Abstract
The lactose permease of Escherichia coli (LacY) is a highly dynamic membrane transport protein. Crystal structures of wild-type and mutant LacY all exhibit an inward-facing conformation with an open cytoplasmic pathway and a tightly packed periplasmic side, which makes the binding site inaccessible from the outside. However, biochemical and biophysical findings provide strong evidence that occupation of the sugar-binding site leads to increased probability of opening of a hydrophilic pathway on the periplasmic side and closing of the cytoplasmic cavity. By this means, the sugar-binding site becomes accessible to either side of the membrane in alternating fashion. In order to extend studies on the relationship between the periplasmic pathway and transport activity, engineered single-Cys replacements in the periplasmic pathway were reacted to completion with thiol reagents, and the effects on transport and sugar binding were tested. Inactivation correlates for the most part with the size of the modifying reagent, although the position of the Cys replacement is also important. However, sugar binding is unaffected. The results suggest that placement of a relatively large moiety in the putative periplasmic cleft of LacY likely prevents closure, an essential step in the transport cycle, without significantly altering access of sugar to the binding site.
The lactose permease of Escherichia coli (LacY), a member of the Major Facilitator Superfamily of membrane transport proteins, couples the stoichiometric translocation of a galactoside and an H+ (reviewed in 1, 2). In this manner, LacY is able to utilize the free energy stored in an electrochemical H+ gradient (Δμ̄H +; interior negative and/or alkaline) to drive accumulation of galactosidic sugars against a concentration gradient. Conversely, in the absence of Δμ̄H +, LacY utilizes the free energy released from downhill translocation of galactosides to drive uphill translocation of H+ with generation of Δμ̄H +, the polarity of which depends on the direction of the sugar gradient.
LacY has been solubilized, purified and reconstituted into proteoliopsomes in a fully functional state (3). X-ray crystal structures of a conformationally restricted mutant (4–9) have been solved in an inward-facing conformation (10, 11), and an X-ray crystal structure of the wild type has the same global fold (12, 13). Each structure exhibits twelve transmembrane helices organized into two pseudo-symmetrical six α-helical bundles surrounding a large interior hydrophilic cavity open to the cytoplasm, which represents an inward-facing conformation. The sugar-binding site and the residues involved in H+ translocation are at the approximate middle of the molecule and distributed such that the side chains important for sugar recognition are in the N-terminal helix bundle, while most of the side chains important for H+ translocation are in the C-terminal bundle. Strikingly, the periplasmic side of LacY is tightly packed, and the sugar-binding site is inaccessible from the periplasm.
Wild-type LacY is dynamic, ligand binding is entropic (7) and induces widespread conformational changes (1, 2, 7, 14, 15). Site-directed alkylation (SDA) (reviewed in 16, 17, 18), single molecule fluorescence resonance energy transfer (smFRET) (8), double electron-electron resonance (DEER) (9) and cross-linking studies (19) provide strong evidence that sugar binding increases the open probability of a relatively wide hydrophilic cleft on the periplasmic side of LacY. Moreover, this cleft must close, as well as open, for translocation of sugar across the membrane to occur (19). Remarkably, despite multiple independent lines of evidence for a hydrophilic pathway that opens upon sugar binding in wild-type LacY, as well as findings demonstrating that the periplasmic pathway in the C154G mutant is paralyzed in the open conformation (8, 9, 18), all X-ray structures of LacY exhibit the same inward-facing conformation. Therefore, it is likely that the crystallization process selects a single conformer of LacY that is in the lowest free energy state.
In this communication, single-Cys replacements in helix VII, one face of which lines the periplasmic pathway (Fig. 1), were labeled with a given thiol reagent (Fig. 2) in the presence of β-D-galactopyranosyl 1-thio-β-D-galactopyranoside (TDG) in order to ensure high accessibility/reactivity of the Cys replacement (16–18). Transport activity or sugar binding to the modified proteins, which were reacted to completion, was then studied in right-side-out (RSO) membrane vesicle preparations that are quantitatively RSO (20–26) and in which each vesicle is active with respect to transport (27). Inhibition of transport activity correlates approximately with the size of the thiol reagent, but shape and/or charge are also probably important. In any case, sugar binding is unaffected despite complete inactivation of transport with the largest thiol reagent. The findings are consistent with the interpretation that when the periplasmic pathway is prevented from closing, the sugar-binding site remains accessible from periplasmic side, but the transport cycle is arrested.
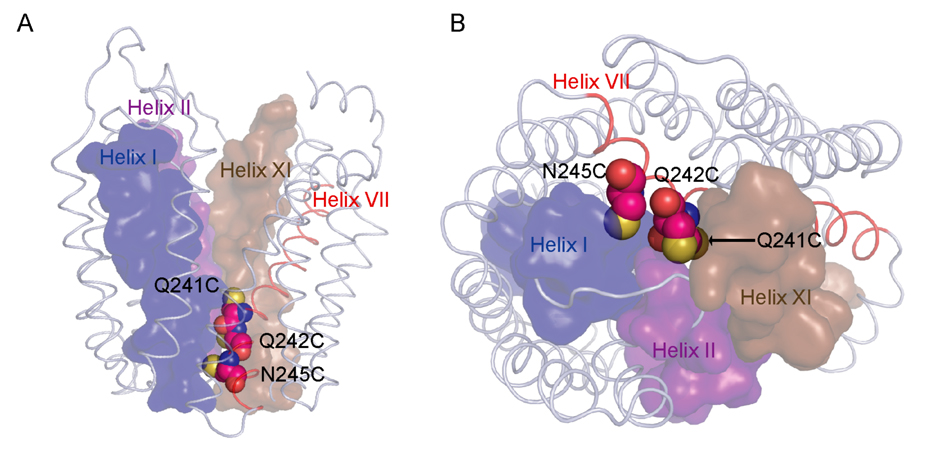
Figure 1.
Q241, Q242 and N245 line the potential periplasmic pathway of LacY. A. LacY is viewed perpendicular to the membrane with the N-terminal helix bundle on the left and the C-terminal bundle on the right. B. LacY is viewed from periplasmic side of the membrane with the N-terminal helix bundle on the left and the C-terminal bundle on the right. Cys replacements (Q241C, Q242C and N245C) at their respective positions are shown as spheres on the backbone of C154G LacY (Protein Data Bank ID code 1PV7; www.pdb.org). Sulfur atoms are shown in yellow. Helices I (blue), II (purple) and XI (light brown) are labeled and shown as surfaces; helix VII (red) is shown as cartoon.
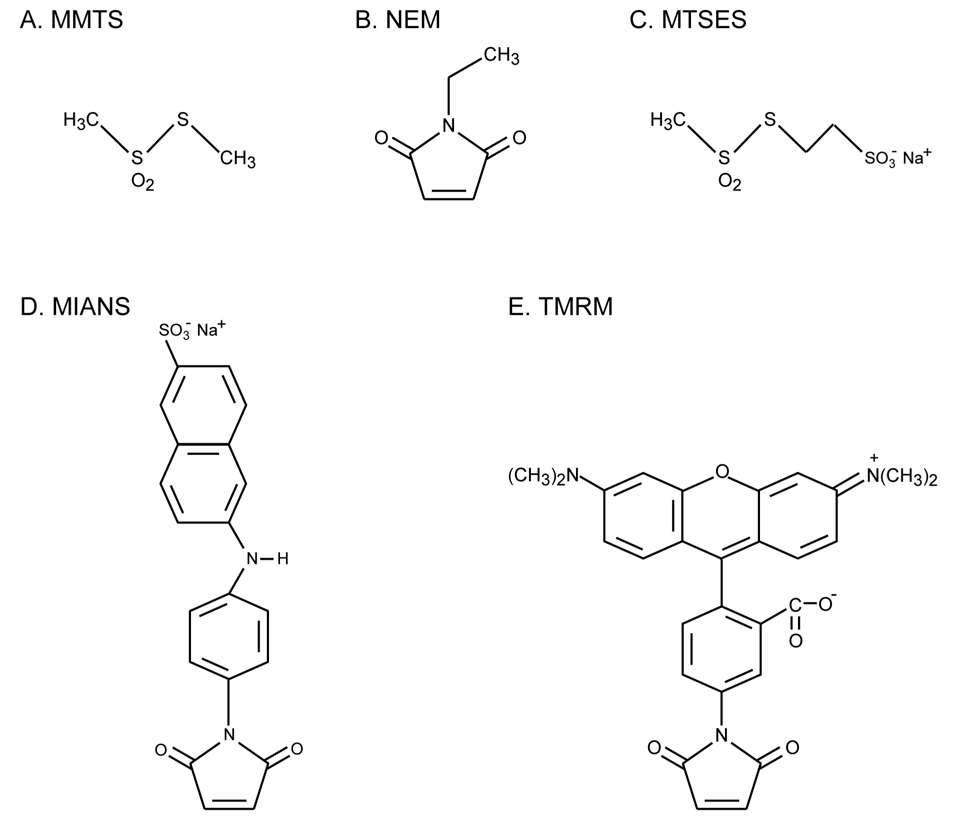
Figure 2.
Thiol reagents
MATERIALS AND METHODS
Materials
Methyl methanethiosulfonate (MMTS, Cat# M321500) and sodium (2-sulfonatoethyl) methanethiosulfonate (MTSES, Cat# S672000) were obtained from Toronto Research Chemicals Inc. (North York, Ontario, Canada). 2-(4'- maleimidylanilino) naphthalene-6-sulfonic acid, sodium salt (MIANS, M-8) and tetramethylrhodamine-5-maleimide (TMRM, T-6027) were obtained from Molecular Probes, Invitrogen Corp. (Carlsbad, CA). N-ethylmaleimide (NEM, Cat# 23030), ImmunoPure immobilized monomeric avidin (Cat# 20228), and avidin-conjugated horseradish peroxidase were obtained from Pierce (Rockford, IL). [1-14C]lactose was purchased from Amersham (Arlington Heights, IL). p-nitrophenyl α-D-[6- 3H]galactopyranoside ([3H]NPG) was kindly provided by Géard Leblanc (Laboratoire J. Maetz/Commissariat a l’Energie Atomique, Ville Franche-sur-Mer, France). Poly(vinylidene difluoride) membranes (Immobilon-PVDF) was from Millipore (Billerica, MA). All other materials were reagent grade and obtained from commercial sources.
Plasmids
Plasmids encoding single-Cys mutants Q241C, Q242C and N245C in Cys-less LacY with a biotin acceptor domain at the C terminus were generated as described (18).
Growth of cells
E. coli T184 (lacY−Z−) transformed with plasmid pT7-5 encoding a given mutant was grown aerobically at 37 °C in Luria-Bertani Broth containing ampicillin (100 µg/ml). Fully grown cultures were diluted 10-fold and grown for 2 h. After induction with 1 mM isopropyl 1-thio-β-D-galactopyranoside for 2 h, cells were harvested and used for the preparation of RSO membrane vesicles.
Preparation of RSO membrane vesicles
RSO membrane vesicles were prepared by lysozyme-ethylenediaminetetraacetic acid (EDTA) treatment and osmotic lysis (20, 21) from 0.8 L cultures of E. coli T184 expressing a given mutant. Vesicles were resuspended to a protein concentration of 10 mg/ml in 100 mM potassium phosphate (KPi, pH 7.5)/10 mM MgSO4, frozen in liquid nitrogen and stored at −80 °C until use.
Effect of thiol reagents on transport activity
Labeling with given thiol reagents was performed following a protocol developed recently (17, 18) with minor modifications. RSO membrane vesicles containing a given single-Cys mutant [1 mg of total protein in 100 µl of 100 mM KPi (pH 7.5)/10 mM MgSO4] were incubated with 1 mM (final concentration) of a given thiol reagent (Fig. 2) in the presence of 10 mM TDG (final concentration) at 25 °C for 30 min. The vesicles were diluted and washed three times with ice-cold 100 mM potassium phosphate (KPi, pH 7.5)/10 mM MgSO4, resuspended in the same buffer and adjusted to an OD600 of 3.0 (3 mg protein/ml). Transport was then assayed by rapid filtration after incubation with 20 mM potassium ascorbate/0.2 mM phenazine methosulfate and 0.4 mM [1-14C]lactose (10 mCi/mmol) under oxygen as described (28).
Thiol labeling of single-Cys mutants
Labeling with TMRM was carried out as described (17, 18). Reaction of non-fluorescent thiol reagents, as well as MIANS, was determined by blockade of TMRM labeling. Briefly, RSO membrane vesicles [0.1 mg of total protein in 50 µl of 100 mM KPi (pH 7.5)/10 mM MgSO4] treated with a given thiol reagent and washed as described above were incubated with 1 mM TMRM in the presence of 10 mM TDG at 25°C for 30 min. Dithiothreitol (10 mM, final concentration) was added to stop the reactions. The membranes were then solubilized in 2% n-dodecyl β-D-maltopyranoside (DDM) and biotinylated LacY was purified with immobilized monomeric avidin sepharose chromatography. Purified proteins (10 µl out of a total of 50 µl) were subjected to sodium dodecyl sulfate-16% polyacrylamide gel electrophoresis (SDS-PAGE), and the wet gels were imaged directly on an Amersham Typhoon™ 9410 Workstation (λex = 532 nm and λem = 580 nm for TMRM).
Western blotting
The SDS-PAGE gels were electroblotted onto poly(vinylidene difluoride) membranes and probed with avidin conjugated to horseradish peroxidase (avidin-HRP) as described (29, Consler, 1993 #95, 30).
Flow Dialysis
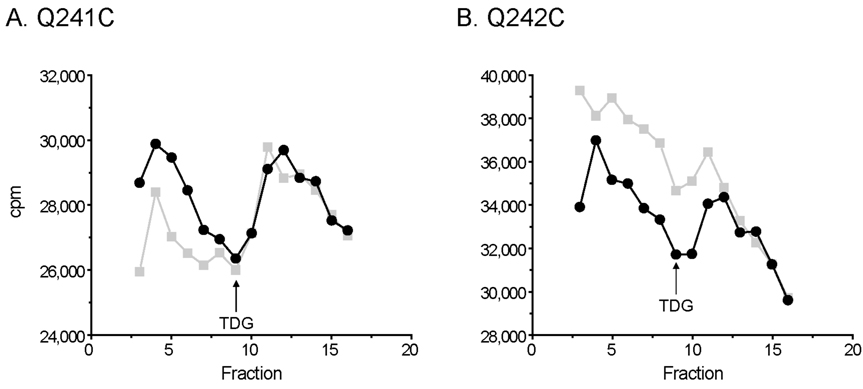
Binding of p-nitrophenyl α-D-[6-3H]galactopyranoside ([3H]NPG) to RSO vesicles containing given LacY mutants was measured by flow dialysis as described (31). RSO vesicles with unlabeled and TMRM-labeled Q241C or Q242C LacY at a protein concentration of 30 mg/ml in the upper chamber of a flow dialysis apparatus were completely de-energized by adding 250 µM valinomycin and 5 µM nigericin. [3H]NPG (840 mCi/mmol; 15 µM, final concentration) was added at fraction 1, and as indicated by the arrows, unlabeled TDG (15 mM, final concentration) was added at fraction 9 in order to displace bound [3H]NPG. One ml fractions were collected, and aliquots (0.9 ml) were assayed for radioactivity by addition of 5 ml of ScintiSafe Econo 2 scintillation mixture and liquid scintillation spectrometry.
RESULTS
Labeling of single-Cys mutants
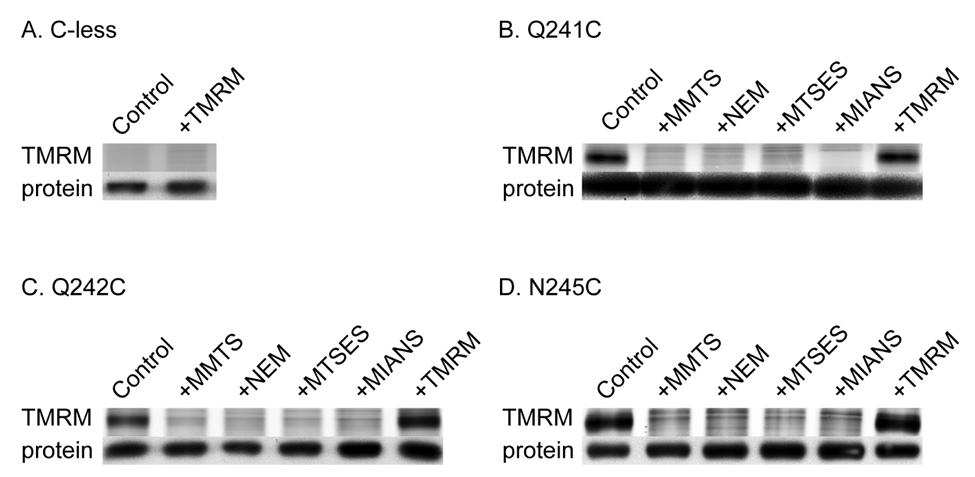
To test labeling of single-Cys mutants Q241C, Q242C and N245C with given thiol reagents (Fig. 2), blockade of TMRM labeling was carried out with RSO membrane vesicles after pretreatment with each of the other thiol reagents as indicated (Fig. 3). No significant labeling of Cys-less LacY is observed with TMRM (panel A). However, with each single-Cys mutant, samples that were not pretreated with reagent are strongly labeled with TMRM (panels B–D, control lanes), while samples pre-treated with MMTS, NEM, MTSES or MIANS exhibit essentially no TMRM labeling (Fig. 3B–3D). In addition, no significant increase in the intensity of TMRM labeling is observed after pretreatment with TMRM (panels B–D, +TMRM lanes), which also indicates that each Cys replacement is labeled to completion.
Figure 3.
Labeling of single-Cys mutants with thiol reagents by blockade of TMRM reactivity. As described in Materials and Methods, RSO membrane vesicles containing Cys-less LacY (A) or single-Cys replacements at positions 241 (B), 242 (C) or 245 (D) were treated with 1 mM MMTS, NEM, MTSES, MIANS, or TMRM, washed and labeled with 1 mM TMRM for 30 min at 25 °C in the presence of 10 mM TDG. Purified proteins were then subjected to SDS-PAGE. LacY bands labeled with TMRM (upper panels) or avidin-conjugated horse radish peroxidase (lower panels) were imaged.
Inhibition of transport activity
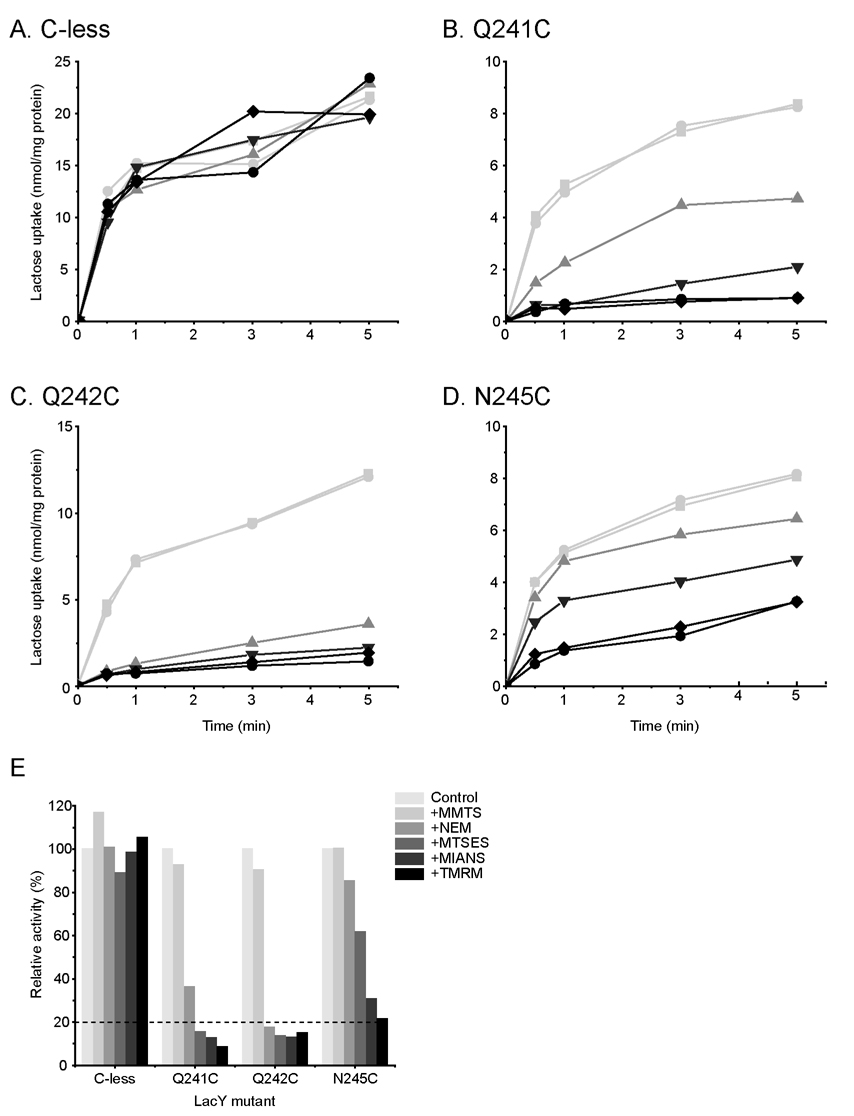
RSO membrane vesicles containing Cys-less LacY or each single-Cys mutant were treated with a given thiol reagent under conditions where reaction is complete and then assayed for Δμ̄H + -driven active lactose transport (Fig. 4). As expected, none of the reagents has a significant effect on the transport activity of Cys-less LacY (panels A & E). With mutant Q241C, transport activity is unaffected by MMTS labeling, but NEM, MTSES, MIANS or TMRM inhibits the rate measured at 30s by ~44%, ~75%, ~87% or ~91%, respectively (panels B & E). With mutant Q242C, activity is not changed significantly by MMTS, but labeling with the other thiol reagents inhibits the rate by more than 80% (panels C & E). With mutant N245C, MMTS again has no effect on the activity, while NEM, MTSES, MIANS or TMRM inhibits by 15%, 38%, 69% or 79%, respectively (panels D & E). Therefore, inactivation of mutant Q241C or N245C correlates for the most part with the size of the reagent and the position of the Cys replacement, although shape and charge differences are probably important as well.
Figure 4.
Transport activity. MMTS-, NEM-, MTSES-, MIANS-, or TMRM-treated RSO membrane vesicles containing Cys-less LacY (A) or single-Cys mutants, Q241C (B), Q242C (C) or N245C (D) were subjected to lactose transport activity assay. (E) Histogram showing relative transport activity. The initial rate of lactose transport at 30 sec is plotted relative to the untreated control value (100%) of each mutant.  , Control;
, Control;  , +MMTS;
, +MMTS;  , +NEM;
, +NEM;  , +MTSES;
, +MTSES;  , +MIANS;
, +MIANS;  , +TMRM. The experiments were performed as described in Materials and Methods.
, +TMRM. The experiments were performed as described in Materials and Methods.
NPG binding
As shown in Fig. 5A, when [3H]NPG is added to de-energized RSO vesicles containing mutant Q241C in the upper chamber of a flow dialysis apparatus, [3H]NPG in the dialysate increases sharply until fraction 4 when the NPG in the medium surrounding the vesicles begins to decrease. At fraction 9, a large excess of non-radioactive TDG is added, and radioactivity in the dialysate increases [from 26,000 cpm at fraction 9 to 29,788 cpm at fraction 11 (~14.6% increase)] as [3H]NPG is displaced from the sugar-binding site in LacY. After labeling with TMRM, the Q241C mutant also shows a similar increase in the dialyzable [3H]NPG concentration after addition of TDG [from 26,354 cpm at fraction 9 to 29,686 cpm at fraction 12 (~12.6% increase)] (panel A). Addition of TDG to mutant Q242C also increases the dialyzable concentration of [3H]NPG [from 34,693 cpm to 36,453 cpm (~5.1% increase)]. After labeling with TMRM, addition of TDG again leads to an increase [from 31,743 cpm to 34,390 cpm (~8.3% increase)] (panel B). The results indicate that modification of mutant Q241C or Q242C with TMRM does not significantly block access of ligand to the sugar-binding site over the time-course of these measurements.
Figure 5.
Effect of TMRM labeling on NPG binding Binding of [3H]NPG to RSO vesicles containing unlabeled and TMRM-labeled Q241C LacY (A) or Q242C LacY mutant (B) was assayed by flow dialysis at a protein concentration of 30 mg/ml as described in Materials and Methods. Only fractions 4 to 16 are shown. [3H]NPG (840 mCi/mmol) at 15 µM (final concentration) was added at fraction 1. As indicated by the arrow, TDG at 15 mM (final concentration) was added at fraction 9 to displace bound NPG.  , unlabeled LacY mutants;
, unlabeled LacY mutants;  , LacY mutants labeled with TMRM.
, LacY mutants labeled with TMRM.
DISCUSSION
In order to catalyze lactose/H+ symport, LacY must exist in at least two conformations to allow access of the sugar-binding site to either side of the membrane. However, all X-ray crystal structures obtained thus far (10, 11, 13) exhibit a structure with a large hydrophilic cavity facing the cytoplasmic side of the molecule in an inward-facing conformation with a tightly packed periplasmic side, which prevents access of sugar to the binding site. Clearly, unless sugar can gain access to the binding site from the outside, translocation across the membrane cannot occur. Recently, four independent lines of evidence—SDA (reviewed in 16, 17, 18), smFRET (8), DEER (9) and thiol cross-linking (19)—support the conclusion that sugar binding results in opening of a relatively wide, hydrophilic cleft on the periplasmic face of LacY. Thus, LacY exists in a minimum of two conformations, inward- and outward-facing, thereby strongly favoring an alternating access model for transport (32–34). In addition, studies with C154G LacY, a conformationally crippled mutant, indicate that the periplasmic cleft in this mutant is fixed in an open position (8, 9, 18). This is not shown in the X-ray crystal structures presumably because cystallization conditions select the lowest free energy conformation (13).
Five thiol reagents were used to label three single-Cys mutants on a face of helix VII that probably lines the periplasmic cleft. Labeling of each mutant to completion in the presence of TDG is demonstrated with each reagent, confirming the increased accessibility of the Cys replacements when the sugar-binding site is occupied (reviewed in 16, 17, 18). The findings also suggest that accessibility/reactivity is independent of the size, shape or charge of the reagent under the conditions tested (i.e., rates were not measured). However, there is an approximate relationship between the size of the reagent and the degree of inhibition of transport with the Q241C and the N245C mutants. No inhibition is observed with the smallest reagent MMTS, which introduces a thiomethyl adduct, with either of the mutants. With NEM or MTSES, the relationship between the size of the adduct and inhibition of transport is reversed. That is, alkylation with NEM, which introduces a larger, but uncharged adduct, is less severe than introduction of the smaller, negatively charged thioethylsulfonate from MMTS. Finally, alkylation with the largest reagents MIANS or TMRM, which are negatively charged or neutral, respectively, is most inhibitory.
With the exception of MMTS, which has no significant effect, the transport activity of the Q242C mutant is markedly inhibited by each of the other reagents. This difference from the other two mutants may be due to variations in the local environment of the three Cys replacements (Fig. 1). Possibly the sulfur atoms are solvated in mutants Q241C and N245C when the periplasmic cleft opens, while the sulfur atom in the Q242C mutant remains in contact with helices II and XI (Fig. 1B). Thus, with mutants Q241C and N245C, the sulfur atoms in the Cys residues of each mutant may be positioned so that there is sufficient space in the open periplasmic cavity to accommodate modification by MTSES or NEM without complete inactivation. However, introduction of any but the smallest adduct inhibits transport in the Q242C mutant perhaps because any adduct larger than a thiomethyl group disturbs the interaction of helix VII the surrounding helices. Remarkably, alkylation of mutant Q241C or Q242C with TMRM, the largest reagent tested, which drastically blocks transport activity, has no significant effect on ligand binding (Fig. 5).
Taken as a whole, the findings suggest that covalent introduction of relatively large adducts into the open periplasmic cleft of LacY can block transport without interfering with access of sugar to the binding site. Therefore, it seems reasonable to deduce that the modifications described interfere with transport by preventing closure of the periplasmic cleft, an essential step in the overall transport cycle. The conclusion is consistent with observations showing that the periplasmic cavity of the crippled mutant C154G LacY, which is paralyzed in an open configuration (9, 18), binds sugar in RSO vesicles (5).
ACKNOWLEDGEMENTS
The authors thank the members of the laboratory for insightful discussions and for critically reading the manuscript.
This work was supported by NIH grants DK051131, DK069463, GM073210 and GM074929, and NSF grant 0450970 to H.R.K.
The abbreviations used are
- LacY
lactose permease
- Cys-less LacY
functional LacY devoid of native Cys residues
- RSO
right-side-out
- TDG
β-D-galactopyranosyl 1-thio-β-D-galactopyranoside
- [3H]NPG
p-nitrophenyl α-D-[6-3H]galactopyranoside
- NEM
N-ethylmaleimide
- MMTS
methyl methanethiosulfonate
- MTSES
sodium (2-sulfonatoethyl) methanethiosulfonate
- MIANS
2-(4'maleimidylanilino) naphthalene-6-sulfonic acid, sodium salt
- TMRM
tetramethylrhodamine-5-maleimide
- DDM
n-dodecyl β-D-maltopyranoside
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis.
REFERENCES
- 1.Kaback HR. Structure and mechanism of the lactose permease. C R Biol. 2005;328:557–567. doi: 10.1016/j.crvi.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Guan L, Kaback HR. Lessons from Lactose Permease. Annu Rev Biophys Biomol Struct. 2006;35:67–91. doi: 10.1146/annurev.biophys.35.040405.102005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viitanen P, Newman MJ, Foster DL, Wilson TH, Kaback HR. Purification, reconstitution, and characterization of the lac permease of Escherichia coli. Methods Enzymol. 1986;125:429–452. doi: 10.1016/s0076-6879(86)25034-x. [DOI] [PubMed] [Google Scholar]
- 4.Menick DR, Sarkar HK, Poonian MS, Kaback HR. Cys154 is important for lac permease activity in Escherichia coli. Biochem Biophys Res Commun. 1985;132:162–170. doi: 10.1016/0006-291x(85)91002-2. [DOI] [PubMed] [Google Scholar]
- 5.Smirnova IN, Kaback HR. A Mutation in the Lactose Permease of Escherichia coli That Decreases Conformational Flexibility and Increases Protein Stability. Biochemistry. 2003;42:3025–3031. doi: 10.1021/bi027329c. [DOI] [PubMed] [Google Scholar]
- 6.Ermolova NV, Smirnova IN, Kasho VN, Kaback HR. Interhelical packing modulates conformational flexibility in the lactose permease of Escherichia coli. Biochemistry. 2005;44:7669–7677. doi: 10.1021/bi0502801. [DOI] [PubMed] [Google Scholar]
- 7.Nie Y, Smirnova I, Kasho V, Kaback HR. Energetics of Ligand-induced Conformational Flexibility in the Lactose Permease of Escherichia coli. J Biol Chem. 2006;281:35779–35784. doi: 10.1074/jbc.M607232200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Majumdar DS, Smirnova I, Kasho V, Nir E, Kong X, Weiss S, Kaback HR. Single-molecule FRET reveals sugar-induced conformational dynamics in LacY. Proc Natl Acad Sci U S A. 2007;104:12640–12645. doi: 10.1073/pnas.0700969104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smirnova I, Kasho V, Choe JY, Altenbach C, Hubbell WL, Kaback HR. Sugar binding induces an outward facing conformation of LacY. Proc Natl Acad Sci U S A. 2007;104:16504–16509. doi: 10.1073/pnas.0708258104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–615. doi: 10.1126/science.1088196. [DOI] [PubMed] [Google Scholar]
- 11.Mirza O, Guan L, Verner G, Iwata S, Kaback HR. Structural evidence for induced fit and a mechanism for sugar/H(+) symport in LacY. Embo J. 2006;25:1177–1183. doi: 10.1038/sj.emboj.7601028. 2006)200251183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan L, Smirnova IN, Verner G, Nagamoni S, Kaback HR. Manipulating phospholipids for crystallization of a membrane transport protein. Proc Natl Acad Sci U S A. 2006;103:1723–1726. doi: 10.1073/pnas.0510922103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan L, Mirza O, Verner G, Iwata S, Kaback HR. Structural determination of wild-type lactose permease. Proc Natl Acad Sci U S A. 2007:15294–15298. doi: 10.1073/pnas.0707688104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.le Coutre J, Kaback HR, Patel CK, Heginbotham L, Miller C. Fourier transform infrared spectroscopy reveals a rigid alpha-helical assembly for the tetrameric Streptomyces lividans K+ channel. Proc Natl Acad Sci U S A. 1998;95:6114–6117. doi: 10.1073/pnas.95.11.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaback HR, Sahin-Toth M, Weinglass AB. The kamikaze approach to membrane transport. Nat Rev Mol Cell Biol. 2001;2:610–620. doi: 10.1038/35085077. [DOI] [PubMed] [Google Scholar]
- 16.Kaback HR, Dunten R, Frillingos S, Venkatesan P, Kwaw I, Zhang W, Ermolova N. Site-directed alkylation and the alternating access model for LacY. Proc Natl Acad Sci U S A. 2007;104:491–494. doi: 10.1073/pnas.0609968104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nie Y, Ermolova N, Kaback HR. Site-directed Alkylation of LacY: Effect of the Proton Electrochemical Gradient. J Mol Biol. 2007;374:356–364. doi: 10.1016/j.jmb.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nie Y, Sabetfard FE, Kaback HR. The Cys154-->Gly mutation in LacY causes constitutive opening of the hydrophilic periplasmic pathway. J Mol Biol. 2008;379:695–703. doi: 10.1016/j.jmb.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Y, Guan L, Freites JA, Kaback HR. Opening and closing of the periplasmic gate in lactose permease. Proc Natl Acad Sci U S A. 2008;105:3774–3778. doi: 10.1073/pnas.0800825105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaback HR. Bacterial Membranes. In: Kaplan NP, Jakoby WB, Colowick NP, editors. Methods in Enzymol. New York: Elsevier; 1971. pp. 99–120. [Google Scholar]
- 21.Short SA, Kaback HR, Kohn LD. Localization of D-lactate dehydrogenase in native and reconstituted Escherichia coli membrane vesicles. J Biol Chem. 1975;250:4291–4296. [PubMed] [Google Scholar]
- 22.Owen P, Kaback HR. Molecular structure of membrane vesicles from Escherichia coli. Proc Natl Acad Sci USA. 1978;75:3148–3152. doi: 10.1073/pnas.75.7.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owen P, Kaback HR. Antigenic architecture of membrane vesicles from Escherichia coli. Biochemistry. 1979;18:1422–1426. doi: 10.1021/bi00575a005. [DOI] [PubMed] [Google Scholar]
- 24.Owen P, Kaback HR. Immunochemical analysis of membrane vesicles from Escherichia coli. Biochemistry. 1979;18:1413–1422. doi: 10.1021/bi00575a004. [DOI] [PubMed] [Google Scholar]
- 25.Sun J, Wu J, Carrasco N, Kaback HR. Identification of the epitope for monoclonal antibody 4B1 which uncouples lactose and proton translocation in the lactose permease of Escherichia coli. Biochemistry. 1996;35:990–998. doi: 10.1021/bi952166w. [DOI] [PubMed] [Google Scholar]
- 26.Sun J, Li J, Carrasco N, Kaback HR. The last two cytoplasmic loops in the lactose permease of Escherichia coli comprise a discontinuous epitope for a monoclonal antibody. Biochemistry. 1997;36:274–280. doi: 10.1021/bi962292f. [DOI] [PubMed] [Google Scholar]
- 27.Short SA, Kaback HR, Kaczorowski G, Fisher J, Walsh CT, Silverstein SC. Determination of the absolute number of Escherichia coli membrane vesicles that catalyze active transport. Proc Natl Acad Sci USA. 1974;71:5032–5036. doi: 10.1073/pnas.71.12.5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Konings WN, Barnes EM, Jr, Kaback HR. Mechanisms of active transport in isolated membrane vesicles. 2. The coupling of reduced phenazine methosulfate to the concentrative uptake of β-galactosides and amino acids. J Biol Chem. 1971;246:5857–5861. [PubMed] [Google Scholar]
- 29.Carrasco N, Herzlinger D, Danho W, Kaback HR. Preparation of monoclonal antibodies and site-directed polyclonal antibodies against the lac permease of Escherichia coli. Methods Enzymol. 1986;125:453–467. doi: 10.1016/s0076-6879(86)25035-1. [DOI] [PubMed] [Google Scholar]
- 30.Consler TG, Persson BL, Jung H, Zen KH, Jung K, Prive GG, Verner GE, Kaback HR. Properties and purification of an active biotinylated lactose permease from Escherichia coli. Proc Natl Acad Sci USA. 1993;90:6934–6938. doi: 10.1073/pnas.90.15.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rudnick G, Schuldiner S, Kaback HR. Equilibrium between two forms of the lac carrier protein in energized and nonenergized membrane vesicles from Escherichia coli. Biochemistry. 1976;15:5126–5131. doi: 10.1021/bi00668a028. [DOI] [PubMed] [Google Scholar]
- 32.Widdas WF. Inability of diffusion to account for placental glucose transfer in the sheep and consideration of the kinetics of a possible carrier transfer. J Physiol. 1952;118:23–39. doi: 10.1113/jphysiol.1952.sp004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell P. Molecule, group and electron transport through natural membranes. Biochem Soc Symp. 1963;22:142–168. [Google Scholar]
- 34.Jardetzky O. Simple allosteric model for membrane pumps. Nature. 1966;211:969–970. doi: 10.1038/211969a0. [DOI] [PubMed] [Google Scholar]