Abstract
The sarcoplasmic reticulum Ca2+ ATPase (SERCA) is redox-regulated by post-translational thiol modifications of cysteine-674 to regulate smooth muscle relaxation and migration. To detect oxidation of cysteine-674 that irreversibly prevents redox-regulation, a polyclonal, sequence-specific antibody was developed towards a peptide containing cysteine-674 sulfonic acid. The antibody stained intact 110 kDa SERCA in pig cardiac SR that was oxidized in vitro by peroxynitrite in a sequence-specific manner, and histochemically stained atherosclerotic pig and rabbit aorta. Surprisingly, immuno-blots of the pig aorta failed to stain intact 110 kDa SERCA protein, but rather, higher molecular mass aggregates and lower molecular mass bands. Of the latter bands at 70 and 60 kDa, the largest were observed in diabetic, hyperlipidemic pigs, and coincided with the most positive histochemical staining. The 70 and 60 kDa molecular mass bands also coincided with the majority of the protein detected by a monoclonal total anti-SERCA antibody, which detected the intact 110 kDa protein in normal pigs. Mass spectrometry identified SERCA in all the major bands detected by the sulfonic acid antibody as well as the oxidation of cysteine-674 in the 70 kDa band. These studies demonstrate a sequence-specific antibody that detects partial degradation products of SERCA, which represent the majority of the protein in some diabetic hypercholesterolemic pig aortae. In addition, the results suggest an association between irreversible oxidation of SERCA and its degradation, and that an important portion of the oxidized protein in tissue samples may be partially degraded.
Keywords: SERCA, thiol, oxidation, peroxynitrite
Introduction
Dysfunction of the sarcoplasmic reticulum Ca2+ ATPase (SERCA) has been reported in many disease states, including heart failure[1], diabetes[2], atherosclerosis[3] and restenosis[4], as well as in aging skeletal muscle[5]. A common feature of these pathological situations is the increased and prolonged production of reactive oxygen species (ROS) [6] to which SERCA is particularly susceptible [7–9]. Many studies have demonstrated that ROS inactivate SERCA, which subsequently results in elevation of cytosolic Ca2+ concentration [10].
Post-translational modification of amino acid residues of SERCA [11] may affect the structure of the Ca2+ transporter and consequently affect its function. The simultaneous production of superoxide anion (O2·−) and nitric oxide from multiple nitric oxide synthase isoforms can promote the formation of peroxynitrite (ONOO−), a potent oxidant that has been implicated in modifying the cysteine, tyrosine and methionine residues in proteins that are most reactive [12]. For example, using immunochemical and mass spectrometric methods, we showed tyrosine nitration of Tyr 294 and 295 in atherosclerotic human and rabbit aorta and aging skeletal muscle [12,13]. However, the precise physiological implications of these nitrotyrosine modifications have not been proven, only that they are associated with SERCA dysfunction in diseased tissues.
In our previous studies, we also demonstrated that cysteine-674, one of the most reactive cysteine residues in SERCA, is critical to NO-mediated regulation of SERCA activity [14]. Under physiological situations, NO stimulates SERCA activity by inducing reversible S-glutathiolation primarily of cysteine-674, which consequently increases SERCA-dependent Ca2+ uptake, decreases Ca2+ influx, lowers cytosolic Ca2+ concentration, and inhibits Ca2+-dependent physiological functions including smooth muscle cell migration [3]. In contrast, in atherosclerotic rabbit aorta in which NO-induced vasodilatation is impaired, SERCA cysteine-674 was irreversibly oxidized by more than 50% and found to be in the sulfonic acid form by mass spectrometry. SERCA with this irreversibly oxidized cysteine was therefore unable to undergo reversible thiol modification, preventing the stimulation of Ca2+ uptake activity[3]. Furthermore, NO-induced inhibition of cell migration was prevented in cells in which SERCA cysteine-674 was mutated to serine, indicating the key physiological importance of this SERCA amino acid residue in calcium regulation by NO [14].
Here we report a sequence-specific polyclonal antibody that detects SERCA cysteine-674 sulfonic acid oxidation. Using this antibody in a model of chronic diabetes and hyperlipidemia in the pig, we find that SERCA with oxidized cysteine-674 is detected primarily in lower molecular mass forms that constitute the majority of total SERCA protein in some of the diseased aortas. These findings suggest an association between irreversible oxidation and degradation of SERCA, and may help to explain decreased SERCA expression and function in various disease states.
Methods
SERCA C674SO3H antibody
Affinity-purified rabbit antibody to detect SERCA cysteine-674 in its sulfonic acid form was made by Bethyl Laboratories (Montgomery, TX). The antigenic peptide against the human SERCA sequence, 669CLNARC(SO3H)FARV678, was chemically synthesized with the constituent amino acids, in which cysteine-674 was substituted with cysteic acid, the sulfonic acid of cysteine. The antigen was injected into rabbits and serum obtained approximately every two weeks over a 5-month period. To eliminate antibodies to the peptide that did not recognize the modified cysteine, antisera were processed over an immuno-sorbent consisting of a peptide of the same sequence as the antigenic peptide but which contained a reduced cysteine-674 immobilized on agarose. Subsequently, specific antibodies against the antigenic peptide were column-purified from the processed antisera using an immuno-sorbent consisting of the antigenic peptide. Final concentration of the antibody was 1 mg/mL.
Diabetic hyperlipidemic pig model
Pig aortic samples were from a diabetic hyperlipidemic pig model developed by Dr. Gerrity[15]. Diabetes was induced in 12 week old (15–20 kg body weight) male pigs by injecting the pancreatic β-cell cytotoxin, streptozotocin (STZ), which resulted in a <80% reduction in β-cells and subsequent increased plasma glucose (Supplemental table). Hyperlipidemia was induced in diabetic pigs by a high cholesterol diet (1.5% cholesterol and 15% lard) for 30 weeks before sacrifice. Some pigs were treated once daily with Lantus insulin (Sanofi Aventis) at dosages sufficient to maintain serum glucose levels at less than 100 mg/dL. Insulin treatment was begun 8 weeks after starting the high cholesterol and fat diet. Control pigs were injected with the citrate buffer used for STZ and kept on a normal chow diet.
Cardiac sarcoplasmic reticulum (SR) membrane preparation
The pig cardiac SR samples were prepared in a buffer containing imidazole. The detailed protocol was previously published [16]. These samples were generously provided by Dr. Diana Bigelow (Pacific Northwest National Laboratory).
Immunohistochemistry
The aorta samples were fixed in 10% buffered formalin acetate and embedded in paraffin. After removal of paraffin and rehydration, tissue sections (5 µm thick) were treated with 10 mmol/L citric acid (pH 6.0), and were heated in a microwave (2 min, 3 times at 700 W) to recover antigenicity. Nonspecific binding was blocked with 10% normal goat or horse serum in phosphate-buffered saline (PBS, pH 7.4) for 30 min before incubation with individual primary antibodies. Anti-SERCA C674-SO3H antibody was used at 2 µg/mL. The secondary antibody, a biotinylated anti-rabbit IgG secondary antibody was used at 1:200. Vector Red alkaline phosphatase substrate (Vector) was used to visualize positive immuno-reactivity. The specificity of the anti-SERCA C674-SO3H antibody was confirmed by preincubating the antibody with C674-SO3H SERCA peptide, reduced C674 SERCA peptide, or the scrambled C674-SO3H SERCA peptide, CRAFNC(SO3H)VRAL, (antibody: peptide = 1:50 by weight) for at least 1 h. Samples of hyperlipidemic rabbit aorta were obtained from a studies published previously [3,17].
Western Blot
Pig cardiac sarcoplasmic reticulum
Peroxynitrite (Cayman) was diluted in 0.1M NaOH and the concentration was derived from the absorbance at 302 nm. Serial stock solutions of peroxynitrite were prepared in 0.1 mmol/L NaOH to reach the final concentrations in 50 µL total volume. One µg of pig cardiac sarcoplasmic reticulum (SR) protein was diluted in 50 µL buffer containing 100 mM K3PO4, pH 7.1. One µL peroxynitrite in NaOH solution was added to the pig cardiac SR protein solution during vigorous vortexing. After separating by SDS-PAGE, the peroxynitrite-treated pig cardiac SR protein was immuno-blotted with anti-SERCA C674-SO3H antibody at a dilution of 1:1000, and anti-rabbit IgG secondary antibody was used at a dilution of 1:5000. The specificity of the anti-SERCA C674-SO3H antibody was confirmed by pre-incubating the antibody with C674-SO3H SERCA peptide, the reduced C674 SERCA peptide, or the scrambled C674-SO3H SERCA peptide in which cysteine-674 was in the sulfonic acid form (antibody: peptide = 1:20 by weight) for 1 h.
Pig aorta
After homogenization, aortic protein from control pigs, diabetic hyperlipidemic pigs, and diabetic pigs treated with insulin were separated by SDS-PAGE. SERCA was immuno-blotted with the monoclonal anti-SERCA monoclonal antibody IID8 (3:10,000, Affinity Bioreagents), by anti-SERCA-C674-SO3H antibody (1:1000), or anti-GAPDH polyclonal antibody (1:1000, Santa Cruz). The secondary antibodies were anti-mouse IgG (1:20,000), and anti-rabbit IgG (1:5,000), respectively.
Immuno-precipitation of SERCA from pig aorta samples
Pig aorta was homogenized on ice in modified RIPA buffer (50 mM Tris-HCl pH 7.1, 150 mmol/L NaOH, 1 mmol/L NaF, 1% NP-40, 1% polyoxyethylene 9 lauryl ether, 100 µmol/L DETAPAC, 2 mmol/L PMSF) with 10 mmol/L iodoacetamide (IAM) to alkylate free thiols. Proteins were separated by centrifugation at 14,000 × g for 20 minutes, and the supernatant was passed through a PD-10 column to remove any unbound IAM. Protein concentration was determined by the Bradford protein assay (BioRad). Five mg of aortic protein lysate was pre-cleared by adding 100 µL protein A Sepharose beads and incubating at 4°C for an hour. After the protein A beads were removed by centrifugation at 8,000 rpm for 2 min, the remaining protein solution was incubated with 15 µL monoclonal anti-SERCA antibody IID8 overnight at 4°C. To capture the immuno-complex, the antigen–antibody mixture was added to 100 µL protein A Sepharose beads, which were pre-incubated with 5 µL rabbit anti-mouse IgG at room temperature for 30 min in order to enhance the capture. The total mixture of antigen, antibody and protein A beads was incubated at 4°C for at least 2 h with gentle agitation. The protein A beads were later collected by centrifuging at 8,000 rpm for 5 min. The supernatant was discarded and the beads were washed three times with ice-cold modified RIPA buffer. SDS (0.1%) was added to the modified RIPA buffer to eliminate non-specific binding on the protein A beads. The captured SERCA protein was released from the beads by incubating with 100 µL Laemmli sample buffer (2% SDS) at 55°C for at least 30 min. After the beads were centrifuged at 8,000 rpm for 5 min, the supernatant was transferred to another tube. 5% β-Mercaptoethanol was added to the supernatant with continued incubation at 55°C for 30 min. The protein was separated on a gradient SDS-PAGE gel (NuPAGE, Invitrogen).
Mass spectrometry analysis
Immuno-precipitated SERCA from control and diabetic pig aortic homogenates was separated by SDS-PAGE gel and stained overnight with Gelcode Blue (Pierce) followed by extensive washing with deionized and distilled H2O. The 220, 70, and 40 kDa bands were excised from the gel and digested with trypsin as described previously[18]. In brief, gel slices were cut into pieces of about 1 mm wide, washed twice for 45 min at 37°C in 0.5 mL of 200 mmol/L NH4HCO3/50% (v/v) acetonitrile with agitation. For cysteine alkylation, the gel slices were incubated with 2 mmol/L DTT in 200 mmol/L NH4HCO3 for 30 min at 50°C, followed by reaction with 5 mmol/L iodoacetamide for 30 min at room temperature. After removal of the solvent, the gel slices were washed for an additional 1 h in 0.5 mL of 200 mmol/L NH4HCO3/50% acetonitrile (v/v) with agitation, and shrunk in pure acetonitrile for 15 min. After removal of acetonitrile and drying under vacuum, the samples were re-swollen with a buffer containing 40 mmol/L NH4HCO3, 1 mmol/L CaCl2, 10% (v/v) acetonitrile, and trypsin at a 10:1 molar ratio of the protein to trypsin (1 µg trypsin per gel band containing an estimated 10 µg protein). The volume of buffer was ca. 1.5-times that of the excised gel band. After the adsorption of trypsin, additional buffer (30–50 µL) was used to cover the gel pieces during overnight digestion (16–18 h) at 37°C. For the extraction of peptides from the gel, the supernatant of the in-gel digest was obtained after sonication for 30 min in a water bath of an Ultrasonic Cleaner ME 4.6 (Mettler Electronics Corp., Anaheim, CA, USA) and short centrifugation, and used for MS analysis. In-gel tryptic digests (7 µl) were submitted to nano-HPLC-electrospray ionization-tandem mass spectrometry (NESI-MS/MS) analysis on either a ThermoElectron LCQ Duo or a ThermoElectron Classic (San Jose, CA), equipped with a nanoelectrospray source (ThermoElectron). Separation of tryptic peptides was achieved on-line prior to MS/MS analysis on in-house packed BioBasic C18 stationary phase (Thermo Electron) nanoflow columns (300Å, 10cm × 75µm, 15 µm tip size) (New Objective, Woburn, MA) with the following chromatographic conditions: mobile phase A: 0.1% formic acid in water, mobile phase B: 0.1% formic acid in MeCN. The flow rate was 0.5 µL/min, delivered by a MicroTech Scientific Ultra Plus II pump (after 1:20 split), or by a MicroTech Xtreme Simple nano-flow pump (direct flow). The following gradient profile was used to increase mobile phase B linearly to the following fractions: from 0 to 5 min gradient held at 10% B, then increased to 60% B within 105 minutes, and continued at 60% B for additional 5 minutes. After each run, the column was washed by a short gradient (0–60% B for 20 min) and allowed to re-equilibrate to the initial conditions for 15 minutes. The following instrumental conditions were used for mass spectrometric analysis: number of microscans = 3, length of microscans = 200 ms, capillary temperature = 160°C, spray voltage = 1.9 kV, capillary voltage = 35 V, tube lens offset = −14V. The mass spectrometer was tuned using the static nanospray setup with a 5 µmol/L solution of angiotensin I (MW 1296.5) infused by a pico-tip emitter (New Objective). Data acquisition was performed in the data-dependent fashion, i.e. an MS scan followed by 3 or 4 MS/MS scans of the 3 or 4 most intense peaks with the normalized collision energy for MS/MS set at 35% and the isolation width of 2.0 m/z. A minimal signal for MS/MS acquisition was set to 2 × 106. Additionally, the dynamic exclusion option was enabled and set with the following parameters: repeat count = 3, repeat duration = 5 min, exclusion list size = 25, exclusion duration = 5, and exclusion mass width = 3. Protein sequence analysis was achieved with the ThermoElectron Bioworks 3.1 software package[19] with the most current non-redundant NCBI protein database downloaded from the ftp.ncbi.nlm.nih.gov/blast/db. For characterization of SERCA modifications, a custom database limited to the protein sequence was created. The following modifications were accounted for during the search: oxidation of Met (+16 amu; amu = atomic mass units), carboxymethylation (+58 amu), carboxyamidomethylation (+57 amu), or oxidation (+32 and +48 amu) of Cys residues. Analysis of the MS/MS spectra was based on a search for the major sequence-indicating ions resulting from the cleavage of the parent ion at specific locations relative to the peptide bond. The Roepstorf-Fohlman nomenclature [20] was used for the annotations of N-terminal (b) and C-terminal (y”) fragments.
Results
Validation of the anti-SERCA C674-SO3H antibody
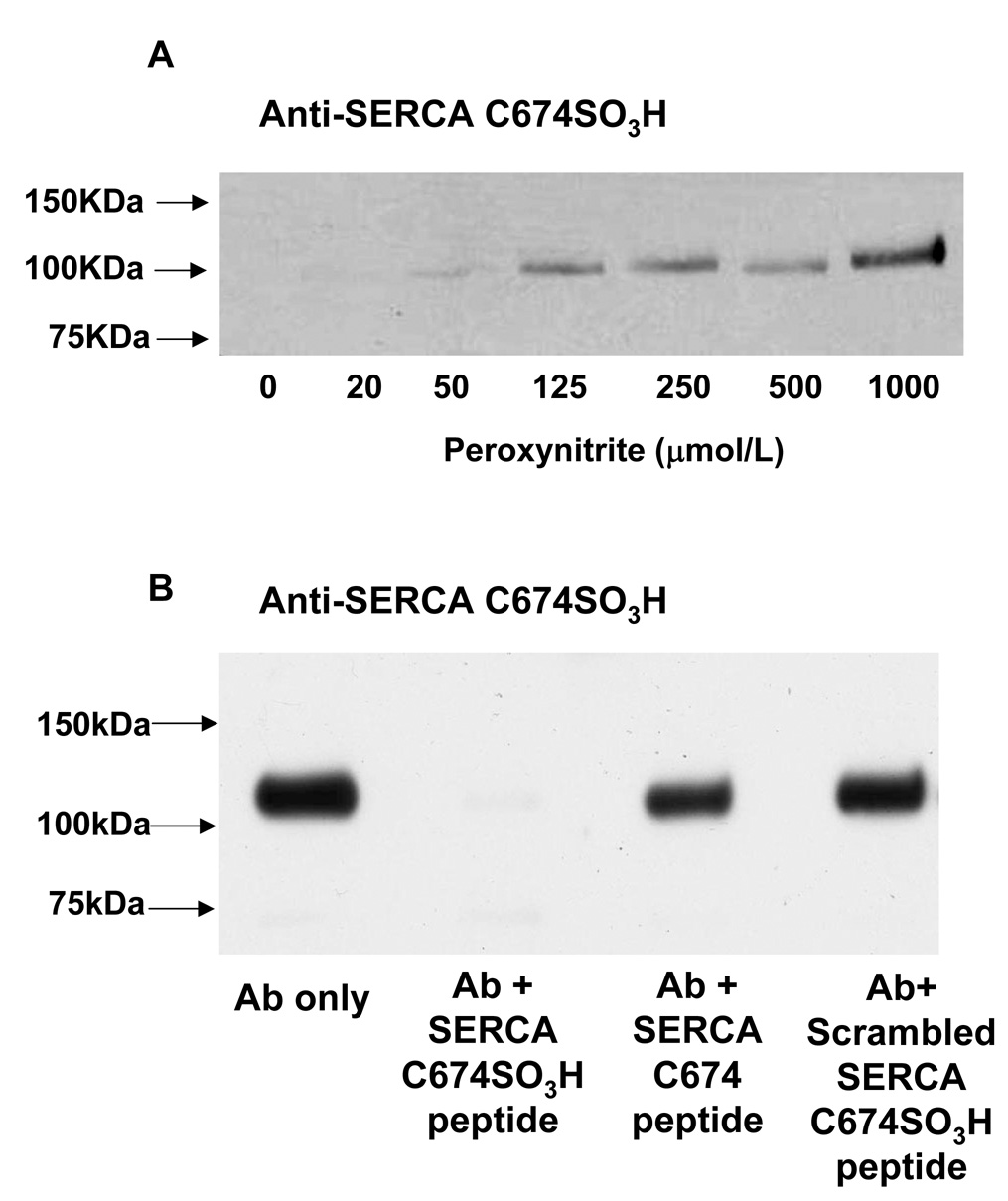
To validate the specificity of the SERCA C674-SO3H antibody, pig cardiac SR membranes that are enriched in SERCA protein were treated with increasing concentrations of peroxynitrite. With equal quantities of SERCA protein in each lane (Supplemental figure 1A), the SERCA C674-SO3H antibody detected a 110 kDa band in cardiac SR samples treated with concentrations of peroxynitrite greater than 50 µmol/L, and the intensity of the signal increased with increasing concentrations of peroxynitrite (Figure 1A). After pre-incubating the SERCA C674-SO3H antibody with the antigenic C674-SO3H SERCA peptide (CLNARC(SO3H)FARV), staining of the 110kDa band detected in pig cardiac SR treated with 1 mM peroxynitrite was eliminated (Figure 1B). However, pre-incubating the antibody either with the peptide of the same sequence with cysteine-674 with reduced thiol (CLNARCFARV), or a peptide containing a scrambled sequence of the antigenic peptide containing the cysteine sulfonic acid (CRAFNC(SO3H)VRAL) did not block the detection of the 110 kDa band. These results indicate that the anti-SERCA C674-SO3H antibody detects only SERCA protein with cysteine sulfonic acid in a sequence-specific manner.
Figure 1. Validation of the specificity of anti-SERCA C674-SO3H antibody.
A: Anti-SERCA C674-SO3H antibody staining of pig cardiac SR membranes treated with different concentrations of peroxynitrite. SR membranes (1µg) purified from pig heart were treated with peroxynitrite (0, 20, 50, 125, 250, 500, 1000 µmol/L) during rapid mixing. SERCA cysteine-674 SO3H was detected by polyclonal anti-SERCA cysteine 674-SO3H antibody at 1:1000. Staining by secondary anti-rabbit IgG antibody alone was negative (data not shown).
B: Pig cardiac SR protein (3 µg) was treated with peroxynitrite (1 mmol/L). The polyclonal anti-SERCA C674-SO3H antibody was incubated with the immunogenic C674-SO3H SERCA peptide, the SERCA peptide containing the reduced cysteine-674, or the scrambled C674-SO3H SERCA peptide for 1 h at room temperature (Ab: peptide =1:20 by weight). The anti-SERCA Cys 674 SO3H antibody was used at 1:1000. Total SERCA was stained with IID8 antibody for control of protein loading in both blots (see Supplemental Figure 1).
SERCA C674-SO3H in diabetic hyperlipidemic pig aorta
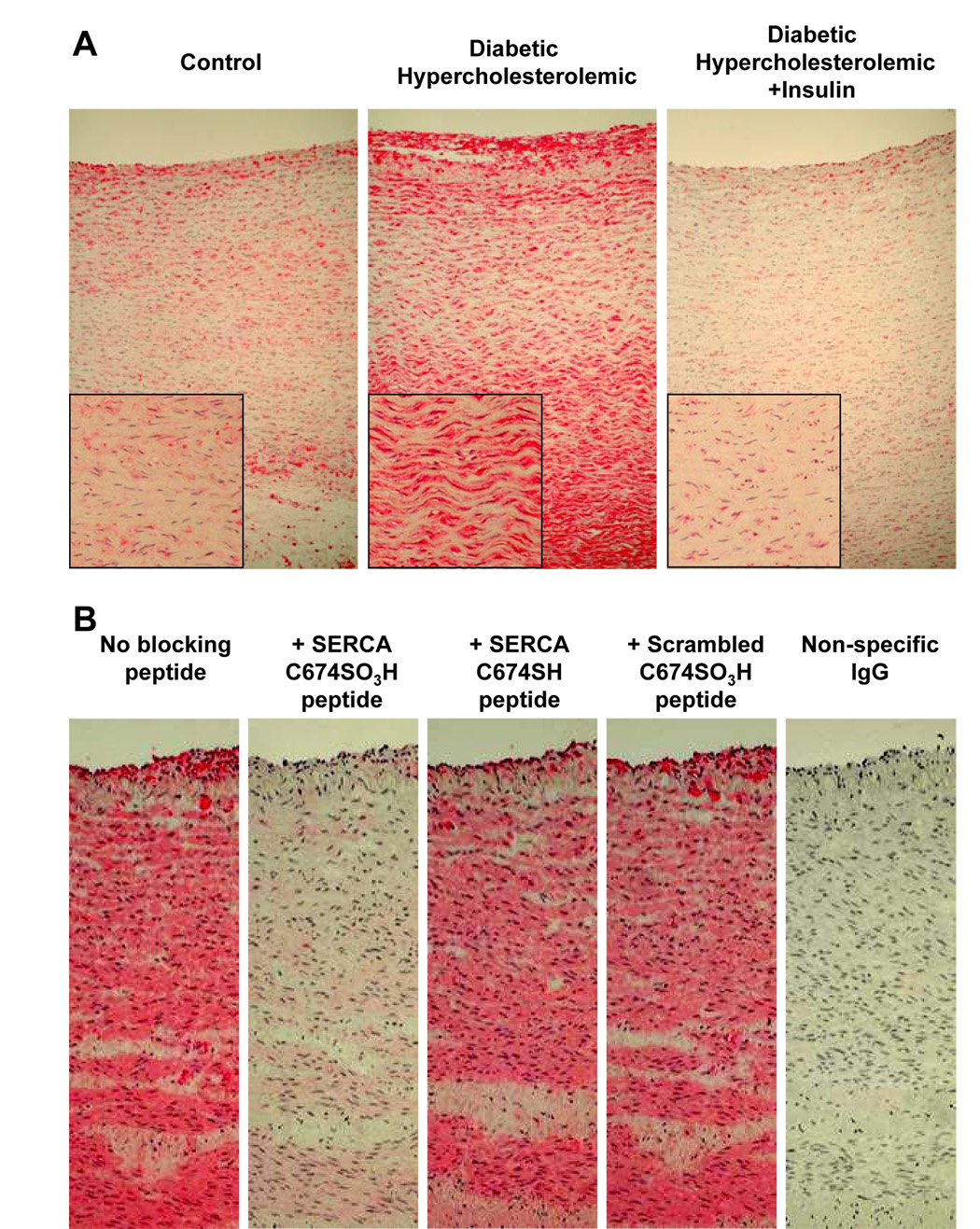
Histochemical staining with anti-SERCA C674-SO3H antibody showed intense staining of neointima, medial smooth muscle and adventitial cells in a diabetic hyperlipidemic pig aorta (Figure 2A). The staining was much less in a non-diabetic control pig fed normal chow, or in a diabetic hyperlipidemic pig treated with insulin. As with the immuno-blot of pig cardiac SR treated with peroxynitrite, immuno-histochemical staining of the diabetic hyperlipidemic pig aorta was prevented by pre-incubating the antibody with the immunogenic peptide (Figure 2B). Neither the peptide containing the cysteine with a reduced thiol, nor the scrambled immunogenic peptide blocked histochemical staining with the antibody. The anti-SERCAC674-SO3H antibody also stained both intimal and medial cells of atherosclerotic rabbit aorta that was previously demonstrated to contain sulfonic acid cysteine-674 by mass spectrometry [3] (Supplemental figure 2A), and the staining of a control rabbit aorta was much less intense.
Figure 2. Immuno-histochemical staining with anti-SERCA C674-SO3H of aorta from control pig (#1), diabetic hyperlipidemic pig (#4) and diabetic pig treated with insulin (#7).
A: Positive staining is shown in diabetic hyperlipidemic pig (#4) with less intense staining observed in other pigs (see Supplemental Figure 2B). Staining can be seen in both the plaque on the lumenal aspect of the diabetic pig aorta as well as in the smooth muscle lamellae.
B: Specificity of staining with anti-SERCA C674-SO3H antibody in aorta of diabetic hyperlipidemic pig #6. Staining was blocked by the C674-SO3H SERCA peptide, but not by the SERCA peptide with reduced cysteine-674, or the scrambled C674-SO3H SERCA peptide, indicating specific staining of SERCA C674-SO3H. Anti-rabbit IgG secondary antibody staining was negative.
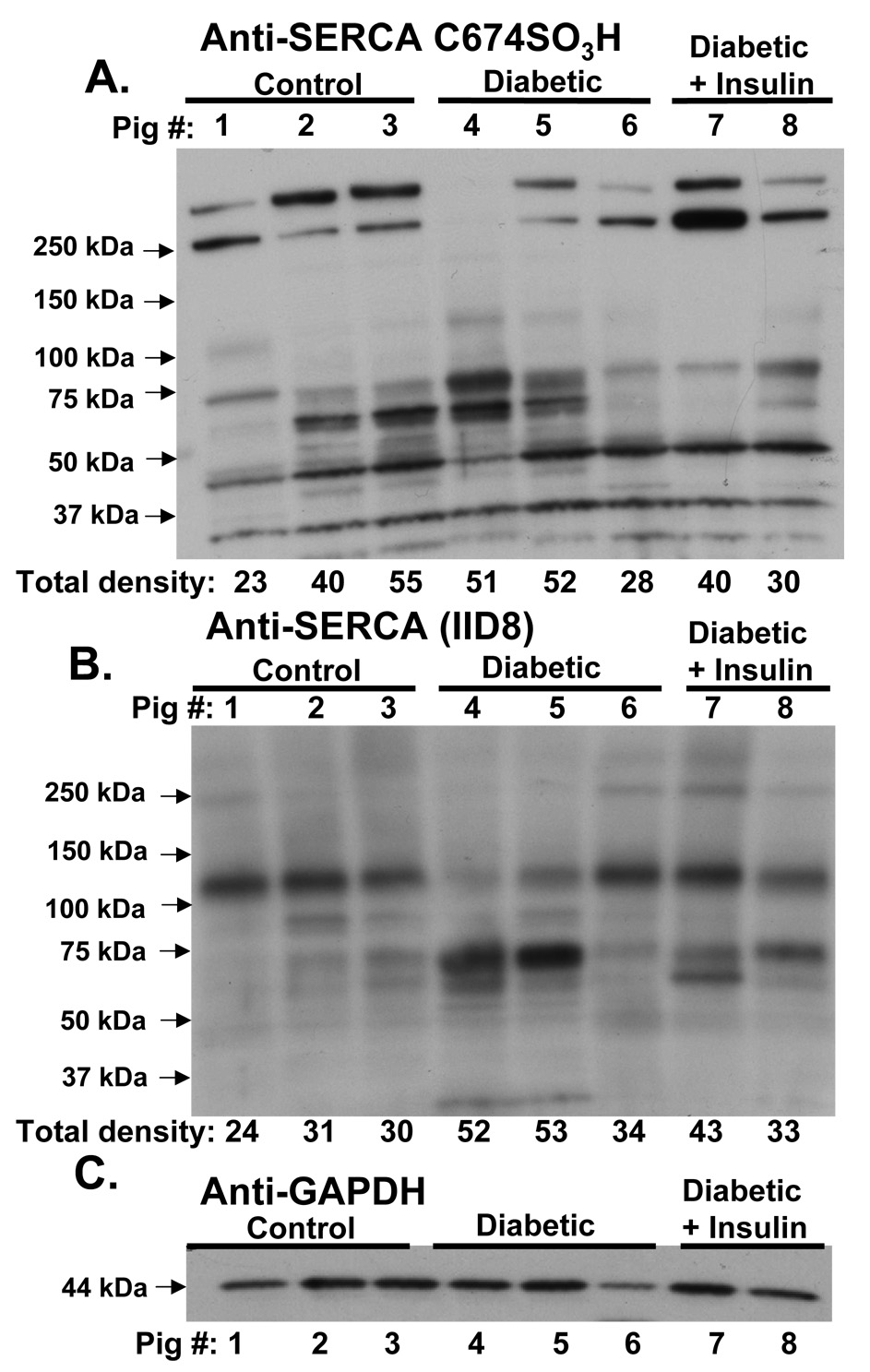
Immuno-blots were performed to detect SERCA cysteine 674 oxidation in pig aortic homogenates from control, diabetic hyperlipidemic, and insulin-treated, diabetic hyperlipidemic pigs. As shown in Figure 3A, the SERCA C674-SO3H antibody detected multiple bands in pig aortas from all groups, notably with only a faint band at 110 kDa in some pigs in each group. Of note were bands at 70 and 60 kDa that were most prominent in two diabetic pigs (#4 and #5). These bands were much less stained in diabetic pig #6, which also showed less histochemical staining of SERCA C674-SO3H (Supplemental Figure 2B). Two bands at or above 250 kDa were detected in all groups, but were less stained in diabetic hyperlipidemic pigs than in controls or treated diabetic pigs. In addition, several bands below 50 kDa were stained similarly in all pigs by the SERCA C674-SO3H antibody.
Figure 3.
Staining of aortic homogenates obtained from control, diabetic, and diabetic pigs treated with insulin with anti-SERCA C674-SO3H antibody (A), anti-SERCA IID8 antibody (B), or anti-GAPDH antibody (C). Aortic homogenate protein (30 µg in A and C, and 100 µg in B was separated by SDS-PAGE. As indicated on each blot are samples from pigs #1–8 listed in the supplemental table from the three groups of pigs. Below each lane in A and B are values of densitometry in arbitrary units integrated over each entire lane. Primary antibody concentrations were A, 1:1000; B, 3:10,000; and C, 1:1000.
As expected, staining for total SERCA of control pig aortic homogenates with the monoclonal anti-SERCA antibody (IID8) detected a single major band at 110 kDa, although faint bands were observed at 60, 70, 80 kDa, and at and above 250 kDa (Figure 3B). In two diabetic hypercholesterolemic pigs (#4 and 5), the anti-SERCA antibody most strongly detected a 70 kDa band with much fainter 110 kDa and lower molecular mass bands. In the insulin-treated diabetic pigs and in one diabetic hypercholesterolemic pig (#6), SERCA was detected strongly at 110 kDa and less so in the lower molecular mass bands at 70 and 60 kDa. These data indicate that in some diabetic pigs, the lower molecular mass bands that stain most strongly with the anti-SERCA IID8 antibody, also stain most strongly with the SERCA C674-SO3H antibody, but that 110 kDa intact SERCA stains weakly.
The identification by NESI-MS/MS of lower molecular mass forms of SERCA with oxidized cysteine-674
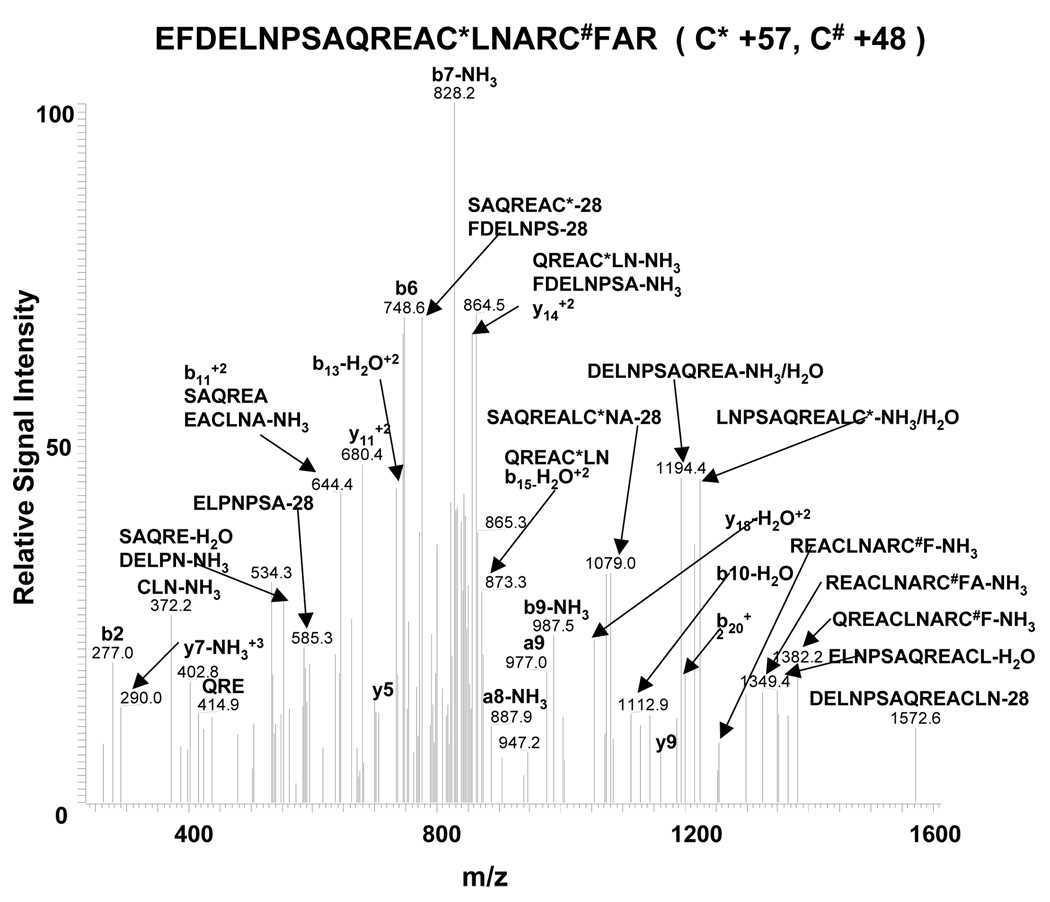
After SERCA immuno-precipitation with the IID8 antibody, Gel code blue stained proteins from both control and diabetic hypercholesterolemic pig aorta appeared primarily at 220, 70, and 40 kDa in addition to heavy and light chain IgG (Supplemental Figure 3). Compared to control pig aortic protein, there was a more intense Gel code blue stained band at 70 kDa, and a less intense band at 220 kDa in the diabetic pig aorta. These bands from aortic homogenates of control (#1) and diabetic hypercholesterolemic (#4) pigs were digested in gel, and NESI-MS/MS was performed. The NESI-MS/MS data proved the identities of the 220, 70 and 40 kDa bands to be SERCA. Representative MS/MS spectra for SERCA peptides detected in the respective bands are shown in Supplemental Figure 4 A–D, and sequence coverage is shown in Supplemental Figure 5 A–C. Furthermore, SERCA C674-SO3H was conclusively demonstrated by MS/MS analysis of the 70 kDa protein from diabetic hyperlipidemic pig # 4 (Figure 4).
Figure 4.
NESI-MS/MS spectrum of the SERCA peptide 656EFDELNPSAQREACLNARCFAR677 containing C674-SO3H. After immuno-precipitation of SERCA from aortic homogenate of diabetic pig #4, the 70 kDa gel band was cut, digested in trypsin, and analyzed by NESI-MS/MS.
* Indicates alkylation of cysteines caused by iodoacetamide present during the in-gel digestion. # Indicates the sulfonic acid modified cysteine.
Discussion
The combined approaches of immuno-histochemisty, immuno-blotting, and mass spectrometry used in this study confirmed irreversible oxidation of the SERCA cysteine-674 thiol in atherosclerotic aorta of a chronic hyperlipidemic diabetic pig model. The sequence-specific antibody provides a practical means with which to monitor SERCA cysteine oxidation in tissue. Remarkably, in all pig aortae, the majority of the SERCA C674-SO3H detected by immuno-blot was not in the intact 110 kDa protein, but in higher and lower molecular mass bands. In some diabetic pigs, the majority of protein detected with a total anti-SERCA antibody appeared in lower molecular mass forms consistent with oxidation of the redox-sensitive cysteine thiol in SERCA being associated with its degradation in the diseased aortae.
The detection of SERCA C674-SO3H in a sequence-specific manner by the antibody used in this study was demonstrated on SERCA in normal pig cardiac SR membranes exposed directly to peroxynitrite by showing that neither the peptide with reduced cysteine-674 nor a peptide containing the sulfonic acid cysteine in a scrambled sequence prevented immuno-detection. In this preparation, the antibody detected C674-SO3H in full-length 110 kDa SERCA, indicating that the antibody does not discriminate against the oxidized intact protein. The same peptides were used to show the specificity of the immuno-histochemistry. A similar antibody was previously developed towards the oxidized catalytic cysteine in protein tyrosine phosphatase-1B in sulfonic acid form, but not used to detect protein oxidation in disease or by immuno-histochemistry [21].
Unlike in the cardiac SR preparation in which cysteine-674 was oxidized in vitro, the SERCA C674-SO3H antibody detected little protein at the molecular mass of the intact protein in tissue homogenates of normal control or diabetic hyperlipidemic pigs. Rather, multiple bands were detected in all pig aortae. In pigs #4 and 5, the most intensely stained bands at 70 and 60 kDa also stained most intensely with the anti-SERCA IID8 monoclonal antibody, which detects primarily full-length 110 kDa molecular mass SERCA in control pigs. A 70 kDa band was also seen in immuno-precipitates used to purify SERCA for mass spectrometry, which confirmed that the band contained SERCA with sulfonic acid cysteine-674. The presence of irreversibly oxidized SERCA cysteine-674 in the major band detected with the total anti-SERCA antibody in some diabetic hyperlipidemic pigs suggests that the majority of SERCA in these aortas is a degraded form of the protein. Notably, even normal pig aortic homogenates, in which most SERCA is in the intact protein at 110 kDa, contained immuno-reactive bands to the SERCA C674-SO3H antibody at both lower and higher molecular mass. These results suggest that oxidation of cysteine-674 occurs in a small percentage of total SERCA protein as part of normal SERCA degradation. Faint bands were detected even in control pigs with the total anti-SERCA antibody in lower as well as higher molecular mass forms. Higher molecular mass aggregates form often during immuno-precipitation of large membrane proteins, and these were confirmed by NESI-MSMS to contain SERCA. The fact that these aggregates stain positively for SERCA C674-SO3H in normal pig aorta is consistent with oxidation of intact SERCA preceding its normal degradation as part of normal protein turnover.
Our results suggest that a fraction of SERCA is present in oxidized, lower and higher molecular mass forms in normal pig aortic homogenates, and that these forms are augmented greatly in the aortic homogenates of some diabetic pigs. This conclusion is supported by the fact that the 70 kDa SERCA band is not intensely stained in the aortic homogenate of one diabetic pig (#6) which also showed less immuno-histochemical staining with the SERCA C674-SO3H antibody, or in aorta of pigs treated with insulin in which the majority of SERCA was detected by the IID8 monoclonal antibody at 110 kDa. Although lower molecular mass forms of SERCA have not been reported associated with disease, decreased 110 kDa SERCA protein expression has been demonstrated in many studies, including those of diabetic animal models[22]. In addition, increased oxidants have been associated with accelerated SERCA degradation seen with cardiac ischemia reperfusion [23], radiation induced apoptosis of HeLa cells[24], and hydrogen peroxide exposure of embryonic heart cells [25]. In the latter study, oxidant-induced SERCA degradation was promoted by overexpression of calreticulin and prevented by the proteosomal inhibitor, lactacystin. The fact that some lower molecular forms of SERCA with oxidized cysteine-674 are present in normal pig aorta is consistent with oxidation and degradation being part of normal protein turnover. Increased oxidative stress in aging and pathological states is associated with the attenuated activity and expression of proteosomal subunits [26–28]. However, some oxidized proteins may be poor substrates for the proteasome inhibiting their degradation[29], possibly explaining why the oxidized 70 kDa SERCA accumulates in some diabetic pig aortae. Therefore, our results illustrate an association of oxidation of SERCA in some diabetic pig aortae with decreased amounts of intact SERCA protein and accumulation of lower molecular mass forms of the protein.
In summary, these studies validate a sequence-specific antibody to detect SERCA C674-SO3H. SERCA C674 oxidation was detected in normal pig aortic homogenates in a small fraction of total SERCA. Oxidized SERCA occurred in the majority of SERCA protein detected at a lower molecular mass form in 2 of 3 chronically diabetic, hyperlipidemic pigs. Although the number of samples that we were able to analyze here is insufficient to determine the frequency with which increased SERCA C674 oxidation or degradation occurs in diseased pigs, the antibody did stain atherosclerotic rabbit aorta in which we previously showed that cysteine-674 oxidation occurred in 50–60% of total SERCA as detected by iodoacetamide binding which measures reduced C674. In addition, our preliminary studies indicate that aortic punch biopsies obtained from diabetic, hyperlipidemic patients undergoing coronary artery bypass surgery stain with the SERCA C674-SO3H antibody reported here, indicating that oxidation of SERCA C674 may be associated with human cardiovascular disease. Oxidation of SERCA cysteine-674 is associated with failure of redox regulation of Ca2+ uptake during smooth muscle relaxation [3] and migration[14], both of which are dysregulated in diabetes. Although further studies are necessary to determine the frequency with which SERCA oxidation and degradation occurs in a major fraction of SERCA protein, as it apparently did in pigs #4 and 5, these studies may suggest an association between oxidation and degradation of SERCA, loss of tissue Ca2+ regulation, and the increased atherogenesis caused by diabetes and hyperlipidemia in this pig model [15].
Supplementary Material
Acknowledgements
The authors wish to acknowledge Eric McIntush of Bethyl Laboratories for invaluable help in providing the sequence-specific SERCA C674-SO3H antibody and SERCA peptides. These studies were supported by R01 HL31607-24 (JY, SX, BJ, VS, CS, RAC), the NIH Boston University Cardiovascular Proteomics Center, N01-HV-28178 (RAC), AG P01 12993 (VS, CS), and an American Heart Association Scientist Development Grant 0435205N (BJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Diaz ME, Graham HK, O'Neill S, Trafford AW, Eisner DA. The control of sarcoplasmic reticulum Ca2+ content in cardiac muscle. Cell Calcium. 2005;38:391–396. doi: 10.1016/j.ceca.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 2.Belke DD, Dillmann WH. Altered cardiac calcium handling in diabetes. Curr Hypertens Rep. 2004;6:424–429. doi: 10.1007/s11906-004-0035-3. [DOI] [PubMed] [Google Scholar]
- 3.Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schoneich C, Cohen RA. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med. 2004;10:1200–1207. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- 4.Lipskaia L, del Monte F, Capiod T, Yacoubi S, Hadri L, Hours M, Hajjar RJ, Lompre AM. Sarco/endoplasmic reticulum Ca2+-ATPase gene transfer reduces vascular smooth muscle cell proliferation and neointima formation in the rat. Circ Res. 2005;97:488–495. doi: 10.1161/01.RES.0000180663.42594.aa. [DOI] [PubMed] [Google Scholar]
- 5.Sharov VS, Dremina ES, Galeva NA, Williams TD, Schoneich C. Quantitative mapping of oxidation-sensitive cysteine residues in SERCA in vivo and in vitro by HPLC-electrospray-tandem MS: selective protein oxidation during biological aging. Biochem J. 2006;394:605–615. doi: 10.1042/BJ20051214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens. 2000;18:655–673. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- 7.Grover AK, Samson SE. Effect of superoxide radical on Ca2+ pumps of coronary artery. Am J Physiol Cell Physiol. 1988;255:C297–C303. doi: 10.1152/ajpcell.1988.255.3.C297. [DOI] [PubMed] [Google Scholar]
- 8.Grover AK, Samson SE, Fomin VP. Peroxide inactivates calcium pumps in pig coronary artery. Am J Physiol Heart Circ Physiol. 1992;263:H537–H543. doi: 10.1152/ajpheart.1992.263.2.H537. [DOI] [PubMed] [Google Scholar]
- 9.Grover AK, Samson SE, Misquitta CM. Sarco(endo)plasmic reticulum Ca2+ pump isoform SERCA3 is more resistant than SERCA2b to peroxide. Am J Physiol Cell Physiol. 1997;273:C420–C425. doi: 10.1152/ajpcell.1997.273.2.C420. [DOI] [PubMed] [Google Scholar]
- 10.Lounsbury KM, Hu Q, Ziegelstein RC. Calcium signaling and oxidant stress in the vasculature. Free Radic Biol Med. 2000;28:1362–1369. doi: 10.1016/s0891-5849(00)00222-7. [DOI] [PubMed] [Google Scholar]
- 11.Schoneich C, Sharov VS. Mass spectrometry of protein modifications by reactive oxygen and nitrogen species. Free Radic Biol Med. 2006;41:1507–1520. doi: 10.1016/j.freeradbiomed.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Viner RI, Ferrington DA, Williams TD, Bigelow DJ, Schoneich C. Protein modification during biological aging: selective tyrosine nitration of the SERCA2a isoform of the sarcoplasmic reticulum Ca2+-ATPase in skeletal muscle. Biochem J. 1999;340:657–669. [PMC free article] [PubMed] [Google Scholar]
- 13.Xu S, Ying J, Jiang B, Guo W, Adachi T, Sharov V, Lazar H, Menzoian J, Knyushko TV, Bigelow D, Schoneich C, Cohen RA. Detection of sequence-specific tyrosine nitration of manganese SOD and SERCA in cardiovascular disease and aging. Am J Physiol Heart Circ Physiol. 2006;290:H2220–H2227. doi: 10.1152/ajpheart.01293.2005. [DOI] [PubMed] [Google Scholar]
- 14.Ying J, Tong XY, Pimental DR, Weisbrod RM, Trucillo MP, Adachi T, Cohen RA. Cysteine-674 of the Sarco/Endoplasmic Reticulum Calcium ATPase Is Required for the Inhibition of Cell Migration by Nitric Oxide. Arterioscl Thromb Vasc Biol. 2007;27:783–790. doi: 10.1161/01.ATV.0000258413.72747.23. [DOI] [PubMed] [Google Scholar]
- 15.Gerrity RG, Natarajan R, Nadler JL, Kimsey T. Diabetes-induced accelerated atherosclerosis in swine. Diabetes. 2001;50:1654–1665. doi: 10.2337/diabetes.50.7.1654. [DOI] [PubMed] [Google Scholar]
- 16.Knyushko TV, Sharov VS, Williams TD, Schoneich C, Bigelow DJ. 3-Nitrotyrosine modification of SERCA2a in the aging heart: a distinct signature of the cellular redox environment. Biochemistry. 2005;44:13071–13081. doi: 10.1021/bi051226n. [DOI] [PubMed] [Google Scholar]
- 17.Adachi T, Matsui R, Xu S, Kirber M, Lazar HL, Sharov VS, Schoneich C, Cohen RA. Antioxidant improves smooth muscle sarco/endoplasmic reticulum Ca2+-ATPase function and lowers tyrosine nitration in hypercholesterolemia and improves nitric oxide-induced relaxation. Circ Res. 2002;90:1114–1121. doi: 10.1161/01.res.0000019757.57344.d5. [DOI] [PubMed] [Google Scholar]
- 18.Sharov VS, Galeva NA, Knyushko TV, Bigelow DJ, Williams TD, Schoneich C. Two-dimensional separation of the membrane protein sarcoplasmic reticulum Ca2+-ATPase for high-performance liquid chromatography-tandem mass spectrometry analysis of posttranslational protein modifications. Anal Biochem. 2002;308:328–335. doi: 10.1016/s0003-2697(02)00261-0. [DOI] [PubMed] [Google Scholar]
- 19.Ducret A, Van Oostveen I, Eng JK, Yates JR, III, Aebersold R. High throughput protein characterization by automated reverse-phase chromatography/electrospray tandem mass spectrometry. Protein Sci. 1998;7:706–719. doi: 10.1002/pro.5560070320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roepstorff P, Fohlman J. Proposal for a common nomenclature for sequence ions in mass spectra of peptides. Biomed Mass Spectrom. 1984;11:601. doi: 10.1002/bms.1200111109. [DOI] [PubMed] [Google Scholar]
- 21.Persson C, Sjoblom T, Groen A, Kappert K, Engstrom U, Hellman U, Heldin CH, den Hertog J, Ostman A. Preferential oxidation of the second phosphatase domain of receptor-like PTP-alpha revealed by an antibody against oxidized protein tyrosine phosphatases. Proc Natl Acad Sci USA. 2004;101:1886–1891. doi: 10.1073/pnas.0304403101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakata S, Lebeche D, Sakata Y, Sakata N, Chemaly ER, Liang L, Nakajima-Takenaka C, Tsuji T, Konishi N, del Monte F, Hajjar RJ, Takaki M. Transcoronary gene transfer of SERCA2a increases coronary blood flow and decreases cardiomyocyte size in a type 2 diabetic rat model. Am J Physiol Heart Circ Physiol. 2007;292:H1204–H1207. doi: 10.1152/ajpheart.00892.2006. [DOI] [PubMed] [Google Scholar]
- 23.French JP, Quindry JC, Falk DJ, Staib JL, Lee Y, Wang KK, Powers SK. Ischemia-reperfusion-induced calpain activation and SERCA2a degradation are attenuated by exercise training and calpain inhibition. Am J Physiol Heart Circ Physiol. 2006;290:H128–H136. doi: 10.1152/ajpheart.00739.2005. [DOI] [PubMed] [Google Scholar]
- 24.Ding X, Xu Q, Liu F, Zhou P, Gu Y, Zeng J, An J, Dai W, Li X. Hematoporphyrin monomethyl ether photodynamic damage on HeLa cells by means of reactive oxygen species production and cytosolic free calcium concentration elevation. Cancer Lett. 2004;216:43–54. doi: 10.1016/j.canlet.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Ihara Y, Kageyama K, Kondo T. Overexpression of calreticulin sensitizes SERCA2a to oxidative stress. Biochem Biophys Res Commun. 2005;329:1343–1349. doi: 10.1016/j.bbrc.2005.02.112. [DOI] [PubMed] [Google Scholar]
- 26.Friguet B. Oxidized protein degradation and repair in ageing and oxidative stress. FEBS Lett. 2006;580:2910–2916. doi: 10.1016/j.febslet.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 27.Versari D, Herrmann J, Gossl M, Mannheim D, Sattler K, Meyer FB, Lerman LO, Lerman A. Dysregulation of the ubiquitin-proteasome system in human carotid atherosclerosis. Arterioscl Thromb Vasc Biol. 2006;26:2132–2139. doi: 10.1161/01.ATV.0000232501.08576.73. [DOI] [PubMed] [Google Scholar]
- 28.Shringarpure R, Davies KJ. Protein turnover by the proteasome in aging and disease. Free Radic Biol Med. 2002;32:1084–1089. doi: 10.1016/s0891-5849(02)00824-9. [DOI] [PubMed] [Google Scholar]
- 29.Grune T, Reinheckel T, Davies KJ. Degradation of oxidized proteins in mammalian cells. FASEB J. 1997;11:526–534. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.