Abstract
Foxp3+ regulatory T (TR) cells limit pathogenic immune responses to self and foreign antigens. An essential role for microRNA (miRNA) in the maintenance and function of TR cells, revealed by the TR-specific Dicer ablation, raised a question as to a specific miRNA contribution. We found that Foxp3 controls the elevated miR155 expression required for maintaining TR proliferative activity and numbers under non-lymphopenic conditions. Moreover, miR155 deficiency in TR cells results in increased SOCS1 expression accompanied by impaired STAT5 activation in response to limiting amounts of IL-2. Our studies suggest Foxp3-dependent regulation of miR155 maintains competitive fitness of TR subset by targeting SOCS1, and provide an experimental support for a proposed role for miRNAs in ensuring the robustness of cellular phenotypes.
Introduction
A class of small non-coding RNA known as microRNAs (miRNA) has been implicated in the regulation of gene expression essential for organ development, cellular differentiation, homeostasis, and functioning through target mRNA degradation or translational control (Bartel, 2004). An important role for the miRNA pathway in the immune system was revealed in studies employing the T cell-specific deletion of a conditional allele of dicer, encoding an RNase III enzyme critical for generation of mature miRNAs (Cobb et al., 2006; Cobb et al., 2005; Muljo et al., 2005). Dicer-deficient T cells exhibit decreased proliferative potential and increased apoptosis in response to activation. Moreover, the Dicer-dependent miRNA pathway influences effector CD4 T cell differentiation (Cobb et al., 2005; Muljo et al., 2005). Recent studies have begun to explore a role for individual miRNA in lymphocyte biology. For example, in B cells, miR150 has been shown to profoundly affect early B cell differentiation and mature B cell responses by targeting the transcription factor c-Myb (Xiao et al., 2007), while the miR17-92 cluster can limit apoptosis through down-regulation of the pro-apoptotic protein Bim (Koralov et al., 2008; Ventura et al., 2008; Xiao et al., 2008). In T cells, miR181a was shown to influence thymic selection by modulating the sensitivity of TCR signaling through down-regulation of several phosphatases involved in the attenuation of signal transduction downstream of the TCR (Li et al., 2007).
Foxp3+ TR cells play a pivotal role in maintaining immunological tolerance. Ablation of these cells in healthy adult mice results in a systemic autoimmune hyperproliferative syndrome, which ultimately leads to death within three weeks (Kim et al., 2007). Disruption of the Dicer-dependent miRNA pathway at the DP stage of thymocyte development upon deletion of a conditional dicer allele resulted in a 2-3-fold reduction in frequency of Foxp3+ TR cells, suggesting a role for miRNA-mediated gene regulation in TR cell differentiation (Cobb et al., 2006). Moreover, miRNAs are important for the elaboration of TR suppressor function, since miRNA depletion restricted to the Foxp3+ TR cell lineage caused fatal autoimmune disease identical to that observed in Foxp3-deficient mice lacking TR cells (Liston et al., 2008). In addition, in healthy mice co-habited by Dicer-deficient and -sufficient TR cells, the former exhibit significantly diminished proliferative response and increased apoptosis. Although a central role for the Dicer-dependent miRNA pathway in various aspects of TR cell biology was made obvious by these genetic studies, an understanding of the role of individual miRNAs in this context is lacking. Previous studies identified a set of miRNAs differentially expressed in TR and “non-TR” CD4 T cells (Cobb et al., 2006). Among them, miR155, a miRNA largely restricted to hematopoietic cells, is of a particular interest, since Foxp3 binds to an intron within the DNA sequence encoding the miR155 precursor mRNA, Bic (Marson et al., 2007; Zheng et al., 2007). High levels of miR155 expression in B cell malignancies in humans, including Hodgkin's and Burkitt lymphomas, implicated this miRNA in hematopoietic cancers (Eis et al., 2005; Metzler et al., 2004; van den Berg et al., 2003). As a corollary to these clinical observations, B cell-restricted expression of a miR155 transgene in mice led to pre-leukemic proliferation of B lineage cells progressing to a severe B cell malignancy (Costinean et al., 2006). Recent gene targeting studies have further demonstrated a broad role for miR155 in cells of the immune system. Depletion of miR155 resulted in diminished germinal center responses as well as impaired B cell memory formation (Thai et al., 2007; Vigorito et al., 2007). Moreover, miR155-deficient dendritic cells (DCs) failed to induce efficient T cell activation in response to an antigen, while activated miR155-deficient T cells were impaired in IL-2 production and displayed skewed differentiation toward Th2 lineage. The observed increase in Th2 cytokine production was potentially due to an increased level of the Th2 transcription factor c-Maf, which was shown to serve as a miR155 target (Rodriguez et al., 2007).
The central role Foxp3 plays in establishing the TR cell lineage, and the potential link between Foxp3 and miR155 expression (Gavin et al., 2007; Marson et al., 2007; Zheng et al., 2007) suggested that miR155 could be involved in regulating TR cell differentiation, maintenance, or function. Indeed, we found that a high level of miR155 in TR cells required continuous Foxp3 expression. While a loss of miR155 did not apparently change the sensitivity of TR cells to induced apoptosis and did not markedly impair TR suppressor function, it resulted in diminished TR cell numbers and negatively impacted TR proliferative potential in a cell-autonomous fashion. Our results suggest that during thymic differentiation, up-regulation of Foxp3 drives high expression of miR155, which in turn promotes the competitive fitness and proliferative potential of TR cells by inducing SOCS1 down-regulation. These results demonstrate that a strategy employed by Foxp3 to control TR cell biology through fixing expression of a number of molecules transiently expressed in conventional T cells upon their activation, extends to a specific microRNA.
Results
Foxp3 regulates miR155 expression in TR cells
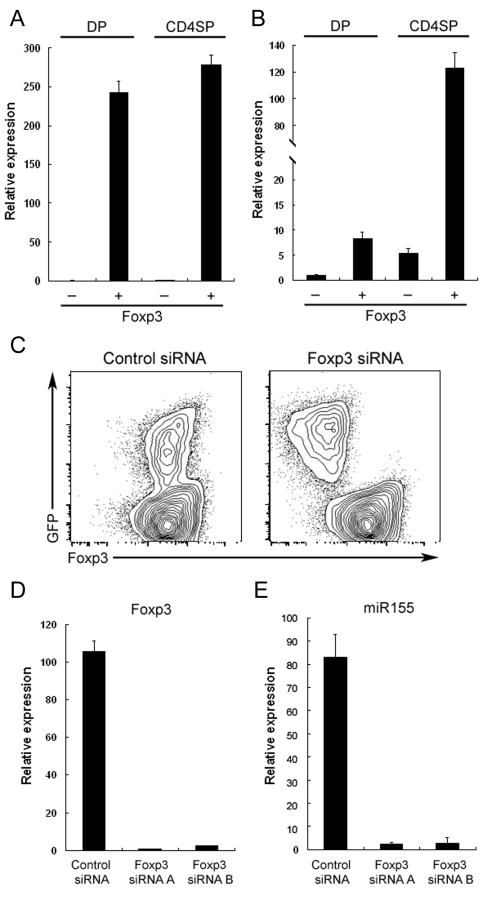
Previous studies showed increased levels of miR155 expression in peripheral Foxp3-expressing TR cells and in activated, but not resting “non-regulatory” T cells and B cells (Thai et al., 2007). We sought to examine whether up-regulation of Foxp3 during thymocyte differentiation coincides with up-regulation of miR155. We have previously shown the bulk of Foxp3+ cells are found within the CD4 single positive (SP) and CD4CD8 double positive thymocyte subsets (DP) (Fontenot et al., 2005). Therefore, we examined miR155 expression in Foxp3+ and Foxp3- SP and DP thymocyte subsets. Like in peripheral TR cells (Fig. S1), the miR155 levels in Foxp3+CD4 SP thymocytes were approximately 20-fold higher than those in the Foxp3- cell population (Fig. 1A, B). Furthermore, Foxp3+DP thymocytes expressed an amount of miR155 approximately 8-fold higher. These results suggested that up-regulation of Foxp3 during thymic differentiation of TR cells is accompanied by increased miR155 expression that is sustained in peripheral TR cells.
Figure 1. High amounts of miR155 expression in TR cells are driven by Foxp3.
Foxp3+ and Foxp3- DP and CDSP thymocyte subsets were isolated and A, Foxp3 and B, miR155 amounts were assessed by real-time PCR. Data are representative of two independent experiments. C, Diminished amounts of miR155 in TR cells upon Foxp3 knockdown with a Foxp3-specific shRNA. Foxp3 shRNA and the corresponding scrambled shRNA (control) were expressed in TR cells using a retroviral vector equipped with a GFP reporter. D and E, GFP+ cells were sorted and the expression of Foxp3 and miR155 were measured by real-time PCR analysis 3 days after retroviral infection.
In agreement with this idea, studies from our lab and others have demonstrated that Foxp3 binds to an intron within the DNA sequence encoding Bic, the precursor transcript of miR155 (Marson et al., 2007; Zheng et al., 2007). These results suggest that Foxp3 might directly control the high miR155 expression in TR cells. Indeed, while forced expression of Foxp3 in peripheral CD4+CD25- T cells resulted in miR155 up-regulation (Cobb et al., 2006), disruption of Foxp3 protein expression upon insertion of the GFP coding sequence into the Foxp3 locus resulted in the loss of miR155 expression (Gavin et al., 2007; Zheng et al., 2007). To establish a role for Foxp3 in sustained miR155 expression in TR cells, we performed Foxp3 knockdown in primary TR cells upon transduction with Foxp3 shRNA-expressing retroviral vectors. Within 3 days after retroviral infection, Foxp3 expression was greatly diminished at both the protein and mRNA levels in GFP+ TR cells transduced with retroviral vectors, expressing two different Foxp3-specific shRNA, as compared to a control vector containing scrambled shRNA sequences (Fig. 1C, D). Similarly, miR155 levels as well as the levels of its precursor transcript (BIC) were diminished upon a decrease in Foxp3 expression in sorted GFP+ TR cells (Fig. 1E and data not shown). These results indicated that continuous Foxp3 expression is indispensable for the maintenance of high amounts of miR155 in TR cells.
miR155 deficiency results in a reduction of TR cell numbers
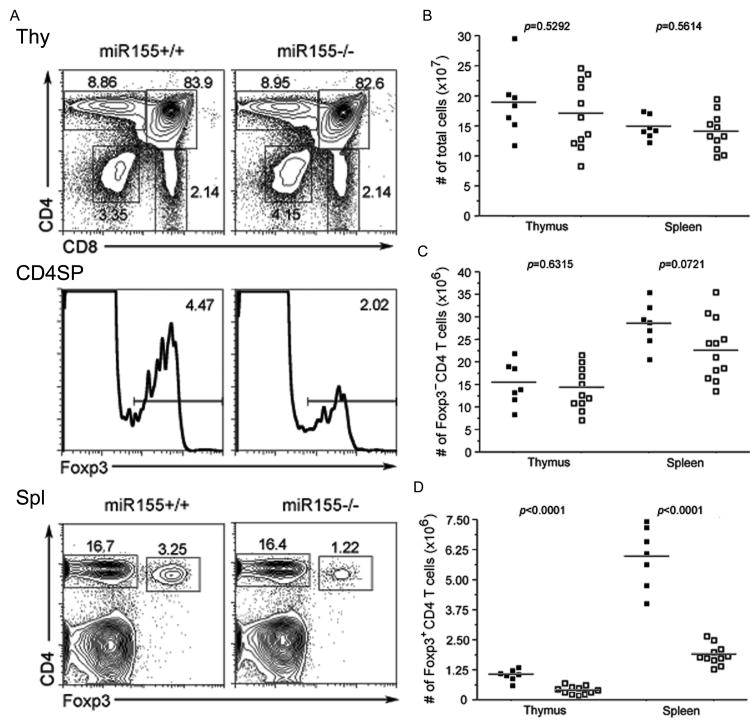
To investigate the role of miR155 in TR cell biology, we examined thymic and peripheral Foxp3+ cell subsets in recently described miR155-deficient mice (Thai et al., 2007). Those mice remained healthy without detectable immune-mediated pathology. While we did not observe significant changes in T cell activation status or numbers, miR155-deficient mice displayed a marked reduction in the proportion and absolute numbers of Foxp3+ cells, both in the thymus and in the periphery (Fig. 2A-D).
Figure 2. Reduced thymic and peripheral TR cell subsets in the absence of miR155.
A, Flow cytometric analysis of Foxp3+TR cell subsets in 6-8 wk-old miR155-deficient mice and wild-type littermates. The proportions of different thymocyte subsets and of Foxp3+ cells within in CD4 SP thymocyte and CD4+ splenic T cell subsets are shown. B-D, Cellularity of the thymus and spleen, and the proportion and absolute numbers of thymic and splenic Foxp3+ and Foxp3-CD4+ T cells in miR155-deficient and -sufficient mice are shown.
A recent study by Rodriguez and colleagues revealed that IL-2 production by T cells and the antigen presenting and co-stimulatory capacity of DC were both impaired in the absence of miR155 (Rodriguez et al., 2007). Since IL-2 plays an important role in maintaining TR homeostasis, it was possible that the numerical reduction in TR cells in miR155-deficient mice was due to the limited supply of IL-2 by non-TR cells. Moreover, other cell extrinsic factors, such as an impaired DC function, might also contribute to the impaired TR homeostasis observed in the miR155-deficient mice.
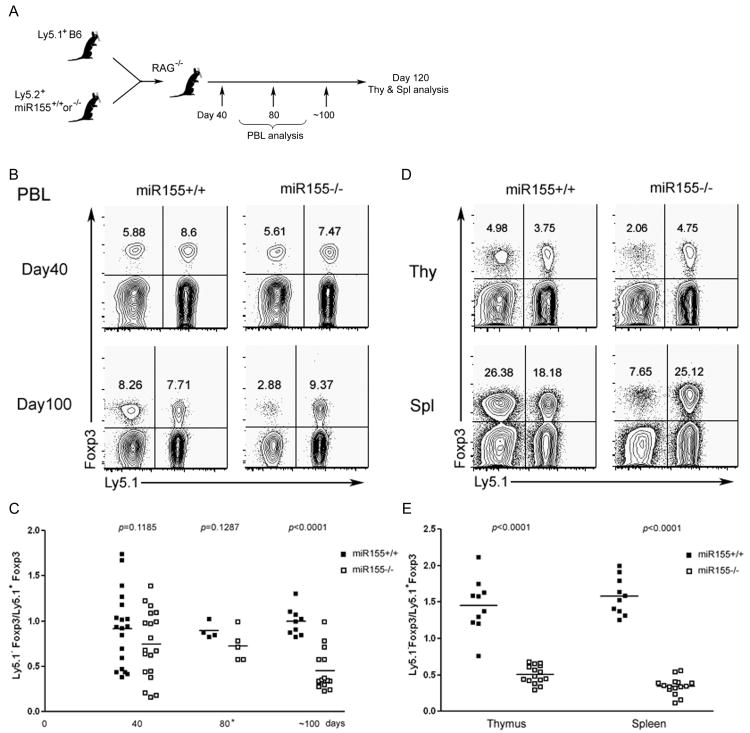
To discriminate between the cell-intrinsic and cell-extrinsic effects of miR155 deficiency in the TR cell lineage, we transferred bone marrow (BM) from miR155-deficient or –sufficient littermates mixed with BM from Ly5.1+ B6 mice at a 1:1 ratio mice into Rag2-deficient recipients. The presence of miR155-sufficient and -deficient Foxp3+ T cells in the peripheral blood of the resulting chimeras was assessed at different time points after BM reconstitution (Fig. 3A). Unexpectedly, we did not detect a marked difference in the frequencies of miR155-deficient and -sufficient TR cells 40 days after BM transfer (Fig. 3B, C). However, 100 days after BM transfer the proportion of Foxp3+ TR within miR155-deficient CD4+ T cell population decreased to about a third of that in the miR155-sufficient CD4+ T cell subset (Fig 3B, C). Further analysis showed a comparable 2-3-fold reduction in the frequencies of Foxp3+ TR cells within the miR155-deficient thymic and peripheral CD4+ T cell subsets compared to those in the corresponding control subsets in the mixed chimeras four months after BM transfer (Fig. 3D, E). The decrease in the proportion of miR155-deficient TR cell subset in the mixed BM chimeras together with a similar numerical decrease of the TR cell subset in un-manipulated miR155 mice suggested that miR155 deficiency affects the TR cell homeostasis in a cell-autonomous manner. Interestingly, despite of the reduced numbers, miR155 was dispensable for TR cell suppression function. miR155-deficient TR cells isolated from BM chimeras or un-manipulated mice displayed only moderately diminished in vitro suppression activity (Fig. S2A). Furthermore, miR155-deficient TR cells were able to maintain immunological tolerance and prevent autoimmune disease in a miR155-sufficient environment, whereas the control group of mice reconstituted only with the Foxp3DTR bone marrow became moribund and succumbed to terminal disease within 10 days of DT treatment (Fig. S2, Fig. S3 and data not shown).
Figure 3. miR155-deficient TR cells exhibit impaired homeostais.
A, Schematic of generation of mixed BM chimeric mice. B, TR cell frequencies within each donor-derived populations of peripheral blood lymphocytes at various time points after BM transfer. C, The ratios of Ly5.1-Foxp3+ (miR155-/- or WT littermates) and Ly5.1+Foxp3+ cells were plotted over time. D, TR cell frequencies within each donor derived T cell population from both the thymus and spleen 120 days after BM transfer. E, The ratio of thymic and splenic Ly5.1-Foxp3+and Ly5.1+Foxp3+cells 120 days after BM transfer. The data in C and E represent three independent experiments.
Diminished proliferative potential of miR155-deficient TR cells
Recent studies of T cells deprived of miRNAs upon ablation of a conditional Dicer allele showed that the Foxp3+ TR cell subset was reduced to a degree comparable to that in miR155-deficient mice and that TGFβ-dependent Foxp3 induction in peripheral Foxp3- T cells was diminished (Cobb et al., 2006). Based on these findings, the miRNA pathway was proposed to be important for TR lineage commitment (Cobb et al., 2006). However, our observation of normal thymic development and the presence of comparable numbers of thymic and peripheral miR155-deficient and -sufficient Foxp3+ TR cells in chimeric mice 6-8 weeks after mixed BM reconstitution (Fig. 3 and Fig. S4) as well as equally efficient TGFβ-dependent induction of Foxp3 in peripheral non-TR cells (Fig. S5) largely discounted a cell-intrinsic role for miR155 role in TR cell differentiation.
Previous studies implicated miR155 in the control of both cellular proliferation and death. Over-expression of miR155 resulted in the up-regulation of over 200 genes related to the regulation of the cell cycle (Costinean et al., 2006) and the inhibition of apoptosis through a blockade of caspase-3 activity (Ovcharenko et al., 2007). The latter was proposed to be associated with down-regulation of pro-apoptotic molecules such as TP53BP1 (Gironella et al., 2007). Therefore, it was possible that high levels of miR155 expression in TR cells were required for their resistance to apoptosis or proliferative capacity, or both. To address these issues, we first examined whether the loss of miR155 led to an increase in apoptosis in TR cells stimulated in vitro by CD3 cross-linking. We found very few apoptotic Foxp3+ TR cells in ex vivo isolated miR155-deficient or -sufficient CD25+ TR cell populations (Fig. 4). Furthermore, we observed comparable progressive increases in apoptotic cell numbers of either mutant or control TR cells upon in vitro CD3 in the presence or absence of IL-2 as well as Fas cross-linking (Fig. 4 and Fig. S6).
Figure 4. miR155 deficiency does not affect TR cell susceptibility to activation induced cell death.
Sorted CD4+CD25- (TE) and CD4+CD25hi (TR) cells from miR155-deficient mice or littermate controls were cultured in vitro with plate-bound CD3 (1μg/ml) antibodies. Apoptotic cells were labeled with the active caspase 3 probe FITC-VAD-FMK at indicated time points and enumerated using flow cytometric analysis. Data are representative of two independent experiments (n=4).
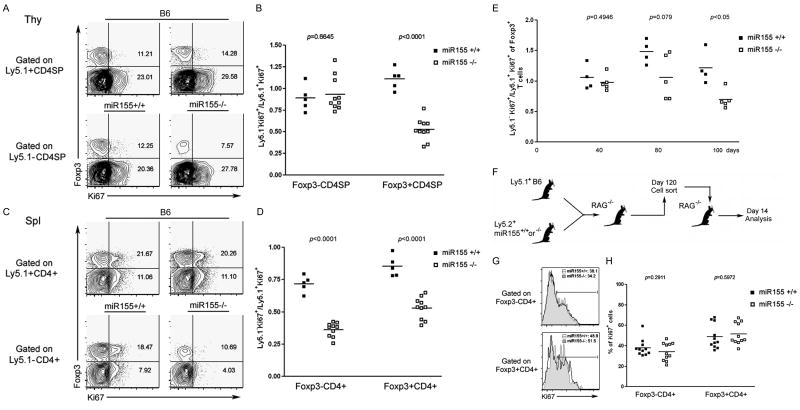
Next, we examined whether miR155 is required for maintaining the TR cell proliferative potential through enumeration of Ki67-expressing cycling cells. As shown in Figure 5A-D, we found a decreased proportion of Ki67+ cells within thymic and peripheral miR155-deficient Ly5.1-Foxp3+ TR cell subsets compared to miR155-sufficient controls (7.57% vs. 14.28% in the thymus and 10.69% vs. 20.26% in the spleen). In contrast, the proportion of cycling Ki67+ cells was reduced only within the peripheral, but not thymic miR155-deficient Foxp3- CD4+ T cell subset, in comparison to wild-type miR155-sufficient cell subsets (29.58% vs. 27.78% in the thymus and 4.03% vs. 11.1% in the spleen). In agreement with these results, we found comparable numbers of miR155-sufficient and -deficient Foxp3- cells at all thymic developmental stages in mixed BM chimeras (Fig. S7A). In contrast, the splenic miR155-deficient CD4+ and CD8+ T cell subsets were markedly decreased in comparison to their wild type counterparts, whereas the numbers of B cells and DCs were unaffected by miR155 deficiency (Fig. S7B). These data suggest that TR homeostasis is impaired in the absence of miR155. In addition, the diminished proliferative activity of peripheral miR155-deficient Foxp3- T cells indicates some involvement of miR155 in non-TR cell homeostasis in the periphery (Fig. S8).
Figure 5. Diminished proliferative activity of TR cell in the absence of miR155 in the competitive environment of lympho-replete, but not lymphopenic mice.
A, Expression of Ki67 in Ly5.1+ (C57/B6) or Ly5.1- (miR155-/- or WT littermates) SP thymocytes 120 days after BM reconstitution. The frequency of Ki67+ cells in either Foxp3+ or Foxp3- population is indicated. B, The ratios of Ly5.1-Ki67+ (miR155-/- or WT littermates) and Ly5.1+Ki67+ cells within either Foxp3+ or Foxp3- CD4SP thymocyte subset. C, Expression of Ki67 in Ly5.1+ or Ly5.1- splenic CD4+ T cells. The frequency of Ki67+ cells within either Foxp3+ or Foxp3- CD4 T cell subsets is indicated. D, The ratios of Ly5.1-Ki67+ and Ly5.1+Ki67+ cells within Foxp3+ and Foxp3- splenic CD4+ T cell subsets. Data are representative of two independent experiments. E, Expression of Ki67 within each donor-derived population of peripheral blood lymphocytes at various time points after BM transfer. The ratios of Ki67+Ly5.1-Foxp3+ (miR155-/- or WT littermates) and Ki67+Ly5.1+Foxp3+ cells were analyzed over time. F, Ly5.1-T cells (miR155-/- or WT littermates) isolated from mixed BM chimeric mice 120 days after BM reconstitution were re-transferred into RAG-deficient mice. G, Expression of Ki67 within Foxp3-CD4+ and Foxp3+CD4+ T cell subsets from miR155-/- (black line) or WT littermates (tinted) 14 days after transfer were assessed by flow cytometric analysis. H, The frequencies of Ki67+ cells in either miR155-sufficient or -deficient population were plotted. Data are representative of two independent experiments.
miR155 maintains competitive fitness of TR subset
The reduction in the proportion and the overall numbers of miR155-deficient TR cells in chimeric mice observed only relatively late after reconstitution with miR155-deficient and -sufficient BM suggested that miR155 is required to maintain proliferative fitness of TR cells in “lympho-replete” mice, where cells compete for limiting growth factors, but not in lymphopenic mice, where competition for growth factors is lacking. To test this notion, we examined the proportion of Ki67+ TR cells at different time points after BM reconstitution. Indeed, we observed the reduction in the proportion of cycling Ki67+ miR155-deficient TR cells only at a later time point after BM reconstitution (Fig. 5E). To further prove that the diminished cycling of miR155-deficient TR cells is due to their inferior ability to compete with miR155-sufficient TR cells, but not due to an irreversible loss of their proliferative potential, we transferred miR155-deficient T cell population, containing few cycling cells four months post BM transfer, into RAG-deficient recipients (Fig. 5F). Upon re-introduction into a lymphopenic environment, both Foxp3+ TR cells and Foxp3- non- TR cells proliferated as robustly as their miR155-sufficient counterparts (Fig. 5G, H). Moreover, the total numbers of miR155-defcient and –sufficient TR cells recovered 2 weeks after transfer into lymphopenic host were fully consistent with the input TR population 120 days after BM transfer (Fig. S9). Thus, miR155 confers proliferative fitness of TR cells in a full lymphoid compartment.
Attenuated IL-2 signaling in miR155-deficient TR cells
IL-2 has long been known for its indispensable role in TR cell homeostasis (Bayer et al., 2007). It was therefore possible that diminished TR cell proliferative activity in the absence of miR155 was due to impaired signaling in response to limiting amounts of IL-2. Indeed, we found comparable STAT5 phosphorylation in miR155-deficient and – sufficient TR cells in response to high amounts of IL-2 (Fig. 6A). In response to a lower concentration of IL-2, however, markedly reduced STAT5 phosphorylation was observed in miR155-deficient TR cells as compared to their miR155-sufficient counterparts (Fig. 6A). This finding was fully consistent with the observation that miR155 confers TR cell proliferative fitness only in the competitive environment of lympho-replete mice where IL-2 is likely limiting.
Figure 6. miR155 modulates sensitivity of TR cells to IL-2 through targeting SOCS1.
A, Flow cytometric analysis of Stat5 phosphorylation in miR155-deficient and -sufficient TR cells induced upon stimulation with IL-2 at the indicated concentrations. B, Expression of the SOCS1 transcript was measured by real-time PCR. TN: naïve CD4+CD25-CD62Lhi cells; TE: non-TR cells activated with anti-CD3/anti-CD28 for 48hrs; TR: CD4+CD25+ cells. C, Western blot analysis of the SOCS1 protein expression. Densitometric SOCS1 expression values normalized based on β-actin expression values are indicated below the corresponding lanes as well as fold increase in normalized SOCS1 expression in the absence of miR155 in the indicated T cell subsets. D, Multiple species sequence alignment of the SOCS1 3′ UTR including the predicted miR155 target site sequence (in bold). Mutation of the miR155 target site sequence is shown below. E, 293T cells were co-transfected with WT or mutated SOCS1 3′UTR and miR155 and assessed for luciferase activity 24hrs after transfection. MiR150 was used as a control miRNA in these experiments. F, The proportion and G, absolute numbers of thymic Foxp3+ TR cells in WT littermate control mice, SOCS1 Tg mice as well as SOCS1 cKO mice. The data shown in every panel are representative of two or more independent experiments (n=3∼6); values represent the mean +/- s.d., *P<0.005.
miR155 maintains TR cell homeostasis by limiting SOCS1 protein expression
To gain an insight into the mechanistic aspect as to how miR155 influences IL-2 signaling pathway, we conducted computational analysis of putative miR155 targets using the PicTar algorithm (Krek et al., 2005). Among the predicted miR155 targets, SOCS1 was the only one with a known inhibitory role in the IL-2 signaling pathway leading to reduced STAT5 phosphorylation (Sporri et al., 2001). Previous studies employing microarray analysis revealed increased amounts of the SOCS1 transcript in TR cells in comparison to non- TR cells (Gavin et al., 2002; McHugh et al., 2002). In agreement with these earlier results, we detected elevated SOCS1 mRNA amounts in miR155-sufficient, but also in –miR155-deficient TR cells (Fig. 6B). However, despite high amounts of the SOCS1 transcript, the SOCS1 protein amounts were low in miR155-sufficient TR cells, whereas miR155-deficient TR cells exhibited approximately a 5-fold increase in the amounts of SOCS1 (Fig. 7C). Moreover, the elevated SOCS1 protein level in miR155-deficient TR cells was diminished when miR155 was re-introduced (Fig. S10). In contrast to TR cells, only a modest (less than 2-fold) increase of the SOCS1 protein expression was observed in non-TR cells lacking Foxp3 protein expression in the absence of miR155 (Fig. 6C). To further examine whether miR155 directly regulates SOCS1, we employed a luciferase reporter construct containing the SOCS1 3′ UTR with an intact or mutated miR155 binding sequence (Fig. 6D). These reporter constructs were co-transfected with miR155 or a control miRNA, miR150, into HEK293T cells. Co-transfection of the intact SOCS1 3′ UTR with miR155 resulted in an appreciable repression of the reporter activity, whereas mutation of the miR155 seed binding-sequence abolished this repression (Fig. 6E). In control, miR150 did not affect expression of the luciferase reporter with the intact or mutant SOCS1 3′ UTR (Fig. 6E). Together, these results suggest that SOCS1 protein expression is directly controlled by miR155.
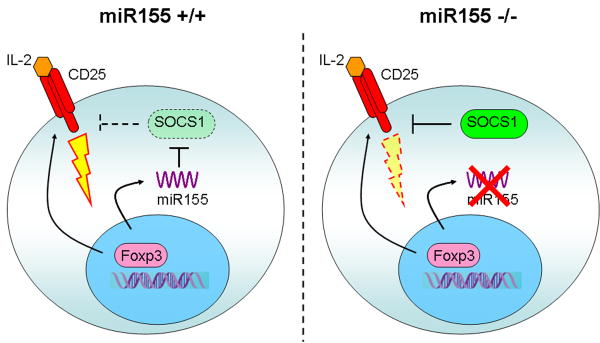
Figure 7. A model for miR155 function in TR cell homeostasis.
In addition to CD25, Foxp3 induces high level of miR155 expression to ensure increased IL-2 responsiveness through miR155-mediated down-regulation of the SOCS1 protein (left panel). In the absence of miR155 (right panel), increased amounts of the SOCS1 protein attenuate IL-2R signaling leading to diminished STAT5 phosphorylation and diminished competitive fitness.
Next, we sought to examine whether increased SOCS1 expression in miR155 sufficient mice can result in a decrease in TR cell numbers. To address this question, we assessed potential changes Foxp3+ subset size in mice expressing SOCS1 transgene under a T cell-specific lck promotor (SOCS1 Tg). As described previously, SOCS1 Tg expression did not lead to detectable abnormalities in thymic differentiation (Hanada et al., 2003). However, it was also demonstrated that another strain of mice with higher amounts of SOCS1 Tg expression developed inflammatory pathology, i.e. spontaneous colitis (Inagaki-Ohara et al., 2006). Therefore, to exclude potential secondary effects of SOCS1 over-expression on peripheral TR cell subsets, we examined Foxp3+ thymocytes in SOCS1 Tg mice and wild-type littermates. As shown in Figure 6F and 6G, SOCS1 Tg mice exhibited a reduced proportion and absolute number of Foxp3+ thymocytes analogous to miR155-deficient mice. Complementing these results, we found an increased proportion and absolute numbers of Foxp3+ thymocytes upon lck-Cre-driven deletion of a conditional Socs1 allele in the T-cell lineage (SOCS1 cKO)(Fig. 6F, G and Fig. S11).
Thus, these experiments strongly suggest that Foxp3-driven miR155 expression defines the size of the Foxp3+ TR subset by conferring competitive fitness to TR cells, at least in part through miR155-mediated control of SOCS1, a negative regulator of IL-2 receptor signaling.
Discussion
The transcription factor Foxp3 orchestrates a specific transcriptional program that is required for the establishment and maintenance of the TR lineage and TR cell function. Previous studies have identified a set of Foxp3 target genes essential for TR homeostasis and function. Among these genes, the Bic gene, which encodes miR155, is of particular interest (Marson et al., 2007; Zheng et al., 2007). The experiments presented here suggest that high amounts of miR155 are induced by Foxp3 in thymic TR precursors and maintained in a Foxp3-dependent manner in peripheral TR cells. We further found that miR155-deficient mice exhibit significantly diminished Foxp3+ TR cell subsets in the thymus and periphery, yet did not manifest enhanced activation of T cells and APCs or clinical signs of autoimmune disease.
A number of factors such as defective microenvironment, impaired lineage commitment, or increased TR cell death could potentially account for the reduced TR cell numbers in miR155-deficient mice. Our studies revealed a cell-intrinsic role for miR155 expression in Foxp3+ TR cell long-term maintenance, but not in thymic or peripheral differentiation. Further experiments suggested the presence or absence of miR155 on its own does not automatically alter the sensitivity of TR cells to induced apoptosis. However, given that miR155 deficiency impairs IL-2 signaling and a well-established role of IL-2 signaling in the survival of TR cells, miR155 deficiency in TR cells very likely leads to reduced survival in vivo in the presence of limited amounts of IL-2. In addition, we observed a marked decrease in the proliferative activity of miR155-deficient TR cells both in the thymus and in the periphery in comparison to their miR155-sufficient counterparts. Thus, the diminished size of miR155-deficient TR subset is most likely due to a combination of diminished thymic output and impaired peripheral homeostasis. Our most intriguing finding was that the reduction in the proportion of cycling Ki67+ cells and in the overall numbers of miR155-deficient TR cells was detectable in chimeric mice only late after reconstitution with miR155-deficient and -sufficient BM. Furthermore, when those cells were re-introduced into a non-competitive lymphopenic environment, they were able to proliferate at a level comparable to their miR155-sufficient counterparts. These results suggested that miR155 is required for competitive fitness of TR cells, whereas it is dispensable in non-competitive lymphopenic settings.
It is well established that IL-2 is required for thymic and peripheral TR cell maintenance, (Bayer et al., 2007; Setoguchi et al., 2005) making IL-2R signaling a likely target of miR155-mediated regulation. Although we cannot formally exclude the possible contribution of other pathways targeted by miR155 to the observed phenomenon, inferior STAT5 phosphorylation in miR155-deficient TR cells upon IL-2 stimulation provided a very likely explanation for the reduced number of TR cells observed in miR155-deficient mice. Moreover, the observation that attenuated IL-2 signaling leading to decreased STAT5 activation can be compensated by high concentrations of IL-2 stimulation further supports the role of miR155 in maintaining TR cell competitive fitness.
Importantly, our study provided a mechanistic insight into the role of miR155 in controlling IL-2 signaling pathway by demonstrating that SOCS1, a negative regulator of the IL-2 signaling cascade, is translationally inhibited by miR155. These results are consistent with previous reports that over-expression of the SOCS1 protein in T cells results in impaired IL-2 signaling, whereas T cells from SOCS-1-deficient mice are hyper-sensitive to IL-2 stimulation (Cornish et al., 2003; Sporri et al., 2001). Further support for our model of the miR155-mediated regulation of the TR subset size is provided by our observation of a comparable reduction in TR cell numbers upon SOCS1 transgene expression. Thus, our study suggests that in addition to increased expression of high affinity IL-2R (Marson et al., 2007; Zheng et al., 2007), Foxp3 drives elevated expression of miR155 expression, leading to translational down-regulation of the SOCS1 protein, which in turn leads to a heightened sensitivity of TR cells to IL-2 stimulation. In the absence of miR155, increased amounts of SOCS1 negatively impact IL-2 dependent TR cell homeostasis by increasing the threshold of IL-2R signaling (Fig. 7).
It is noteworthy that a notable, albeit less pronounced effect of miR155 deficiency extends to the homeostasis of peripheral Foxp3- T cells, which are known to up-regulate miR155 upon activation (Thai et al., 2007). Therefore, the questions have been raised as to how different is miR155-mediated SOCS1 regulation in non-TR cells and in TR cells. As demonstrated by previous studies, Foxp3 fixes and enhances expression of a number of genes only transiently expressed in activated non-TR cells to benefit TR homeostasis. In addition to miR155, this group of genes includes CTLA4 and CD25. Consistent with miR155 transient expression in non-TR cells, miR155 deficiency also affected this cell subset as suggested by reduction in miR155-deficient Foxp3- T cells in comparison to miR155-sufficient counterparts in mixed chimeras late after BM reconstitution. We also observed increased SOCS1 protein level in non-TR T cells in the absence of miR155. Therefore, it is possible that miR155/SOCS1 regulation also contributes to the homeostasis of non-TR cells. However, while in the absence of miR155 the bulk non-TR population exhibited increased SOCS1 levels, it never reached the SOCS1 amounts in miR155-deficient TR cells (∼2-fold vs. ∼5-fold increase, respectively). Furthermore, in activated T cells (TE) a modest increase in the SOCS1 level in the absence of miR155 suggested a difference in SOCS1 regulation between TR and non-TR cells. While low SOCS1 expression in TR cells is controlled by miR155 at a protein, but not mRNA level (SOCS1 mRNA level remains high in TR cells regardless of the presence or absence of miR155), the level of SOCS1 in activated non-TR cells is regulated at both mRNA and protein levels. Finally, homeostasis of TR and non-TR is different in its reliance on different growth factors. While IL-2 is essential for TR cells, non-TR cells are not solely dependent on IL-2 or IL-15 signaling. Thus, it is not surprising that Foxp3+ TR cells were affected by miR155 deficiency more profoundly and that we consistently observed a reduced frequency of miR155-deficient TR cells within the total miR155-deficient T cell population in both miR155 null mice or in mixed BM chimeras.
In addition to IL-2R, miR155-dependent SOCS1 regulation in TR cells could also impact signaling through other cytokine receptors, e.g. IL-15R, and potentially non-cytokine signaling pathways such as LPS/TLR4 (Yoshimura et al., 2007). Moreover, miR155-dependent SOCS1 regulation is also likely applicable to other cell types, and helps explain some of the immune defects previously described in miR155-deficient mice. For example, miR155-deficent DCs fail to efficiently activate T cells, whereas miR155-deficient CD4 T cells exhibit increased Th2 polarization and produce high amounts of Th2 cytokines (Rodriguez et al., 2007; Thai et al., 2007). In this regard, it has been previously shown that SOCS1 negatively regulates the antigen presenting capacity of DC (Evel-Kabler et al., 2006). In T cells, the amount of SOCS1 affects the balance between Th1 and Th2 differentiation. Higher amounts of SOCS1 protein suppress IL-12 and IFNγ signaling leading to a Th1 cell differentiation blockade, while promoting Th2 cell induction (Harada et al., 2007; Yoshimura et al., 2007).
In conclusion, miR155 is required for TR cell homeostasis in the presence of limiting amounts of IL-2, whereas it is dispensable in non-competitive lymphopenic settings. Foxp3 dependent up-regulation and maintenance of high amounts of miR155 in TR cells maintains TR cell homeostasis by targeting SOCS1 and by ensuring increased sensitivity of these cells to their principal growth factor, interleukin-2. Unlike the pleiotropic effects of the bulk miRNA depletion resulting from the Dicer ablation, the focused effect of miR155 deficiency on TR cells suggests that multiple miRNAs affect distinct facets of TR biology. Finally, our studies provide experimental support for a proposed role for miRNA-dependent gene regulation in ensuring robustness of the cellular phenotypes (Hornstein et al., 2006).
Material and Methods
Mice
miR155-/- (Thai et al., 2007), Foxp3DTR (Kim et al., 2007), SOCS1 Tg (Hanada et al., 2003) and SOCS1 cKO (Tanaka et al., 2008)mice were described elsewhere. Experimental mice were age-matched and housed under specific pathogen-free conditions. All mice were used in accordance with guidelines from the University of Washington Institutional Animal Care Committee.
shRNA knock-down and quantitative PCR analysis
For Foxp3 shRNA knockdown, shRNA sequences were designed using commonly used algorithms. The sequences targeted by two Foxp3 specific shRNAs are A: CACTATCACACATAGGTGT; B: CAGACACCATCCTAATATTT. These sequences were cloned into a retroviral vector pRNAT-H1.1-Retro, packaged using Phoenix-E cells and used for transduction of MACS purified CD4+CD25+ cells stimulated overnight with plate bound anti-CD3 (1μg/ml), anti-CD28 (1μg/ml), and IL-2 (200U/ml). Cells were maintained in IL2 for 60-72h post-transduction, followed by analysis of Foxp3 expression by flow cytometry or quantitative PCR.
For quantitative PCR analysis, total RNA (including microRNA) was prepared from FACS purified cells previously treated with Foxp3-specific or control shRNA using Trizol (Invitrogen). First strand complementary DNA was synthesized by using NCode miRNA First-Strand cDNA Kit (Invitrogen). Amounts of miRNA were measured by qPCR according to manufactural instructions. Ubiquitously expressed U6 small nuclear RNA was used for normalization.
Generation of BM chimeras
Single suspensions of BM isolated extracted from femurs and tibias were depleted of T cells using Thy1 antibody-coated magnetic beads. 3-5×106 T-depleted BM cells were transferred individually or at a 1:1 ratio into lethally irradiated (950 rads) Rag1-/- recipients. BM engraftment was examined using flow cytometric analysis of peripheral blood lymphocyte at indicated times. Chimeric mice were euthanized and analyzed 120 days after BM transfer. Ly5.1-CD4+CD25-CD62Lhi TE cells and Ly5.1-CD4+CD25hi TR cells originating from miR155-/- or miR155+/+ BM were sorted and used in functional assays described below.
In vitro apoptosis assays
For apoptosis study, 1×105 CD4+CD25- and CD4+CD25hi T cells isolated from miR155-/- and miR155+/+ mice were cultured in 96-well plates with 1 μg/ml CD3 (2C11) antibody at 37°C for the indicated time. FITC-VAD-FMK (Promega) was used to measure caspase-3 activity according to the manufacturer's instructions. Cells were harvested and the presence of apoptotic cells was assessed by flow cytometric analysis.
Flow cytometric analysis
Cell were stained using CD4 (clone L3T4), CD8 (clone 53-6.7), CD25 (clone PC61), Ly5.1 (clone A20) antibodies, followed by intracellular staining using Foxp3 (clone FJK-16s, eBioscience), GFP/YFP (polyclonal rabbit, Invitrogen) and Ki67 (polyclonal rabbit, BD Biosciences) antibodies and subjected to flow cytometric analysis using a FACS Canto flow cytometer (Becton Dickinson). Fixation and permeabilization of cells were performed using the reagents from the eBioscience Foxp3 staining kit.
For phospho-STAT5 staining, cells were stimulated for 20 min with indicated IL-2 concentrations in 37°C, equal volume of 3.2% formaldehyde/PBS was added directly into the culture for fixation. After 15 min incubation at room temperature, cells were harvested and washed twice with PBS, followed by permeabilization with ice-cold 100% MtOH for 25 min on ice. Cells were then washed twice and stained with the phospho-Stat5 antibody (clone 47, BD Biosciences) and antibodies specific for other cell surface molecules.
Luciferase reporter assay
HEK293T cells were cultured at 3.5×105 cells/well in 6-well plate 1 day prior to transfection. psiCheck2 luciferase reporter plasmids (Promega) containing either WT or mutated SOCS1 3′ UTR and miR155- or control miR150-expressing pMDH-PGK-EGFP plasmids (Zhou et al., 2007) were co-transfected into HEK293T cells using Fugene 6 transfection reagent (Roche). Cells were harvested 24 hr later and luciferase activity was assessed using Dual-Luciferase Reporter Assay System (Promega) according to manufacturer's protocol.
Western blot analysis
Isolated TR cells (5×105) were lysed using a chilled lysis buffer (50 mM Tris-Hcl, pH 7.4, 1% NP-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 1X Protease Inhibitor Cocktail). Cell debris was removed by centrifugation at 14,000 rpm for 15 min at 4°C and the supernatant was immediately transferred to a fresh tube. Solubilized proteins were separated using SDS-PAGE and transferred to a nitrocellulose membrane. SOCS1 and β-actin (loading control) were visualized using monoclonal antibodies ab62584 (Abcam) and AC-74 (Sigma), correspondingly. Protein quantitation was performed using NIH Image J software (http://rsb.info.nih.gov/ij/).
Supplementary Material
Acknowledgments
We thank S. Josefowicz for the help in key experiments, T. Chu, L. Karpik, and K. Forbush for superb technical assistance, and all members of our laboratory for discussions. This work was supported by grants from the NIH (A.Y.R. and K.R.), from European Union through MUGEN (K.R.) and from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (A.Y.). L.L.F. is a Leukemia and Lymphoma Society Fellow. D.P.C. is supported from the Portuguese Foundation for Science and Technology (FCR-MCES). A.Y.R. is a Howard Hughes Medical Institute investigator. The authors declare they have no conflicting financial interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Bayer AL, Yu A, Malek TR. Function of the IL-2R for thymic and peripheral CD4+CD25+ Foxp3+ T regulatory cells. J Immunol. 2007;178:4062–4071. doi: 10.4049/jimmunol.178.7.4062. [DOI] [PubMed] [Google Scholar]
- Cobb BS, Hertweck A, Smith J, O'Connor E, Graf D, Cook T, Smale ST, Sakaguchi S, Livesey FJ, Fisher AG, Merkenschlager M. A role for Dicer in immune regulation. J Exp Med. 2006;203:2519–2527. doi: 10.1084/jem.20061692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb BS, Nesterova TB, Thompson E, Hertweck A, O'Connor E, Godwin J, Wilson CB, Brockdorff N, Fisher AG, Smale ST, Merkenschlager M. T cell lineage choice and differentiation in the absence of the RNase III enzyme Dicer. J Exp Med. 2005;201:1367–1373. doi: 10.1084/jem.20050572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish AL, Chong MM, Davey GM, Darwiche R, Nicola NA, Hilton DJ, Kay TW, Starr R, Alexander WS. Suppressor of cytokine signaling-1 regulates signaling in response to interleukin-2 and other gamma c-dependent cytokines in peripheral T cells. J Biol Chem. 2003;278:22755–22761. doi: 10.1074/jbc.M303021200. [DOI] [PubMed] [Google Scholar]
- Costinean S, Zanesi N, Pekarsky Y, Tili E, Volinia S, Heerema N, Croce CM. Pre-B cell proliferation and lymphoblastic leukemia/high-grade lymphoma in E(mu)-miR155 transgenic mice. Proc Natl Acad Sci U S A. 2006;103:7024–7029. doi: 10.1073/pnas.0602266103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eis PS, Tam W, Sun L, Chadburn A, Li Z, Gomez MF, Lund E, Dahlberg JE. Accumulation of miR-155 and BIC RNA in human B cell lymphomas. Proc Natl Acad Sci U S A. 2005;102:3627–3632. doi: 10.1073/pnas.0500613102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evel-Kabler K, Song XT, Aldrich M, Huang XF, Chen SY. SOCS1 restricts dendritic cells' ability to break self tolerance and induce antitumor immunity by regulating IL-12 production and signaling. J Clin Invest. 2006;116:90–100. doi: 10.1172/JCI26169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Gavin MA, Clarke SR, Negrou E, Gallegos A, Rudensky A. Homeostasis and anergy of CD4(+)CD25(+) suppressor T cells in vivo. Nat Immunol. 2002;3:33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- Gironella M, Seux M, Xie MJ, Cano C, Tomasini R, Gommeaux J, Garcia S, Nowak J, Yeung ML, Jeang KT, et al. Tumor protein 53-induced nuclear protein 1 expression is repressed by miR-155, and its restoration inhibits pancreatic tumor development. Proc Natl Acad Sci U S A. 2007;104:16170–16175. doi: 10.1073/pnas.0703942104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada T, Yoshida H, Kato S, Tanaka K, Masutani K, Tsukada J, Nomura Y, Mimata H, Kubo M, Yoshimura A. Suppressor of cytokine signaling-1 is essential for suppressing dendritic cell activation and systemic autoimmunity. Immunity. 2003;19:437–450. doi: 10.1016/s1074-7613(03)00240-1. [DOI] [PubMed] [Google Scholar]
- Harada M, Nakashima K, Hirota T, Shimizu M, Doi S, Fujita K, Shirakawa T, Enomoto T, Yoshikawa M, Moriyama H, et al. Functional polymorphism in the suppressor of cytokine signaling 1 gene associated with adult asthma. Am J Respir Cell Mol Biol. 2007;36:491–496. doi: 10.1165/rcmb.2006-0090OC. [DOI] [PubMed] [Google Scholar]
- Hornstein E, Shomron N. Canalization of development by microRNAs. Nat Genet. 2006;38(Suppl):S20–4. doi: 10.1038/ng1803. [DOI] [PubMed] [Google Scholar]
- Inagaki-Ohara K, Sasaki A, Matsuzaki G, Ikeda T, Hotokezaka M, Chijiiwa K, Kubo M, Yoshida H, Nawa Y, Yoshimura A. Suppressor of cytokine signalling 1 in lymphocytes regulates the development of intestinal inflammation in mice. Gut. 2006;55:212–219. doi: 10.1136/gut.2004.062653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- Koralov SB, Muljo SA, Galler GR, Krek A, Chakraborty T, Kanellopoulou C, Jensen K, Cobb BS, Merkenschlager M, Rajewsky N, Rajewsky K. Dicer ablation affects antibody diversity and cell survival in the B lymphocyte lineage. Cell. 2008;132:860–874. doi: 10.1016/j.cell.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, Rajewsky N. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- Li QJ, Chau J, Ebert PJ, Sylvester G, Min H, Liu G, Braich R, Manoharan M, Soutschek J, Skare P, et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129:147–161. doi: 10.1016/j.cell.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Liston A, Lu LF, O'Carroll D, Tarakhovsky A, Rudensky AY. Dicer-dependent microRNA pathway safeguards regulatory T cell function. J Exp Med. 2008;205:1993–2004. doi: 10.1084/jem.20081062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marson A, Kretschmer K, Frampton GM, Jacobsen ES, Polansky JK, MacIsaac KD, Levine SS, Fraenkel E, von Boehmer H, Young RA. Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature. 2007;445:931–935. doi: 10.1038/nature05478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–323. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- Metzler M, Wilda M, Busch K, Viehmann S, Borkhardt A. High expression of precursor microRNA-155/BIC RNA in children with Burkitt lymphoma. Genes Chromosomes Cancer. 2004;39:167–169. doi: 10.1002/gcc.10316. [DOI] [PubMed] [Google Scholar]
- Muljo SA, Ansel KM, Kanellopoulou C, Livingston DM, Rao A, Rajewsky K. Aberrant T cell differentiation in the absence of Dicer. J Exp Med. 2005;202:261–269. doi: 10.1084/jem.20050678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovcharenko D, Kelnar K, Johnson C, Leng N, Brown D. Genome-scale microRNA and small interfering RNA screens identify small RNA modulators of TRAIL-induced apoptosis pathway. Cancer Res. 2007;67:10782–10788. doi: 10.1158/0008-5472.CAN-07-1484. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska EA, et al. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316:608–611. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporri B, Kovanen PE, Sasaki A, Yoshimura A, Leonard WJ. JAB/SOCS1/SSI-1 is an interleukin-2-induced inhibitor of IL-2 signaling. Blood. 2001;97:221–226. doi: 10.1182/blood.v97.1.221. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Ichiyama K, Hashimoto M, Yoshida H, Takimoto T, Takaesu G, Torisu T, Hanada T, Yasukawa H, Fukuyama S, et al. Loss of suppressor of cytokine signaling 1 in helper T cells leads to defective Th17 differentiation by enhancing antagonistic effects of IFN-gamma on STAT3 and Smads. J Immunol. 2008;180:3746–3756. doi: 10.4049/jimmunol.180.6.3746. [DOI] [PubMed] [Google Scholar]
- Thai TH, Calado DP, Casola S, Ansel KM, Xiao C, Xue Y, Murphy A, Frendewey D, Valenzuela D, Kutok JL, et al. Regulation of the germinal center response by microRNA-155. Science. 2007;316:604–608. doi: 10.1126/science.1141229. [DOI] [PubMed] [Google Scholar]
- van den Berg A, Kroesen BJ, Kooistra K, de Jong D, Briggs J, Blokzijl T, Jacobs S, Kluiver J, Diepstra A, Maggio E, Poppema S. High expression of B-cell receptor inducible gene BIC in all subtypes of Hodgkin lymphoma. Genes Chromosomes Cancer. 2003;37:20–28. doi: 10.1002/gcc.10186. [DOI] [PubMed] [Google Scholar]
- Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, Newman J, Bronson RT, Crowley D, Stone JR, et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132:875–886. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigorito E, Perks KL, Abreu-Goodger C, Bunting S, Xiang Z, Kohlhaas S, Das PP, Miska EA, Rodriguez A, Bradley A, et al. microRNA-155 Regulates the Generation of Immunoglobulin Class-Switched Plasma Cells. Immunity. 2007;27:847–859. doi: 10.1016/j.immuni.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Calado DP, Galler G, Thai TH, Patterson HC, Wang J, Rajewsky N, Bender TP, Rajewsky K. MiR-150 controls B cell differentiation by targeting the transcription factor c-Myb. Cell. 2007;131:146–159. doi: 10.1016/j.cell.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Xiao C, Srinivasan L, Calado DP, Patterson HC, Zhang B, Wang J, Henderson JM, Kutok JL, Rajewsky K. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol. 2008 doi: 10.1038/ni1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007;445:936–940. doi: 10.1038/nature05563. [DOI] [PubMed] [Google Scholar]
- Zhou B, Wang S, Mayr C, Bartel DP, Lodish HF. miR-150, a microRNA expressed in mature B and T cells, blocks early B cell development when expressed prematurely. Proc Natl Acad Sci U S A. 2007;104:7080–7085. doi: 10.1073/pnas.0702409104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.