Abstract
Background
Major depression (MDD) occurs in a subset of patients receiving interferon-alpha treatment, although many are resilient to this side effect. Genetic differences in the serotonin reuptake transporter promoter (5-HTTLPR) may interact with the inflammatory system and may influence depression risk.
Methods
A cohort of 71 non-depressed hepatitis C patients about to receive interferon-alpha was prospectively followed, employing a diagnostic structured clinical interview (SCID; DSM-IV) and self-report questionnaires. Patients were genotyped for the 5-HTTLPR (LG, LA, and S) and the variable number of tandem repeats (VNTR) polymorphism in the second intron. Kaplan-Meier analyses were used to compare major depression incidence. Genotype effects on sleep quality (Pittsburgh Sleep Quality Index) and Beck Depression Inventory (BDI) were assessed using mixed-effect repeated-measure analyses.
Results
The LA allele was associated with a decreased rate of developing MDD (Mantel-Cox Log Rank Test p<0.05) with the LA/LA genotype being the most resilient. This genotype was also associated with better sleep quality (F(61.2,2) = 3.3 p<0.05). The ability of baseline sleep quality to predict depression incidence disappeared when also including genotype in the model. Conversely, the relationship of neuroticism with depression incidence (B=0.07 SE = 0.02 p<0.005) was not mitigated when including genotype.
Conclusions
Using a prospective design, 5-HTTLPR is associated with MDD incidence during interferon-alpha treatment. Preliminary evidence that this effect could be mediated by effects on sleep quality was observed. These findings provide support for a possible interaction between inflammatory cytokine (interferon-alpha) exposure and 5-HTTLPR variability in MDD.
Keywords: Depression, cytokine, polymorphism, serotonin, interferon, prospective
Introduction
Although major depression (MDD) is common (1), determining the role of genetic vulnerability is complicated. There is difficulty with depression measurement and heterogeneity (2, 3), and the power of prospective studies is limited by an incidence of about 1–3% per year (4). Nonetheless, meta-analyses (5) and large association studies (6) indicate that the short/short (S/S) genotype in the serotonin transporter length promoter region (5-HTTLPR) increases MDD risk. 5-HTTLPR likely interacts with ‘stressful life events’ to influence MDD (7–9), though this is not universally replicated (10).
One approach to prospectively examining the role of genetics is to focus on specific, homogenous depression-inducing incidents. As one example, a potent MDD trigger is interferon-alpha (IFN-α), an inflammatory cytokine, with the occurrence of depression ranging up to 30–50%, depending upon study design (11–15). For some individuals, depression symptoms develop over the first few months of therapy and include sadness, anhedonia, irritability, anxiety, social withdrawal, and suicidal ideation. IFN-α -induced depression can respond to antidepressant treatments, ranging from selective serotonin reuptake inhibitors to electroconvulsive therapy (16), and is distinct from general somatic effects such as fatigue and aching (12, 14). Abnormalities in endogenous cytokines have been associated with idiopathic MDD, prompting speculation regarding their involvement in MDD’s etiology (17–19). Although the mechanism is unknown, IFN-α may affect frontal lobe and anterior cingulate function (20, 21), influence transcription of multiple genes (22, 23), and involve neurotransmitters including dopamine (24), glutamate (25, 26), apoptotic systems (27), and multiple targets in the 5-HT system (28–31).
We therefore examined 5-HTTLPR in patients without current MDD but who were about to be treated with IFN-α for hepatitis C. 5-HTTLPR has been associated with transcriptional efficiency (32), serotonin 1A receptor binding (33), activity in the amygdala (34), and connective interaction between the amygdala and anterior cingulate (35). Thus, 5-HTTLPR could influence resiliency when facing a depressogenic trigger such as an inflammatory cytokine like IFN-α.
A polymorphism within the L allele (LG) may result in lower transcription efficiency, functionally comparable to the S allele (7, 36), warranting its inclusion in our analyses. We also assessed another polymorphism in 5-HTT that may affect transcriptional regulation (37), a variable number of tandem repeats (VNTR) polymorphism in the second intron (5). We additionally examined both sleep quality and neuroticism. Neurotic traits and poor sleep quality may be influenced by 5-HTTLPR (38, 39); and both are risk factors for MDD (40, 41) and IFN-induced MDD (12, 42).
Methods
Seventy-eight non-depressed patients with Hepatitis C were started on pegylated-IFN-α2 and oral ribavirin and followed for four months of treatment. Using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID), patients were excluded from this study if they had active mood, anxiety, psychotic, or drug/alcohol use disorders within six months prior to starting IFN-a. Subjects could have a resolved, past history of mood or substance use disorder. Seven participants who were euthymic but taking antidepressants were excluded, as antidepressant use may confound the results. The study was approved by the University of Pittsburgh Institutional Review Board.
An abbreviated SCID specifically focused on mood disorders was used to diagnose categorical MDD during IFN-α treatment. It was obtained either monthly (by F.L. or a single research assistant) or sooner at the request of the treating hepatologist (requesting that the psychiatrist (F.L.) promptly evaluate the patient). Once MDD was diagnosed, immediate psychiatric intervention was provided (typically starting an antidepressant, or sometimes discontinuing IFN-α treatment). A subset of patients also developed severe irritability with depression symptoms. Although they did not meet formal criteria for MDD, psychiatric intervention to prevent potentially injurious behavior was deemed ethically appropriate and therefore instituted. Thus, time until intervention for any ‘mood disorder’ was a secondary endpoint. Although, diagnoses were made blind to genotype, independent raters were not used.
The Beck Depression Inventory-II (BDI) assessed depressive symptoms. Sleep quality was measured with the Pittsburgh Sleep Quality Index (PSQI) (43), a well-validated 19-item questionnaire consisting of 7 components that yield a global score (0–21; higher numbers indicating poorer sleep quality). Both were administered prior to IFN-α, and at weeks 2, 4, 8, 12, and 16 during treatment. Neuroticism from the NEO-Five Factor Inventory (44) and medical illness burden (Cumulative Illness Rating Scale-Geriatric) (45) were assessed at baseline.
Genetics
DNA was isolated from blood lymphocytes using the PureGene™ kit (Gentra Systems, Minneapolis, MN). The serotonin transporter gene (SLC6A4) promoter polymorphism was typed by DNA amplification (PCR) using flanking primers 5′-TCCTCCGCTTTGGCGCCTCTTCC-3′ (forward) and 5′-TGGGGGTTGCAGGGGAGATCCTG-3′ (reverse) (46). The L allele was subtyped for rs25531. This A>G single nucleotide polymorphism was concurrently detected by digesting the amplified fragments with MspI (New England Biolabs, Beverly, Massachusetts), where the A>G substitution creates an additional MspI site. Amplification products were simultaneously resolved by electrophoresis on 3.5% agarose gels. For the second intron VNTR, PCR used the following primers: 5′-GGGCAATGTCTGGCGCTTCCCCTACATA-3′ (forward) and 5′-TTCTGGCCTCTCAAGAGGACCTACCAGC-3’ (reverse), examining the 10/10 10/12 or 12/12 repeat genotypes -- where we combined the rare 9s with 10s.
Data Analysis
We used SPSS 16.0 for all analyses. Based on equivalence of function (36), the S and LG alleles were grouped together for analyses, unless otherwise indicated (7% of the L alleles were LG and 93% were LA). Baseline measures were assessed using ANOVA or chi-square tests (Table 1). Kaplan-Meier survival analyses using the Mantel-Cox log-rank test, initially stratifying by race and comparing factors pooled over strata, was used to examine MDD incidence. For the survival analyses, eight participants were censored before 16 weeks for either discontinuing or holding IFN-α treatment; seven for medical complications and one for severe fatigue.
Table 1.
Baseline characteristics of subjects (mean +/− S.D.)
| LA/LA | S/LA or LG/LA | S/S or S/LG | ||
|---|---|---|---|---|
| N=18 | N=39 | N=14 | ||
| Age | 47.6 +/− 12.2 | 49.0 +/− 11.0 | 47.7 +/− 13.5 | n.s. |
| Percent female | 33.3% | 20.5% | 35.7% | n.s. |
| Percent African-American | 11.1% | 12.8% | 14.3% | n.s. |
| History of mood disorder | 33% | 23% | 43% | n.s. |
| History of drug/alcohol disorder | 50% | 56% | 57% | n.s. |
| Beck Depression Inventory | 6.3 +/− 6.2 | 6.0 +/− 5.1 | 8.6 +/− 7.5 | n.s. |
| Pittsburgh Sleep Quality Index | 4.4 +/− 2.0 | 7.1 +/− 3.9 | 8.1 +/− 6.1 | p<0.1 |
| Neuroticism | 15.3 +/− 8.3 | 14.9 +/− 6.8 | 18.5 +/− 8.0 | n.s. |
| Cumulative Illness Rating Scale | 2.8 +/− 1.2 | 3.7 +/− 2.1 | 3.1 +/− 1.6 | n.s. |
(n.s. indicates p>0.1 in either ANOVA or chi-square tests)
To assess the relationship between genotype and symptoms (BDI and PSQI), we used repeated-measures mixed-effects models, with last observation carried forward. Because of the repeated measure design, a first-order ante-dependence covariance structure was used. Cox proportional hazards models were then employed in exploratory analyses, and included baseline neuroticism, BDI, or PSQI in conditional stepwise regression models with genotype.
Results
The 5-HTTLPR genotypes were in Hardy-Weinberg equilibrium, with no significant differences between subjects at baseline (Table 1). There was a non-significant trend for LA to be associated with lower PSQI scores (Table 1).
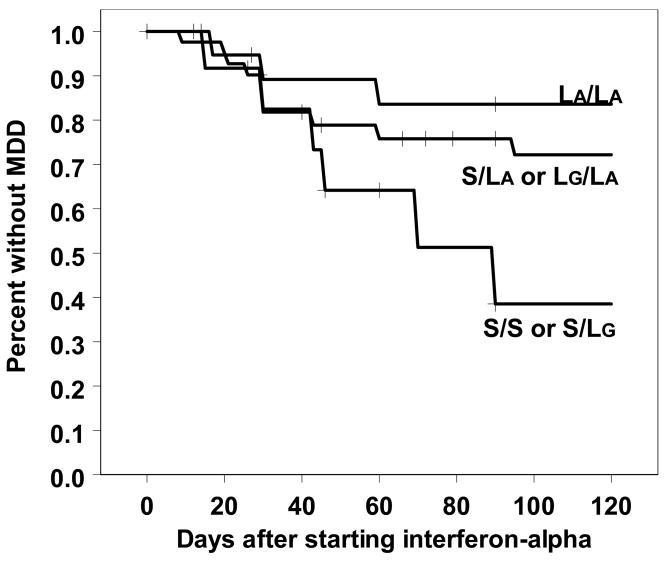
The LA allele was associated with lower rates of MDD (Mantel-Cox Log Rank χ2(1) = 4.3; p < 0.05), with the S/S genotype being the least resilient (Figure 1). Pair-wise comparisons indicated that LA/LA was different from S/S but not from S/LA. Notably, the overall pattern was similar for both Caucasians and the nine African-Americans, with the LA/LA genotype being the most resilient and S/S the most vulnerable in both groups. However, when solely examining Caucasians, the results only trended towards significance (χ2(1) = 3.12; p = 0.077). When specifically comparing the S/S genotype vs. LA in just Caucasians, the results were again significant (χ2 (1) = 4.5; p < 0.05), consistent with association studies in which the S/S genotype is the most vulnerable (5, 6).
Figure 1.
Development of MDD in patients starting interferon-alpha treatment.
Given uncertainty regarding the functional role of the rs25531 G allele, we combined LA and LG together. MDD incidence was similar (χ2 (1) = 4.06; p < 0.05) comparing 22 L/L 37 S/L and 12 S/S. Thus, rs25531 may have a small but present role. The effect was significantly mitigated when solely examining Caucasians (χ2 (1) = 2.46; p = 0.12). There was no relationship between the second intron VNTR and MDD incidence (Mantel-Cox Log Rankχ2 (1) = 0.005; n.s.).
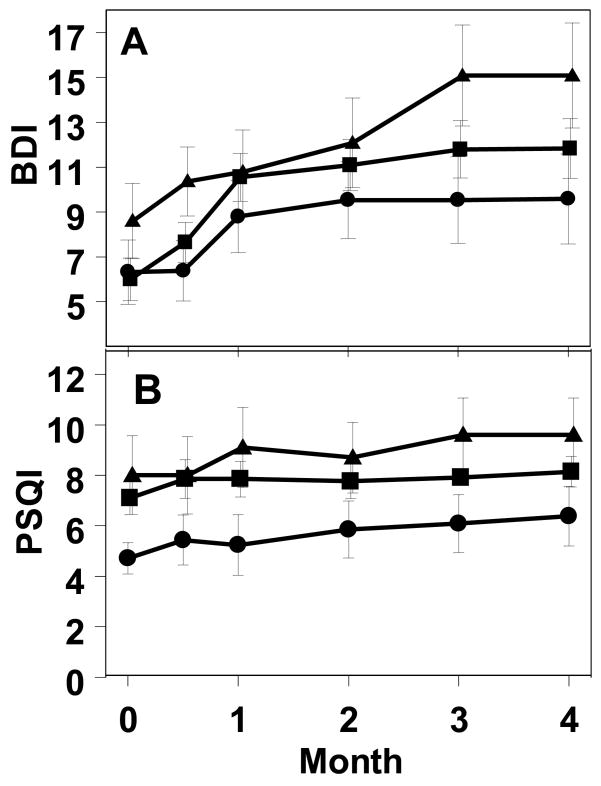
We next examined BDI scores using a mixed-effect repeated-measures analysis. Individuals developing MDD received antidepressant treatment and/or had their INF-α held, potentially limiting any further increase in symptoms over time and resulting in a likely ‘ceiling’ effect. Despite this limitation, time was highly significant (F(137.8,5) = 3.7; p<0.005) with BDI increasing in all three genotypes during treatment (Figure 2A). Genotype was not significantly influential (F(75.8,2) = 1.7), nor was the interaction.
Figure 2.
Development of increased BDI and PSQI self-report scores in patients starting interferon-alpha treatment. (Means +/− S.E.M. LA/LA = circles; S/LA or LA/LG = squares; S/S or S/LG=triangles).
Conversely, when assessing sleep quality (Figure 2B), genotype was influential (F(61.2,2) = 3.3; p<0.05), but time was not (F(95.5,5) =1.7), nor was the interaction. That is, sleep did not significantly worsen during interferon-alpha treatment, but those with the LA/LA genotype had better sleep quality throughout the study (Figure 2B).
Using Cox Regression analysis, we confirmed that genotype was related to MDD incidence (B=0.62 SE=0.27 p<0.05). We also confirmed that baseline neuroticism was strongly associated with MDD incidence (B=0.07 SE = 0.02 p<0.005). When genotype was included with neuroticism in the Cox Regression, both continued to be significantly associated with MDD incidence; with both neuroticism’s (B=0.07 SE = 0.02 p<0.005) and genotype’s (B=0.53 SE = 0.27 p<0.05) relationship relatively unchanged, indicating that neuroticism does not mediate the effect of 5-HTTLPR. Clinically, neuroticism could predict MDD (χ2 (2) = 9.7 p < 0.005; −2 Log Likelihood = 233.04), and genotyping slightly improved this predictive ability (χ2 (4) = 14.23 p < 0.005; −2 Log Likelihood = 228.41).
In Cox Regression analyses, both baseline PSQI and BDI were also associated with MDD incidence (PSQI: B=0.153 SE = 0.074 p<0.05 and BDI:B=0.067 SE = 0.034 p<0.05). When either PSQI or BDI was included with genotype using stepwise Cox Regression (both forward and backward conditional LR), only genotype remained significant (B = 0.73 SE = .37 p<0.05). Forcing all three into the same model, again only genotype remained associated (B=0.66 SE = 0.34 p<0.05), with the effects of PSQI (B=0.08 SE = 0.05 n.s.) and BDI (B=0.02 SE = 0.03 n.s.) no longer remaining. However, because baseline PSQI only trended toward a significant association with genotype (Table 1), actual mediation could not be concluded.
To ensure that we weren’t biasing our analyses by focusing purely on a DMS-IV based MDD diagnosis, we next examined the rate of development of all diagnosed “mood disorders” combined. After starting IFN- α, 24% ultimately developed categorical MDD by month four, but 45% total developed mood symptoms severe enough to prompt treatment (including one episode of mania). This group had “subsyndromal depression/irritability” as they did not have grandiosity, racing thoughts, increased goal-oriented activity, or decreased need for sleep – but did have insomnia, elevated anger, and irritability. When stratified by race; the LA allele remained dose-dependently associated with resiliency to this broader diagnosis (χ2 (1) = 5.74 p < 0.05). This association remained when examining solely Caucasians (χ2 (1) = 4.8 p < 0.05), but was lost when ignoring the role of the rs25531A/G SNP (χ2 (1) = 2.96 p = 0.085).
Discussion
The LA allele was associated with a lower rate of depression development during INF-α treatment. This relationship was notable despite heterogeneity in presentation and diagnosis used (i.e. MDD or ‘subsyndromal depression with irritability’). The S/S genotype was the least resilient. Thus, with a homogenous influence (INF-α treatment), in a uniform set of patients (non-depressed patients with hepatitis C), followed prospectively, the effects of genotype can be discernable, even with a relatively small sample size (n=71). Nonetheless, given the sample size, these results require replication.
Neuroticism may also influence development of MDD (12). However, neuroticism does not appear to mediate the effect of 5-HTTLPR; as both lower neuroticism and 5-HTTLPR were independently associated with MDD. Conversely, 5-HTTLPR was associated with sleep quality, a relationship observed by others (39), and consistent with serotonin’s role as a regulator of sleep. Poor sleep may predict future depression (41). In the current study, the relationship between PSQI scores and MDD disappeared when 5-HTTLPR was included in the model. However, (i) we do not have the statistical power to test a mediation model, (ii) PSQI baseline scores only trended towards an association with genotype, and (iii) the PSQI is a self-report rather than direct measure of sleep. The results are therefore only suggestive that poor sleep could potentially mediate the influence of 5-HTTLPR, and require further examination. We did not assess whether increased stress, interpersonal conflict, or changes in social support during treatment could have mediated the influence of interferon-alpha. These possibilities also await further examination. Also, whether 5-HTTLPR may influence a variety of phenotypes relevant to mental health, or a single widely influential factor such as sleep is not yet determined (38).
The findings were more pronounced when including LG and S together as low expressing alleles, consistent with observations in the literature (7, 36), although the actual role of the LG remains under debate (47). Moreover, we cannot rule out the possibility the 5-HTTLPR is simply in linkage with another nearby functional region. But we did not find any trend toward association of the second intron VNTR with MDD risk, ruling out this site. We did observe that the relationship between 5-HTTLPR and MDD risk was similar in both Caucasians and African-Americans, making racial differences less likely contributors to the findings. Nonetheless, the results will need to be confirmed in a larger sample that includes genomic control.
A study recently reported that 5-HTTLPR and Hospital Anxiety and Depression Scale (HADS) scores in IFN-α patients were not associated (48), using a cross-tabs X2 analysis. It was not stated whether any patients were on antidepressants before or during IFN-α treatment. Other differences with that study relate to patient population (whether patients with a past history of MDD were included is not indicated), and depression diagnosis (SCID vs. a HADS cutoff).
Regardless, this study is consistent with a growing literature on the relationship between inflammatory cytokines and the central 5-HT system. IFN-α and other cytokines can alter expression of the 5-HT transporter in vitro (49) and affect central 5-HT efflux in vivo (50). IFN-α can affect expression of 5-HT1A receptors (28), consistent with 5-HT1A findings in depressed humans (33). In humans, IFN-α also decreases peripheral tryptophan levels, an effect correlated with depression (51). Abnormalities in the inflammatory system can also be observed in many depressed patients (17, 18), prompting the biologically plausible possibility for an interaction between endogenous IFN-α and 5-HT variability in the pathogenesis of depression.
In summary, in a cohort of patients at risk for depression because of exposure to exogenous IFN-α, the role of 5-HTTLPR in influencing resiliency was demonstrable. 5-HTTLPR influenced MDD incidence independently from neuroticism. Exploratory analyses suggested that poor sleep quality at baseline could potentially mediate this genetic effect. Regardless, one pathogenic pathway towards the development of depression may involve an interaction between the 5-HT system and increases in inflammatory cytokines such as IFN-α. Explicating the details underlying this interaction may be informative in delineating the pathophysiologic pathways that lead to MDD.
Acknowledgments
This research was supported by NIMH grants K23MH074012, K24MH065416, P30MH071944.
Footnotes
Disclosures
Bruce Pollock has received research support from Janssen Pharmaceuticals and is a consultant to Lundbeck and Forest Pharmaceuticals. Francis Lotrich, Robert Ferrell, and Mordechai Rabinovitz have no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stoudemire A, Frank R, Hedemark N, Kamlet M, Blazer DG. The economic burden of depression. Gen Hosp Psychiatry. 1986;8:387–94. doi: 10.1016/0163-8343(86)90018-6. [DOI] [PubMed] [Google Scholar]
- 2.Merikangas KR, Chakravarti A, Moldin SO, Araj H, Blangero J, Burmeister M, et al. Future of genetics of mood disorders research. Biol Psychiatry. 2002;52:457–77. doi: 10.1016/s0006-3223(02)01471-3. [DOI] [PubMed] [Google Scholar]
- 3.Schuckit MA, Tipp JE, Bucholz KK, Nurnberger JIJ, Hesselbrock VM, Crowe RR, et al. The life-time rates of three major mood disorders and four major anxiety disorders in alcoholics and controls. Addiction. 1997;92:1289–304. [PubMed] [Google Scholar]
- 4.Blazer DG. Mood disorders: Epidemiology. In: Sadock BJ, Sadock VA, editors. Comprehensive Textbook of Psychiatry. 7. Lippincott Williams & Williams; 2000. pp. 1298–307. [Google Scholar]
- 5.Lotrich FE, Pollock BG. Meta-analysis of serotonin transporter polymorphisms and affective disorder. Psychiatr Genet. 2004;14:121. doi: 10.1097/00041444-200409000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Hoefgen B, Schulze TG, Ohlraun S, von Widdern O, Hofels S, Gross M, et al. The power of sample size and homogenous sampling: association between the 5-HTTLPR serotonin transporter polymorphism and major depressive disorder. Biol Psychiatry. 2005;57:247–51. doi: 10.1016/j.biopsych.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 7.Zalsman G, Huang Y, Oquendo MA, Burke AK, Hu X, Brent DA, et al. Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. Am J Psychiatry. 2006;163:1588–93. doi: 10.1176/ajp.2006.163.9.1588. [DOI] [PubMed] [Google Scholar]
- 8.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Sci. 2003;301:368–89. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 9.Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Arch Gen Psychiatry. 2005;62:529–35. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- 10.Chipman P, Jorm AF, Prior M, Sanson A, Smart D, Tan X, et al. No interaction between the serotonin transporter polymorphism (5-HTTLPR) and childhood adversity or recent stressful life events on symptoms of depression: results from two community surveys. Am J Medl Genet Part B, Neuropsychiatr Genet. 2007;144:561–5. doi: 10.1002/ajmg.b.30480. [DOI] [PubMed] [Google Scholar]
- 11.Malaguarnera M, Laurino A, Di Fazio I, Pistone G, Castorina M, Guccione N, et al. Neuropsychiatric effects and type of IFN-a in chronic hepatitis C. J Interferon Cytokine Res. 2001;21:273–8. doi: 10.1089/107999001300177457. [DOI] [PubMed] [Google Scholar]
- 12.Lotrich FE, Rabinovitz F, Gironda P, Pollock BG. Depression following pegylated interferon-alpha: characteristics and vulnerability. J Psychosom Res. 2007;63:131–5. doi: 10.1016/j.jpsychores.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trask PC, Esper P, Riba M, Redman B. Psychiatric side effects of interferon therapy: prevalence, proposed mechanisms, and future directions. J Clin Oncol. 2000;18:2316–26. doi: 10.1200/JCO.2000.18.11.2316. [DOI] [PubMed] [Google Scholar]
- 14.Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, et al. Neurobehavioral effects of interferon-a in cancer patients: Phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacol. 2002;26:643–52. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 15.Capuron L, Miller AH. Cytokines and psychopathology: Lessons from Interferon-a. Biol Psychiatry. 2004;56:819–908. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Raison CL, Demetrashvili M, Capuron L, Miller AH. Neuropsychiatric Adverse Effects of Interferon-a: Recognition and Management. CNS Drugs. 2005;19:105–23. doi: 10.2165/00023210-200519020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maes M. Evidence for an immune response in major depression: a review and hypothesis. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:11–38. doi: 10.1016/0278-5846(94)00101-m. [DOI] [PubMed] [Google Scholar]
- 18.Rothermundt M, Arolt V, Fenker J, Gutbrodt H, Peters M, Kirchner H. Different immune patterns in melancholic and non-melancholic major depression. Eur Arch Psychiatry Clin Neurosci. 2001;251:90–7. doi: 10.1007/s004060170058. [DOI] [PubMed] [Google Scholar]
- 19.Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry. 2008;65:409–15. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juengling FD, Ebert D, Gut O, Engelbrecht MA, Rasenack J, Nitzsche EU, et al. Prefrontal cortical hypometabolism during low-dose inteferon alpha treatment. Psychopharmacol (Berl) 2001;152:383–9. doi: 10.1007/s002130000549. [DOI] [PubMed] [Google Scholar]
- 21.Capuron L, Pagnoni G, Demetrashvili M, Woolwine BJ, Nemeroff CB, Berns GS, et al. Anterior cingulate activation and error processing during interferon-alpha treatment. Biol Psychiatry. 2005;58:190–6. doi: 10.1016/j.biopsych.2005.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Campbell IL, Zhang H. Systemic interferon- regulates interferon-stimulated genes in the central nervous system. Mol Psychiatry. 2008;13:293–302. doi: 10.1038/sj.mp.4002013. [DOI] [PubMed] [Google Scholar]
- 23.Geiss GK, Carter VS, He Y, Kwieciszewski BK, Holzman T, Korth MJ, et al. Gene expression profiling of the cellular transcriptional network regulated by alpha/beta interferon and its partial attenuation by the hepatitis C virus nonstructural 5A protein. J Virol. 2003;77:6367–75. doi: 10.1128/JVI.77.11.6367-6375.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shuto H, Kataoka Y, Horikawa T, Fujihara N, Oishi R. Repeated interferon-a administration inhibits dopaminergic neural activity in the mouse brain. Brain Res. 1997;747:348–51. doi: 10.1016/s0006-8993(96)01371-6. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Samuel CE. Editing of glutamate receptor subunit B pre-mRNA by splice-site variants of interferon-inducible double-stranded RNA-specific adenosine deaminase ADAR1. J Biol Chem. 1999;274:5070–7. doi: 10.1074/jbc.274.8.5070. [DOI] [PubMed] [Google Scholar]
- 26.Muller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry. 2007;12:988–1000. doi: 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- 27.Chawla-Sarkar M, Lindner DJ, Liu YF, Williams BR, Sen GC, Silverman RH, et al. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis. 2003;8:237–49. doi: 10.1023/a:1023668705040. [DOI] [PubMed] [Google Scholar]
- 28.Abe S, Hori T, Suzuki T, Baba A, Shiraishi H, Yamamoto T. Effects of chronic administration of interferon alpha A/D on serotonergic receptors in rat brain. Neurochemical Research. 1999;24:359–63. doi: 10.1023/a:1020929415443. [DOI] [PubMed] [Google Scholar]
- 29.Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, et al. Interferon-alpha-induced changes in tryptophan metabolism. relationship to depression and paroxetine treatment. Biol Psychiatry. 2003;54:906–14. doi: 10.1016/s0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- 30.Dunn AL, Crnic LS. Repeated injections of interferon-alpha A/D in Balb/c mice: behavioral effects. Brain Behav Immun. 1993;7:104–11. doi: 10.1006/brbi.1993.1011. [DOI] [PubMed] [Google Scholar]
- 31.Wichers MC, Maes M. The role of indoleamine 2,3-dioxygenase (IDO) in the pathophysiology of interferon-alpha-induced depression. J Psychiatry Neurosci. 2004;29:11–7. [PMC free article] [PubMed] [Google Scholar]
- 32.Heils A, Mossner R, Lesch KP. The human serotonin transporter gene polymorphism - basic research and clinical implication. J Neural Transm. 1997;104:1005–14. doi: 10.1007/BF01273314. [DOI] [PubMed] [Google Scholar]
- 33.Lee M, Bailer UF, Frank GK, Henry SE, Meltzer CC, Price JC, et al. Relationship of a 5-HT transporter functional polymorphism to 5-HT1A receptor binding in healthy women. Mol Psychiatry. 2005;10:715–6. doi: 10.1038/sj.mp.4001680. [DOI] [PubMed] [Google Scholar]
- 34.Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, et al. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005;62:146–52. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- 35.Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–34. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 36.Hu X, Lipsky R, Zhu G, Akhtar L, Taubman J, Greenberg D, et al. Serotonin transporter promoter gain of function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78:815–26. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaiser R, Muller-Oerlinghausen B, Filler D, Tremblay P, Berghofer A, Roots I, et al. Correlation between serotonin uptake in human blood platelets with the 44-bp polymorphism and the 17-bp variable number of tandem repeat of the serotonin transporter. Am J Med Genet. 2002;114:323–8. doi: 10.1002/ajmg.10119. [DOI] [PubMed] [Google Scholar]
- 38.Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nat Neurosci. 2007;10:1103–9. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- 39.Brummett BH, Krystal AD, Ashley-Koch A, Kuhn CM, Zuchner S, Siegler IC, et al. Sleep quality varies as a function of 5-HTTLPR genotype and stress. Psychosom Med. 2007;69:621–4. doi: 10.1097/PSY.0b013e31814b8de6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kendler KS, Kuhn J, Prescott CA. The intererlationship of neuroticism, sex, and stressful life events in the perdiction of episodes of major depression. Am J Psychiatry. 2004;161:631–6. doi: 10.1176/appi.ajp.161.4.631. [DOI] [PubMed] [Google Scholar]
- 41.Riemann D, Voderholzer U. Primary insomnia: a risk factor to develop depression? J Affect Disord. 2003;76:255–9. doi: 10.1016/s0165-0327(02)00072-1. [DOI] [PubMed] [Google Scholar]
- 42.Capuron L, Ravaud A, Miller AH, Dantzer R. Baseline mood and psychosocial characteristics of patients developing depressive symptoms during interleukin-2 and/or interferon-alpha cancer therapy. Brain Behav Immun. 2004;18:205–13. doi: 10.1016/j.bbi.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 43.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatric Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 44.Costa PT, McCrae RR. NEO PI-R Professional Manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- 45.Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, et al. Rating chronic medical illness burden in geropsychiatric practice and research: Application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41:237–48. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 46.Wendland JR, Martin BJ, Kruse MR, Lesch KP, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Mol Psychiatry. 2006;11:224–6. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- 47.Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Mol Psychiatry. 2008;13:131–46. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- 48.Kraus MR, Al-Taie O, Schefer A, Pfersdorff M, Lesch KP, Scheurlen M. Serotonin-1A receptor gene (HTR1A) vairation predicts interferon-induced depression chronic hepatitis C. Gastroenterol. 2007;132:1279–86. doi: 10.1053/j.gastro.2007.02.053. [DOI] [PubMed] [Google Scholar]
- 49.Morikawa O, Sakai N, Obara H, Saito N. Effects of interferon-alpha, interferon-gamma and cAMP on the transcriptional regulation of the serotonin transporter. Eur J Pharmacol. 1998;349:317–24. doi: 10.1016/s0014-2999(98)00187-3. [DOI] [PubMed] [Google Scholar]
- 50.De La Garza RI, Asnis GM. The non-steroidal anti-inflammatory drug diclofenac sodium attenuates IFN-alpha induced alterations to monoamine turnover in prefrontal cortex and hippocampus. Brain Res. 2003;977:70–9. doi: 10.1016/s0006-8993(03)02757-4. [DOI] [PubMed] [Google Scholar]
- 51.Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry. 2002;7:468–73. doi: 10.1038/sj.mp.4000995. [DOI] [PubMed] [Google Scholar]