Abstract
This study examined the development of joint attention in 95 infants assessed between 9 and 18 months of age. Infants displayed significant test – retest reliability on measures of following gaze and gestures (responding to joint attention, RJA) and in their use of eye contact to establish social attention coordination (initiating joint attention, IJA). Infants displayed a linear, increasing pattern of age-related growth on most joint attention measures. However, IJA was characterized by a significant cubic developmental pattern. Infants with different rates of cognitive development exhibited different frequencies of joint attention acts at each age, but did not exhibit different age-related patterns of development. Finally, 12-month RJA and 18-month IJA predicted 24-month language after controlling for general aspects of cognitive development.
The human capacity for social attention coordination has been referred to as joint attention (Bakeman & Adamson, 1984; Bruner & Sherwood, 1983). Different behavioral manifestations of joint attention begin to emerge in the first 6 months of life (D’Entremont, Hains, & Muir, 1997; Farroni, Massaccesi, & Francesca, 2002; Morales, Mundy, & Rojas, 1998) and continue to develop at least through 3 years of age (Adamson, Bakeman, & Dekner, 2004; Carpenter, Nagell, & Tomasello, 1998). These different infant joint attention behaviors may be used for declarative and instrumental-imperative functions (Bates, Benigni, Bretherton, Camaioni, & Volterra, 1979), as well as to initiate or respond to bids in interactions with social partners (Seibert, Hogan, & Mundy, 1982).
Various labels have been used to refer to these behavioral dimensions of infant joint attention (Carpenter et al., 1998). Here we adopt the nomenclature initially suggested by Seibert et al. (1982) that is currently widely used in the research literature as well as in multidimensional assessments of early social communication development (e.g., Fidler, Philofsky, Hepburn, & Rogers, 2005; Henderson, Yoder, Yale, & McDuffie, 2002; Laing et al., 2002; Lord et al., 2002; Mundy, Sigman, Kasari, & Yirmiya, 1988; Smith & Ulvund, 2003; Wetherby, Allen, Cleary, Kublin, & Goldstein, 2002). Accordingly, responding to joint attention (RJA) refers to the ability to follow the direction of gaze and gestures of others, initiating joint attention (IJA) refers to the ability to use direction of gaze and gestures to direct the attention of others to spontaneously share experiences, initiating behavior regulation/requests (IBR) refers to the ability to use gaze and gestures to elicit aid from a social partner to obtain an object or event, and responding to behavior requests (RBR) refers to the ability to correctly respond to a “Give it to me!” statement presented with a gesture (see Figure 1).
Figure 1.

Illustrations of (a) responding to joint attention; (b) initiating joint attention — IJA – PS, “pointing”; (c1, c2, c3) IJA – EC, “alternating gaze”; (d) initiating behavior requests — IBR – PG, “pointing”; and (e) responding to behavior request from the Early Social Communication Scales (Mundy,Delgado et al., 2003; Seibert et al., 1982).
Observations of joint attention behaviors provide important information about the development of mental processes in infancy that are critical to subsequent aspects of human social and cognitive development (e.g., Bates et al., 1979; Moore & Corkum, 1994; Mundy & Sigman, 2006; Tomasello, Carpenter, Call, Behne, & Moll, 2005). This hypothesis has been supported by numerous studies that indicate that individual differences in joint attention skills among infants are related to subsequent language and cognitive development (e.g., Adamson et al., 2004; Carpenter et al., 1998; Delgado et al., 2002; Mundy & Gomes, 1998; Smith & Ulvund, 2003; Tomasello & Todd, 1983), “A not B” paradigm measures of learning processes and inhibition (Dawson et al., 2002; Nichols, Fox, & Mundy, 2005), and measures of social competence and self-regulation in preschool and older children (e.g., Morales, Mundy, Crowson, Neal, & Delgado, 2005; Sheinkopf, Mundy, Claussen, & Willoughby, 2004; Sigman & Ruskin, 1999; Vaughan Van Hecke et al., in press). Thus, the developmental continuity between infant joint attention and early childhood social and cognitive abilities is reasonably well supported in the literature. A detailed understanding of the nature of these continuities, however, is lacking. At least three models, though, may guide research in this regard. These models are complementary rather than mutually exclusive. However, they are also characterized by different assumptions about the nature and development of joint attention.
What may be referred to as the universal cognitive model (UCM) suggests that infant joint attention is an expression of general aspects of cognitive development and that this is the source of continuity between joint attention and later outcomes (e.g., Bates et al., 1979; Seibert et al., 1982). For example, protoimperatives and protodeclaratives may be viewed as the expressions of aspects of early representational development in social interactions (Bates et al., 1979; Leslie & Happe, 1989). Moreover, measures of joint attention and related skills have been observed to be associated with infant novelty responding and visual information processing ability (Mundy, Seibert, & Hogan, 1984), as well as IQ (Smith & Ulvund, 2003). Thus, this model suggests that all four dimensions of infant joint attention illustrated in Figure 1 should primarily share and reflect a common source of cognitive variance in early development.
A variant of the UCM is the social cognitive model (SCM; e.g., Tomasello, 1995). This model suggests that joint attention reflects specific, rather than general, components of cognition, in particular, the development of infants’ early understanding that others have intentions (i.e., social cognition). In turn, this epistemological component of joint attention provides a unique part of the cognitive foundation for advances in infants’ referential communication and subsequent language development (Bretherton, 1991; Brooks & Meltzoff, 2005; Bruner, 1985; Tomasello, 1995; Tomasello et al., 2005). This model suggests that social cognition becomes a prominent feature of joint attention during a developmental shift between 9 and 12 months (Brooks & Meltzoff, 2005; Tomasello, 1995), and that after that time individual differences on dimensions of joint attention in infancy may be expected to reflect common social-cognitive sources of variance (Tomasello, 1995). Research on a small sample of infants has provided impressive support for the latter assumption (Carpenter et al., 1998), but these observations were not replicated in a recent study of a larger sample of infants (Slaughter & McConnell, 2003).
The multiple process model (MPM) provides a third perspective on the nature of joint attention development (Mundy, Card, & Fox, 2000). This model suggests that infant joint attention development is influenced by several so-called “hot” (Zelazo, Qu, & Muller, 2005) or “social” executive processes (Mundy, 2003), which contribute to the early acquisition of the capacity for social sharing and subsequent social-cognitive development. Pertinent research here indicates that dimensions of joint attention are differentially related to measures of frontal brain activity (Caplan et al., 1993; Henderson et al., 2002; Mundy et al., 2000), inhibiting and switching behavioral responses (Dawson et al., 2002; Griffith, Pennington, Wehner, & Rogers, 1999; Nichols et al., 2005), attention regulation (Morales et al., 2005), self-monitoring (Nichols et al., 2005), the expression of positive affect associated with social motivation (Kasari, Sigman, Mundy, & Yirmiya, 1990; Mundy, Kasari, & Sigman, 1992; Vaughan et al., 2003) as well as learning and reward sensitivity (Corkum & Moore, 1998; Dawson et al., 2002; Nichols et al., 2005). Based on this research, an assumption of the MPM is that different constellations of executive processes may contribute to different dimensions of joint attention development. Thus, different dimensions of joint attention may reflect unique as well as common processes (Mundy et al., 2000; Mundy & Sigman, 2006; Mundy & Vaughan Van Hecke, in press).
One approach to the study of these various models is to examine individual differences as well as the patterns of development exhibited by infants on measures of the different dimensions of joint attention. The study of individual differences in behavior development may be critical to the formulation of comprehensive psychological theory in many areas (Kosslyn et al., 2002; Underwood, 1975), including theory on the development of attention-related processes in infancy (Bornstein & Sigman, 1986; Colombo, 2002). Indeed, three distinct hypotheses about individual differences and the development of joint attention skills in infancy may be derived and tested based on the three models of joint attention described above.
If the UCM (Bates et al., 1979) is correct in that all dimensions of joint attention reflect variability in common aspects of cognitive development in infancy, then all measures of joint attention should also be significantly associated with infant performance on a general measure of cognitive development. In addition, individual differences on all measures of joint attention should be intercorrelated, and there should be little difference between correlations within dimensions (intradimensional test – retest reliability) versus those observed across dimensions (interdimensional correlations). All measures of infant joint attention should also display similar patterns of growth over the 9- to 18-month period. Finally, infant individual differences on all dimensions of joint attention should display similar predictions to language outcomes and these associations would be explained by shared common variance with general cognitive development.
The SCM (e.g., Tomasello, 1995) also suggests that all aspects of joint attention development reflect a common source of variance. Therefore, all measures of joint attention should be correlated, such that intradimensional and interdimensional correlations would be comparable, and the patterns of growth across ages would be comparable across dimensions of joint attention. However, this model suggests that the sources of common variance are specific to the development of the understanding of intentionality in others. This aspect of cognitive development is not a clear focus of assessment in general measures of infant cognitive development (e.g., Bayley, 1994). Therefore, joint attention measures may only display modest associations with general cognitive development, and measures of the latter should not be able to explain all of the variance shared between measures of infant joint attention and later language development. Nevertheless, common social cognitive variance should lead all dimensions of joint attention to display equivalent paths of predictive validity for early language development.
Alternatively, if the MPM (Mundy et al., 2000) provides a valid perspective, and different dimensions of joint attention reflect different constellations and combinations of a variety of executive and social motivation processes, then all dimensions of joint attention would not be expected to be intercorrelated in development. Indeed, in this case, there would be more evidence of within-dimension correlations (stability or test – retest reliability) rather than intradimension correlations. Furthermore, the different dimensions of joint attention would not necessarily be expected to display identical patterns of growth during infancy. Finally, different dimensions of joint attention may display different degrees or patterns of predictive validity for early language development. Furthermore, where significant associations are observed, variance associated with general cognitive development would not be expected to explain all of the variance shared between infant joint attention and later language outcomes.
This study was designed to examine these alternative hypotheses in one of the largest longitudinal studies of joint attention development to date. Joint attention development was observed in 95 infants at 9, 12, 15, and 18 months of age, and 24-month language development data were also collected. The 9-to 18-month age range was chosen for this study because this is a formative period of infant joint attention development during which important age-related shifts in social cognition are thought to be a primary influence on joint attention development (Brooks & Meltzoff, 2005; Carpenter et al., 1998; Tomasello, 1995). The main goals of the study were as follows: (1) to compare patterns of age-related development across different dimensions of infant joint attention, (2) to examine the degree to which infants display stable individual differences in the development of different dimensions of joint attention between 9 and 18 months, (3) to investigate the intradimensional correlations of joint attention measures at 9 – 18 months, (4) to examine the predictive associations between different dimensions of infant joint attention at different ages and 24-month language development, and (5) to explore the degree to which variability in general cognitive development may affect individual differences and the patterns of development of joint attention in infancy.
Method
Participants
One hundred and forty-nine infants were recruited from public Miami-Dade County birth records for this study. The inclusion criteria included 5-min Apgar scores > 6, birth weight > 2,499 g, and no history of seizure conditions or congenital and/or chromosomal abnormalities. Mothers of the infants were 18 years or older. The data for this study were drawn from 95 infants who had complete data relevant to this study at all points in the longitudinal assessment: 9, 12, 15, 18, and 24 months. To evaluate attrition-related sample effects, the group of 54 infants who had incomplete data were compared with those with complete data on all 9-month joint attention variables, as well as measures of gestational age, birth weight, maternal education, number of languages spoken in the home, and number of siblings (see variable descriptions below). Mothers of the “incomplete data” sample had lower education scores, 3.4 versus 4.1, F(1, 147) = 16.2, p < .01, but infants in this group had higher Responding to Behavior Request scores on the Early Social Communication Scales (ESCS), 33.4% versus 23.2%, F(1, 147) = 3.8, p < .054. None of the other comparisons were significant (all ps > .20).
The primary sample (N = 95) comprised infants who had typical Bayley Mental Developmental Index scores at 18 months (MDI > 85, n = 63) as well as infants who had scores in the “at-risk” or developmentally delayed range at 18 months (MDI < 86, n = 32). The ethnicity and multiple language exposure of the infants reflected the general demographic characteristics of the Miami metropolitan area (see Table 1). The infants with typical development (TD group) and those at-risk or developmentally delayed (ARDD group) did not differ on birth weight, gestational age, mothers’ education, or number of languages that infants were reportedly exposed to in their homes at 9 months (see Table 1). However, the groups did differ on gender (χ2 = 7.5, p < .006), number of siblings (χ2 = 14.2, p < .007), and ethnicity (Hispanic vs. non-Hispanic; χ2 = 15.4, p < .03, see Table 1). Preliminary analyses indicated that neither number of siblings nor ethnicity were associated with major effects on infant joint attention or the relations of joint attention to language. Gender, however, was associated with some effects that were considered more completely in the data analyses. Infants were recruited to the laboratory for assessment within 2 weeks (± 14 days) of their 9-, 12-, 15-, 18-, and 24-month birthdays. The mean chronological ages were 8.9 months (standard deviation [SD] = 10.1 days), 11.6 months (10.7 days), 15.3 months (10.4 days), 17.8 months (12.5 days), and 24.7 months (13.3 days), respectively. The ages for 7 TD and 2 ARDD children were corrected throughout for gestational ages below 37 weeks (range 33 – 36 weeks).
Table 1.
Demographic Characteristics of Infants and Families in the Typical Development (TD) Group and the Group at Risk for Developmental Delays (ARDD)
| Variables | ARDD group | TD group |
|---|---|---|
| n = 32 | n = 63 | |
| MDI at 18 months | 77.7 (SD = 5.04) | 98.9 (SD = 98.9) |
| Gender | ||
| Female | 10 (31%) | 38 (61%) |
| Male | 22 (69%) | 25 (39%) |
| Birth weight (g) | 3280 (SD = 519) | 3339 (SD = 532) |
| Gestational age (weeks) | 38.6 (SD = 1.2) | 38.1 (SD = 5.2) |
| No. of languages in the home | ||
| 1 | 8 (25%) | 19 (28%) |
| 2 | 19 (59%) | 37 (57%) |
| 3 | 5 (16%) | 7 (10.9) |
| Race/ethnicity | ||
| White, non-Hispanic | 12 (38%) | 39 (61%) |
| White, Hispanic | 18 (56%) | 22 (35%) |
| Black | 1 (3%) | 1 (2%) |
| Asian | 1 (3%) | 1 (2%) |
| Mother’s level of education | ||
| High school incomplete = 1 | 0 | 0 |
| High school graduate = 2 | 4 (13%) | 3 (5%) |
| Some college = 3 | 6 (19%) | 12 (19%) |
| College degree = 4 | 11 (34%) | 16 (25%) |
| Graduate/professional = 5 | 11 (34%) | 33 (51%) |
| Siblings | ||
| 0 | 11 (34%) | 44 (69%) |
| 1 | 14 (44%) | 11 (17%) |
| 2 | 5 (16%) | 9 (14%) |
| 3 or more | 2 (6%) | 0 |
Procedures and Measures
Assessments were conducted in an observation laboratory containing a testing table, several chairs, a couch, a TV/computer cart, and an intercom, and decorated with posters and photographs. Fluent bilingual testers used the language that parents reported was primary for their infants (either English or Spanish for all children).
ESCS (Mundy, Delgado et al., 2003)
The ESCS is a 20-min assessment designed to measure the development of different dimensions of nonverbal communication. The tester and the child sat face to face across a small table, with the infant seated on a caregiver’s lap, if necessary (see Figure 1). A set of toys, visible to the child, was placed to the right of the experimenter, but out of reach of the infant. Four posters were placed on the walls at a distance of 4 feet from the table. Two were 90° to the child’s right or left, and two were placed 165° behind the child (behind the child’s right and left shoulders). Video recording through a one-way mirror captured a 3/4 full-face view of the child and a 1/4 profile view of the tester.
Infants were presented with a series of three active wind-up toys (three trials each), three hand-operated toys (three trials each), and opportunities to play a turn-taking game with a toy car or ball (two trials), interact with the experimenter with a hat, comb, and glasses (one trial each), play a tickle turn-taking game (three trials), and look at a book with the tester (one trial). In addition, the tester presented the child with two sets of four gaze-following trials. In these trials, the tester gained the child’s attention by emphatically calling out the child’s name three times, while turning toward, pointing to, and remaining visually fixated on a poster located in the room. Left, left-behind, right, and right-behind trials were presented (a maximum of two trials for each direction). In addition, the tester also requested toys from the child throughout the assessment.
Data from the ESCS measures of IJA, RJA, and IBR, as well as a measure of RBR, were examined in this study. The RJA variable reflects the percentage of gaze-following trials on which a child’s first response was to correctly turn their visual regard at least 45° off of midline on left and right trials, or more than 90° on behind trials, in the direction of the tester’s visual regard and pointing gesture. Infants did not need to fixate the target to be rated as “correct” on RJA trials. The three initiating joint attention variables analyzed in this study included a measure that reflected the total frequency of four behaviors (IJA) and subscale scores reflecting either the frequency of two eye-contact behaviors (IJA – EC) or two conventional gestures: pointing and showing (IJA – PS). The four specific items were (1) making eye contact with the examiner while manipulating a toy (IJA – EC), (2) alternating eye contact between an active mechanical toy and the tester (IJA – EC), (3) pointing to an active mechanical toy or distal objects in the room with or without eye contact (IJA – PS), and (4) showing by raising objects toward the tester’s face with eye contact (IJA – PS).
The three initiating behavior request variables analyzed in this study included a measure of the combined frequency of five infant behaviors (IBR), as well as subscale scores for the combinations of three eye contact and reaching behaviors (IBR – ECR), or observations of two behaviors involving pointing or giving (IBR – PG). The five specific items were (1) making eye contact after an object has been moved out of reach (IBR – ECR), (2) reaching for a toy that is out of reach (IBR – ECR), (3) making eye contact while reaching for a toy (IBR – ECR), (4) pointing to a toy that is out of reach (IBR – PG), and (5) giving a toy to the tester (IBR – PG). RBR provided an index of infants’ responses to the tester’s request for objects. Analogous to RJA, it is scored as the percentage of trials administered on which the child correctly responds to the tester’s palm-up, hand-extended gesture with the accompanying verbal request of “Give it to me!” For a more complete description of the ESCS procedures and scoring, see the ESCS manual available on the Internet (Mundy, Delgado et al., 2003).
The interrater reliability of the ESCS measures has been shown to equal or exceed typical standards in numerous previous studies across ages and laboratories, in typical as well as atypical samples (e.g., Fidler et al., 2005; Griffith et al., 1999; Mundy et al., 1988, 2000; Mundy, Sigman, & Kasari, 1994; Mundy, Kasari, Sigman, & Ruskin, 1995; Mundy & Gomes, 1998; Smith & Ulvund, 2003; Sheinkopf et al., 2004). Interrater reliability was reexamined in this study, with intraclass correlations computed across three independent coder ratings of data from two separate samples of 10 randomly selected children at 9 and 15 months of age. At 9 months, the interrater reliabilities were IJA = .95, IJA–EC = .97, IJA–PS = .32, IBR = .77, IBR–ECR = .95, IBR–PG = .59, RJA = .97, and RBR = .88. At 15 months, the interrater reliabilities were IJA = .93, IJA–EC = .92, IJA–PS = .89, IBR = .94, IBR–ECR = .93, IBR–PG = .94, RJA = .95, and RBR = .83. All of these coefficients were significant (p < .005) except for the 9-month IJA–PS and IBR–PG coefficients that occurred infrequently (M = 0.44, SD = 1.26 and M = 2.7, SD = 3.4, respectively). At 15 months, both IJA–PS and IBR–PG ratings were reliable and their mean scores were significantly higher (M = 1.55, SD = 2.3 and M = 15.95, SD = 9.6, respectively; 9- vs. 15-month t tests exceed 3.10, ps < .01).
Mental Scale of the Bayley Scales of Infant Development – II (BSID – II; Bayley, 1994)
The Bayley – II provided a standardized index of general cognitive development. This is a commonly used, standardized developmental assessment with normative data for children aged 0 – 4 years old. It measures aspects such as problem solving, memory, understanding of numbers, classification, generalization, verbal skills, social skills, and habituation (Bayley, 1994). During administration, the child and tester sat across from each other at a small table. The parent sat nearby in the same room, remaining silent. To limit practice effects, the BSID – II was administered at 12, 18, and 24 months of age, but not at 9 or 15 months. The BSID – II administration was initiated at 12 rather than 9 months because of better-reported test validity at 12 months (Bayley, 1994). The BSID – II yields mental age estimates and a standardized MDI, with a mean of 100 and a standard deviation of 15.
Reynell Developmental Language Scales (RDLS; Reynell & Gruber, 1990)
The RDLS was administered at 24 months and is a standardized test of receptive and expressive language skills for children aged 1 year to 6 years 11 months. The RDLS includes objects that the child must identify, name, answer questions about, and perform tasks with, simple pictures that the child must name, and complex pictures that the child must describe. The RDLS provided standardized estimates of receptive and expressive language development with a mean of 100 and a standard deviation of 15.
MacArthur Communication Development Inventory –Short Form Level 2 (MCDI; Fenson et al., 1994)
The MCDI is a parent-report checklist. It consists of 100 words, which the parent rates for the child’s comprehension and expression. The critical variable in this study was the parent report of the numbers of words that children were able to say at 24 months. The combination of Reynell and MCDI measures was used because early receptive and expressive language development is often dissociated (Bates, Thal, Whitesell, & Fenson, 1989) and convergent parent-report and direct observation data provide a more valid assessment of individual differences in early language development (Tamis-Lemonda & Bornstein, 1994).
Results
Age-Related Patterns of Development
A sequence of 4(ages of assessment) × 2(cognitive group: TD vs. ARDD groups) × 2(gender) × 2(maternal education: less than college vs. college or more) mixed-design ANOVAs were used to examine age-related changes on the ESCS variables (see Table 2).
Table 2.
Comparison of Age and Cognitive Group Effects on the Early Social Communication Scale (ESCS) Variables
| Variables | Total group | ARDD group | TD group |
|---|---|---|---|
| N = 95 | n = 32 | n = 63 | |
| 9 months | |||
| RJA (%) | 23.4 (20.5)L,Q | 20.8 (20.1) | 26.9 (22.2) |
| IJA | 17.5 (10.5)C | 14.4(9.2) | 18.4 (10.9)ˆ |
| IBR | 12.4 (7.7)L,Q | 10.6 (7.6) | 13.4 (7.1)ˆ |
| RBR (%) | 27.0 (31.4)L, Q | 18.9 (26.6) | 28.8 (33.2) |
| 12 months | |||
| RJA (%) | 48.2 (24.1) | 40.2 (23.4) | 51.7 (23.1)* |
| IJA | 18.3 (10.5) | 15.6 (9.2) | 19.2 (10.7) |
| IBR | 25.8 (13.0) | 21.6 (12.4) | 29.6 (12.9)** |
| RBR (%) | 66.8 (34.8) | 58.7 (35.5) | 66.3 (26.7) |
| 15 months | |||
| RJA (%) | 63.2 (23.6) | 53.3 (26.9) | 66.8 (21.9)* |
| IJA | 14.8 (8.0) | 11.7 (7.9) | 16.0 (7.8)* |
| IBR | 28.4 (11.1) | 28.4 (12.3) | 28.9 (10.7) |
| RBR (%) | 70.1 (32.2) | 77 .9 (34.7) | 71.4 (31.1) |
| 18 months | |||
| RJA (%) | 67.9 (22.9) | 59.4 (26.8) | 72.0 (18.8)** |
| IJA | 17.5 (9.4) | 14.5 (10.1) | 19.0 (8.9)* |
| IBR | 28.4 (10.7) | 26.0 (10.9) | 30.0 (10.4)ˆ |
| RBR (%) | 77.8 (30.1) | 78.0 (32.4) | 77.8 (29.1) |
Note. ARDD = at risk or developmentally delayed; IBR = initiating behavior regulation/requests; IJA = initiating joint attention; RBR = responding to behavior requests; RJA = responding to joint attention; TD = typical development.
L, Q, or C indicate Significant linear, quadratic, or cubic main effects for age, respectively, for the ESCS variables across the 9-, 12-, 15-, and 18-month assessment intervals.
p < .075;
p < .05;
p < .01 indicate significant differences between the TD and ARDD cognitive groups.
Analyses of RJA data revealed a significant linear main effect for age, F(1, 77) = 159.45, p < .001, partial eta2 or ηp2 = .68, as well as a quadratic age effect, F(1, 77) = 13.7, p < .001, ηp2 = .15 (see Table 2 and Figure 2). Pairwise, post hoc Bonferroni comparisons indicated that 12-month RJA was significantly higher than 9-month RJA (p < .001) and 15-month RJA was significantly higher than 12-month RJA (p < .002), but 18-month RJA was not significantly higher than 15-month RJA (p < .91). A significant main effect for cognitive group was also observed, F(1, 77) = 8.54, p < .005, ηp2 = .10. Post hoc comparisons indicated that the TD group displayed significantly higher RJA scores than the ARDD groups at 12, 15, and 18 months (see Table 2). A significant main effect of maternal education was not observed, F(1, 77) = 1.45, p < .23, but this variable did interact with age of assessment of RJA, F(1, 77) = 7.6, p < .007, ηp2 = .09. Surprisingly, post hoc comparisons indicated that infants of mothers with less education displayed more advanced RJA skills at 12 and 15 months, M = 54.7% (3.8), M = 69.2% (3.8), compared with infants of mothers with more education, M = 41.7 (5.4), M = 55.7 (5.3), respectively (ps < .05), but no maternal education effects were observed at 9 or 18 months. There was no main effect of gender on RJA, F(1, 77) = 0.01, p > .50, or any significant two-way or three-way interactions involving gender, range of F(1, 77) = 0.01 – 0.24, p > .50.
Figure 2.
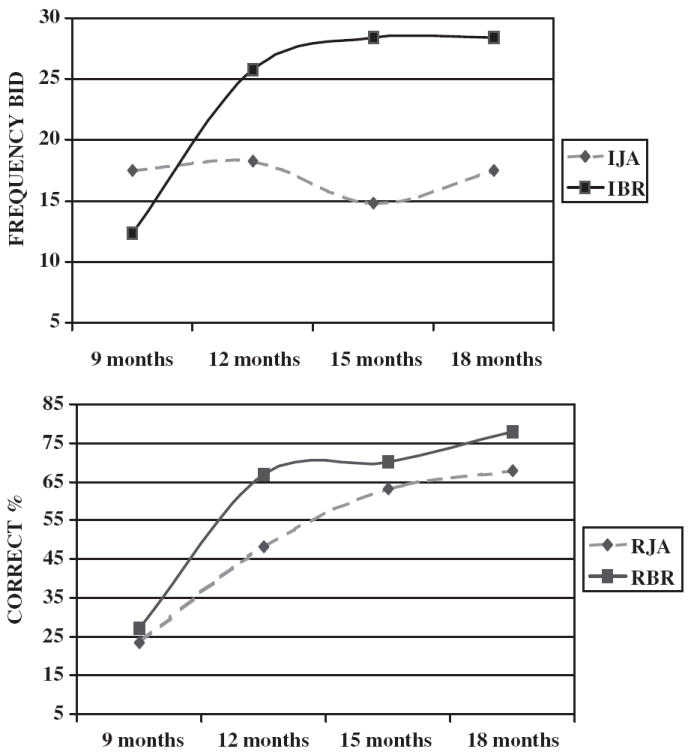
Comparison of age-related growth patterns for the frequency of initiating joint attention (IJA) and initiating behavior requests (IBR) bids (top) and a comparison of the age-related growth patterns for the frequency of responding to joint attention (RJA) and responding to behavior request (RBR) bids (bottom).
Infant IJA performance displayed a significant cubic main effect for age, F(1, 77) = 4.96, p < .03, ηp2 = .06 (see Table 2 and Figure 2). However, neither the linear term, F = 0.071 (p < .50), nor the quadratic term was significant, F = 2.07 (p < .20). Pairwise Bonferroni comparisons indicated that there was a marginally significant decline in IJA between 12 and 15 months (p < .08) and a marginally significant rebound of IJA at 18 months (p < .09). This pattern of development was apparent in data for both the IJA – EC measures (9-month M = 17.43, 12-month M = 16.00, 15-month M = 13.17, and 18-month M = 15.95) and the IJA – PS measures (9-month M = 0.44, 12-month M = 2.45, 15-month M = 1.55, and 18-month M = 2.14). There was also a main effect of cognitive group, F(1, 77) = 5.23, p < .025, ηp2 = .065, with marginal to significant advantages on IJA development observed for the TD group at 9, 15, and 18 months (see Table 2). No significant main effects of gender, F(1, 77) = 1.64, p < .25, or interactions involving gender were observed. However, there was an apparent advantage for girls on IJA at 9 months, and this was significant when examined post hoc, M = 19.6 (9.8) versus M = 15.8 (1.9), p < .015. There was no main effect of maternal education, F(1, 77) = 0.46, p > .50, on IJA or significant interactions involving this variable.
Performance on IBR displayed both a linear main effect, F(1, 77) = 84.81, p < 001, ηp2 = .57, and a quadratic main effect for age, F(1, 77) = 45.10, p < .001, ηp2 = .34 (see Table 2 and Figure 2). Post hoc comparisons revealed that IBR was significantly lower at 9 months than at 12, 15, and 18 months (ps < .001). However, none of the pairwise comparisons between 12-, 15-, and 18-month scores were significant. There was also a significant main effect of cognitive group on IBR, F(1, 77) = 5.19, p < .03, ηp2 = .064. The TD group displayed marginally to significantly better IBR skills at 9, 12, and 18 months (see Table 2). There was no main effect of gender, F(1, 77) = 0.26, p > .50, but there was a significant Age of Assessment × Gender interaction involving IBR, F(1, 77) = 8.65, p < .004; ηp2 = .10. Pairwise comparisons revealed a marginally significant advantage for girls on IBR performance at 12 months, girls’ M = 28.6 (2.3) versus boys’ M = 22.5 (2.5), and 15 months, girls’ M = 31.2 (2.1) versus boys’ M = 28.3 (2.2) (ps < .095). IBR was not affected by maternal education, F(1, 77) = 0.22, p > .50, and there were no significant interactions involving this variable.
Analyses of RBR yielded a significant linear main effect for age, F(1, 77) = 80.5, p < .001, ηp2 = .51, and a quadratic effect for age, F(1, 77) = 12.24, p < .001, ηp2 = .14 (see Table 2 and Figure 2). Pairwise comparisons indicated significant improvements in RBR between 9 and 12 months and 12 and 15 months (ps < .05). There were no main effects for cognitive group, F(1, 77) = 2.16, p < .15 (see Table 2), gender, F(1, 77) = 1.48, p < .25, or maternal education, F(1, 77) = 0.09, p > .50, nor any significant interactions involving these variables.
Individual Differences: Stability and Intrascale Correlations of the ESCS Measures
Individual differences in infants’ IJA and RJA performance displayed significant stability across the consecutive 9- to 12-month, 12- to 15-month, and 15- to 18-month periods of development (see Table 3). All of these test – retest coefficients remained significant at p < .005 (one-tailed) after controlling for variance associated with 18-month Bayley MDI, except for the 9- to 12-month association of RJA, partial r = .17, p < .075, one-tailed. Individual differences on RJA at 9 and 12 months were also associated with 18-month RJA performance, rs = .22 and .23, p < .05, respectively. No evidence of test – retest reliability was observed for RBR, and limited evidence of stability was observed for IBR (see Table 3). However, 9- and 12-month IBR predicted 18-month IBR, rs = .22 and .21, p < .05, respectively.
Table 3.
Test – Retest Reliability of the Early Social Communication Scale (ESCS) Variables
| Variables | 9 – 12 months | 12 – 15 months | 15 – 18 months |
|---|---|---|---|
| RJA | .21* | .39*** | .25* |
| IJA | .26* | .30** | .48*** |
| IBR | .39*** | .07 | .14 |
| RBR | .12 | .03 | .09 |
| IJA – PS | .06 | .10 | .12 |
| IJA – EC | .26* | .32** | .46*** |
| IBR – PG | .26* | .02 | .14 |
| IBR – ECR | .16 | .07 | .22* |
Note. IBR = initiating behavior regulation/requests; IJA = initiating joint attention; RBR = responding to behavior requests; RJA = responding to joint attention.
p < .05;
p < .01;
p < .001.
The data in Table 3 also indicated that infants displayed stable individual differences on IJA – EC across all consecutive ages (see means provided above), but not on the IJA – PS measures. The 9- to 12-month test – retest reliability may have been attenuated by the low interrater reliability of IJA – PS at 9 months, but this possibility does not account for low test – retest reliabilities of the 12- to 15- and 15- to 18-month measures. Thus, there was more evidence of individual stability in the use of eye contact rather than conventional gestures in observation of IJA behaviors in this sample of infants. Moreover, the stability in the use of eye contact was more apparent for IJA than IBR functions, where a significant correlation was only apparent between 15 and 18 months on the IBR – ECR measure (Ms = 9.9, 14.9, 13.2, 13.9, respectively, for 9, 12, 15, and 18 months). Thus, this sample of 9- to 18-month-olds displayed stable individual differences in their tendency to initiate eye contact for social sharing or to respond to the gaze, head turn, and the gesture of a social partner.
A second issue concerned whether infants’ performance across different dimensions of joint attention was correlated. IBR and RBR displayed significant concurrent correlations at 9, 12, 15, and 18 months, rs = .26, .26, .28, .22, ps < .05, respectively. In contrast, no significant concurrent associations were observed across the IJA and RJA measures of joint attention at any age, with rs ranging from .17 at 9 months to .10 at 18 months. Thus, there was little evidence of common processes associated with individual differences on IJA and RJA measures at 9, 12, 15, and 18 months in this study. Alternatively, there was some evidence of commonality of processes associated with individual differences in the development of requesting skills in this sample of infants.
Individual Differences: Predictions of 24-Month Language Scores
Twenty-four-month language data from the RDLS and the McArthur Communication Development Inventory (MCDI) were available for 72 of the 95 infants who participated in this study. There were no differences in the ESCS scores, 18-month MDI scores, or demographic characteristics of the 23 infants (24%) who dropped out after 18 months versus the 72 who attended the 24-month assessment session.
The mean 24-month language scores were Reynell Receptive Language = 81.65 (SD = 17.5), Reynell Expressive Language = 84.88 (SD = 18.5), and MCDI Expressive score = 55.00 (SD = 26.5). As expected, the parent-report MCDI and direct observation from the Reynell scale scores were correlated, with rs ranging from .50 to .73 (ps < .001). Therefore, following methods from previous research (Tamis-LeMonda & Bornstein, 1994), the three language scores were Z transformed and averaged to form a composite 24-month language variable.
To evaluate the predictive validity of infant joint attention for early language development, the correlations of the ESCS scores and the 24-month language scores were examined. Significant associations with language development were only observed for the IJA and RJA scales (see Table 4). Consistent predictive correlations were observed for 9- and 12-month RJA as well as for 9-, 15-, and 18-month IJA scores with 24-month Reynell Receptive Language. Only RJA at 9 months and IJA at 18 months were significantly correlated with the MCDI Expressive Index or the Composite Language score (see Table 4).
Table 4.
Correlations of the Early Social Communication Scale (ESCS) Variables With the 24-Month Language Outcome Scores (n = 72)
| Variables | MCDI | Reynell Receptive | Reynell Expressive | Combined Z |
|---|---|---|---|---|
| 9 months | ||||
| RJA | .24* | .36** | .16 | .29* |
| IJA | .13 | .25* | .04 | .17 |
| IBR | .20 | .20 | .14 | .21 |
| RBR | .06 | .11 | .09 | .15 |
| 12 months | ||||
| RJA | .12 | .24* | .04 | .15 |
| IJA | .02 | .22 | .18 | .18 |
| IBR | .21 | .19 | .01 | .18 |
| RBR | .06 | .05 | .04 | .01 |
| 15 months | ||||
| RJA | .14 | .05 | .04 | .10 |
| IJA | .07 | .38** | .05 | .10 |
| IBR | .07 | .05 | .12 | .06 |
| RBR | .06 | − .14 | − .03 | − .09 |
| 18 months | ||||
| RJA | .18 | .12 | .16 | .21 |
| IJA | .26* | .36** | .16 | .30* |
| IBR | − .11 | .03 | .03 | − .02 |
| RBR | − .04 | .10 | − .02 | .01 |
| Bayley MDI-12 | .37** | .23* | .29* | .35** |
| Bayley MDI-18 | .71** | .50** | .54** | .67** |
Note. IBR = initiating behavior regulation/requests; IJA = initiating joint attention; MCDI = McArthur Communication Development Inventory; MDI = Mental Developmental Index; RBR = responding to behavior requests; RJA = responding to joint attention.
p < .05;
p < .01.
Analyses of IJA components indicated that IJA – EC scores at 9 and 15 months significantly predicted Reynell Receptive Language scores, rs = .26 and .38, p < .02 and .008, respectively. IJA – EC at 18 months also predicted both the MCDI Expressive scores, r = .33, p < .01, and the Composite Language score, r = .33, p < .01. It was also the case that IJA – PS at 12 months was correlated with Reynell Expressive Language scores, r = .33, p < .005, and with the Composite Language scores, r = .29, p < .01. IJA – PS at 18 months was related to Reynell Receptive Language at 24 months, r = .26, p < .03.
The 24-month language scores were associated with 12- and 18-month performance on the Bayley Scales of Infant Development, as well as some of the demographic variables (see Table 4). Gender was associated with 24-month MCDI and Composite Language scores, rs = − .31 and − .24, ps < .005 and .05, with girls displaying higher scores than boys, and children who experienced multiple languages in the home tended to perform less well on all the 24-month language measures, even with assessments conducted in their primary language, rs = − .27 to − .35, ps < .015. Maternal education, however, was not significantly correlated with 24-month language performance, rs = .17 – .22, p < .25. Because of this covariance matrix, multiple hierarchical regression analyses were computed.
Steps 1, 2, and 3 were designed to provide data relevant to the hypotheses of this study.
In Step 1, data from relevant measures of infant joint attention were entered as a block to examine whether different types of joint attention variables displayed unique paths of association with language outcomes. In Steps 2 and 3, data from the 12- and 18-month Bayley MDI scores were entered to examine the degree to which joint attention measures maintained significant paths of predictive association with 24-month language scores (e.g., displayed incremental validity) after controlling for variance on standardized measures of cognitive development. A final step in these analyses provided an exploratory analysis of the degree to which variance shared with gender or home language affected any of the effects observed on preceding steps of the regression equations. To limit the number of analyses, regression equations were only computed for the Reynell Receptive Language standard score and the Composite Language score. To limit the number of variables, only the total ESCS scores with significant zero-order correlations with these respective language measures were considered in these analyses (see Table 4). Preliminary analyses of receptive language indicated that both IJA at 15 and 18 months contributed significantly and equally to this multiple regression when considered individually, but neither contributed significantly when considered together because of their covariance (see Table 3). Therefore, only IJA-18 was entered in the analysis of receptive language because of this variable’s more reliable and consistent relations with other language measures (see Table 4).
Regression analyses for Reynell Receptive Language scores indicated that RJA at 9 months, IJA at 18 months, and Bayley MDI at 18 months all made significant and unique contributions to the prediction of 24-month Reynell Receptive scores (see Table 5). The addition of gender and home language information on the fourth step was not significant, F = 0.89, p < .50, β = .01 and .10, respectively. Post hoc analyses revealed no significant effects for the Bayley MDI at 18 months by RJA or IJA interaction terms.
Table 5.
Regression Analyses of Predictors of the 24-Month Reynell Receptive Language Outcomes (n = 72)
| Steps and variables | R | Adjusted R2 | β | F change |
|---|---|---|---|---|
| Step 1 (df 4/67) | .53** | .28 | ||
| RJA 9 months | .30** | |||
| IJA 9 months | .07 | |||
| RJA 12 months | .13 | |||
| IJA 18 months | .31* | |||
| Step 2 (df 5/66) | .57** | .32 | 5.81** | |
| RJA 9 months | .31** | |||
| IJA 9 months | .10 | |||
| RJA 12 months | .06 | |||
| IJA 18 months | .30** | |||
| Bayley MDI 12 months | .23* | |||
| Step 3 (df 6/65) | .63** | .39 | 6.45** | |
| RJA 9 months | .22* | |||
| IJA 9 months | .07 | |||
| RJA 12 months | .07 | |||
| IJA 18 months | .24* | |||
| Bayley MDI 12 months | .06 | |||
| Bayley MDI 18 months | .33** |
Note. IJA = initiating joint attention; MDI = Mental Developmental Index; RJA = responding to joint attention.
p < .05;
p < .025.
The analyses of the 24-month Composite Language score revealed that both RJA at 9 months and IJA at 18 months contributed to the prediction of the Composite Language measure above and beyond variance associated with performance on the 12-month standardized Bayley cognitive scale (see Table 6). However, once data for the Bayley at 18 months were entered, only IJA at 18 months remained as a significant and unique predictor of the Composite Language scores. The addition of gender and home language in Step 4 did not make a significant contribution to this equation, F = 1.79, p < .16. There were no significant effects associated with the RJA or IJA and Bayley Scale interaction terms.
Table 6.
Regression Analyses of the Predictors of the 24-Month Combined Z-Language Scores (n = 72)
| Steps and variables | R | Adjusted R2 | β | F change |
|---|---|---|---|---|
| Step 1 (df 2/69) | .41** | .15 | ||
| RJA 9 months | .22ˆ | |||
| IJA 18 months | .33** | |||
| Step 2 (df 3/68) | .52** | .23 | 8.18** | |
| RJA 9 months | .22* | |||
| IJA 18 months | .32** | |||
| Bayley MDI 12 months | .31** | |||
| Step 3 (df 4/67) | .72** | .48 | 31.07** | |
| RJA 9 months | .05 | |||
| IJA 18 months | .18* | |||
| Bayley MDI 12 months | .01 | |||
| Bayley MDI 18 months | .62** |
Note. IJA = initiating joint attention; MDI = Mental Developmental Index; RJA = responding to joint attention.
p < .075 (two-tailed);
p < .05 (two-tailed);
p < .025 (two-tailed).
Discussion
Observations from this study indicated that infants not only displayed systematic age-related changes in some types of joint attention behaviors but also displayed a wide range of meaningful individual differences in joint attention development that were stable across development from the 1st through 2nd year of life. Developmental continuity of individual differences from 9 to 18 months was especially apparent for the use of eye contact for IJA, and the response to deictic gestures and gaze on RJA trials. Although performance differences on both of these dimensions of joint attention displayed similar stability, several aspects of the data also suggested that these two dimensions of joint attention reflected different processes during infant development. Individual differences in IJA and RJA were not correlated within ages, although comparable measures of requesting (IBR and RBR) were correlated within ages. In addition, variance in RJA and IJA from different points in development (9 and 18 months, respectively) demonstrated unique paths of associations with 24-month language outcomes that were also independent of covariance with general cognitive development. Finally, the frequency of infants’ use of IJA bids did not display the type of linear increase with age that was observed on RJA, IBR, or RBR.
Understanding the nature and meaning of these differences in joint attention among infants is an important goal for developmental science. Differences in the frequency of use of joint attention behaviors among infants reflect constitutional and/or environmental processes that are related to subsequent language, intellectual, and social development in typical and atypical samples (e.g., Sheinkopf et al., 2004; Sigman & Ruskin, 1999; Smith & Ulvund, 2003; Vaughan Van Hecke et al., in press). Therefore, explaining the factors that influence individual differences in joint attention may be critical to a more comprehensive theory of joint attention, as well as early social and social-cognitive development (Mundy & Sigman, 2006; Tomasello et al., 2005; cf. Underwood, 1975). Current theory and research, however, rarely directly addresses the meaning of individual differences in infant joint attention. The results of this study, though, illustrate that a deeper empirical appreciation of individual differences in infant behaviors may assist in evaluating current perspectives on the development of joint attention.
As noted in the introduction, the UCM led to the expectation that infant performance on all measures of joint attention should be significantly associated with their performance on a comprehensive assessment of cognitive development. This tenet of the model was supported by the observation that the TD cognitive group of children, defined by higher Bayley Infant MDI scores at 18 months, displayed higher frequencies of joint attention behaviors across ages than did the AARD group, defined by significantly lower 18-month Bayley MDI scores. Research suggests that these group differences may have occurred because joint attention is affected to some extent by basic cognitive processes such as representation, memory, speed of information processing, learning, and response inhibition (e.g., Bates et al., 1979; Mundy et al., 1984; Nichols et al., 2005; Smith & Ulvund, 2003). Work by Landry, Miller-Loncar, and Smith (2002) also suggests that differences in cognitive level may be associated with a passive versus active interactive style that may affect the frequency of expression of social-communication bids in children. However, too little work has been devoted to these possible cognitive and behavioral influences to fully understand the connections between joint attention and rate of cognitive development. A more precise understanding of the connections between joint attention and rate of cognitive development remains a goal of considerable significance for future research on joint attention (Mundy & Newell, in press).
Several observations in this study were also inconsistent with critical aspects of the UCM. Contrary to expectations, all joint attention measures were not intercorrelated. Furthermore, all measures of joint attention did not display similar patterns of age-related growth, and all measures of joint attention did not display relations comparable to language outcome. Last, but perhaps most importantly, associations between infant joint attention and language could not be completely explained in terms of the variance associated with general cognitive development.
Unlike the UCM, the SCM suggests that joint attention reflects the development of specific, rather than general, aspects of cognition that involve understanding intentionality in others. Therefore, measures of joint attention should provide meaningful information about early social-cognitive development that is related to language acquisition but is not redundant with information from a measure of general cognition. This tenet of the SCM was supported by observations that indicated that RJA and IJA at 12 and 18 months predicted 24-month language scores after controlling for either 12- or 18-month Bayley scores. Thus, the IJA and RJA measures provided valid and unique sources of information about infant development that are not clearly indexed in general measures of infant cognitive development. It is likely that both measures provided unique information about social-cognitive processes because at least two studies have empirically linked differences on infant/toddler measures comparable to the ESCS IJA–EC measure with performance of pre-school children on false belief or theory-of-mind paradigms (Charman et al., 2001; Clifford, 2006). Research has also documented a 10-month social-cognitive shift in RJA (Brooks & Meltzoff, 2005).
The observations of this study, however, also provided data on joint attention developmental phenomena that lie beyond the easy explanation of current versions of the SCM. Similar to the UCM, the SCM suggests that all measures of joint attention should be intercorrelated within ages during development and comparable in their prediction of language development (Carpenter et al., 1998; Tomasello, 1995). This, though, was not the case in this study, or in previous work reported by Slaughter and McConnell (2003). Unlike IJA and RJA, measures of requesting (IBR and RBR) did not display incremental validity in the prediction of language in this study, and have been less consistently related to language, cognition, executive functions, and social outcomes in previous research (e.g., Griffith et al., 1999; Mundy & Gomes, 1998; Smith & Ulvund, 2003; Vaughan Van Hecke et al., in press). Moreover, IBR and RBR variables displayed a pattern of interdimensional correlations indicative of common underlying variance across these measures at each age, but less consistent intradimensional correlations indicative of developmental stability across ages (i.e., test – retest reliability).
Alternatively, IJA and RJA were characterized by the opposite pattern of inter- and intradimensional correlations. These variables displayed consistent evidence of intradimensional correlations across ages, but little evidence of common interdimensional variance at any age. Thus, RJA and IJA appeared to reflect stable but unique aspects of joint attention and social development in the 9- to 18-month period. These data also validate the Bates et al. (1979) distinction between a protodeclarative dimension of infant social attention coordination assessed with IJA and RJA measures, and a behaviorally similar, but functionally distinct, protoimperative dimension assessed with IBR and RBR measures. Finally, these observations suggest that, in interpreting data from studies conducted on one or another measure (e.g., IJA or RJA), caution should be exercised in generalizing the interpretation of the data to all facets of infant joint attention development.
The SCM also suggests that all measures of joint attention reflect social cognition, which displays significant age-related developments between 9 and 18 months (Brooks & Meltzoff, 2005; Tomasello, 1995). However, while several joint attention measures displayed clear monotonic age-related developmental trends, infants’ use of IJA did not display a monotonic increase with age. This was another inconsistent finding relative to the SCM. These inconsistencies encourage the refinement of the current SCM and/or the need to consider alternative models.
The MPM provides one alternative that was consistent with several aspects of the data in this study, as well as some of the data presented by Slaughter and McConnell (2003). This model suggests that the development of IJA, RJA, and other joint attention behaviors reflects divergent as well as convergent processes. The divergent processes reduce the associations between joint attention measures at any age, and also mean that different measures of joint attention provide somewhat different information about psychological processes at various points in early development (Mundy & Vaughan Van Hecke, in press). Thus, joint attention measures may display divergent patterns of age-related development and unique patterns of associations with outcome measures (Mundy & Sigman, 2006). In particular, this model suggests that varied combinations of executive functions involved in attention regulation, the intentional control of behavior, rapid integrated self-and other-monitoring, and social motivation contribute to different aspects of joint attention development and social cognition (see Mundy 1995, 2003; Mundy et al., 2000; Zelazo et al., 2005; Mundy & Newell, in press).
For example, the development of RJA may involve relatively involuntary forms of social orienting behavior (Moore & Corkum, 1994; Mundy et al., 2000) and imitation (Mundy & Vaughan Van Hecke, in press). Neuropsychological research suggests that RJA is associated with parietal activation (Mundy et al., 2000) that is associated with the posterior attention system, which serves to regulate the development of reflexive orienting to biologically meaningful stimuli (Posner & Petersen, 1990; Rothbart, Posner, & Rosicky, 1994). This relatively reflexive system develops early in the first year (D’Entremont et al., 1997; Rothbart et al., 1994). On the other hand, research suggests that IJA may not involve imitation and is regulated by activation of frontal systems (Caplan et al., 1993; Henderson et al., 2002; Mundy et al., 2000; Mundy, 2003; Mundy & Vaughan Van Hecke, in press) that are associated with functions of the more volitional and intentional anterior attention system, which develops later in infancy (e.g., Rothbart et al., 1994).
These varied neurobehavioral components play a role in differentiating the development of IJA from RJA, as well as differentiating these two variables from IBR and RBR, which research suggests are not as clearly associated with activation of the posterior or anterior attention systems (Caplan et al., 1993; Henderson et al., 2002; Mundy et al., 2000). Moreover, the distinction between reflexive versus intentional orienting systems may help to explain why RJA at 12 months and IJA at 18 months appeared to reflect unique facets of early social development that were associated with 24-month language development. Variability in the early-developing, posterior RJA system may be connected to language development because it reflects differences in an inherent, or acquired, reflexive social attention bias among infants. This bias prioritizes processing and reacting to gaze, which ultimately leads to a social-cognitive understanding of the referential meaning of gaze shifts of other people (e.g., Brooks & Meltzoff, 2005; Corkum & Moore, 1998; D’Entremont et al., 1997). In turn, this allows infants to better self-organize social information processing in early incidental language learning opportunities by following gaze and decreasing representational mapping errors (Baldwin, 1995; Corkum & Moore, 1998; Mundy & Vaughan Van Hecke, in press).
Alternatively, infants’ experience with the intentional control of their own social attention coordination acts in IJA may provide additional and unique types of learning opportunities that are not so available in reflexive RJA (Mundy, Sigman, & Kasari, 1993; Mundy, 1995). This experience with the intentional control of social attention coordination contributes to a broader and deeper understanding of commonalities between self and other (e.g., shared affective experience in addition to shared direction of gaze; see Figure 1) and, consequently, to the further growth and consolidation of social cognition in the second year (Mundy et al., 1993; Mundy & Sigman, 2006). Hence, later IJA development may be related to language via frontal functions that are more closely associated with understanding intentionality in self and others than is the case in RJA (Mundy, Fox, & Card, 2003; Tomasello, 1995).
The difference between RJA and IJA in intentional control is likely to be associated with another point of differentiation between these two forms of joint attention. Internal motivational constraint is thought to have a greater influence on the intentional rather than the reflexive control of attention (e.g., Rothbart et al., 1994). Related to this, several lines of theory suggest that social motivation may contribute to individual differences in IJA, but less so in RJA or IBR and RBR (Bates, 1976; Bruner, 1985; Moore & Corkum, 1994; Mundy et al., 1992; Rheingold, Hay, & West, 1976). IJA may reflect a relative enthusiasm for social engagement and eliciting attention to the self for the purpose of sharing affective experiences with others (Bates, 1976; Mundy & Sigman, 2006) that is associated with reward-based approach tendencies (Mundy, 1995). Similarly, Tomasello et al. (2005) have cogently argued that social motivation may be fundamental to the evolution and development of human social cognition and the tendency to share psychological states, and Langston (1994) suggests that individual differences in the motivation to share information contributes to differences in adult social behavior and personality. Empirically, support for the differential involvement of motivation in joint attention is provided by data that indicate that sharing of positive affective experiences is a prominent feature of joint attention development (Adamson & Bakeman, 1985), and that this sharing is specifically associated with IJA rather than RJA or IBR (Kasari et al., 1990; Mundy et al., 1992; Vaughan et al., 2003; see Figure 1). This feature of IJA is present by 9 – 10 months of age (Venezia, Messinger, Thorp, & Mundy, 2004).
The social motivation perspective also offers at least one plausible explanation of the unique pattern of development displayed on the IJA measure. Motivation differences may reflect a stable temperament-like feature of IJA development (Vaughan et al., 2003). As a consequence of motivation differences, some infants may display more interest in social events and engage and display more IJA – EC (Mundy, 1995). This higher rate of early social attention may be associated with increased opportunities for certain types of social information processing, which stimulate the development of better cognitive facilities for social problem solving (i.e., social cognition) among these infants (Mundy et al., 1993). Thus, variability in infant IJA may be related to stable differences in social motivation, which in turn are related to subsequent differences in the development of social cognition (Mundy, 1995). However, while the social cognitive awareness inherent to an IJA bid may deepen with age, the frequency of use of IJA in early infancy may be more affected by social motivation rather than a social-cognitive process.
The motivation hypothesis, though, does not offer a clear explanation for the pattern of decline and rebound that was observed for the development of IJA. A similar pattern of IJA development has been observed in an earlier study (Mundy et al., 2000). Nevertheless, given the marginal nature of pairwise comparisons involved in the observations in this study, the interpretation of these data should be circumscribed. The “U”-shaped pattern of decline and rebound of IJA between 12, 15, and 18 months may reflect an important phase of consolidation in learning new and relatively complicated behaviors (Rogers, Rakinson, & McClelland, 2004; Touwen, 1998). Alternatively, the decline and rebound could reflect perturbations of the course of IJA as its development begins to interact with advances in motor skills (walking) or verbal communication (talking) that occur in the 12- to 15-month interval. Additional research to confirm or disconfirm the validity of regression and rebound in IJA development would be useful. Verification may contribute to a deeper understanding of the development of joint attention that could also provide clues about the nature of autism, which is often characterized by impairments in IJA and developmental declines in the 2nd year of life (Mundy et al., 1994; Rogers, 2004).
The data in this study also indicated that gender has some effect on joint attention development in infancy. Gender was associated with IJA – EC at 9 months and also affected IBR development across ages, with girls displaying a transient early advantage. In previous research, gender-related processes have been observed to contribute to early differences in infants’ social eye contact (Leeb & Rejskind, 2004; Lutchmaya, Baron-Cohen, & Raggatt, 2002). Such differences may especially affect the development of IJA in the 1st year. Gender may also contribute to the development and meaning of individual differences across measures of joint attention development, as a more consistent pattern of gender relations was observed for IBR, relative to the other dimensions of joint attention measured in this study.
It is also important to consider the environmental influences on individual differences in joint attention development. Previous research has suggested that culture and ethnicity may impact joint attention in an infant – caregiver measure (Chavajay & Rogoff, 1999). In this study, though, there was little indication that language environments that may be associated with cultural/ethnic differences in the home environment of the infants impacted joint attention. However, maternal education, a variable that may also be related to environmental effects, had a significant conditional effect on RJA development. Children of mothers with lower educational achievement displayed an earlier pattern of age-related growth on RJA than children of mothers with higher levels of education. This effect was unexpected. One post hoc possibility was that mothers with less education worked outside of the home less frequently than mothers with more education. This may have had an early positive, but transient, effect on RJA development. However, more research on this effect will be needed before it can be deemed a reliable phenomenon in joint attention research.
In this regard, it should be noted that constitutional differences in social behavior may be more apparent in measures of infants’ interactions with an unfamiliar rather than familiar person (Plomin & Rowe, 1979). Therefore, the ESCS may be more sensitive to constitutional differences among infants in joint attention development than are infant– caregiver joint attention paradigms (e.g., Adamson et al., 2004). In future research, it may be useful to combine paradigms to best understand the unique and complementary contributions of infants and their caregiving environments to the development of joint attention (see Vaughan et al., 2003). It may also be vital to include greater heterogeneity in family education and socioeconomic status in future research to acquire a more comprehensive view of typical infant joint attention development.
In conclusion, two methodological caveats warrant consideration in interpreting the results of this study. First, it is important to note that the sample in this study was self-selected, to the extent that some parents chose to continue through the course of the longitudinal study and others did not. Although attrition analyses did not reveal large differences between the two groups, the possibility that sample selection may have affected the results of this study cannot be excluded. Second, in relating the results of this study to much of the previous literature on infant joint attention development, it is essential to recognize that previous studies have often used dichotomous measures of the presence or absence of a skill at an age (e.g., Brooks & Meltzoff, 2005; Carpenter et al., 1998; Corkum & Moore, 1998). This study, however, used interval levels of measurement to examine the frequency or consistency with which infants used a variety of joint attention behaviors in structured social interactions. The processes that influence whether a particular joint attention behavior is present or absent in a sample of infants at a given age, or whether a behavior is used more or less by infants at a given age, may be different. Nevertheless, individual differences in the utilization of a behavior by infants at a given age, as well as the presence or absence of a behavior, are both essential phenomena to study to acquire a more definitive and veridical model of infant joint attention development.
Acknowledgments
The research reported in this article was supported by NIH Grant HD38052 (P. M.).
Contributor Information
Peter Mundy, University of Miami.
Jessica Block, University of Miami.
Christine Delgado, University of Miami.
Yuly Pomares, University of Miami.
Amy Vaughan Van Hecke, University of Illinois at Chicago.
Meaghan Venezia Parlade, University of Pittsburgh.
References
- Adamson L, Bakeman R. Affect and attention: Infants observed with mothers and peers. Child Development. 1985;56:582–593. [Google Scholar]
- Adamson L, Bakeman R, Dekner D. The development of symbol infused joint engagement. Child Development. 2004;75:1171–1187. doi: 10.1111/j.1467-8624.2004.00732.x. [DOI] [PubMed] [Google Scholar]
- Bakeman R, Adamson L. Coordinating attention to people and objects in mother – infant and peer – infant interaction. Child Development. 1984;55:1278–1289. [PubMed] [Google Scholar]
- Baldwin D. Understanding the link between joint attention and language. In: Moore C, Dunham P, editors. Joint attention: Its origins and role in development. Hillsdale, NJ: Erlbaum; 1995. pp. 131–158. [Google Scholar]
- Bates E. Language and context: The acquisition of performatives. New York: Academic Press; 1976. [Google Scholar]
- Bates E, Benigni L, Bretherton I, Camaioni L, Volterra V. The emergence of symbols: Cognition and communication in infancy. New York: Academic Press; 1979. [Google Scholar]
- Bates E, Thal D, Whitesell K, Fenson L. Integrating language and gesture in infancy. Developmental Psychology. 1989;25:1004–1019. [Google Scholar]
- Bayley N. Bayley Scales of Infant Development. 2. San Antonio: Psychological Corporation; 1994. [Google Scholar]
- Bornstein M, Sigman M. Continuity in mental development from infancy. Child Development. 1986;57:251–274. doi: 10.1111/j.1467-8624.1986.tb00025.x. [DOI] [PubMed] [Google Scholar]
- Bretherton I. Intentional communication and the development of an understanding of mind. In: Frye D, Moore C, editors. Children’s theories of mind: Mental states and social understanding. Hillsdale, NJ: Lawrence Erlbaum Associates Inc; 1991. pp. 49–75. [Google Scholar]
- Brooks R, Meltzoff A. The development of gaze following and its relations to language. Developmental Science. 2005;8:535–543. doi: 10.1111/j.1467-7687.2005.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner J. Child’s talk: Learning to use language. New York: Norton; 1985. [Google Scholar]
- Bruner J, Sherwood V. Thought, language and interaction in infancy. In: Call J, Galenson E, Tyson R, editors. Frontiers in infant psychiatry. New York: Basic Books; 1983. pp. 38–55. [Google Scholar]
- Caplan R, Chugani H, Messa C, Guthrie D, Sigman M, Traversay J, et al. Hemispherectomy for early onset intractable seizures: Presurgical cerebral glucose metabolism and postsurgical nonverbal communication patterns. Developmental Medicine and Child Neurology. 1993;35:574–581. doi: 10.1111/j.1469-8749.1993.tb11695.x. [DOI] [PubMed] [Google Scholar]
- Carpenter M, Nagell K, Tomasello M. Social cognition, joint attention, and communicative competence from 9- to 15-months of age. Monographs of the Society for Research in Child Development. 1998;63(4 Serial No255) [PubMed] [Google Scholar]
- Charman T, Swettenham J, Baron-Cohen S, Baird G, Cox A, Drew A. Testing joint attention, imitation, and play as infancy precursors to language and theory of mind. Cognitive Development. 2001;15:481–498. [Google Scholar]
- Chavajay P, Rogoff B. Cultural variation in management of attention by children and their caregivers. Developmental Psychology. 1999;35:1079–1090. doi: 10.1037//0012-1649.35.4.1079. [DOI] [PubMed] [Google Scholar]
- Clifford S. A dissertation to the faculty of the School of Psychological Science, La Trobe University, Bundoora, Australia. 2006. The early development of joint attention and its contribution to social understanding and responsiveness in preschool children with autism. [Google Scholar]
- Colombo J. Visual attention in infancy: Process and product in early cognitive development. In: Posner M, editor. Cognitive neuroscience of attention. New York: Guilford Publications Inc; 2002. pp. 329–341. [Google Scholar]
- Corkum V, Moore C. Origins of joint visual attention in infants. Developmental Psychology. 1998;34:28–38. doi: 10.1037/0012-1649.34.1.28. [DOI] [PubMed] [Google Scholar]
- Dawson G, Munson J, Estes A, Osterling J, McPartland J, Toth K, et al. Neurocognitive function and joint attention ability in young children with autism spectrum disorder versus developmental delay. Child Development. 2002;73:345–358. doi: 10.1111/1467-8624.00411. [DOI] [PubMed] [Google Scholar]
- Delgado C, Mundy P, Crowson M, Markus J, Yale M, Schwartz H. Responding to joint attention and language development: A comparison of target locations. Journal of Speech, Language and Hearing Research. 2002;45:1715–1719. doi: 10.1044/1092-4388(2002/057). [DOI] [PubMed] [Google Scholar]
- D’Entremont B, Hains S, Muir D. A demonstration of gaze following in 3- to 6- month-olds. Infant Behavior and Development. 1997;20:569–572. [Google Scholar]
- Farroni T, Massaccesi S, Francesca S. Can the direction of gaze of another person shift the attention of a neonate? Giornole-Italiano-di-Psicologia. 2002;29:857–864. [Google Scholar]
- Fenson L, Dale PS, Reznick JS, Bates E, Thal DJ, Pethick SJ. Variability in early communicative development. Monographs of the Society for Research in Child Development. 1994;59(5 Serial No 242) [PubMed] [Google Scholar]
- Fidler D, Philofsky A, Hepburn S, Rogers S. Nonverbal requesting and problem solving by toddlers with Down syndrome. American Journal on Mental Retardation. 2005;110:312–322. doi: 10.1352/0895-8017(2005)110[312:NRAPBT]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith E, Pennington B, Wehner E, Rogers S. Executive functions in young children with autism. Child Development. 1999;70:817–832. doi: 10.1111/1467-8624.00059. [DOI] [PubMed] [Google Scholar]
- Henderson L, Yoder P, Yale M, McDuffie A. Getting the point: Electrophysiological correlates of protodeclarative pointing. International Journal of Developmental Neuroscience. 2002;20:449–458. doi: 10.1016/s0736-5748(02)00038-2. [DOI] [PubMed] [Google Scholar]
- Kasari C, Sigman M, Mundy P, Yirmiya N. Affective sharing in the context of joint attention interactions of normal, autistic, and mentally retarded children. Journal of Autism and Developmental Disorders. 1990;20:87–100. doi: 10.1007/BF02206859. [DOI] [PubMed] [Google Scholar]
- Kosslyn S, Cacioppo J, Davidson R, Hugdahl K, Lovallo W, Rose R, et al. Bridging psychology and biology: The analysis of individuals in groups. American Psychologist. 2002;57:341–351. [PubMed] [Google Scholar]
- Laing E, Butterworth G, Ansari D, Gsodl M, Longhi E, Panagiotake G, et al. Atypical development of language and social communication in toddlers with Williams syndrome. Developmental Science. 2002;5:233–246. [Google Scholar]
- Landry S, Miller-Loncar C, Smith K. Individual differences in the development of social communication competency in very low birthweight children. In: Molfese D, Molfese V, editors. Developmental variations in learning: Applications to social, executive function, language, and reading skills. Mahwah, NJ: Lawrence Erlbaum; 2002. pp. 81–112. [Google Scholar]
- Langston CA. Capitalizing on and coping with daily-life events: Expressive responses to positive events. Journal of Personality and Social Psychology. 1994;67:1112–1125. [Google Scholar]
- Leeb R, Rejskind G. Here’s looking at you, kid! A longitudinal study of perceived gender differences in mutual gaze behavior in young infants. Sex Roles. 2004;50:1–5. [Google Scholar]
- Leslie A, Happe F. Autism and ostensive communication: The relevance of meta-representation. Development and Psychopathology. 1989;1:205–212. [Google Scholar]
- Lord C, Lambrecht L, Cook E, Leventhal B, DiLavore P, Pickles A, et al. The autism diagnostic observation schedule-generic: A standard measure of social communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lutchmaya S, Baron-Cohen S, Raggatt P. Foetal testosterone and eye contact in 12-month-old infants. Infant Behavior and Development. 2002;25:327–335. [Google Scholar]
- Moore C, Corkum V. Social understanding at the end of the first year of life. Developmental Review. 1994;14:349–372. [Google Scholar]
- Morales M, Mundy P, Crowson M, Neal R, Delgado C. Individual differences in infant attention skills, joint attention, and emotion regulation behavior. International Journal of Behavioral Development. 2005;29:259–263. [Google Scholar]
- Morales M, Mundy P, Rojas J. Following the direction of gaze and language development in 6-month olds. Infant Behavior and Development. 1998;21:373–377. [Google Scholar]
- Mundy P. Joint attention and social-emotional approach behavior in children with autism. Development and Psychopathology. 1995;7:63–82. [Google Scholar]
- Mundy P. The neural basis of social impairments in autism: The role of the dorsal medial-frontal cortex and anterior cingulate system. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2003;44:793–809. doi: 10.1111/1469-7610.00165. [DOI] [PubMed] [Google Scholar]
- Mundy P, Card J, Fox N. EEG correlates of the development of infant joint attention skills. Developmental Psychobiology. 2000;36:325–338. [PubMed] [Google Scholar]
- Mundy P, Delgado C, Block J, Venezia M, Hogan A, Seibert J. A manual for the Abridged Early Social Communication Scales (ESCS) University of Miami Psychology Department; Coral Gables, Florida: 2003. Retrieved 2003, from http://www.psy.miami.edu/faculty/pmundy/main.phtml. [Google Scholar]
- Mundy P, Fox N, Card J. Joint attention, EEG coherence and early vocabulary development. Developmental Science. 2003;6:48–54. [Google Scholar]
- Mundy P, Gomes A. Individual differences in joint attention skill development in the second year. Infant Behavior and Development. 1998;21:469–482. [Google Scholar]
- Mundy P, Kasari C, Sigman M. Nonverbal communication, affective sharing, and intersubjectivity. Infant Behavior and Development. 1992;15:377–381. [Google Scholar]
- Mundy P, Kasari C, Sigman M, Ruskin E. Nonverbal communication and early language acquisition in children with Down syndrome and in normally developing children. Journal of Speech and Hearing Research. 1995;38:157–167. doi: 10.1044/jshr.3801.157. [DOI] [PubMed] [Google Scholar]
- Mundy P, Newell L. Joint attention, social cognition and the anterior/posterior attention systems. Current Directions in Psychological Science. doi: 10.1111/j.1467-8721.2007.00518.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Seibert J, Hogan A. Novelty responding and behavioral development in young handicapped and at risk children. Intelligence. 1984;8:11–29. [Google Scholar]
- Mundy P, Sigman M. Joint attention, social competence and developmental psychopathology. In: Cicchetti D, Cohen D, editors. Developmental psychopathology, 2nd ed., Vol. 1: Theory and methods methods. Hoboken, NJ: Wiley; 2006. pp. 293–332. [Google Scholar]
- Mundy P, Sigman M, Kasari C. The autistic person’s theory of mind and early nonverbal joint attention deficits. In: Baron-Cohen S, Tager-Flusberg H, Cohen D, Volkmar F, editors. Understanding other minds: Perspectives from autism. Oxford, UK: Oxford University Press; 1993. pp. 181–201. [Google Scholar]
- Mundy P, Sigman M, Kasari C. Joint attention, developmental level, and symptom presentation in children with autism. Development and Psychopathology. 1994;6:389–401. [Google Scholar]
- Mundy P, Sigman M, Kasari C, Yirmiya N. Nonverbal communication skills in Down syndrome children. Child Development. 1988;59:235–249. [PubMed] [Google Scholar]
- Mundy P, Vaughan Van Hecke A. Neural systems, gaze following and the development of joint attention. In: Nelson C, Luciana M, editors. Handbook of developmental cognitive neuroscience. New York: Oxford University Press; 2007. pp. 17–51. [Google Scholar]
- Nichols KE, Fox N, Mundy P. Joint attention, self-recognition and neurocognitive functioning. Infancy. 2005;7:35–51. doi: 10.1207/s15327078in0701_4. [DOI] [PubMed] [Google Scholar]
- Plomin R, Rowe D. Genetic and environmental etiology of social behavior in infancy. Developmental Psychology. 1979;15:62–72. [Google Scholar]
- Posner M, Petersen S. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Reynell JK, Gruber CP. Reynell Developmental Language Scales. Los Angeles: Western Psychological Services; 1990. [Google Scholar]
- Rheingold H, Hay D, West M. Sharing in the second year of life. Child Development. 1976;47:1148–1158. [Google Scholar]
- Rogers S. Developmental regression in autism spectrum disorders. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10:139–143. doi: 10.1002/mrdd.20027. [DOI] [PubMed] [Google Scholar]
- Rogers T, Rakinson D, McClelland J. U-shaped curves in development: A PDP approach. Journal of Cognition and Development. 2004;5:137–145. [Google Scholar]
- Rothbart M, Posner M, Rosicky J. Orienting in normal and pathological development. Development and Psychopathology. 1994;6:635–652. [Google Scholar]
- Seibert JM, Hogan AE, Mundy PC. Assessing interactional competencies: The early social-communication scales. Infant Mental Health Journal. 1982;3:244–258. [Google Scholar]
- Sheinkopf S, Mundy P, Claussen A, Willoughby J. Infant joint attention skill and preschool behavioral outcomes in at-risk children. Development and Psychopathology. 2004;16:273–293. doi: 10.1017/s0954579404044517. [DOI] [PubMed] [Google Scholar]
- Sigman M, Ruskin E. Continuity and change in the social competence of children with autism, Down syndrome, and developmental delays. Monographs of the Society for Research in Child Development. 1999;64(1 Serial No 256) doi: 10.1111/1540-5834.00002. [DOI] [PubMed] [Google Scholar]
- Slaughter V, McConnell D. Emergence of joint attention: Relationships between gaze following, social referencing, imitation, and naming in infancy. Journal of Genetic Psychology. 2003;164:54–71. doi: 10.1080/00221320309597503. [DOI] [PubMed] [Google Scholar]
- Smith L, Ulvund L. The role of joint attention in later development among preterm children: Linkages between early and middle childhood. Social Development. 2003;12:222–234. [Google Scholar]
- Tamis-LeMonda C, Bornstein M. Specificity in mother – toddler language – play relations across the second year. Developmental Psychology. 1994;30:283–292. [Google Scholar]
- Tomasello M. Joint attention as social cognition. In: Moore C, Dunham P, editors. Joint attention: Its origins and role in development. Hillsdale, NJ: Lawrence Erlbaum; 1995. pp. 103–130. [Google Scholar]
- Tomasello M, Carpenter M, Call J, Behne T, Moll H. Understanding sharing intentions: The origins of cultural cognition. Brain and Behavior Sciences. 2005;28:675–690. doi: 10.1017/S0140525X05000129. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Todd J. Joint attention and lexical acquisition style. First Language. 1983;4:197–211. [Google Scholar]
- Touwen B. The development of brain function. Developmental Review. 1998;18:504–526. [Google Scholar]
- Underwood B. Individual differences as a crucible in theory construction. American Psychologist. 1975;30:128–134. [Google Scholar]
- Vaughan A, Mundy P, Block J, Burnette C, Delgado C, Gomez Y, et al. Child, caregiver, and temperament contributions to infant joint attention. Infancy. 2003;4:603–616. [Google Scholar]
- Vaughan Van Hecke A, Mundy P, Acra F, Block J, Delgado C, Parlade MV, et al. Infant joint attention, temperament, and social competence in preschool children. Child Development. 2007;78:53–69. doi: 10.1111/j.1467-8624.2007.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venezia M, Messinger D, Thorp D, Mundy P. Timing changes: The development of anticipatory smiling. Infancy. 2004;6:397–406. doi: 10.1207/s15327078in0603_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherby A, Allen L, Cleary J, Kublin K, Goldstein H. Validity and reliability of the communication and symbolic behavior scales developmental profile with very young children. Journal of Speech, Language, and Hearing Research. 2002;45:1202–1218. doi: 10.1044/1092-4388(2002/097). [DOI] [PubMed] [Google Scholar]
- Zelazo P, Qu L, Muller U. Hot and cool aspects of executive function: Relations in early language development. In: Schneider W, Schumann-Hegsteler R, Sodian B, editors. Young children’s cognitive development: Interrelations among executive functioning, working memory, verbal ability and theory of mind. Mahwah, NJ: Lawrence Erlbaum Associates; 2005. pp. 71–93. [Google Scholar]


