraedt SR, Goethals EJ. Polymer International. 1995;38:89–94. [Google Scholar]
- 16.Measurement of Suspended Particles by Quasi-Elastic Light Scattering. New York: Wiley-Interscience; 1983. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed procedures and data referenced but not shown in this article. This material is available free of charge via the internet at http://pubs.acs.org/