Abstract
Objective
Antiretroviral (ARV) therapies fail when behavioral or biologic factors lead to inadequate medication exposure. Currently available methods to assess ARV exposure are limited. Levels of ARVs in hair reflect plasma concentrations over weeks to months and may provide a novel method for predicting therapeutic responses.
Design/methods
The Women's Interagency HIV Study, a prospective cohort of HIV-infected women, provided the basis for developing and assessing methods to measure commonly-prescribed protease inhibitors (PIs) - lopinavir (LPV) and atazanavir (ATV) - in small hair samples. We examined the association between hair PI levels and initial virologic responses to therapy in multivariate logistic regression models.
Results
ARV concentrations in hair were strongly and independently associated with treatment response for 224 women starting a new PI-based regimen. For participants initiating LPV/RTV, the odds ratio (OR) for virologic suppression was 39.8 (95%CI 2.8–564) for those with LPV hair levels in the top tertile (>1.9ng/mg) compared to the bottom (≤0.41ng/mg) when controlling for self-reported adherence, age, race, starting viral load and CD4, and prior PI experience. For women starting ATV, the adjusted OR for virologic success was 7.7 (95%CI 2.0-29.7) for those with hair concentrations in the top tertile (>3.4ng/mg) compared to the lowest (≤1.2ng/mg).
Conclusions
PI levels in small hair samples were the strongest independent predictor of virologic success in a diverse group of HIV-infected adults. This noninvasive method for determining ARV exposure may have particular relevance for the epidemic in resource-poor settings due to the ease of collecting and storing hair.
Keywords: Hair levels, therapeutic drug monitoring, antiretroviral exposure, virologic response, protease inhibitors, atazanavir, lopinavir, WIHS cohort
Introduction
Highly active antiretroviral therapies (HAART) continue to markedly reduce the morbidity and mortality of HIV infection in treated populations[1]. Treatment outcomes on an individual basis, however, vary widely. Response to treatment is influenced by interindividual differences in drug exposure, but an optimal method to determine antiretroviral exposure has not been elucidated.
Although commonly used as surrogates for antiretroviral exposure, adherence measures are imperfect indicators of the amount of drug that eventually reaches the site of viral activity. Not only are methods of evaluating adherence limited by patient recollection and accuracy[2], but adherence measurements do not account for interindividual biologic variability in the amount of drug that is absorbed and eliminated[3-5]. Measurement of plasma drug levels as a method of therapeutic drug monitoring (TDM) has had inconsistent success in predicting treatment outcomes[6-9], probably because single plasma levels only represent a small window of exposure. Other limitations of blood levels for TDM include significant intraindividual variation in plasma ARV levels[10], “white-coat” effects[11], in which adherence just prior to medical appointments improves above average, and imprecision in reporting the timing of recent doses.
Many drugs are incorporated into hair as it grows, but use of hair specimen testing has largely been limited to forensic applications[12]. Recently, the potential utility of hair specimen assays in the assessment of exposure to chronically administered medications has been reported[13-15], including testing for indinavir[16-19], an HIV protease inhibitor. In a manner analogous to glycosylated hemoglobin A1C providing information on average glucose levels over prolonged periods of time[20], the concentration of medications in hair reflects drug uptake from the systemic circulation over weeks to months. Therefore, drug levels in hair provide an advantage over single plasma drug concentrations in estimating an average level of medication exposure[13].
The use of hair specimens for therapeutic drug monitoring could have broad applicability because hair collection and storage is simple and noninvasive, and hair levels can provide information about cumulative exposure to chronically administered medications in a single assay. While this method would have a clear utility for HIV treatment monitoring in developed counties, it also may also provide a unique approach for monitoring treatment outcomes in resource poor settings where virologic monitoring can be prohibitively expensive.
We developed new laboratory techniques for measuring the most commonly-used protease inhibitors (PIs) in HIV treatment using very small amounts of hair and simple methods of sample preparation. We then examined the relationship between concentrations of these PIs (lopinavir/ritonavir and atazanavir) in hair and virologic outcomes in a cohort of treatment-experienced HIV-infected women.
Methods
Study sample
For the purposes of assay development, we collected full sets of scalp hair from ten HIV-positive volunteers on HAART who exhibited optimal virologic responses to therapy and consented to have their heads shaved[21]. Most of these participants were recruited by providers at the San Francisco Veteran's Administration (VA) Hospital as approved by both the University of California, San Francisco (UCSF) and VA Medical Center review boards. Testing of the relationship between antiretroviral levels measured in hair and viral load outcomes utilized specimens collected longitudinally from participants in the Women's Interagency HIV study (WIHS), an ongoing multicenter, prospective cohort of HIV-infected (and at-risk uninfected) women established in 1994[22, 23]. Every six months, WIHS participants are seen for a study visit comprised of an extensive interviewer-administered questionnaire, physical examination, and specimen collection.
Between October 2003 and October 2005, 70 women in WIHS initiated a lopinavir/ritonavir (LPV/RTV)-based regimen for the first time. Between October 2004 and October 2006, 154 women in WIHS initiated an atazanavir (ATV)-based regimen. Of the 154 women on atazanavir, 129 were concomitantly taking ritonavir. We analyzed hair levels of LPV, RTV, and ATV in these women at the semiannual WIHS visit following the initiation of the target PI and analyzed the relationship between drug levels in hair and virologic success. WIHS study protocols and consent materials were reviewed and approved by institutional review boards at all of the participating institutions.
WIHS hair specimen collection and processing
Hair was collected in the following manner: (1) 10-20 strands of hair (approximately 1-3 milligrams) are cut from underneath the top layer of hair (to eliminate environmental effects[24]) and from the occiput, an area with less variability in hair growth rates than other regions [25, 26]; (2) The small thatch of hair is cut with clean scissors as close to the scalp as possible. The distal portion is labeled since drug concentrations may be highest in proximal specimens, depending on the date medications were started; (3) The hair sample is placed in aluminum foil to avoid excessive light exposure and stored at room temperature in a plastic bag with a desiccant until analysis.
The development of methods for measuring ARVs in small samples of hair is summarized in a separate laboratory methods paper[21] and includes assessment of the most efficient means of hair extraction and analysis, as well as details on calibration standards and quality assurance controls. Briefly, the methods established for lopinavir, ritonavir and atazanavir extraction and analysis are as follows: two milligrams (mg) of cut hair (∼5-10 strands) are placed into a test tube and 1 milliliter (ml) of methanol (MeOH) is added as the organic solvent for lopinavir and ritonavir. For the maximally efficient extraction of atazanavir, a more acidified organic solvent, specifically methanol/trifluoroacetic acid (TFA) at 9:1, was used. After the initial extraction with organic solvent at 37°C overnight (∼14 hours), the samples are then extracted under weak alkaline conditions using methyl tertiary-butyl ether/ethyl acetate (1/1).
After the second extraction, the samples are reconstituted with 0.2 ml of 50% methanol and 10 microliters (μl) are injected into a liquid chromatography/tandem mass spectrometer (LC/MS/MS) system for analyzing drug concentrations in the same manner as in plasma[27-31]. The extracted ritonavir, atazanavir and lopinavir from hair are separated by reversed phase chromatography and detected by tandem mass spectrometry in electrospray positive ionization with multiple reaction monitoring (MRM) mode. Using 2 mg of human hair, RTV is detected as low as 0.01ng/mg hair, while LPV and ATV are detected as low as 0.05 ng/mg hair. This method has been validated from 0.01 to 4.0 ng/mg hair for RTV and 0.05 to 20 ng/mg hair for LPV and ATV with good linearity (R2>0.99) and reproducibility (coefficient of variation or CV < 17% for LPV; CV < 14% for ATV and RTV). No significant matrix ionization suppression was observed.
Statistical Analysis
The aim of our study was to evaluate the association between antiretroviral levels in hair and initial virologic outcome in WIHS participants initiating a new LPV or ATV-based regimen. Virologic success was defined as achieving a viral load of <80 copies/mL or more than a 2 log10 (100-fold) drop in viral load from the time of regimen initiation to when the drug was measured in hair at the subsequent WIHS visit (∼six months)[32].
The Wilcoxon rank-sum test was used to assess whether the distribution of hair levels in virologic successes differed from those in the failures. Multivariate logistic regression models were used to estimate the association of hair drug levels with the dichotomous outcome of virologic response. Included in these models were variables that could impact virologic response, including age, race, viral load at the time of regimen initiation (continuous or dichotomized), prior ARV experience (dichotomized as yes/no) and degree of PI experience (categorized into naïve to PIs, experience with one PI, or experience with two or more PIs in past), nadir and pre-treatment CD4 cell counts, and self-reported adherence. Core WIHS visits are approximately 6 months apart, but time between visits vary by participant, so the total time on drug was calculated and also assessed as a covariate. Adherence to the target PI was reported by the participant as the percentage of prescribed doses consumed over 6 months, 30 days or 3 days; visual analog scales were used to aid women in estimating percentages[33]. The level of adherence was either analyzed as a continuous measure or dichotomized into ≥95% or <95% over the time interval assessed.
Because LPV/RTV is a combination tablet, hair levels of each of these agents are substantially collinear, so separate models were run with LPV and RTV. Separate models were also run for the 129 women taking RTV in the group on ATV-based HAART.
Results
Hair collection
Collection of hair specimens in WIHS for participants on treatment was implemented in April 2003 and is ongoing. Of note, 87% of women consent to sampling of these small amounts (10-20 strands) of hair at each visit, indicating a high degree of acceptance for this noninvasive specimen collection. Providing information that the scalp normally loses approximately 100 hairs per day[34] has aided in acceptability of collection.
Participant demographics
Table 1 summarizes the demographic and covariate data for the 224 participants (70 women initiating LPV/RTV and 154 women initiating ATV-based regimens), including the distribution in tertiles of PI concentrations in hair at the subsequent WIHS visit. The racial/ethnic distribution of the study sample mirrors the demographics of HIV among U.S. women[35], with approximately 60% African-Americans, 23% Hispanics, and 17% Caucasians. Approximately 95% of the combined cohort had experience with one or more ARV in the past, and less than one-third of participants were naïve to PIs. The median time on the new HAART regimen prior to the measurement of hair concentrations collectively was 4.5 (range 2.0-11.3) months.
Table 1.
Distribution of variables in the participants on lopinavir or atazanavir
| Variable | Lopinavir (n = 70) N (%) |
Atazanavir (n = 154) N (%) |
||
|---|---|---|---|---|
| Time period of study | 10/03-10/05 | 2/04-10/06 | ||
| Age in years | ||||
| ≤ 29 | 7 (10) | 9 (6) | ||
| 30 – 39 | 25 (36) | 51 (33) | ||
| 40 – 49 | 29 (41) | 61 (40) | ||
| ≥ 50 | 9 (13) | 33 (21) | ||
| Ethnicity | ||||
| African American | 38 (54) | 29 (19) | ||
| Latina/Hispanic | 22 (32) | 35 (23) | ||
| White | 9 (13) | 88 (57) | ||
| Other | 1 (1) | 2 (1) | ||
| Pre-treatment HIV RNA level | ||||
| ≥ 100,000 copies/ml | 10 (14) | 22 (14) | ||
| < 100,000 copies/ml | 60 (86) | 132 (86) | ||
| log10viral load median (range) | 4.18 (1.90-6.49) | 3.96 (1.90-6.40) | ||
| Past protease inhibitor experience | ||||
| Naïve to PIs | 22 (31) | 36 (23) | ||
| Exposure to single PI | 27 (39) | 60 (39) | ||
| Exposure to 2 or more PIs | 21 (30) | 58 (38) | ||
| Adherence over past 6 months (self-report) | ||||
| <95% | 25 (36) | 34 (22) | ||
| ≥95% | 45 (64) | 120 (78) | ||
| Pre-treatment CD4 count (cells/mm3) | ||||
| <200 | 16 (23) | 41 (27) | ||
| ≥200 | 54 (77) | 113 (73) | ||
| CD4 median (range) | 278 (9-884) | 274 (5-2046) | ||
| Number in each tertile of hair level | Hair level (ng/mg) |
Hair level (ng/mg) |
||
| Lowest tertile | 23 (33) | ≤ 0.41 | 51 (33) | ≤ 1.19 |
| Middle tertile | 23 (33) | 0.41-1.86 | 52 (34) | 1.19-3.43 |
| Highest tertile | 24 (34) | >1.86 | 51 (33) | >3.43 |
Distribution of PI hair concentrations by virologic outcome
Patients were classified as virologic successes or failures based on their viral load response at the visit following initiation of regimens containing the PI of interest. Based on this definition, 52 women (74%) on LPV/RTV and 122 women (79%) on ATV had a successful virologic response. Figures 1a and 1b show the distribution of LPV and ATV hair concentrations, respectively, in the virologic successes versus the failures. LPV concentrations in hair were significantly higher in virologic successes versus failures (median 1.58ng/mg versus 0.290ng/mg, Wilcoxon rank-sum test p 0.0008). The distribution of ATV concentrations was also significantly higher in virologic successes versus failures (median 2.60ng/mg versus 0.669ng/mg, Wilcoxon rank-sum test p < 0.0001). Hair concentrations of RTV in the 70 patients on LPV/RTV and the 129 participants on ATV who were also on RTV were also significantly higher in the virologic successes than failures (Wilcoxon rank-sum test p = 0.005 for LPV/RTV; p 0.0017 for ATV/RTV).
Figure. 1.
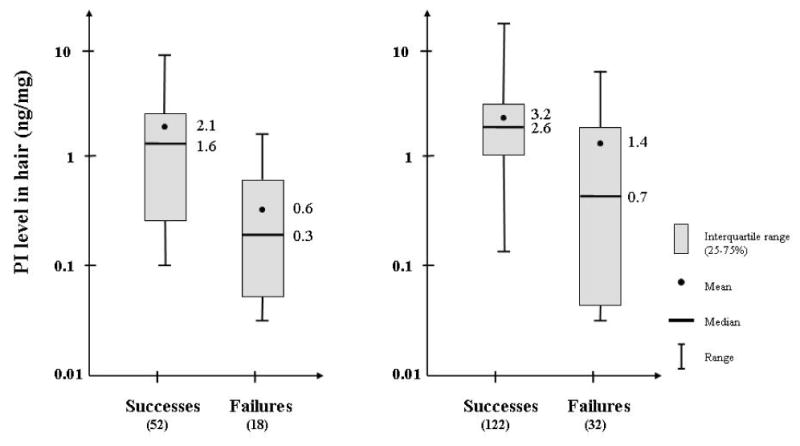
Boxplots showing distribution of lopinavir (Figure 1a) and atazanavir (Figure 1b) hair concentrations (mean, median, interquartile ranges, ranges) in virologic successes versus failures
Multivariate logistic regression models of virologic response
Participants on LPV/RTV
Table 2 shows the estimated associations of various covariates, including LPV or ATV levels in hair, with the outcome of virologic response early during therapy. Hair levels of the protease inhibitor showed the strongest association with virologic response in each multivariate model. For each doubling of LPV level, the odds ratio for virologic response was 2.1 (95% CI 1.32-3.5, p=0.002). The linearity assumption for logarithmically transformed hair levels was assessed by addition of a quadratic term, which did not show strong evidence for non-linearity (p=0.99). Therefore, we examined the association between category of hair level, as assessed in tertiles, and virologic response. The adjusted odds ratios for virologic success increased by tertile of hair concentration: the odds ratio for virologic success was 39.8 (95% CI 2.8 – 564, p 0.006) for those with LPV hair levels in the top tertile for the group (>1.86ng/mg) compared to the bottom tertile (≤0.41ng/mg) when controlling for self-reported adherence, age, race, starting viral load and CD4 count, total time on drug, and prior antiretroviral experience.
Table 2.
Multivariate Logistic Regression Models of Virologic Response
| Variable | Lopinavir (n=70) | Atazanavir (n=154) | ||
|---|---|---|---|---|
| Adjusted odds ratios1 (±95%CI) | p-value | Adjusted odds ratios1 (±95%CI) | p-value | |
| Pre-treatment HIV RNA level ≥ 100,000 copies/ml vs <100,000 | 0.11 (0.013 –0.93) | 0.042 | 1.38 (0.37 – 5.11) | 0.63 |
| Adherence over past 6 months (self-report) ≥95% vs <95% | 2.9 (0.74 – 11.3) | 0.13 | 2.7 (1.04 – 6.9) | 0.042 |
| Pre-treatment CD4 count (cells/mm3) <200 vs ≥200 | 0.15 (0.033 – 0.66) | 0.012 | 0.36 (0.14 – 0.93) | 0.036 |
| Per tertile of hair level | ||||
| Lowest tertile | 1.00 | 1.00 | ||
| Middle tertile | 2.6 (0.59 – 11.9) | 0.21 | 2.7 (1.00 – 7.3) | 0.050 |
| Highest tertile | 39.8 (2.8 - 564) | 0.006 | 7.7 (2.0 – 29.7) | 0.003 |
Adjusted for age, race, exact number of months on therapy and degree of PI experience
Models using RTV levels from participants on LPV/RTV or ATV/RTV yield similar results
Self reported adherence was moderately associated with treatment outcome. The odds of achieving virologic success were 2.89 (95% CI 0.74-11.3, p 0.13) times greater in women with self-reported adherence of ≥95% than women with adherence levels <95%. Models with measures of adherence presented continuously or assessed over shorter time intervals produced similar results. An HIV RNA level above 100,000 copies/ml and a CD4 cell count < 200 cells/mm3 upon treatment initiation were each associated with a lower odds of virologic success. Age, race, and past experience with PIs did not reach statistical significance in terms of association with response. Similar results were seen for the RTV hair levels in the patients treated with LPV/RTV, where adherence measurements, pre-treatment HIV viral loads and CD4 cell counts, and hair concentrations of RTV were independently associated with virologic response.
Because there were only 18 virologic non-responders among the women who reported taking LPV/RTV, we also fitted more parsimonious models with both medications by dropping the variables with the largest p-values, one by one, until only adherence and LPV (or RTV) level remained. In each of the resulting models, results were similar.
Participants on ATV-based regimens
In multivariate logistic regression models, the odds of achieving virologic success increased by a factor of 1.61 (95% CI 1.28-2.03, p<0.0001) for each doubling of ATV level in hair. Since the linearity assumption for logarithmically transformed hair levels was verified (p=0.84 for non-linearity), we then looked at the association of category of hair level with response. The adjusted odds ratios for virologic success increased by tertile of hair concentrations: for women starting ATV, the OR for virologic success was 7.74 (2.01-29.7, p 0.003) for those in the third tertile (>3.43ng/mg) compared to the lowest (≤1.19ng/mg). Self-reported adherence and pre-treatment CD4 cell counts were also significantly associated with treatment outcome. Age, race, pre-treatment HIV RNA level, and degree of experience with PIs appeared to have little association with the outcome of virologic success. Similar results were seen for the 129 patients in the ATV-treated group who were concomitantly on RTV and again, more parsimonious models with fewer covariates yielded similar results.
Discussion
In a cohort of HIV-infected women initiating new PI-based combination regimens, the strongest independent predictor for virologic response in adjusted analyses was hair concentrations of the anchor drug. Monitoring drug exposure may be important for chronically-administered medications when drug exposure is variable and the consequences of treatment failure are high. Interest in therapeutic drug monitoring using hair levels has recently extended to anticonvulsant[15, 36, 37] and psychotropic[38-41] medications. Similar to ARVs, the therapeutic index of these medications is narrow, intra-individual variation in drug levels is significant[10, 42], patient adherence greatly influences efficacy, drug-drug interactions alter pharmacokinetics[43], and administration of these medications is often lifelong, making dose optimization important.
Despite its potential importance, there is no gold standard or even optimal method for the assessment of drug exposure in the field of HIV therapeutics. Self-reported adherence can be a poor surrogate for assessing ARV exposure in situations where inaccuracy of reporting is likely, drug formulations are unpredictable and parameters of bioavailability or clearance vary. Single or infrequent plasma levels of ARVs represent only a brief snapshot of drug exposure and have not consistently contributed to improving treatment outcomes. Hair levels of drug may be superior to plasma measurements in providing a prolonged assessment of drug exposure, analogous to the advantage of hemoglobin A1C (HbA1C) monitoring over single glucose levels in predicting long term outcomes of diabetes mellitus[20].
Another research group has examined levels of a indinavir in hair samples and correlated these levels with virologic responses[16-19]; concentrations of indinavir (IDV) in hair showed a stronger correlation with virologic suppression than plasma IDV levels in 43 HIV-infected patients [19]. This study had several limitations: relatively large thatches of hair were required to measure IDV concentrations, which may limit the acceptability of repeated collection; lengthy sample preparation procedures were required prior to hair analysis; the relative value of hair concentrations versus self-reported adherence to HAART was not assessed; and PIs in more prevalent use in the current era were not studied.
Our study is the first to demonstrate quantification of the most commonly prescribed PIs in small hair samples (approximately 1-5mg or between 10-20 strands) using similar methods to measuring plasma levels. LC-tandem MS is a well-described method for drug level determination[27], available in commercial laboratories, and can be performed in laboratories distant from sites of hair collection. The collection of small amounts of hair may be more acceptable to patients and our sample preparation and analysis methods are simple and inexpensive.
Unlike phlebotomy, hair collection is noninvasive and does not require specific skills, sterile or designated equipment, or storage materials. The collection of hair samples for analysis of ARV levels merely requires a pair of scissors and aluminum foil for storage. The drug-protein complex in hair is highly stable, so that hair does not require immediate processing after collection. Hair can be stored for indefinite periods of time at room temperature and shipped without precautions for biohazardous materials, offering additional feasibility advantages over blood levels for TDM. These features of this monitoring tool may make hair measurement of ARVs a useful method for assessing exposure in the developing world, where hair levels can be collected on-site and sent to outside laboratories for analysis. This approach may also be helpful when specimen collection is difficult, such as in pediatrics or when drug exposure is unpredictable, such as during pregnancy[44] or with multiple drug-drug interactions.
Assessing hair exposure of ARVs in resource-constrained settings may be cost-effective when HIV RNA quantification is too expensive for routine monitoring. In these settings, treatment failure may be detected late, after the accumulation of multiple viral resistance mutations[45]. The cost of hair collection is nominal; non-biohazardous shipping costs are inexpensive; and a high through-put hair analysis laboratory can perform the test economically. One possible algorithm for testing would involve measuring hair ARV levels a few months after starting a new ARV regimen and only performing HIV viral load testing if the hair levels fall below the range observed to predict virologic success in treated populations. After a patient is on stable HIV therapy, hair ARV measurements need not be performed routinely, but only when clinical disease progression is observed or when an alteration in drug exposure is predicted, such as a new drug-drug interaction, pregnancy, change in dietary patterns, change in liver or renal function, etc.
The association of both adherence and hair levels of protease inhibitors with virologic control in our models indicates that exposure is a function of both behavioral and biologic factors. Drug levels measured in hair therefore add unique information not provided by measures of adherence. Careful assessment of adherence is indicated, and consideration of biologic factors that impede bioavailability or increase clearance should be considered, such as concomitant use of an interacting drug. Low levels of drug in hair in patients who profess optimal adherence may also prompt more comprehensive pharmacokinetic evaluation.
Prolonging the success of current and novel HIV medications is important in treating the burgeoning HIV epidemic worldwide. We have developed methods to monitor HIV drug exposure in hair and shown these levels to be the strongest predictor of treatment response in multivariate modeling. The field of HIV diagnostics lacks an optimal method for assessing exposure to medications, and hair levels have the potential of addressing that gap. Further study of this tool for therapeutic drug monitoring is indicated to demonstrate its utility in enhancing treatment responses in the global HIV setting.
Acknowledgments
Authors' contributions: MG developed the study protocol, provided study oversight, designed the analysis plan, interpreted the data, and wrote the paper. MG and RMG contributed to the study concept. NA and PB provided data management, performed most of the statistical analyses, and edited the draft. RMG, SJG, KA, AL, CHL, MC, MY contributed to the data interpretation and critically revised the manuscript. YH developed the laboratory methods for analysis of antiretroviral levels in hair.
Funding: Data in this manuscript were collected by the Women's Interagency HIV Study (WIHS) Collaborative Study Group. The WIHS is funded by the National Institute of Allergy and Infectious Diseases (NAIAD) with supplemental funding from the National Cancer Institute, the National Institute on Drug Abuse (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590). Funding is also provided by the National Institute of Child Health and Human Development (UO1-CH-32632) and the National Center for Research Resources (MO1-RR-00071, MO1-RR-00079, MO1-RR-00083). Dr. Gandhi was supported by a Mentored Patient-Oriented Research Career Development Award (K23 A1067065) from NIAID. A research grant from Bristol Meyers Squibb partially funded the development of hair methods for atazanavir.
Footnotes
All authors declare they have no conflict of interests. Data was previously presented as an oral abstract at the 14th Conference on Retroviruses and Opportunistic Infections, February 25-28, Los Angeles, CA 2007 [Gandhi et al. Concentrations of Lopinavir and Ritonavir in Hair Are Strongly Correlated with Virologic Success (oral presentation, paper#51)]
References
- 1.Lima VD, Hogg RS, Harrigan PR, Moore D, Yip B, Wood E, Montaner JS. Continued improvement in survival among HIV-infected individuals with newer forms of highly active antiretroviral therapy. AIDS. 2007;21:685–692. doi: 10.1097/QAD.0b013e32802ef30c. [DOI] [PubMed] [Google Scholar]
- 2.Berg KM, Arnsten JH. Practical and conceptual challenges in measuring antiretroviral adherence. J Acquir Immune Defic Syndr. 2006;43 Suppl 1:S79–87. doi: 10.1097/01.qai.0000248337.97814.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Back D, Khoo S, Gibbons S, Merry C. The role of therapeutic drug monitoring in treatment of HIV infection. Br J Clin Pharmacol. 2001;52:89S–96S. doi: 10.1046/j.1365-2125.2001.0520s1089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marzolini C, Buclin T, Decosterd L, Biollaz J, Telenti A. Nelfinavir plasma levels under twice-daily and three-times-daily regimens: high interpatient and low intrapatient variability. Ther Drug Monit. 2001;23:394–398. doi: 10.1097/00007691-200108000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Stahle L, Moberg L, Svensson JO, Sonnerborg A. Efavirenz plasma concentrations in HIV-infected patients: inter- and intraindividual variability and clinical effects. Ther Drug Monit. 2004;26:267–270. doi: 10.1097/00007691-200406000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Clevenbergh P, Boulme R, Kirstetter M, Dellamonica P. Efficacy, safety and predictive factors of virological success of a boosted amprenavir-based salvage regimen in heavily antiretroviral-experienced HIV-1-infected patients. HIV Med. 2004;5:284–288. doi: 10.1111/j.1468-1293.2004.00222.x. [DOI] [PubMed] [Google Scholar]
- 7.Clevenbergh P, Garaffo R, Durant J, Dellamonica P. PharmAdapt: a randomized prospective study to evaluate the benefit of therapeutic monitoring of protease inhibitors: 12 week results. AIDS. 2002;16:2311–2315. doi: 10.1097/00002030-200211220-00011. [DOI] [PubMed] [Google Scholar]
- 8.Wertheimer BZ, Freedberg KA, Walensky RP, Yazdanapah Y, Losina E. Therapeutic drug monitoring in HIV treatment: a literature review. HIV Clin Trials. 2006;7:59–69. doi: 10.1310/hct.2006.7.2.004. [DOI] [PubMed] [Google Scholar]
- 9.Demeter L, Jiang H, Mukherjee L, Morse G, DiFrancesco R, Klingman K, et al. A Prospective, Randomized, Controlled, Open-label Trial Evaluating the Effect of Therapeutic Drug Monitoring and Protease Inhibitor Dose Escalation on Viral Load Responses in Antiretroviral-experienced, HIV-infected Patients with a Normalized Inhibitory Quotient. 15th Conference on Retroviruses and Opportunistic Infections; February 3-6; Boston, MA. 2008. [Google Scholar]
- 10.Nettles RE, Kieffer TL, Parsons T, Johnson J, Cofrancesco J, Jr, Gallant JE, et al. Marked intraindividual variability in antiretroviral concentrations may limit the utility of therapeutic drug monitoring. Clin Infect Dis. 2006;42:1189–1196. doi: 10.1086/501458. [DOI] [PubMed] [Google Scholar]
- 11.Podsadecki T, Vrijens B, Touseet E, Rode R, Hanna G. White Coat Compliance Patterns Make Therapeutic Drug Monitoring (TDM) a Potentially Unreliable Tool for Assessing Long-Term Drug Exposure. 13th Conference on Retroviruses and Opportunistic Infections; Denver. February 5-8; 2006. [Google Scholar]
- 12.Pepin G, Gaillard Y. Concordance between self-reported drug use and findings in hair about cocaine and heroin. Forensic Sci Int. 1997;84:37–41. doi: 10.1016/s0379-0738(96)02046-4. [DOI] [PubMed] [Google Scholar]
- 13.Beumer JH, Bosman IJ, Maes RA. Hair as a biological specimen for therapeutic drug monitoring. Int J Clin Pract. 2001;55:353–357. [PubMed] [Google Scholar]
- 14.Nakahara Y. Hair analysis for abused and therapeutic drugs. Journal of Chromatography B, Biomedical Sciences and Applications. 1999;733:161–180. doi: 10.1016/s0378-4347(99)00059-6. [DOI] [PubMed] [Google Scholar]
- 15.Tsatsakis AM, Psillakis T, Paritsis N. Phenytoin concentration in head hair sections: a method to evaluate the history of drug use. Journal of Clinical Psychopharmacology. 2000;20:560–573. doi: 10.1097/00004714-200010000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Bernard L, Vuagnat A, Peytavin G, Hallouin MC, Bouhour D, Nguyen TH, et al. Relationship between levels of indinavir in hair and virologic response to highly active antiretroviral therapy. Annals of Internal Medicine. 2002;137:656–659. doi: 10.7326/0003-4819-137-8-200210150-00009. [DOI] [PubMed] [Google Scholar]
- 17.Bernard L, Peytavin G, Vuagnat A, de Truchis P, Perronne C. Indinavir concentrations in hair from patients receiving highly active antiretroviral therapy. Lancet. 1998;352:1757–1758. doi: 10.1016/S0140-6736(05)79831-7. [DOI] [PubMed] [Google Scholar]
- 18.Servais J, Peytavin G, Arendt V, Staub T, Schneider F, Hemmer R, et al. Indinavir hair concentration in highly active antiretroviral therapy-treated patients: association with viral load and drug resistance. AIDS. 2001;15:941–943. doi: 10.1097/00002030-200105040-00019. [DOI] [PubMed] [Google Scholar]
- 19.Duval X, Peytavin G, Breton G, Ecobichon JL, Descamps D, Thabut G, Leport C. Hair versus plasma concentrations as indicator of indinavir exposure in HIV-1-infected patients treated with indinavir/ritonavir combination. AIDS. 2007;21:106–108. doi: 10.1097/QAD.0b013e3280118486. [DOI] [PubMed] [Google Scholar]
- 20.Koenig RJ, Peterson CM, Jones RL, Saudek C, Lehrman M, Cerami A. Correlation of glucose regulation and hemoglobin AIc in diabetes mellitus. N Engl J Med. 1976;295:417–420. doi: 10.1056/NEJM197608192950804. [DOI] [PubMed] [Google Scholar]
- 21.Huang Y, Gandhi M, Greenblatt RM, Gee W, Lin E, Messenkoff N. Sensitive Analysis of Anti-HIV Drugs, Efavirenz, Lopinavir and Ritonavir, in Human Hair Samples by Liquid Chromatography Coupled with Tandem Mass Spectrometry. Rapid Communications in Mass Spectrometry. 2008 Nov;22(21):3401–9. doi: 10.1002/rcm.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, et al. The Women's Interagency HIV Study. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- 23.Bacon MC, von Wyl V, Alden C, Sharp G, Robison E, Hessol N, et al. The Women's Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12:1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skopp G, Pötsch L, Mauden M. Stability of cannabinoids in hair samples exposed to sunlight. Clinical Chemistry. 2000;46:1846–1848. [PubMed] [Google Scholar]
- 25.Cassani M, Da Re N, Giuliani L, Sesana F. Experience with hair testing in the clinical biochemistry laboratory of Ca' Granda Niguarda Hospital, Milan, Italy. Forensic Science International. 1997;84:17–24. doi: 10.1016/s0379-0738(96)02044-0. [DOI] [PubMed] [Google Scholar]
- 26.Kosuge K, Uematsu T, Araki SI, Matsuno H, Ohashi K, Nakashima M. Comparative dispositions of ofloxacin in human head, axillary, and pubic hairs. Antimicrobial Agents and Chemotherapy. 1998;42:1298–1302. doi: 10.1128/aac.42.5.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egge-Jacobsen W, Unger M, Niemann CU, Baluom M, Hirai S, Benet LZ, Christians U. Automated, fast, and sensitive quantification of drugs in human plasma by LC/LC-MS: quantification of 6 protease inhibitors and 3 nonnucleoside transcriptase inhibitors. Ther Drug Monit. 2004;26:546–562. doi: 10.1097/00007691-200410000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Villani P, Feroggio M, Gianelli L, Bartoli A, Montagna M, Maserati R, Regazzi MB. Antiretrovirals: simultaneous determination of five protease inhibitors and three nonnucleoside transcriptase inhibitors in human plasma by a rapid high-performance liquid chromatography--mass spectrometry assay. Ther Drug Monit. 2001;23:380–388. doi: 10.1097/00007691-200108000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Chi J, Jayewardene AL, Stone JA, Motoya T, Aweeka FT. Simultaneous determination of five HIV protease inhibitors nelfinavir, indinavir, ritonavir, saquinavir and amprenavir in human plasma by LC/MS/MS. J Pharm Biomed Anal. 2002;30:675–684. doi: 10.1016/s0731-7085(02)00357-6. [DOI] [PubMed] [Google Scholar]
- 30.Chi J, Jayewardene AL, Stone JA, Aweeka FT. An LC-MS-MS method for the determination of nevirapine, a non- nucleoside reverse transcriptase inhibitor, in human plasma. J Pharm Biomed Anal. 2003;31:953–959. doi: 10.1016/s0731-7085(02)00708-2. [DOI] [PubMed] [Google Scholar]
- 31.Frerichs VA, DiFrancesco R, Morse GD. Determination of protease inhibitors using liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;787:393–403. doi: 10.1016/s1570-0232(02)01002-4. [DOI] [PubMed] [Google Scholar]
- 32.Department of Health and Human Services and Henry J. Kaiser Family Foundation living document; Guidelines for the use of antiretroviral agents in HIV-infected adolescents and adults. Available on http://aidsinfo.nih.gov. Updated 12/1/07. [Google Scholar]
- 33.Giordano TP, Guzman D, Clark R, Charlebois ED, Bangsberg DR. Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clin Trials. 2004;5:74–79. doi: 10.1310/JFXH-G3X2-EYM6-D6UG. [DOI] [PubMed] [Google Scholar]
- 34.Shapiro J. Clinical practice. Hair loss in women. N Engl J Med. 2007;357:1620–1630. doi: 10.1056/NEJMcp072110. [DOI] [PubMed] [Google Scholar]
- 35.HIV and AIDS in the United States: A Picture of Today's Epidemic (CDC) 2008 http://www.cdc.gov/hiv/topics/surveillance/united_states.htm.
- 36.Williams J, Patsalos PN, Mei Z, Schapel G, Wilson JF, Richens A. Relation between dosage of carbamazepine and concentration in hair and plasma samples from a compliant inpatient epileptic population. Therapeutic Drug Monitoring. 2001;23:15–20. doi: 10.1097/00007691-200102000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Williams J, Patsalos P, Wilson J. Hair analysis as a potential index of therapeutic compliance in the treatment of epilepsy. Forensic Sci Int. 1997;84:113–122. doi: 10.1016/s0379-0738(96)02053-1. [DOI] [PubMed] [Google Scholar]
- 38.Shen M, Xiang P, Wu H, Shen B, Huang Z. Detection of antidepressant and antipsychotic drugs in human hair. Forensic Sci Int. 2002;126:153–161. doi: 10.1016/s0379-0738(02)00051-8. [DOI] [PubMed] [Google Scholar]
- 39.Thieme D, Sachs H. Examination of a long-term clozapine administration by high resolution segmental hair analysis. Forensic Sci Int. 2007;166:110–114. doi: 10.1016/j.forsciint.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 40.Kronstrand R, Nystrom I, Josefsson M, Hodgins S. Segmental ion spray LC-MS-MS analysis of benzodiazepines in hair of psychiatric patients. J Anal Toxicol. 2002;26:479–484. doi: 10.1093/jat/26.7.479. [DOI] [PubMed] [Google Scholar]
- 41.Cirimele V, Kintz P, Gosselin O, Ludes B. Clozapine dose-concentration relationships in plasma, hair and sweat specimens of schizophrenic patients. Forensic Sci Int. 2000;107:289–300. doi: 10.1016/s0379-0738(99)00172-3. [DOI] [PubMed] [Google Scholar]
- 42.Banh HL, Burton ME, Sperling MR. Interpatient and intrapatient variability in phenytoin protein binding. Ther Drug Monit. 2002;24:379–385. doi: 10.1097/00007691-200206000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Spina E, Perucca E. Clinical significance of pharmacokinetic interactions between antiepileptic and psychotropic drugs. Epilepsia. 2002;43 Suppl 2:37–44. doi: 10.1046/j.1528-1157.2002.043s2037.x. [DOI] [PubMed] [Google Scholar]
- 44.Roustit M, Jlaiel M, Leclercq P, Stanke-Labesque F. Pharmacokinetics and therapeutic drug monitoring of antiretrovirals in pregnant women. Br J Clin Pharmacol. 2008;66:179–195. doi: 10.1111/j.1365-2125.2008.03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marcelin AG, Jarrousse B, Derache A, Ba M, Dakouo ML, Doumbia A, et al. HIV drug resistance after the use of generic fixed-dose combination stavudine/lamivudine/nevirapine as standard first-line regimen. AIDS. 2007;21:2341–2343. doi: 10.1097/QAD.0b013e328235a527. [DOI] [PubMed] [Google Scholar]


