TO THE EDITOR
Serum-stimulation of quiescent (G0) keratinocytes initiates a temporally regulated program of transcriptional activity required for G0/G1 transit and subsequent entry into the proliferative cycle (Qi and Higgins, 2003). Expression profiling of such “activated” keratinocytes identified physiologically relevant subsets of cell cycle/growth state-regulated genes (Gromov et al., 2002; Gazel et al., 2003). Indeed, non-cycling human (HaCaT) keratinocytes express a differentiated (i.e., super-basal) genetic signature, whereas the serum-stimulated transcriptome approximates that of transient amplifying cells (Pivarcsi et al., 2001; Lemaitre et al., 2004). Clearly, the associated transcriptional responses dictate epidermal cell lineage commitments by impacting the expression of pathway-relevant genes (Banno et al., 2004; Lemaitre et al., 2004).
This report provides early evidence regarding the comprehensive inventory of genes expressed by human HaCaT-II4 keratinocytes during the initial stage of cell-cycle re-entry. Re-introduction of serum to quiescent HaCaT-II4 cells stimulates G0 exit and residence in a short-lived “activated G0 substate” (i.e., the kinetically defined G0→G1 transition state) (Qi et al., 2006). Microarray analysis of quiescent and 2 hours fetal bovine serum (FBS)-“ activated” HaCaT-II4 cells defined the transcriptional signature of this early G0→G1 window. A total of 54,675 expressed sequence-tagged genes were analyzed with 41,083 directly compared for groups A (quiescent) and B (2 hours FBS-stimulated) and a total of 35,991 reproducibly assessed for all three experimental conditions (i.e., 66% of the total sequences available; this includes group C [FBS for 2 hours in the presence of puromycin included as a first approximation of the immediate-early response cluster]). Genes exhibiting statistically significant (analysis of variance) changes (two-fold increase or decrease) distributed as follows: 1151 for A versus B, 1241 for A versus C, and 1319 for B versus C. Among the most prominently upregulated mRNA transcripts were those encoding proteins involved in the initial growth response (EGR1-4), extracellular matrix remodeling and tissue invasion (uPA, uPAR, tPA, SERPINE1 (PAI-1), PAI-2, MMP-2, MMP- 12, CYR61), transcription (Myc, Fos, Jun, KLF4, AT3, p300/pTAF), signal transduction (DUSP1, -4, -8, -10, MAPK3, TGF-α), proliferation (GADD45a, GADD45b, CDK7, cyclins), and apoptosis (CASP9, MCL2) (e.g., Figure 1a). Reverse transcription-PCR and northern blotting validated the expression data for selected genes. When more stringent criteria were applied to data filtering (i.e., set threshold of ≥ 10-fold increase), 79 genes were identified (Table 1) of which 75 also partitioned to the puromycin-resistant subset. Rank order analysis indicated that PAI-1 (SERPINE1) and the uPA receptor (PLAUR) were the most significantly elevated transcripts. uPA increased (by 12-fold) as well (by microarray and northern analyses), although maximal uPA expression occurred several hours later than PAI-1. Additional genes that comprise the “tissue repair” subset and that were upregulated > 10-fold within the first 2 hours included DTR, IL8, EREG, HBEGF, IL11, CTGF, LIF, IL6, IL1A, HAS3, SERPINB1, and TGFA. Northern blotting confirmed that PAI-1 transcripts were low to undetectable in quiescent HaCaT-II4 cells, peaked in puromycin-resistant manner 2 hours after serum addition (during residence in the initial activated G0 substate; Qi et al., 2006), and then rapidly decreased (Figure 1b and c). Expression required EGFR/MEK/rho-ROCK signaling during the G0→G1 transition (Figure 1d and e). Actinomycin chase/mRNA decay and temporal assessments of mRNA abundance indicated, moreover, that PAI-1 transcripts were substantially reduced (from a maximum at 2 hours) as early as 4 hours post-stimulation decreasing further by 6–8 hours post-stimulation (i.e., approximately mid-G1) likely due to E2F1-mediated suppression (Koziczak et al., 2001), consistent with a narrow window of serum-initiated transcription and short mRNA half-life (1.5–2 hours) (Mu et al., 1998; White et al., 2000).
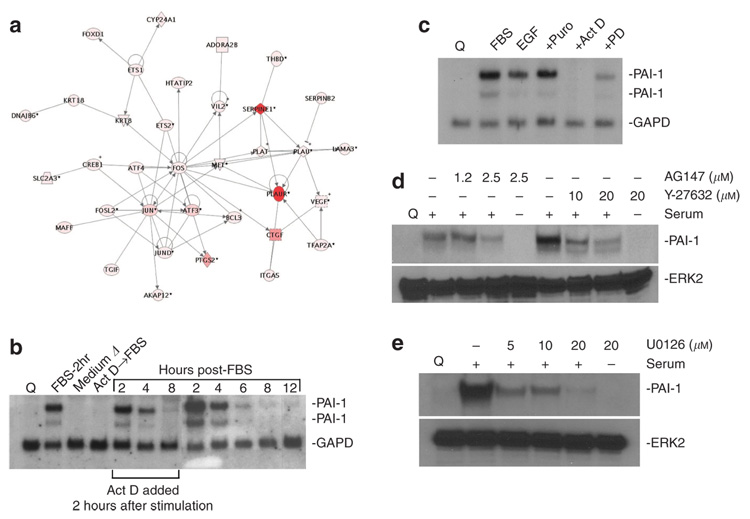
Figure 1. A significant fraction of FBS-induced transcripts encode proteins involved in cell proliferation, transcriptional reprogramming, and control of pericellular proteolysis.
The clustergram plot (a) illustrates functional groupings for several highly expressed (≥ 10-fold; red to orange shading) relative to more moderately stimulated (> 2- to < 10-fold; light to dark pink colored) genes (mapped using Ingenuity Pathways software). (b, c) PAI-1 transcripts (both the 3.0 and 2.2 kb species) are low to undetectable in quiescent (Q) HaCaT-II4 keratinocytes and induced within 2 hours of serum re-introduction (FBS-2 hours) or EGF, but not by replacement with serum-free medium (medium Δ). (b, c) PAI-1 expression is effectively inhibited by prior incubation with actinomycin D (Act D) or the MEK inhibitor PD98059 (PD) but not by puromycin (Puro). (b) PAI-1 mRNA decay rates in cultures treated with Act D 2 hours after FBS addition were virtually identical to control mRNA decay profiles suggesting that PAI-1 repression was initiated between 2 and 4 hours post-serum stimulation. (d, e) Y-27632 and U0126 inhibited PAI-1 induction implicating both the rho GTPase effector ROCK and MEK, respectively, in gene control. (d) Pretreatment of quiescent cells with the EGFR inhibitor AG1478 similarly blocked PAI-1 expression indicating that EGFR ligands were major contributors to the serum-responsiveness of the PAI-1 gene. Glyceraldehyde-3-phosphate dehydrogenase (GAPD) hybridization provided a normalizing signal for (b and c) northern analysis (Mu et al., 1998; Qi et al., 2006 for details), whereas (d, e) western blots were stripped and re-probed with antibodies to ERK2 to confirm protein loading levels (described in Providence and Higgins, 2004).
Table 1.
Genes exhibiting a ≥ 10-fold increase in expression 2 hours after serum stimulation of quiescent HaCaT-II4 cells
| Gene symbol | Fold-increased expression | Description |
|---|---|---|
| SERPINE1 | 97.7 | Plasminogen activator inhibitor type-1 |
| PLAUR | 76.6 | Urokinase plasminogen activator receptor |
| PLAUR | 70.3 | Urokinase plasminogen activator receptor |
| DTR | 67.9 | Heparin-binding epidermal growth factor-like precursor |
| NR4A3 | 65.7 | Nuclear receptor subfamily 4, group A, member 3 |
| CLC | 62.0 | Cardiotrophin-like cytokine |
| C8FW | 61.1 | Phosphoprotein regulated by mitogenic pathways |
| IL8 | 56.3 | Interleukin 8 |
| CLDN4 | 49.5 | Claudin 4 |
| GEM | 48.5 | GTP-binding protein overexpressed in skeletal muscle |
| EREG | 48.0 | Epiregulin |
| SPRR2B | 46.7 | Small proline-rich protein 2B |
| EGR2 | 46.3 | Early growth response 2 (Krox-20 homolog) |
| HB-EGF | 41.3 | Heparin-binding epidermal growth factor |
| PHLDA1 | 40.0 | Pleckstrin homology-like domain, family A, member 1 |
| IL11 | 38.1 | Interleukin 11 |
| CTGF | 37.8 | Connective tissue growth factor |
| KRTAP3-1 | 37.4 | Keratin-associated protein 3-1 |
| LIF | 36.9 | Leukemia inhibitory factor |
| EDN1 | 36.6 | Endothelin 1 |
| FOSL1 | 35.8 | FOS-like antigen 1 |
| TNFAIP3 | 34.4 | Tumor necrosis factor, α-induced protein 3 |
| TNFAIP3 | 32.9 | Tumor necrosis factor, α-induced protein 3 |
| EGR3 | 32.9 | Early growth response 3 |
| PTGS2 | 32.5 | Prostaglandin-endoperoxide synthase 2 |
| COPEB | 31.1 | Core promoter element-binding protein |
| ZFP36 | 30.6 | Zinc finger protein 36 |
| APOBEC3A | 30.2 | Apolipoprotein B mRNA editing enzyme |
| COPEB | 28.9 | Core promoter element-binding protein |
| DUSPI | 28.3 | Dual specificity phosphatase 1 |
| NR4A2 | 28.0 | Nuclear receptor subfamily 4, group A, member 2 |
| FOXA1 | 27.4 | Forkhead box A1 |
| EDN1;ET1 | 26.7 | Endothelin 1 |
| DUSP10 | 25.3 | Dual specificity phosphatase 10 |
| IL6 | 25.1 | Interleukin 6 |
| SOCS3 | 24.8 | Suppressor of cytokine signaling 3 |
| KLF4 | 24.1 | Kruppel-like factor 4 |
| NR4A2 | 23.4 | Nuclear receptor subfamily 4, group A, member 2 |
| PTGS2 | 23.1 | Prostaglandin-endoperoxide synthase 2 |
| C20oorf16 | 21.4 | Chromosome 20 open reading frame 16 |
| DUSP4 | 21.0 | Dual specificity phosphatase 4 |
| ATF3 | 19.3 | Activating transcription factor 3 |
| PIM1 | 19.1 | Pim-1 oncogene |
| MAFF-like | 18.6 | v-maf-like |
| JUN | 18.3 | v-jun sarcoma virus 17 oncogene homolog |
| NR4A2 | 18.1 | Nuclear receptor subfamily 4, group A, member 2 |
| IL1A | 18.0 | Interleukin 1, alpha |
| GADD45B | 17.4 | Growth arrest and DNA-damage-inducible, beta |
| HAS3 | 17.3 | Hyaluronan synthase 3 |
| EMP1 | 16.8 | Epithelial membrane protein 1 |
| SLC20A1 | 16.8 | Solute carrier family 20 (phosphate transporter), member 1 |
| GADD45B | 16.8 | Growth arrest and DNA-damage-inducible, beta |
| DSCR1 | 16.7 | Down syndrome critical region gene 1 |
| GADD45B | 16.6 | Growth arrest and DNA-damage-inducible, beta |
| ARTN | 16.6 | Artemin |
| B3GNT5 | 16.1 | UDP-GlcNAc:betaGal β-1,3-N-acetylglucosaminyltransferase 5 |
| RGC32 | 16.0 | RGC32 protein |
| JUN | 15.0 | v-jun sarcoma virus 17 oncogene homolog |
| TRIF | 14.9 | TIR domain containing adaptor-inducing interferon-β |
| KLF4 | 14.6 | Kruppel-like factor 4 |
| IER3 | 14.5 | Immediate early response 3 |
| SERPINB1 | 14.4 | Serine (or cysteine) proteinase inhibitor, clade B, member 1 |
| RFX2 | 14.4 | Regulatory factor X, 2 |
| SGK | 13.5 | Serum/glucocorticoid-regulated kinase |
| ADM | 13.5 | Adrenomedulin |
| IL1B | 12.9 | Interleukin 1-β |
| SPRR3 | 12.8 | Small proline-rich protein 3 |
| HSPC159 | 12.6 | Human galectin-related protein |
| EMP1 | 12.5 | Epithelial membrane protein 1 |
| PTGER4 | 12.3 | Prostaglandin E, receptor 4 |
| PLEKHC1 | 12.2 | Pleckstrin homology domain containing, family C, member 1 |
| LM07 | 12.1 | LIM domain only 7 |
| PLEKHC1 | 11.9 | Pleckstrin homology domain containing, family C, member 1 |
| ATF3 | 11.7 | Activating transcription factor 3 |
| PDCD1L1 | 11.7 | Programmed cell death 1 ligand 1 |
| CNK | 11.6 | Cytokine-inducible kinase |
| PPP1R15A | 11.5 | Protein phosphatase 1, regulatory (inhibitor) subunit 15A |
| FST | 11.5 | Follistatin |
| MAFF | 11.1 | v-maf fibrosarcoma oncogene avian homolog F |
| IFRD1 | 11.0 | Interferon-related developmental regulator 1 |
| SNARK | 10.9 | Likely ortholog of rat SNF1/AMP-activated protein kinase |
| ODC1 | 10.7 | Ornithine decarboxylase 1 |
| SLC2A3 | 10.4 | Solute carrier family 2 (facilitated glucose transporter), member 3 |
| TGFA | 10.4 | Transforming growth factor, alpha |
| GADD45A | 10.3 | Growth arrest and DNA-damage-inducible, alpha |
| B3GNT5 | 10.2 | UDP-GlcNAac:betaGal β-1,3-N-acetylglucosaminyltransferase 5 |
| SLC2A14 | 10.2 | Solute carrier family 2 (facilitated glucose transporter), member 14 |
| DUSP5 | 10.1 | Dual specificity phosphatase 5 |
| CUL1 | 10.1 | Cullin 1 |
| PPP1R3C | 10.0 | Protein phosphatase 1, regulatory (inhibitor) subunit 3C |
ANOVA, analysis of variance; FBS, fetal bovine serum; cDNA, complementary DNA. RNA isolated from quiescent or 2 hours FBS-stimulated keratinocytes was converted into single-stranded cDNA using Superscript II reverse transcriptase and the GeneChip T7 promoter primer kit. Double-stranded cDNA was prepared, biotinylated cRNA generated, hydrolyzed to 35–200 base fragments, and hybridized to the Affymetrix Human Genome U133 Plus 2.0 oligonucleotide array. Arrays were washed, stained with streptavidin-phycoerythrin, scanned and images analyzed qualitatively with Affymetrix GCOS software; probe signal outputs (pivot tables) were imported as text files into GeneSpring v6.1. Values below 0.01 were set to 0.01 and each was divided by the 50th percentile of all measurements in that sample. Individual gene data points, in each of the experimental groups, were divided by the median value in the corresponding control sets. ANOVA analysis (95% confidence) compared groupings as follows: B versus A, C versus A, and B versus C. The statistically significant genes (in triplicate assessments) were filtered to obtain lists based on expression levels (for this paper, 10× increased relative to the corresponding control). Signal reproducibility is evidenced by expression level values for duplicated genes on each array (indicated by color coding in bold font).
Activation of a wound repair transcript profile appears to be a general response to serum addition (Iyer et al., 1999; this study). The present findings indicate, furthermore, that PAI-1 is the most prominent member of the keratinocyte “serum response transcriptome”. Several SERPINS (i.e., PAI-1, protease nexin-1), in fact, modulate the complex process of injury resolution through control of focalized plasminmediated matrix remodeling, cell migration, and apoptosis (e.g., Bajou et al., 2001; Li et al., 2000; Deng et al., 2001; Degryse et al., 2004; Rossignol et al., 2004; Wang et al., 2005). Targeted PAI-1 knockdown/overexpression and protein add-back approaches, moreover, support the contention that PAI-1 participates within the global program of injury to coordinate cycles of cell-to-substrate adhesion/detachment and/or maintain a stromal “scaffold” to satisfy the prerequisites for both G1/S transition and effective cellular migration (Planus et al., 1997; Chazaud et al., 2002; Palmeri et al., 2002; Providence et al., 2002; Czekay et al., 2003; Providence and Higgins, 2004). PAI-1 is also expressed at high levels in senescent cells where it likely interferes with uPA-dependent growth factor activation (Mu et al., 1998; Kortlever et al., 2006). Certain “senescence-associated” genes (i.e., p16INK4a, PAI-1) may actually function in the wound repair program by inhibiting proliferation while promoting migration (Chan et al., 2001; Ploplis et al., 2004; Darbro et al., 2005; Kortlever et al., 2006; Natarajan et al., 2006). Indeed, keratinocytes at the leading edge during wound re-epithelialization are less mitotically active than cells more displaced from the motile front and express relatively high levels of PAI-1 (Garlick and Taichman, 1994; Jensen and Lavker, 1996; Providence and Higgins, 2004). Collectively, these data suggest that PAI-1 may regulate the temporal cadence of cell-cycle progression in replicatively competent cells as part of the injury repair program.
ACKNOWLEDGMENTS
Supported by NIH grants GM57242 and GM42461.
Abbreviations
- FBS
fetal bovine serum
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Bajou K, Masson V, Gerard RD, Schmitt PM, Albert V, Praus M, et al. The plasminogen activator inhibitor PAI-1 controls in vivo tumor vascularization by interaction with proteases, not vitronectin. Implications for antiangiogenic strategies. J Cell Biol. 2001;152:777–784. doi: 10.1083/jcb.152.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banno T, Gazel A, Blumenberg M. Effects of tumor necrosis factor-α (TNFα) in epidermal keratinocytes using global transcriptional profiling. J Biol Chem. 2004;279:32633–32642. doi: 10.1074/jbc.M400642200. [DOI] [PubMed] [Google Scholar]
- Chan JC, Duszczyszyn DA, Castellino FJ, Ploplis VA. Accelerated skin wound healing in plasminogen activator inhibitor-1-deficient mice. Am J Path. 2001;159:1681–1688. doi: 10.1016/S0002-9440(10)63015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazaud B, Ricoux R, Christov C, Plonquet A, Gherardi RK, Barlovatz-Meimon G. Promigratory effect of plasminogen activator inhibitor-1 on invasive breast cancer populations. Am J Path. 2002;160:237–246. doi: 10.1016/S0002-9440(10)64367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czekay R-P, Aertgeerts K, Curriden SA, Loskutoff DJ. Plasminogen activator inhibitor-1 detaches cells from extracellular matrices by inactivating integrins. J Cell Biol. 2003;160:781–791. doi: 10.1083/jcb.200208117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbro BW, Schneider GB, Klingelhutz AJ. Co-regulation of p16INK4a and migratory genes in culture conditions that lead to premature senescence in human keratinocytes. J Invest Dermatol. 2005;125:499–509. doi: 10.1111/j.0022-202X.2005.23844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degryse B, Neels JG, Czekay R-P, Aertgeerts K, Kamikubo T, Loskutoff DJ. The low density lipoprotein receptor-related protein is a motogenic receptor for plasminogen activator inhibitor-1. J Biol Chem. 2004;279:22595–22604. doi: 10.1074/jbc.M313004200. [DOI] [PubMed] [Google Scholar]
- Deng G, Curriden SA, Hu G, Czekay R-P, Loskutoff DJ. Plasminogen activator inhibitor-1 regulates cell adhesion by binding to the somatomedin B domain of vitronectin. J Cell Physiol. 2001;189:23–33. doi: 10.1002/jcp.1133. [DOI] [PubMed] [Google Scholar]
- Garlick JA, Taichman LB. Fate of human keratinocytes during reepithelialization in an organotypic culture model. Lab Invest. 1994;70:916–924. [PubMed] [Google Scholar]
- Gazel A, Ramphal P, Rosdy M, De Wever B, Tornier C, Hosein N, et al. Transcriptional profiling of epidermal keratinocytes: comparison of genes expressed in skin, cultured keratinocytes, and reconstituted epidermis, using large DNA microarrays. J Invest Dermatol. 2003;121:1459–1468. doi: 10.1111/j.1523-1747.2003.12611.x. [DOI] [PubMed] [Google Scholar]
- Gromov PS, Ostergaard M, Gromova I, Celis JE. human proteomic databases: a powerful resource for functional genomics in health resource for functional genomics in health and disease. Prog Biophys Biol. 2002;80:3–22. doi: 10.1016/s0079-6107(02)00005-6. [DOI] [PubMed] [Google Scholar]
- Iyer VR, Eisen MB, Ross DT, Schuler G, Moore T, Lee JC, et al. The transcriptional program in the response of human fibroblasts to serum. Science. 1999;283:83–87. doi: 10.1126/science.283.5398.83. [DOI] [PubMed] [Google Scholar]
- Jensen PJ, Lavker RM. Modulation of the plasminogen activator cascade during enhanced epidermal proliferation in vivo. Cell Growth Differ. 1996;7:1793–1804. [PubMed] [Google Scholar]
- Kortlever RM, Higgins PJ, Bernards R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat Cell Biol. 2006;8:877–884. doi: 10.1038/ncb1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziczak M, Muller H, Helin K, Nagamine Y. E2F1-mediated transcriptional inhibition of the plasminogen activator inhibitor type 1 gene. Eur J Biochem. 2001;268:4969–4978. doi: 10.1046/j.0014-2956.2001.02428.x. [DOI] [PubMed] [Google Scholar]
- Lemaitre G, Lamartine J, Pitaval A, Vaigot P, Garin J, Bouet S, et al. Expression profiling of genes and proteins in HaCaT keratinocytes: proliferating versus differentiated state. J Cell Biochem. 2004;93:1048–1062. doi: 10.1002/jcb.20212. [DOI] [PubMed] [Google Scholar]
- Li F, Goncalves J, Faughnan K, Steiner MG, Pagan-Charry I, Esposito D, et al. Targeted inhibition of wound-induced PAI-1 expression alters migration and differentiation in human epidermal keratinocytes. Exp Cell Res. 2000;258:245–253. doi: 10.1006/excr.2000.4918. [DOI] [PubMed] [Google Scholar]
- Mu XC, Staiano-Coico L, Higgins PJ. Increased transcription and modified growth state-dependent expression of the plasminogen activator inhibitor type-1 gene characterize the senescent phenotype in human diploid fibroblasts. J Cell Physiol. 1998;174:90–98. doi: 10.1002/(SICI)1097-4652(199801)174:1<90::AID-JCP10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Natarajan E, Omobono JD, Guo Z, Hopkinson S, Lazar AJF, Brenn T, et al. A keratinocyte hypermotility/growth-arrest response involving laminin 5 and p16INK4A activated in wound healing and senescence. Am J Path. 2006;168:1821–1837. doi: 10.2353/ajpath.2006.051027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmeri D, Lee JW, Juliano RL, Church FC. Plasminogen activator inhibitor-1 and -3 increase cell adhesion and motility in MDA-MB-435 breast cancer cells. J Biol Chem. 2002;277:40950–40957. doi: 10.1074/jbc.M202333200. [DOI] [PubMed] [Google Scholar]
- Pivarcsi A, Szell M, Kemeny L, Dobozy A, Bata-Csorgo Z. Serum factors regulate the expression of the proliferation-related genes α5 integrin and keratin 1, but not keratin 10, in HaCaT keratinocytes. Arch Dermatol Res. 2001;293:206–213. doi: 10.1007/s004030100217. [DOI] [PubMed] [Google Scholar]
- Planus E, Barlovatz-Meimon G, Rogers RA, Bonavaud S, Ingber DF, Wang N. Binding of urokinase to plasminogen activator inhibitor type-1 mediates cell adhesion and spreading. J Cell Sci. 1997;110:1091–1098. doi: 10.1242/jcs.110.9.1091. [DOI] [PubMed] [Google Scholar]
- Ploplis VA, Balsara R, Sandoval-Cooper MJ, Yin ZJ, Batten J, Modi N, et al. Enhanced in vitro proliferation of aortic endothelial cells from plasminogen activator inhibitor-1-deficient mice. J Biol Chem. 2004;279:6143–6151. doi: 10.1074/jbc.M307297200. [DOI] [PubMed] [Google Scholar]
- Providence KM, Higgins PJ. PAI-1 expression is required for epithelial cell migration in two distinct phases of in vitro wound repair. J Cell Physiol. 2004;200:297–308. doi: 10.1002/jcp.20016. [DOI] [PubMed] [Google Scholar]
- Providence KM, White LA, Tang J, Gonclaves J, Staiano-Coico L, Higgins PJ. Epithelial monolayer wounding stimulates binding of USF-1 to an E-box motif in the plasminogen activator inhibitor type 1 gene. J Cell Sci. 2002;115:3767–3777. doi: 10.1242/jcs.00051. [DOI] [PubMed] [Google Scholar]
- Qi L, Allen RR, Lu Q, Higgins CE, Garone R, Staiano-Coico L, et al. PAI-1 transcriptional regulation during the G0→G1 transition in human epidermal keratinocytes. J Cell Biochem. 2006;99:495–507. doi: 10.1002/jcb.20885. [DOI] [PubMed] [Google Scholar]
- Qi L, Higgins PJ. Use of chromatin immunoprecipitation to identify E box-binding transcription factors in the promoter of the growth state-regulated human PAI-1 gene. Recent Res Dev Mol Biol. 2003;1:1–12. [Google Scholar]
- Rossignol P, Ho-Tin-Noe B, Vranckx R, Bouton MC, Meilhac O, Lijnen HR, et al. Protease nexin-1 inhibits plasminogen activation-induced apoptosis of adherent cells. J Biol Chem. 2004;279:10346–10356. doi: 10.1074/jbc.M310964200. [DOI] [PubMed] [Google Scholar]
- Wang Z, Sosne G, Kurpakus-Wheater M. Plasminogen activator inhibitor-1 (PAI-1) stimulates human corneal epithelial cell adhesion and migration in vitro. Exp Eye Res. 2005;80:1–8. doi: 10.1016/j.exer.2004.06.006. [DOI] [PubMed] [Google Scholar]
- White LA, Bruzdzinski C, Kutz SM, Gelehrter TD, Higgins PJ. Growth state-dependent binding of USF-1 to a proximal promoter E box element in the rat plasminogen activator inhibitor type 1 gene. Exp Cell Res. 2000;260:127–135. doi: 10.1006/excr.2000.5001. [DOI] [PubMed] [Google Scholar]