Abstract
Background:
Pulmonary complications following injury significantly contribute to subsequent mortality. Obese patients have preexisting risk factors for pulmonary complications, and are at risk for these complications following elective surgery. Whether or not obesity contributes to pulmonary complications after critical injury is poorly understood.
Methods:
A secondary analysis of a prospective cohort study of critically injured adults requiring at least 48 h of intensive care was performed. Patients were classified into the following body mass index groups: ≤ 18.5 kg/m2 (underweight); 18.5 to 24.9 kg/m2 (normal); 25 to 29.9 kg/m2 (overweight); 30.0 to 39.9 kg/m2 (obese); and ≥ 40.0 kg/m2 (severely obese). Outcomes included the rates of ARDS and pneumonia, the placement of a tracheostomy tube, and in-hospital mortality rate.
Results:
A total of 1,291 patients were available for analysis, and 30% of these patients were classified as either obese or severely obese. The age-, gender-, and severity-adjusted rate of ARDS was lower in severely obese patients (odds ratio, 0.36; 95% confidence interval [CI], 0.13 to 0.99) compared to normal weight patients. The rates of pneumonia (37%), tracheostomy (10%), and in-hospital mortality (11%) did not differ among the groups. Despite no difference in pulmonary complications, the severely obese group had an ICU length of stay that was 4.8 days (95% CI, 1.8 to 7.7 days) longer than the normal weight group.
Conclusion:
Obesity does not appear to be an independent risk factor for increased pulmonary complications after critical injury, but severely obese patients are likely to require longer ICU stays.
Trial registration:
Clinicaltrials.gov Identifier: NCT00170560
Keywords: ARDS, epidemiology, obesity, pneumonia, trauma
Obesity is a pervasive disease affecting > 30% of Americans of all ages and socioeconomic groups.1,2 Its implications include increased risks of cancer, diabetes, dyslipidemia, heart disease, hypertension, insulin resistance, and death.3 While the association between obesity and these chronic conditions is clear, there is still considerable debate regarding the role of obesity on outcomes in the critical care setting and after trauma. A number of authors have documented worse outcomes in obese patients in the critical care setting4 and after trauma,5-8 but others9-13 have been unable to demonstrate differences in outcomes related to obesity.
An equal amount of ambiguity surrounds the relationship between pulmonary complications after trauma and during critical illness. At baseline, obese patients have a compromised pulmonary status that is characterized by reduced lung volumes and compliance, as well as ventilation-perfusion mismatching.14 Artificial airways are often more difficult to maintain in obese patients,15 and radiographic imaging may have reduced reliability and delay recognition of impending complications.16 Obese patients may also experience higher rates of chest trauma, rib fractures, and pulmonary contusions, and a trend toward more severe chest injury.10,17 Despite the presumption that obese patients are at high risk for pulmonary complications, very few studies have specifically addressed these outcomes. Our objective was to determine whether obesity represents an independent risk factor for the development of pulmonary complications after critical injury. We hypothesized that obese and severely obese patients would be at increased risk for pulmonary complications following critical injury.
Materials and Methods
Study Design and Participating Centers
This study represents a secondary analysis of a prospective cohort study of critically injured adults. The detailed methods of this study have been previously outlined.18 Briefly, patients ≥ 18 years of age, who had been admitted to the trauma ICUs of either Vanderbilt University Medical Center or the University of Virginia Health Systems for at least 48 h were eligible for study enrollment. Patients who died before 48 h were excluded as were patients who had been discharged from the ICU prior to 48 h. The minimum stay of 48 h was intended to exclude postoperative surgical patients with short observational stays as well as patients who died rapidly from illness beyond the aid of modern critical care. Patient care was at the discretion of the attending physician according to established critical care protocols in the respective ICUs.
Measure of Obesity
Body mass index (BMI) was determined at hospital admission by dividing the weight in kilograms by the height in meters squared. Patients were classified into the following BMI groups according to the National Heart, Lung, and Blood Institute guidelines19: ≤ 18.5 kg/m2 (underweight); 18.5 to 24.9 kg/m2 (normal); 25 to 29.9 kg/m2 (overweight); 30.0 to 39.9 kg/m2 (obese); and ≥ 40.0 kg/m2 (severely obese).
Measures of Injury Severity
The injury severity score (ISS) is based on an anatomic grading of injury severity.20 Each of six body regions (head and neck, face, chest, abdomen, extremity, and external) is assigned an abbreviated injury score (AIS). The three most severely injured regions have their scores squared and summed to produce the ISS. ISS ranges from 0 to 75. The trauma-related ISS (TRISS) determines the probability of survival from the ISS and revised trauma score weighted for patient age and mechanism of injury. The revised trauma score is a physiologic score based on the Glasgow coma scale, the systolic BP, and the respiratory rate on first contact with the patient.21,22
Outcome Measures
The primary outcomes of interest were ARDS, pneumonia, placement of a tracheostomy tube, and the number of ventilator days. ARDS was classified according to the following standard definition23: the presence of bilateral patchy infiltrates seen on a chest radiograph; a Pao2/fraction of inspired oxygen ratio of < 200; and the absence of cardiogenic pulmonary edema. Radiographs were classified by critical care specialists who were not a part of the patient care team. Hospital-acquired pneumonia was diagnosed when a predominant organism was isolated from an appropriately obtained culture in the setting of purulent sputum production, a new or changing infiltrate seen on chest radiograph, and systemic evidence of infection. Quantitative endotracheal suction was routinely used at the University of Virginia (a concentration of > 105 organisms per milliliter was considered to be positive for infection), and quantitative BAL was routinely used at Vanderbilt University (a concentration of > 104 organisms per milliliter was considered to be positive for infection). The placement of a tracheostomy tube was defined as any new tracheostomy tube that was placed during hospitalization, the need for which was determined by a multidisciplinary team of critical care physicians. Secondary outcomes included all-cause in-hospital mortality, and hospital and ICU length of stay (LOS).
Statistical Analysis
Normally distributed continuous variables were summarized by reporting the mean and SD. Continuous variables that were not normally distributed were presented by reporting the median and interquartile ranges (IQRs). For comparisons among multiple groups, analysis of variance was used with a Bonferroni correction. To estimate the relationship between outcomes and BMI group, multivariate linear and logistic regression was used to determine regression coefficients and odds ratios, respectively. In these models, BMI was fit in the logistic models as a dummy variable (BMI groups). Variables that were found to have statistically significant associations (p < 0.10 or parameter estimates outside of the 95% confidence interval [CI] of the comparison group) with BMI group were selected for inclusion in the multivariate logistic models. To further explore the nonlinear relationship between BMI and outcomes, restricted cubic spline covariates were determined using a statistical software program.
Detectable Alternatives Calculations
Detectable alternatives were calculated given the following available data and assumptions: 1,219 patients total; 236 patients with a BMI > 30 kg/m2; a case (obese patient)-to-control (nonobese patient) ratio of 2.3:1; a type I error of 0.05; and a type II error of 0.20 (80% power). Given the observed rates of pulmonary outcomes in nonobese patients, we had 80% power to detect a difference in ARDS rates of ± 8%, a difference in pneumonia rates of ± 9%, and a difference in the rate of tracheostomy tube placement of ± 5%. The analysis is powered to detect a ± 6% difference in mortality.
A statistical software program (Stata, version 9.2; Stata Corp; College Station, TX) was used for analysis. Tests for statistical significance were two sided with an α of 0.05. The study was approved by the institutional review board of Vanderbilt University Medical Center. All data are maintained in a secure, password protected database that is compliant with the Health Insurance Portability and Accountability Act of 1996 (or HIPAA). All patient information is deidentified prior to analysis and reporting.
Results
A total of 2,291 patients were enrolled into the original cohort. Patients admitted to the ICU for a diagnosis other than trauma (n = 883) were excluded from the study. Of the 1,406 trauma patients, 187 (15%) were excluded for missing BMI data. The demographics and clinical characteristics of the 1,219 remaining patients by BMI group are displayed in Table 1. The majority of patients fell into the normal (35%) and overweight categories (33%), but the prevalence of obesity (23%) and severe obesity (7%) was also high. The overweight group was made up of significantly more male patients (p < 0.001), while the patients in the normal weight group were younger (p < 0.001). There was no difference among the groups according to blunt or penetrating mechanism, or acute physiology and chronic health evaluation (APACHE) II score. Anatomic injury severity (ISS) was statistically higher (p = 0.008) and the predicted survival (TRISS) was significantly lower (p = 0.006) in the normal weight group. Anatomic injury pattern did not significantly differ between the groups with the exception of head injuries. The underweight and normal weight groups had a higher AIS head and neck score than the other groups (p < 0.001). With regard to pre-ICU comorbidities, underweight patients were more likely to experience chronic renal insufficiency and dialysis dependence, while severely obese patients were more likely to experience diabetes mellitus, cardiac disease, hyperlipidemia, and hypertension. Individual comorbidities were not included in the regression models since they are summarized by the chronic health score of the APACHE II score. Table 2 summarizes demographic and clinical characteristics of patients by pulmonary outcomes.
Table 1.
Demographic and Clinical Characteristics of Patients by BMI Group*
| BMI Groups |
|||||||
|---|---|---|---|---|---|---|---|
| Variables | Underweight (n = 23) |
Normal (n = 426) |
Overweight (n = 403) |
Obese (n = 286) |
Severely Obese (n = 81) |
p Value† | |
| Age, yr | 44 ± 23 | 46 ± 19 | 45 ± 19 | 47 ± 19 | 44 ± 18 | < 0.001 | |
| Male gender, % | 48 | 72 | 79 | 73 | 62 | 0.001 | |
| BMI, kg/m2 | 18 | 23 | 27 | 33 | 45 | < 0.001 | |
| Blunt injury, % | 87 | 89 | 87 | 89 | 93 | 0.65 | |
| APACHE II score | 17 ± 7 | 18 ± 6 | 17 ± 6 | 18 ± 6 | 16 ± 5 | 0.35 | |
| TRISS | 0.82 (0.63–0.94) | 0.73 (0.30–0.94) | 0.80 (0.52–0.95) | 0.87 (0.46–0.96) | 0.90 (0.52–0.97) | 0.006 | |
| ISS | 28 ± 9 | 31 ± 12 | 30 ± 12 | 29 ± 12 | 26 ± 11 | 0.008 | |
| AIS | |||||||
| Face | 0 (0–2) | 0 (0–2) | 0 (0–2) | 0 (0–2) | 0 (0–1) | 0.21 | |
| Head | 3 (0–4) | 3 (2–4) | 3 (0–4) | 3 (0–4) | 1 (0–3) | < 0.001 | |
| Chest | 3 (0–3) | 3 (0–4) | 3 (0–4) | 3 (2–4) | 3 (2–4) | 0.68 | |
| Abdomen | 0 (0–3) | 2 (0–3) | 2 (0–3) | 2 (0–3) | 2 (0–3) | 0.46 | |
| Extremity | 2 (0–2) | 0 (0–3) | 2 (0–3) | 2.5 (0–3) | 3 (0–3) | 0.26 | |
| External | 0 (0–1) | 0 (0–1) | 0 (0–0) | 0 (0–0) | 0 (0–1) | 0.91 | |
| Pre-ICU admission disease prevalence, % |
|||||||
| Diabetes mellitus | 9 | 3 | 8 | 11 | 26 | < 0.001 | |
| Cardiac disease | 22 | 7 | 15 | 13 | 26 | < 0.001 | |
| Cerebrovascular disease | 4 | 3 | 3 | 2 | 2 | 0.90 | |
| Pulmonary disease | 13 | 9 | 10 | 10 | 9 | 0.38 | |
| Chronic renal insufficiency | 9 | 0 | 1 | 1 | 4 | 0.001 | |
| Dialysis dependence | 4 | 0 | 0 | 0 | 1 | 0.006 | |
| HIV/AIDS | 0 | 0 | 1 | 0 | 0 | 0.74 | |
| Malignancy | 0 | 2 | 3 | 4 | 0 | 0.20 | |
| Hepatic insufficiency | 0 | 0 | 0 | 1 | 0 | 0.86 | |
| Hyperlipidemia | 9 | 2 | 2 | 6 | 10 | 0.001 | |
| Hypertension | 26 | 11 | 20 | 31 | 33 | < 0.001 | |
| Peripheral vascular disease | 0 | 3 | 2 | 1 | 4 | 0.30 | |
| Rheumatic/connective tissue disorder |
4 | 1 | 1 | 1 | 1 | 0.65 | |
| Thyroid disease | 9 | 1 | 2 | 2 | 0 | 0.08 | |
| Long-term corticosteroid use | 4 | 0 | 1 | 2 | 1 | 0.07 | |
| Inflammatory bowel disease | 0 | 0 | 0 | 0 | 0 | 0.77 | |
Values are given as the mean ± SD or median (IQR), unless otherwise indicated.
Comparisons made using one-way analysis of variance with Bonferroni correction.
Table 2.
Demographic and Clinical Characteristics by Outcome*
| Variables | No ARDS |
ARDS | p Value | No Pneumonia |
Pneumonia | p Value | No Tracheostomy |
Tracheostomy | p Value† | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age, yr | 43 ± 19 | 43 ± 19 | 0.48 | 44 ± 19 | 42 ± 18 | 0.36 | 44 ± 19 | 45 ± 19 | 0.40 | |
| Male gender, % | 76 | 71 | 0.15 | 71 | 78 | 0.002 | 73 | 81 | 0.07 | |
| BMI, kg/m2 | 28 ± 7 | 28 ± 6 | 0.58 | 28 ± 8 | 28 ± 7 | 0.74 | 28 ± 7 | 27 ± 6 | 0.06 | |
| Blunt injury, % | 86 | 89 | 0.23 | 88 | 89 | 0.39 | 89 | 86 | 0.34 | |
| APACHE II score | 17 ± 6 | 18 ± 6 | < 0.001 | 15 ± 6 | 17 ± 6 | < 0.001 | 16 ± 6 | 18 ± 6 | 0.004 | |
| TRISS | 0.81 | 0.82 | 0.58 | 0.85 | 0.78 | 0.33 | 0.83 | 0.79 | 0.51 | |
| ISS | 31 ± 11 | 33 ± 13 | 0.02 | 29 ± 12 | 31 ± 12 | 0.001 | 29 ± 12 | 31 ± 12 | 0.21 | |
| AIS | ||||||||||
| Face | 0 (0–2) | 0 (0–1) | 0.06 | 0 (0–2) | 0 (0–2) | 0.37 | 0 (0–1) | 1 (0–2) | < 0.001 | |
| Head | 3 (0–4) | 3 (0–4) | 0.01 | 3 (0–4) | 3 (0–4) | 0.62 | 3 (0–4) | 3 (0–5) | 0.05 | |
| Chest | 3 (0–4) | 4 (3–4) | < 0.001 | 3 (2–4) | 3 (2–4) | 0.002 | 3 (1–4) | 3 (0–4) | 0.75 | |
| Abdomen | 2 (0–3) | 2 (0–3) | 0.005 | 2 (0–3) | 2 (0–3) | 0.39 | 2 (0–3) | 2 (0–3) | < 0.001 | |
| Extremity | 2 (0–3) | 3 (0–3) | 0.07 | 2 (0–3) | 2 (0–3) | 0.05 | 2 (0–3) | 2 (0–3) | 0.002 | |
| External | 0 (0–1) | 0 (0–1) | 0.88 | 0 (0–1) | 0 (0–1) | 0.81 | 0 (0–1) | 0 (0–1) | 0.64 | |
| Mortality, % | 13 | 14 | 0.79 | 12 | 9 | 0.07 | 12 | 10 | 0.62 | |
| Time spent receiving ventilation, d |
6 | 10 | < 0.001 | 6 | 11 | < 0.001 | 7 | 7 | 0.88 | |
| ICU LOS, d | 8 | 11 | < 0.001 | 7 | 14 | < 0.001 | 9 | 9 | 0.58 | |
| Hospital LOS, d | 16 | 19 | < 0.001 | 15 | 23 | < 0.001 | 18 | 19 | 0.27 | |
Values are given as the mean ± SD or median (IQR), unless otherwise indicated.
Comparisons were made using two-sample t test, χ2 test, or Wilcoxon rank sum test.
Unadjusted rates of primary and secondary outcomes by BMI group are summarized in Table 3. The overall rate of ARDS was 27%. The unadjusted rate of ARDS was significantly lower in the severely obese group (11%) compared to the obese group (32%; p = 0.02). While a difference was also suggested between the severely obese group and the underweight, normal weight, and overweight groups, this difference was not statistically significant. When compared to the normal weight group, the age-, gender-, and severity-adjusted odds of ARDS remained lower in the severely obese group (odds ratio, 0.36; 95% CI, 0.13 to 0.99) [Table 4]. The rates of pneumonia, tracheostomy tube placement, and mortality were 37%, 10%, and 11%, respectively (Table 5). There was no difference in these rates by BMI group in either the univariate or multivariate analyses.
Table 3.
Clinical Outcomes by BMI Group
| BMI Groups |
||||||
|---|---|---|---|---|---|---|
| Variables | Underweight (n = 23) |
Normal Weight (n = 426) |
Overweight (n = 403) |
Obese (n = 286) |
Severely Obese (n = 81) |
p Value* |
| ARDS, % | 21 | 26 | 28 | 32 | 11 | 0.04 |
| Pneumonia, % | 22 | 37 | 39 | 36 | 36 | 0.57 |
| Tracheostomy, % | 10 | 12 | 10 | 8 | 4 | 0.18 |
| Mortality rate, % | 13 | 11 | 12 | 10 | 11 | 0.94 |
| Time spent receiving ventilation, d | 7.5 | 7 | 7 | 8 | 8 | 0.04 |
| ICU LOS, d | 8 | 9 | 9 | 10 | 10 | < 0.001 |
| Hospital LOS, d | 14 | 17 | 18 | 19 | 20 | 0.14 |
Comparisons were made using one-way analysis of variance with Bonferroni correction. p Values represent composite analysis of variance.
Table 4.
Adjusted Values for Pulmonary Outcomes and Death by BMI Group*
| BMI Groups |
|||||||
|---|---|---|---|---|---|---|---|
| Variables | Underweight (n = 23) |
Normal (n = 426) |
Overweight (n = 403) |
Obese (n = 286) |
Severely Obese (n = 81) |
HL GOF† | Area Under ROC Curve |
| ARDS | 0.86 (0.22–3.4) | 1.0 | 0.97 (0.63–1.5) | 1.0 (0.64–1.7) | 0.36 (0.13–0.99) | 576.9; 0.41 | 0.58 |
| Pneumonia | 0.36 (0.10–1.3) | 1.0 | 1.1 (0.75–1.5) | 0.96 (0.64–1.4) | 0.89 (0.46–1.7) | 776.3; 0.41 | 0.55 |
| Tracheostomy | 0.44 (0.06–3.4) | 1.0 | 0.83 (0.48–1.4) | 0.67 (0.36–1.3) | 0.30 (0.07–1.3) | 744.3; 0.44 | 0.61 |
| Mortality | 1.5 (0.31–7.1) | 1.0 | 1.5 (0.83–2.6) | 0.90 (0.46–1.8) | 1.5 (0.5–4.2) | 751.0; 0.66 | 0.72 |
Values are given as odds ratio (95% CI) adjusted for age, gender, TRISS, and AIS head score. ROC = receiver operating characteristic; HL-GOF = Hosmer-Lemeshow goodness of fit.
Values are given as χ2 statistic; p value.
Table 5.
Adjusted Continuous Outcomes by BMI Group*
| BMI Groups |
|||||
|---|---|---|---|---|---|
| Variables | Underweight (n = 23) |
Normal (n = 426) |
Overweight (n = 403) |
Obese (n = 286) |
Severely Obese (n = 81) |
| Time spent receiving ventilation, d | 0.10 (−3.7 to 3.9) | 0 | 0.33 (−0.98 to 1.7) | 1.4 (0.09 to 2.8) | 1.3 (−0.97 to 3.6) |
| ICU LOS, d | −1.6 (−6.3 to 3.1) | 0 | 0.60 (−1.1 to 2.3) | 1.6 (−0.23 to 3.5) | 4.8 (1.8 to 7.7) |
| Hospital LOS, d | −2.0 (−17 to 13.0) | 0 | −2.3 (−7.6 to 3.1) | 5.2 (−0.83 to 11) | 2.1 (−7.4 to 12) |
Values are given as β-coefficient (95% CI) adjusted for age, gender, TRISS, and AIS head score.
The median mechanical ventilation requirement of the cohort was 7 days (IQR, 4 to 12 days), and the number of days spent receiving mechanical ventilation did not differ by BMI group. These relationships did not differ when nonsurvivors were excluded. The median ICU LOS was 9 days, and the median hospital LOS was 18 days. After adjusting for age, gender, head injury severity, and predicted survival, the severely obese group had an ICU LOS that was 4.8 days (95% CI, 1.8 to 7.7) longer than the normal weight group (Table 3).
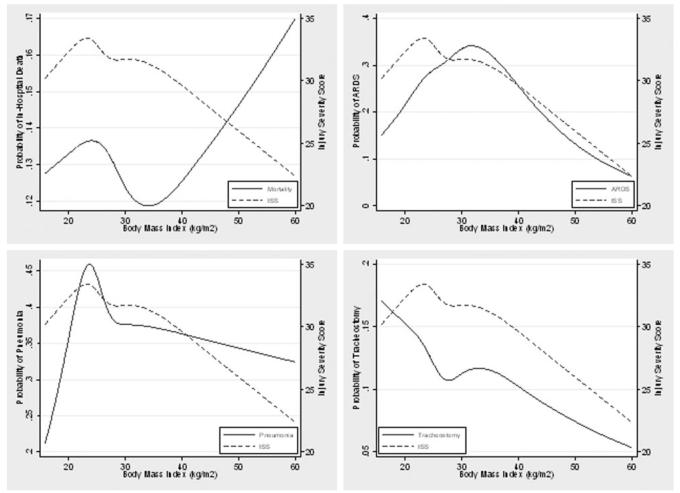
Figure 1 summarizes the unadjusted relationship between BMI and dichotomous outcomes with a concurrent summary of anatomic injury severity (as estimated by the ISS). The unadjusted relationships among ARDS, pneumonia, and tracheostomy tube placement closely parallel anatomic injury severity across the entire spectrum of BMI, suggesting no independent relationship between BMI and these outcomes. In contrast, for patients with a BMI of > 35 kg/m2, mortality increases while injury severity decreases. This trend was detected in the BMI group analysis, but it was not significant since patients with the highest mortality rates (ie, those with a BMI of > 50 kg/m2) represent only 26% of patients (21 of 81 patients) in the severely obese group.
Figure 1.
Associations among BMI, mortality, and pulmonary complications after injury.
Discussion
Conventional wisdom holds that obesity increases the number of adverse outcomes during critical illness, but an independent effect of obesity on outcome from critical illness has never been conclusively demonstrated. Early reports5-7 suggested that obesity played a large role in determining outcomes in the ICU and after trauma, but more recently reports9,10,12,13 have suggested no relationship. The conclusions from the published literature are limited by mostly retrospective studies and by varying definitions and rates of obesity. We sought to describe the relationship of obesity to pulmonary complications in a large, prospective population of trauma patients with a prevalence of obesity that reflects the general population.
Despite these underlying risk factors for pulmonary complications,14 we did not detect a difference in pulmonary complications that was related to BMI group. Some have speculated that since obesity is a chronic inflammatory condition, severe injury may incite a second-hit phenomenon, which would pre-dispose obese patients to “inflammatory” complications such as ARDS and multiple system organ failure.10 In this study, we demonstrate the opposite phenomenon; that severely obese patients may have a lower rate of ARDS. It is not clear from these data what accounts for this observation. It is possible that an abundance of subcutaneous fat provides a cushion and lessens pulmonary injury; we, however, did not detect a difference in chest injury by BMI group as measured by the AIS chest score. It is also possible that this represents a type I error.
We did not detect differences in pneumonia related to BMI group or the placement of a tracheostomy tube. The need for ventilator support was longer for the obese group (but not the severely obese group). One might speculate that obese patients continue to receive mechanical ventilation for longer periods of time because clinicians fear airway complications associated with reintubation. With standard ventilator weaning protocols and strategies, this is unlikely to account for differences among groups. Severely obese patients had a longer ICU LOS (independent of the requirements for mechanical ventilation), but LOS is often confounded by bed availability and insurance status; these results should be interpreted with these limitations in mind.
We were not able to detect a difference in mortality in the severely obese group of patients compared to the normal weight group of patients using multivariate analysis. We also did not detect a difference in mortality when classifying the patients as either nonobese (BMI < 30 kg/m2) or obese (BMI > 30 kg/m2). This is similar to some reports9,13 that have suggested that obesity is not associated with mortality in the injured patient. At least one other report6 demonstrated similar findings, suggesting that while a cutoff BMI of > 30 kg/m2 may be important in the chronic setting, it may not be an accurate indicator of increased risk in the critically ill patient. The graph (Fig 1) derived using restricted cubic splines suggests a higher mortality in patients with extreme BMIs (ie, > 50 kg/m2), but this is difficult to demonstrate statistically because of the small number of patients with a BMI in this range. As the prevalence of obesity has increased, hospitals and health-care providers have become more accustomed to caring for obese patients, and these findings may reflect this difference. Hospitals are more likely to have special equipment such as specialty beds and larger sized radiology and physical therapy equipment. In addition, improved management of associated chronic morbidities in the outpatient setting (eg, the aggressive use of antiplatelet agents and lipid agents, and strict glucose control) and a focus on hyperglycemia in the ICU may reduce the extra morbidity and mortality that has historically been associated with obesity.
The strengths of this study include its large size and prospective classification of pulmonary outcomes in injured patients. Despite these strengths, there are several important limitations. Although BMI correlates with chronic disease, its use in the critical care setting has been criticized. Despite these criticisms, the alternatives are limited, and BMI is correlated with more complicated measures of obesity (ie, waist circumference and electrical impedance).24,25 Another limitation is that smoking status was not measured, and this could confound the results. Finally, this study was of injured patients only, and these results cannot be generalized to other ICU populations.
Conclusion
Neither obesity nor severe obesity appears to be an independent risk factor for pulmonary complications after injury. Improvements in the care of the obese patient both in the inpatient and outpatient settings may account for this observation.
Abbreviations
- AIS
abbreviated injury score
- APACHE
acute physiology and chronic health evaluation
- BMI
body mass index
- CI
confidence interval
- IQR
interquartile range
- ISS
injury severity score
- LOS
length of stay
- TRISS
trauma-related injury severity score
Footnotes
Portions of these data were presented in poster form at the 2008 Society for Critical Care Medicine Congress, Honolulu, HI.
This work was supported by National Institutes of Health grant RO1 AI49989-01 and Agency for Healthcare Research and Quality grant T32 HS 013833.
The authors have reported to the ACCP that no significant conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/misc/reprints.shtml).
References
- 1.Kuczmarski RJ, Flegal KM, Campbell SM, et al. Increasing prevalence of overweight among US adults: the National Health and Nutrition Examination Surveys, 1960 to 1991. JAMA. 1994;272:205–211. doi: 10.1001/jama.272.3.205. [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AH, Serdula MK, Dietz WH, et al. The continuing epidemic of obesity in the United States. JAMA. 2000;284:1650–1651. doi: 10.1001/jama.284.13.1650. [DOI] [PubMed] [Google Scholar]
- 3.Calle EE, Thun MJ, Petrelli JM, et al. Body-mass index and mortality in a prospective cohort of US adults. N Engl J Med. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 4.Bercault N, Boulain T, Kuteifan K, et al. Obesity-related excess mortality rate in an adult intensive care unit: a risk-adjusted matched cohort study. Crit Care Med. 2004;32:998–1003. doi: 10.1097/01.ccm.0000119422.93413.08. [DOI] [PubMed] [Google Scholar]
- 5.Neville AL, Brown CV, Weng J, et al. Obesity is an independent risk factor of mortality in severely injured blunt trauma patients. Arch Surg. 2004;139:983–987. doi: 10.1001/archsurg.139.9.983. [DOI] [PubMed] [Google Scholar]
- 6.Byrnes MC, McDaniel MD, Moore MB, et al. The effect of obesity on outcomes among injured patients. J Trauma. 2005;58:232–237. doi: 10.1097/01.ta.0000152081.67588.10. [DOI] [PubMed] [Google Scholar]
- 7.Choban PS, Weireter LJ, Jr, Maynes C. Obesity and increased mortality in blunt trauma. J Trauma. 1991;31:1253–1257. doi: 10.1097/00005373-199109000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Bochicchio GV, Joshi M, Bochicchio K, et al. Impact of obesity in the critically ill trauma patient: a prospective study. J Am Coll Surg. 2006;203:533–538. doi: 10.1016/j.jamcollsurg.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Duane TM, Dechert T, Aboutanos MB, et al. Obesity and outcomes after blunt trauma. J Trauma. 2006;61:1218–1221. doi: 10.1097/01.ta.0000241022.43088.e1. [DOI] [PubMed] [Google Scholar]
- 10.Brown CV, Rhee P, Neville AL, et al. Obesity and traumatic brain injury. J Trauma. 2006;61:572–576. doi: 10.1097/01.ta.0000200842.19740.38. [DOI] [PubMed] [Google Scholar]
- 11.Morris JA, Jr, MacKenzie EJ, Edelstein SL. The effect of preexisting conditions on mortality in trauma patients. JAMA. 1990;263:1942–1946. [PubMed] [Google Scholar]
- 12.Milzman DP, Boulanger BR, Rodriguez A, et al. Pre-existing disease in trauma patients: a predictor of fate independent of age and injury severity score. J Trauma. 1992;32:236–243. [PubMed] [Google Scholar]
- 13.Alban RF, Lyass S, Margulies DR, et al. Obesity does not affect mortality after trauma. Am Surg. 2006;72:966–969. [PubMed] [Google Scholar]
- 14.Varon J, Marik P. Management of the obese critically ill patient. Crit Care Clin. 2001;17:187–200. doi: 10.1016/s0749-0704(05)70159-7. [DOI] [PubMed] [Google Scholar]
- 15.Adams JP, Murphy PG. Obesity in anaesthesia and intensive care. Br J Anaesth. 2000;85:91–108. doi: 10.1093/bja/85.1.91. [DOI] [PubMed] [Google Scholar]
- 16.Boulanger BR, Milzman DP, Rodriguez A. Obesity. Crit Care Clin. 1994;10:613–622. [PubMed] [Google Scholar]
- 17.Boulanger BR, Milzman D, Mitchell K, et al. Body habitus as a predictor of injury pattern after blunt trauma. J Trauma. 1992;33:228–232. doi: 10.1097/00005373-199208000-00011. [DOI] [PubMed] [Google Scholar]
- 18.May AK, Dossett LA, Norris PR, et al. Estradiol is associated with mortality in critically ill trauma and surgical patients. Crit Care Med. 2008;36:62–68. doi: 10.1097/01.CCM.0000292015.16171.6D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Institutes of Health, National Heart, Lung, and Blood Institute . The practical guide: identification, evaluation, and treatment of overweight and obesity in adults. National Institutes of Health; Rockville, MD: 2000. (NIH Publication No. 00–4084). [Google Scholar]
- 20.Baker SP, O'Neill B, Haddon W, Jr, et al. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187–196. [PubMed] [Google Scholar]
- 21.Champion HR, Sacco WJ, Carnazzo AJ, et al. Trauma score. Crit Care Med. 1981;9:672–676. doi: 10.1097/00003246-198109000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Champion HR, Sacco WJ, Copes WS, et al. A revision of the Trauma Score. J Trauma. 1989;29:623–629. doi: 10.1097/00005373-198905000-00017. [DOI] [PubMed] [Google Scholar]
- 23.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 24.Keys A, Aravanis C, Blackburn H, et al. Coronary heart disease: overweight and obesity as risk factors. Ann Intern Med. 1972;77:15–27. doi: 10.7326/0003-4819-77-1-15. [DOI] [PubMed] [Google Scholar]
- 25.Deurenberg P, Weststrate JA, Seidell JC. Body mass index as a measure of body fatness: age- and sex-specific prediction formulas. Br J Nutr. 1991;65:105–114. doi: 10.1079/bjn19910073. [DOI] [PubMed] [Google Scholar]