Abstract
Objective:
Sexual dimorphism (variation in outcome related to sex) after trauma–hemorrhage and sepsis is well documented in animals, with the pro-estrus state being proinflammatory and associated with a survival advantage. Although some observational studies confirm this pattern in humans, others demonstrate no difference in mortality. Estrogens are important modulators of the inflammatory response and insulin resistance in humans and have been linked to increased mortality during sepsis. Our objective was to determine whether sex hormone levels were associated with outcomes in critically ill surgical patients.
Design:
Prospective cohort.
Patients:
A total of 301 adult critically ill or injured surgical patients remaining in the intensive care unit for ≥48 hrs at two academic medical centers.
Interventions:
None.
Measurements:
Blood was collected 48 hrs after intensive care unit admission and assayed for sex hormones (estradiol, testosterone, prolactin, and progesterone) and cytokines (tumor necrosis factor-α and interleukin-1, -2, -4, -6, -8, and -10). Demographic and outcome data were also collected.
Main Results:
Estradiol was significantly higher in nonsurvivors (p < .001). Analysis by quartiles of estradiol demonstrated greater than a three-fold increase in the mortality rate for the highest vs. the lowest estradiol quartiles (29% vs. 8%, p < .001). Estradiol was also higher in nonsurvivors. An estradiol level of 100 pg/mL was associated with an odds ratio for death of 4.60 (95% confidence interval, 1.56–13.0) compared with a reference estradiol level of 45 pg/mL.
Conclusions:
We conclude that serum estradiol correlates with mortality in critically ill and injured surgical patients and discuss potential mechanisms for this observation.
Keywords: estradiol, mortality, trauma, critical care, sexual dimorphism, aromatase
In-hospital death after a severe traumatic, surgical, or septic insult often results from the sequential development of organ dysfunction (multiple organ dysfunction syndrome, or MODS). Although progression to MODS largely depends on the magnitude of tissue injury, considerable variation exists in an individual's susceptibility to this syndrome (1-3). Sex has been suggested as one determinant of this variability.
In animal models, the pro-estrous state is proinflammatory, with improved survival after untreated trauma–hemorrhage and sepsis, whereas the proandrogenic state leads to immune depression and decreased survival (4-10). Observational studies in humans have been less conclusive (9, 11-40). Although a number of human observational studies confirm this pattern, others have been conflicting or failed to demonstrate sex-related outcomes (9, 13, 15, 20-27, 29, 30). Men are more frequently diagnosed with sepsis and admitted to intensive care units (ICUs) (25, 41), and they develop bacteremia, healthcare-associated pneumonia, sepsis, and major postsurgical infections after trauma more frequently than women (5, 7, 8, 10, 41). Some studies, however, have found a higher incidence of infectious complications in women after both cardiac surgery and burns (9, 22, 27). Although most studies suggest an increased susceptibility to infectious complications among men, they generally demonstrate a higher mortality rate for women from infections and sepsis (6, 7, 42, 43), but again, this is not universal (4, 44, 45).
The uncertainty regarding the role of sex in influencing outcomes in humans may simply result from the clinical, phenotypic, and genetic diversity among populations and the difficulty in detecting a difference due to these confounders. Two differences between animal models and humans are of particular interest, however. First, although a robust inflammatory response conveys a survival advantage in animal models of untreated sepsis, it can lead the systemic inflammatory response syndrome and MODS in humans long after the septic insult is treated. Second, the differences in estrogen biosynthesis between humans and animal models contribute to less sex-dependent differences in endogenous estrogen levels, particularly in older patients and after stress.
Although estrogen biosynthesis in nonprimates is limited to the gonads, primates (including humans) possess peripheral aromatase activity—and the resulting ability to convert androgens to estrogens—in adipocytes, fibroblasts, and osteoblasts. This peripheral production of estrogens is under tissue-specific biosynthesis (31-33), which in contrast to gonadal estrogen biosynthesis, is stimulated by stress.
Given the properties of human peripheral aromatase under stress and the known deleterious effects of an exaggerated inflammatory response during critical illness, we hypothesized that estradiol (E2) would be increased under conditions of surgical or traumatic stress and would correlate with subsequent mortality, making it a candidate biomarker for mortality in these settings.
MATERIALS AND METHODS
All patients ≥18 yrs of age admitted to the surgical or trauma ICUs of Vanderbilt University Medical Center and the University of Virginia Health Sciences Center for ≥48 hrs were eligible for enrollment. The entry criteria of a 48-hr ICU stay was utilized to eliminate those patients with early deaths and those who were not critically ill enough to require prolonged ICU care. Sex, age, date of hospital and ICU admission, Acute Physiology and Chronic Health Evaluation (APACHE) II score (38) at ICU admission, and hospital mortality were recorded. Measures of outcome were also collected, including in-hospital mortality, ICU and hospital length of stay, MODS score (3), and ventilator days. Patient care was at the discretion of the attending physician according to established critical care protocols in the respective ICUs.
One 10-mL blood sample was collected 48 hrs after ICU admission (at the time of study entry) for analysis of hormone and cytokine levels. Plasma was separated from whole blood and stored at −70°C until analysis. Assays for 17β-estradiol (E2), progesterone, total testosterone, and prolactin were performed in the General Clinical Research Center at the University of Virginia Health Sciences Center. E2 was measured by kit enzyme immunoassay, utilizing a competitive immunoassay strategy and alkaline phosphatase/p-Npp chromogenic reaction (estradiol, 90108, Assay Designs, Ann Arbor, MI). Total testosterone, prolactin, and progesterone were measured by enzyme-linked immunosorbent assay kits and manufacturer's directions (testosterone enzyme immunoassay, 700C-081; prolactin enzyme immunoassay, 30MC-009; progesterone enzyme immunoassay, 700C-079; Alfa Scientific Designs, San Diego, CA). Samples were also assayed for interleukin (IL)-1, IL-2, IL-4, IL-6, IL-8, and IL-10 and tumor necrosis factor-α. This was accomplished by using a LINCOplex custom kit, following the manufacturer's instructions (Linco Research, St. Charles, MO). From each collection time point on all patients, 25 μL of human serum was run in duplicate on a Luminex (100) System (Miraibio, Almeda, CA) to determine the concentration of the cytokines of interest. The Luminex system is an enzyme-linked immunosorbent assay–based technology allowing multi-analyte detection in a single well. Data reduction was performed with STATLia (Brendan Scientific, Grosse Pointe, MI) and subsequently incorporated into the database.
Categorical variables were compared using Pearson's chi-square test for independence or Fisher's exact test. The Shapiro-Wilk W test was used to evaluate the distributions of all continuous variables for normality. Normally distributed continuous variables were summarized by reporting the mean and 95% confidence interval and compared using Student's t-test. Non–normally distributed continuous variables were presented by reporting the median and interquartile ranges and compared using Wilcoxon's rank-sum test. We performed a restricted cubic spline regression analysis to model the relationship between E2 and the log-odds of death. Spline covariates were derived using five knots located at the default values recommended. We then used multiple logistic regression to regress mortal outcome against these spline covariates. Stata version 8.0 (Stata Corporation, College Station, TX) was used for the entire analysis. The cubic spline covariates were derived using the rc_spline program. Tests for statistical significance were two sided, with a level of significance of .05.
The study was approved by the institutional review boards of both Vanderbilt University Medical Center and the University of Virginia Health Sciences Center. A wavier of consent was obtained from both institutional review boards for the blood draw at 48 hrs and study inclusion because the study posed minimal risk to subjects. Families were assented during the critical illness phase, and patients were consented after their critical illness resolved when possible. Patients who died or were discharged before consent being obtained remained in the study, with institutional review board approval. Patients who were able to consent, but refused, could either withdraw completely or refuse subsequent participation. All data are maintained in a secure, password-protected database that is Health Insurance Portability and Accountability Act (HIPAA) compliant, and all patient information is de-identified before analysis and reporting.
RESULTS
A total of 301 critically ill patients were enrolled from November 2001 to September 2003. Consent or assent was subsequently obtained from 141 patients. Before consent could be obtained, 156 patients either died (34 patients) or were discharged (122 patients). Three patients refused consent and were withdrawn completely. One patient refused consent for future data collection but allowed the use of already existing blood samples and clinical data.
There were 192 male patients (63.8%) and 109 female patients (36.2%). The mean age was 55.1 yrs (95% confidence interval, 47.5–62.8 yrs), the mean APACHE II score was 18.0 (95% confidence interval, 17.3–18.8), 61% were traumatically injured, and 45 patients died, for an overall mortality rate of 15.0%. Demographic and outcome variables by sex are shown in Table 1. Male and female patients were similar with respect to age and APACHE II scores, but as expected, a higher portion of men were trauma victims (68.2% vs. 49.5% of women). The unadjusted mortality rates for men and women was not statistically different (17.2% vs. 11.0%, p = .15). E2 and prolactin were significantly higher in critically ill women (p < .01 and p < .01, respectively), but no differences were demonstrated in progesterone or testosterone levels between sexes. No differences were observed in serum cytokines between sexes.
Table 1.
Demographics and clinical characteristics by sex
| Male (n = 192) | Female (n = 109) | p Value | |
|---|---|---|---|
| Age, yrsa | 47.3 (44.6–49.9) | 49.3 (45.7–52.8) | .37 |
| APACHE IIa | 18.0 (17.0–18.9) | 18.2 (17.0–19.4) | .80 |
| Trauma victims, n (%)b | 131 (68.2) | 54 (49.5) | <.01 |
| Deaths, n (%)b | 33 (17.2) | 12 (11.0) | .15 |
| BMIc | 26.9 (24–31.1) | 28.3 (22.7–34.6) | .56 |
| Estradiol, pg/mLc | 29.2 (10.0–54.6) | 49.2 (16.3–97.1) | <.01 |
| Progesterone, ng/mLc | 0.64 (0.44–0.94) | 0.66 (0.42–1.14) | .37 |
| Prolactin, ng/mLc | 13.3 (9.6–19.1) | 18.4 (10.8–33.4) | <.01 |
| Testosterone, ng/mLc | 36 (24–59) | 41.0 (17.8–50.0) | .08 |
| IL-1c | 18.6 (2.9–34.4) | 22.6 (3.6–38) | .43 |
| IL-2c | 3.7 (2.7–12.5) | 2.9 (2.7–9.3) | .33 |
| IL-4c | 32.8 (14.1–91) | 35.5 (10–112.2) | .82 |
| IL-6c | 152.9 (65.6–304.7) | 192.7 (68.7–406.7) | .52 |
| IL-8c | 35.3 (14.6–60.5) | 39.5 (16.1–78) | .24 |
| IL-10c | 36.3 (22.1–96.7) | 51.6 (20–101.3) | .51 |
| TNF-αc | 8.9 (5.1–14.3) | 7.7 (2.9–14.4) | .38 |
APACHE II, Acute Physiology and Chronic Health Evaluation II; BMI, body mass index; IL, interleukin; TNF, tumor necrosis factor.
Mean (95% confidence interval), compared by Student's t-test
proportion, compared by Pearson's chi-square test
median (interquartile range), compared using Wilcoxon's rank-sum test. A p value of <.05 is considered statistically significant.
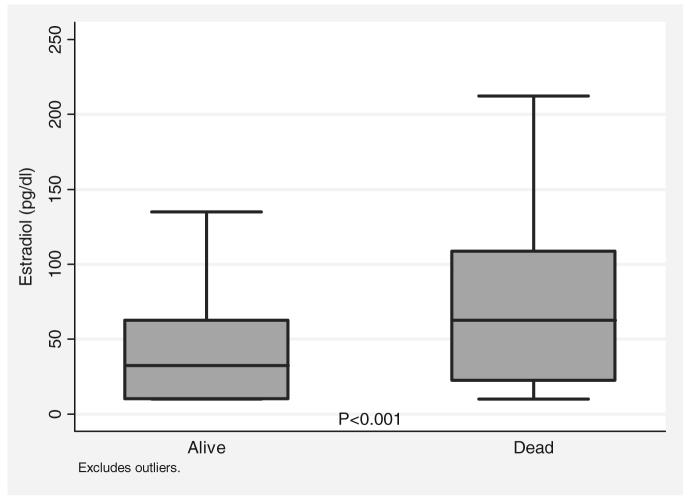
Demographic and clinical characteristics by outcome are shown in Table 2. As expected, age (p < .001) and APACHE II (p < .001) were higher for nonsurvivors. Body mass index was not associated with mortality (p = .21). Trauma victims had lower mortality rates than patients who were critically ill after other surgical illnesses (11% vs. 21%, p < .01). The mortality rate for patients <40 vs. those >65 yrs of age increased from 6% to 20% (p = .001). Likewise, the mortality rate for patients with APACHE II scores above the mean was 22%, and the mortality rate for patients with scores below the mean was 8% (p = .001). Of the sex hormones, only E2 was significantly higher in nonsurvivors, with the median E2 for nonsurvivors twice the median E2 for survivors (66.9 pg/mL vs. 32.4 pg/mL, p < .001) (Fig. 1). As expected, serum cytokines (IL-1, IL-2, IL-4, IL-6, IL-8, IL-10, and tumor necrosis factor-α) were higher in nonsurvivors vs. survivors, with most of these differences reaching statistical significance.
Table 2.
Demographics and clinical characteristics by outcome
| Survivors (n = 256) | Nonsurvivors (n = 45) | p Value | |
|---|---|---|---|
| Age, yrsa | 46.2 (44.0–48.5) | 57.9 (52.7–63.1) | <.001 |
| APACHE IIa | 17.3 (16.5–18.0) | 22.5 (20.0–25.0) | <.001 |
| Trauma victims, n (%)b | 164 (64.1) | 21 (46.7) | .03 |
| Men, n (%)b | 159 (62.1) | 33 (73.3) | .15 |
| BMIc | 27.0 (23.6–31.3) | 28.8 (24.3–33.1) | .21 |
| Estradiol, pg/mLc | 32.4 (10.1–62.7) | 66.9 (21.4–115.3) | <.001 |
| Progesterone, ng/mLc | 0.65 (0.43–0.94) | 0.66 (0.48–1.03) | .11 |
| Prolactin, ng/mLc | 14.6 (10.1–23.1) | 18.4 (8.5–34.7) | .14 |
| Testosterone, ng/mLc | 35.0 (20.3–57.0) | 41.0 (26.8–58.0) | .29 |
| IL-1c | 19.3 (3.5–34.5) | 27 (2.8–43.3) | .29 |
| IL-2c | 2.7 (2.7–8.6) | 4.2 (2.7–12.2) | .21 |
| IL-4c | 31.4 (13.1–87.6) | 66.7 (26.3–176.1) | .04 |
| IL-6c | 162.9 (67.4–398.4) | 247.4 (76.9–566.3) | .20 |
| IL-8c | 36.1 (14.4–58.0) | 60 (334.1–108.1) | <.01 |
| IL-10c | 33.4 (19.9–67.7) | 69 (230.4–110.4) | .05 |
| TNF-αc | 8.3 (3.8–13.4) | 14 (65.3–24.6) | .02 |
APACHE II, Acute Physiology and Chronic Health Evaluation II; BMI, body mass index; IL, interleukin; TNF, tumor necrosis factor.
Mean (95% confidence interval), compared by Student's t-test
proportion, compared by Pearson's chi-square test
median (interquartile range), compared using Wilcoxon's rank-sum test. A p value of <.05 is considered statistically significant.
Figure 1.
Median estradiol level by outcome in critically ill and injured patients. The median estradiol level for survivors was 32.4 pg/mL, whereas the median for nonsurvivors was 66.9 pg/mL (p < .001 by Wilcoxon's rank-sum test).
Given the highly significant association between E2 and mortality, we focused additional analysis on this relationship. Mortality for the highest quartile of E2 levels is greater than three times that of the lowest quartile (28% vs. 8%, p = .001). Median E2 levels for survivors and nonsurvivors in both men and women, in several age categories, and in patients above and below the mean APACHE II score, are presented in Table 3. In all subgroups, except that for patients aged <40 yrs, E2 for nonsurvivors is roughly twice that of survivors, values that reach significance for men, age >40 yrs, and APACHE II scores of ≥18. Other outcome variables were also analyzed with respect to median E2 values. For those patients with E2 values below the median as compared with those above the median, MODS score (4 vs. 6, p < .001) and ICU length of stay (7 vs. 10 days, p = .002) were significantly less and the ventilator-free days at day 14 significantly greater (4 vs. 3, p = .04).
Table 3.
Estradiol level (picograms per milliliter) by outcome for various subgroups
| Survivors (n = 256) | Nonsurvivors (n = 45) | p Value | |
|---|---|---|---|
| Men | 27.0 (9.99–48.2) | 59.2 (22.2–114.3) | <.001 |
| Women | 44.6 (16.4–87.5) | 76.4 (21.6–105.2) | .28 |
| Age <40 yrs | 26.5 (9.99–50.1) | 19.8 (9.99–62.7) | .76 |
| Age 40–65 yrs | 33.8 (9.99–65.2) | 67.7 (20.6–122.1) | .01 |
| Age >65 yrs | 39.3 (15.0–72.7) | 75.1 (29.2–118.3) | <.01 |
| APACHE II <18 | 26.1 (9.99–50.7) | 29.3 (22.2–60.9) | .24 |
| APACHE II ≥ 18 | 40.7 (13.4–74.4) | 76.4 (23.9–124.1) | <.01 |
APACHE II, Acute Physiology and Chronic Health Evaluation II.
Data provided as median (interquartile range); compared using Wilcoxon's rank-sum test; p values of <.05 are considered statistically significant.
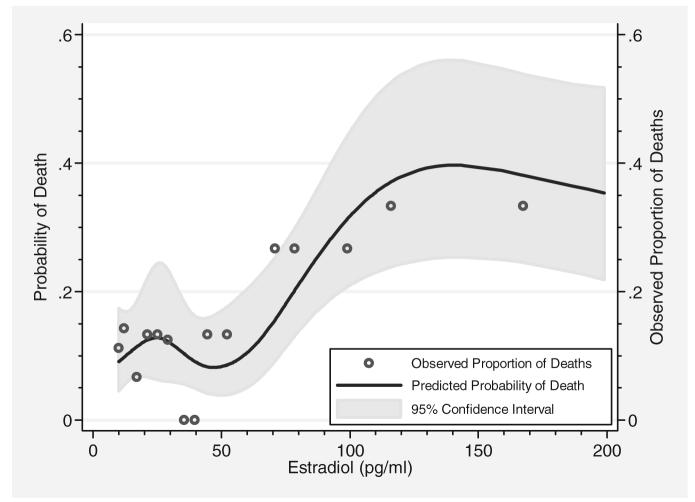
To further evaluate the relationship of E2 to mortality, logistic regression modeling was performed. A restricted cubic spline analysis yielded a better model (p = .03); we therefore rejected the model for simple logistic regression. As shown in Figure 2, the predicted probability of death is lowest within the physiologic range of E2 but increases sharply outside of this range. The resulting logistic regression model using the spline covariates of E2 yielded an odds ratio for mortality of 4.60 (95% confidence interval, 1.56–13.0) associated with an E2 level of 100 pg/mL (compared with a reference value of 45 pg/mL). The predictive value of these logistic regression models (as determined by the area under the receiver operating characteristic curve) was compared with the predictive value of several of the variables individually, and these values are presented in Table 4. APACHE II was the best single-variable predictor of mortality (area under the curve, 0.70) and comparable with the restricted cubic spline model of E2 (0.70). IL-8 (area under the curve, 0.68) was the best cytokine predictor. The best overall predictor of mortality was a multivariate regression model combining E2, age, sex, and APACHE II (area under the curve, 0.79).
Figure 2.
Relationship between mortality and increasing serum estradiol levels, adjusted for age and Acute Physiology and Chronic Health Evaluation II. Regression model using restricted cubic spline analysis demonstrating probability of death with increasing serum estradiol levels. Although estradiol seems to be protective within the physiologic range (<45 pg/mL), the probability of death increases sharply with rising levels.
Table 4.
Area under the receiver operating characteristic curve (ROC) for selected individual variables and multivariate logistic regression models
| Variable | Area Under ROC |
|---|---|
| APACHE II | 0.70 |
| E2 | 0.70 |
| Age | 0.68 |
| IL-8 | 0.68 |
| E2 + age + sex + APACHE II | 0.79 |
| E2 + APACHE II + age | 0.78 |
| E2 + APACHE II | 0.75 |
| Sex + APACHE II + age | 0.75 |
| Age + sex + E2 | 0.75 |
| APACHE II + age | 0.74 |
| E2 + age | 0.74 |
| Sex + APACHE II | 0.72 |
| Sex + age | 0.70 |
APACHE II, Acute Physiology and Chronic Health Evaluation II; E2, estradiol; IL-8, interleukin-8.
DISCUSSION
Sex is hypothesized to be a determinant of the immunologic variability after severe traumatic and surgical stress and, at least in animal models, accounts for differences in outcomes (4, 5, 7, 10, 39, 40). Within our cohort of critically ill surgical and trauma patients, we did not detect a difference in unadjusted mortality related to sex, although the study population is small and not powered to detect this difference.
Despite no difference in outcome related to sex, serum E2 was independently associated with death when controlling for age, sex, and body mass index. The median E2 level for survivors was 32.4 pg/mL, which falls within the normal range of E2 for both men and postmenopausal women (<45 pg/mL), whereas the median E2 level for nonsurvivors was almost twice that of the survivors (66.0 pg/mL). In nearly all subgroups, median E2 values for nonsurvivors were roughly twice the median E2 values for survivors. The mortality rate for patients with E2 levels in the highest quartile was three times the mortality rate of those in the lowest quartile. The nonlinear relationship demonstrated by the restricted cubic spline analysis further supports the notion that although estrogens may be beneficial within the normal physiologic range, markedly elevated levels can be detrimental. There is physiologic basis for this observation, with a similar relationship being observed between estrogens and insulin resistance. Postmenopausal women (very low estrogens) develop insulin resistance, which improves with hormone replacement therapy (“normal” levels), but the excess estrogen associated with pregnancy also induces insulin resistance. In many basic science models of the relationship between E2 and insulin resistance, this same dose-dependent relationship is observed.
The source of elevated serum estrogens in critically ill patients is unknown but is thought to be related to increased peripheral conversion of androgens to estrogens via aromatase activity (31-34, 46, 47). These peripheral sites of aromatization account for the majority of estrogens in men and postmenopausal women (31) and may be an important source of estrogens during critical illness. A recent study of elective cardiac surgery patients demonstrated that the elevation in estrogens present postoperatively was due to increased peripheral aromatase activity and not related to reduced clearance (46). A nonspecific increase in the production of all sex hormones via the hypothalamic-pituitary axis seems unlikely because luteinizing hormone and follicle-stimulating hormone levels are suppressed in critically ill patients and other precursor hormones seem to be low (36). Although few data are available regarding estrogen metabolism during critical illness, the predominant pathways of estrogen clearance seem to be maintained unless severe hepatic failure is present (39).
Unlike gonadal or brain aromatase, the peripheral aromatase promoter is specifically stimulated by tumor necrosis factor-α and class 1 cytokines in the presence of glucocorticoids, thus increasing estrogen synthesis in settings of stress (34, 35). In light of the known immunomodulating properties of E2, this non–sex-dependent increased conversion could contribute to the divergence of results obtained between animal models and human observational studies. In addition, this peripheral aromatization, as the main source of estrogens in men and postmenopausal women, provides the rationale for our finding that elevated serum E2 was associated with both age and body mass index. An important feature of aromatase expression, specifically in adipose tissue, is that it increases with advancing age (31). One possible explanation is that cytokines such as IL-6 are elevated in older patients, at least in serum (48). Likewise, aromatase activity is known to increase with increasing adiposity, also likely due to increased levels of cytokines (31). In our cohort, body mass index was not associated with mortality, although the study is likely under-powered to detect a difference in outcome.
Despite the known link between cytokines and aromatase expression, we did not demonstrate markedly elevated levels of serum cytokines that correlated with E2 levels. One possible explanation, and a limitation of this study, is the single value of sex hormones and serum cytokines. Because blood samples were drawn 48 hrs after ICU admission, they are unlikely to have been timed with peak cytokine values. It is impossible to know the trend of cytokines or hormones in these patients without serial samples. In addition, serum levels of these hormones and cytokines will not fully reveal their potential interactions and effects. As opposed to an endocrine system, in which hormones must be released into the circulation to act on a distant target organ, E2 can work in a paracrine, autocrine, and even an intracrine fashion (exerting an effect within the cell of its synthesis). As in the postmenopausal breast cancer model, serum estrogens may not reflect the intracellular and interstitial concentrations of these hormones. Because hormones working within a local system are not metabolized before they can exert their effect, only tiny concentrations are required to generate maximal effects. To fully understand the role of estrogens during critical illness, further studies must be carried out at the tissue and cellular level.
Whether E2 contributes physiologically to adverse outcomes in critically ill patients or simply serves as a biomarker for inflammation is unknown. Some data do support a pathophysiologic role in adverse outcomes. E2 is a well-documented immunomodulator (39, 40, 49-54), altering monocyte and macrophage function and life span (54), cytokine and chemokine production (50, 55), myeloperoxidase production, and apoptosis of various populations of macrophages (5, 40, 51, 56). E2 also reduces the expression of E-selectin by endothelial cells and both basal- and cytokine-induced monocyte adhesion to endothelial cells (52, 57). In addition, E2 modulates lymphocyte and monocyte numbers, increases B-cell differentiation, and decreases CD8+ (suppressor/cytotoxic) cells in the spleen, thymus, and lymph nodes (58-60). All of these effects may lead to an exaggerated inflammatory response and, ultimately, MODS and death.
In addition to its inflammatory properties, E2 has also been shown to induce and activate nitric oxide synthase in endothelial cells, which may contribute to low peripheral resistance in septic shock (61, 62). As already mentioned, estrogens are also known to modulate insulin resistance, an important contributor to outcomes during critical illness. Perhaps the best evidence of the pathophysiologic role of E2 in outcomes is that high doses of E2 pretreatment in mice increases mortality from subsequent lethal challenge with lipopolysaccharide (63) and that aromatase inhibitors decrease subsequent mortality from endotoxin in animals (6, 64).
This preliminary analysis has several limitations. Currently, this study is underpowered to allow appropriate analysis controlling for confounding variables or to perform subgroup analysis. Further subgroup analysis, including serial hormone and cytokine values, awaits accrual of an adequate numbers of patients. As previously mentioned, we report only a single value of E2 and have not examined either timing of E2 elevation or trends in level. In addition, the single analysis of serum cytokines and the timing of serum collection limits the ability to determine the relationship between cytokines and E2.
CONCLUSION
E2 is associated with illness severity and mortality in critically ill and injured surgical patients. Taken together with the recent finding that E2 correlates with mortality in critically ill medical patients with primary infection (11), these data make a case for further investigations into the role of E2 in critical illness. Further research is needed to define the relationship between E2, inflammation, and mortality and to determine the variables that contribute to enhanced estrogen biosynthesis in the critically ill patient. Ultimately, E2 may prove to be a useful predictor of mortality during critical illness or a therapeutic target.
Acknowledgments
Supported, in part, by grant RO1 AI49989-01 from the National Institutes of Health (Clinical Trials.gov identifier, NCT00170560).
Footnotes
The authors have not disclosed any potential conflicts of interest.
This work was performed at Vanderbilt University Medical Center, Nashville, TN, and the Hospitals of the University of Virginia Health System, Charlottesville, VA. All authors took part in the interpretation of the findings and contributed to the preparation of the manuscript.
REFERENCES
- 1.Awad SS. State-of-the-art therapy for severe sepsis and multisystem organ dysfunction. Am J Surg. 2003;186:23S–30S. doi: 10.1016/j.amjsurg.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Lee CC, Marill KA, Carter WA, et al. A current concept of trauma-induced multiorgan failure. Ann Emerg Med. 2001;38:170–176. doi: 10.1067/mem.2001.114313. [DOI] [PubMed] [Google Scholar]
- 3.Marshall JC, Cook DJ, Christou NV, et al. Multiple organ dysfunction score: A reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Angele MK, Wichmann MW, Ayala A, et al. Testosterone receptor blockade after hemorrhage in males: Restoration of the depressed immune functions and improved survival following subsequent sepsis. Arch Surg. 1997;132:1207–1214. doi: 10.1001/archsurg.1997.01430350057010. [DOI] [PubMed] [Google Scholar]
- 5.Knoferl MW, Angele MK, Diodato MD, et al. Female sex hormones regulate macrophage function after trauma-hemorrhage and prevent increased death rate from subsequent sepsis. Ann Surg. 2002;235:105–112. doi: 10.1097/00000658-200201000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider CP, Nickel EA, Samy TS, et al. The aromatase inhibitor, 4-hydroxyandrostenedione, restores immune responses following trauma-hemorrhage in males and decreases mortality from subsequent sepsis. Shock. 2000;14:347–353. doi: 10.1097/00024382-200014030-00019. [DOI] [PubMed] [Google Scholar]
- 7.Wichmann MW, Zellweger R, DeMaso CM, et al. Mechanism of immunosuppression in males following trauma-hemorrhage: Critical role of testosterone. Arch Surg. 1996;131:1186–1191. doi: 10.1001/archsurg.1996.01430230068012. [DOI] [PubMed] [Google Scholar]
- 8.Wichmann MW, Angele MK, Ayala A, et al. Flutamide: A novel agent for restoring the depressed cell-mediated immunity following soft-tissue trauma and hemorrhagic shock. Shock. 1997;8:242–248. [PubMed] [Google Scholar]
- 9.Wichmann MW, Inthorn D, Andress HJ, et al. Incidence and mortality of severe sepsis in surgical intensive care patients: The influence of patient gender on disease process and outcome. Intensive Care Med. 2000;26:167–172. doi: 10.1007/s001340050041. [DOI] [PubMed] [Google Scholar]
- 10.Zellweger R, Wichmann MW, Ayala A, et al. Females in proestrus state maintain splenic immune functions and tolerate sepsis better than males. Crit Care Med. 1997;25:106–110. doi: 10.1097/00003246-199701000-00021. [DOI] [PubMed] [Google Scholar]
- 11.Angstwurm MW, Gaertner R, Schopohl J. Outcome in elderly patients with severe infection is influenced by sex hormones but not gender. Crit Care Med. 2005;33:2786–2793. doi: 10.1097/01.ccm.0000190242.24410.17. [DOI] [PubMed] [Google Scholar]
- 12.Bowles BJ, Roth B, Demetriades D. Sexual dimorphism in trauma? A retrospective evaluation of outcome. Injury. 2003;34:27–31. doi: 10.1016/s0020-1383(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 13.Crabtree TD, Pelletier SJ, Gleason TG, et al. Gender-dependent differences in outcome after the treatment of infection in hospitalized patients. JAMA. 1999;282:2143–2148. doi: 10.1001/jama.282.22.2143. [DOI] [PubMed] [Google Scholar]
- 14.Croce MA, Fabian TC, Malhotra AK, et al. Does gender difference influence outcome? J Trauma. 2002;53:889–894. doi: 10.1097/00005373-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Eachempati SR, Hydo L, Barie PS. Gender-based differences in outcome in patients with sepsis. Arch Surg. 1999;134:1342–1347. doi: 10.1001/archsurg.134.12.1342. [DOI] [PubMed] [Google Scholar]
- 16.Gannon CJ, Pasquale M, Tracy JK, et al. Male gender is associated with increased risk for postinjury pneumonia. Shock. 2004;21:410–414. doi: 10.1097/00024382-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 17.George RL, McGwin G, Jr, Metzger J, et al. The association between gender and mortality among trauma patients as modified by age. J Trauma. 2003;54:464–471. doi: 10.1097/01.TA.0000051939.95039.E6. [DOI] [PubMed] [Google Scholar]
- 18.George RL, McGwin G, Jr, Windham ST, et al. Age-related gender differential in outcome after blunt or penetrating trauma. Shock. 2003;19:28–32. doi: 10.1097/00024382-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 19.George RL, McGwin G, Jr, Schwacha MG, et al. The association between sex and mortality among burn patients as modified by age. J Burn Care Rehabil. 2005;26:416–421. doi: 10.1097/01.bcr.0000176888.44949.87. [DOI] [PubMed] [Google Scholar]
- 20.Kollef MH, Sharpless L, Vlasnik J, et al. The impact of nosocomial infections on patient outcomes following cardiac surgery. Chest. 1997;112:666–675. doi: 10.1378/chest.112.3.666. [DOI] [PubMed] [Google Scholar]
- 21.Morris JA, Jr, MacKenzie EJ, Damiano AM, et al. Mortality in trauma patients: The interaction between host factors and severity. J Trauma. 1990;30:1476–1482. [PubMed] [Google Scholar]
- 22.Napolitano LM, Greco ME, Rodriguez A, et al. Gender differences in adverse outcomes after blunt trauma. J Trauma. 2001;50:274–280. doi: 10.1097/00005373-200102000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Oberholzer A, Keel M, Zellweger R, et al. Incidence of septic complications and multiple organ failure in severely injured patients is sex specific. J Trauma. 2000;48:932–937. doi: 10.1097/00005373-200005000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Offner PJ, Moore EE, Biffl WL. Male gender is a risk factor for major infections after surgery. Arch Surg. 1999;134:935–938. doi: 10.1001/archsurg.134.9.935. [DOI] [PubMed] [Google Scholar]
- 25.Rello J, Ollendorf DA, Oster G, et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122:2115–2121. doi: 10.1378/chest.122.6.2115. [DOI] [PubMed] [Google Scholar]
- 26.Romo H, Amaral AC, Vincent JL. Effect of patient sex on intensive care unit survival. Arch Intern Med. 2004;164:61–65. doi: 10.1001/archinte.164.1.61. [DOI] [PubMed] [Google Scholar]
- 27.Schroder J, Kahlke V, Staubach KH, et al. Gender differences in human sepsis. Arch Surg. 1998;133:1200–1205. doi: 10.1001/archsurg.133.11.1200. [DOI] [PubMed] [Google Scholar]
- 28.Valentin A, Jordan B, Lang T, et al. Gender-related differences in intensive care: A multiple-center cohort study of therapeutic interventions and outcome in critically ill patients. Crit Care Med. 2003;31:1901–1907. doi: 10.1097/01.CCM.0000069347.78151.50. [DOI] [PubMed] [Google Scholar]
- 29.Vuorisalo S, Haukipuro K, Pokela R, et al. Risk features for surgical-site infections in coronary artery bypass surgery. Infect Control Hosp Epidemiol. 1998;19:240–247. doi: 10.1086/647802. [DOI] [PubMed] [Google Scholar]
- 30.Wisplinghoff H, Perbix W, Seifert H. Risk factors for nosocomial bloodstream infections due to Acinetobacter baumannii: A case-control study of adult burn patients. Clin Infect Dis. 1999;28:59–66. doi: 10.1086/515067. [DOI] [PubMed] [Google Scholar]
- 31.Simpson ER, Merrill JC, Hollub AJ, et al. Regulation of estrogen biosynthesis by human adipose cells. Endocr Rev. 1989;10:136–148. doi: 10.1210/edrv-10-2-136. [DOI] [PubMed] [Google Scholar]
- 32.Simpson ER, Mahendroo MS, Means GD, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev. 1994;15:342–355. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- 33.Simpson ER. Aromatase: Biologic relevance of tissue-specific expression. Semin Reprod Med. 2004;22:11–23. doi: 10.1055/s-2004-823023. [DOI] [PubMed] [Google Scholar]
- 34.Simpson ER, Ackerman GE, Smith ME, et al. Estrogen formation in stromal cells of adipose tissue of women: Induction by glucocorticosteroids. Proc Nat Acad Sci U S A. 1981;78:5690–5694. doi: 10.1073/pnas.78.9.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao Y, Mendelson CR, Simpson ER. Characterization of the sequences of the human CYP19 (aromatase) gene that mediate regulation by glucocorticoids in adipose stromal cells and fetal hepatocytes. Mol Endocrinol. 1995;9:340–349. doi: 10.1210/mend.9.3.7776980. [DOI] [PubMed] [Google Scholar]
- 36.Christeff N, Benassayag C, Carli-Vielle C, et al. Elevated oestrogen and reduced testosterone levels in the serum of male septic shock patients. J Steroid Biochem. 1988;29:435–440. doi: 10.1016/0022-4731(88)90254-3. [DOI] [PubMed] [Google Scholar]
- 37.Christeff N, Carli A, Benassayag C, et al. Relationship between changes in serum estrone levels and outcome in human males with septic shock. Circ Shock. 1992;36:249–255. [PubMed] [Google Scholar]
- 38.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 39.Homo-Delarche F, Fitzpatrick F, Christeff N, et al. Sex steroids, glucocorticoids, stress and autoimmunity. J Steroid Biochem Mol Biol. 1991;40:619–637. doi: 10.1016/0960-0760(91)90285-d. [DOI] [PubMed] [Google Scholar]
- 40.Yokoyama Y, Kuebler JF, Matsutani T, et al. Mechanism of the salutary effects of 17beta-estradiol following trauma-hemorrhage: Direct downregulation of Kupffer cell proinflammatory cytokine production. Cytokine. 2003;21:91–97. doi: 10.1016/s1043-4666(03)00014-0. [DOI] [PubMed] [Google Scholar]
- 41.Fourrier F, Jallot A, Leclerc L, et al. Sex steroid hormones in circulatory shock, sepsis syndrome, and septic shock. Circ Shock. 1994;43:171–178. [PubMed] [Google Scholar]
- 42.Lephart ED, Baxter CR, Parker CR., Jr Effect of burn trauma on adrenal and testicular steroid hormone production. J Clin Endocrinol Metab. 1987;64:842–848. doi: 10.1210/jcem-64-4-842. [DOI] [PubMed] [Google Scholar]
- 43.Majetschak M, Christensen B, Obertacke U, et al. Sex differences in posttraumatic cytokine release of endotoxin-stimulated whole blood: Relationship to the development of severe sepsis. J Trauma. 2000;48:832–839. doi: 10.1097/00005373-200005000-00006. [DOI] [PubMed] [Google Scholar]
- 44.Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 45.Schroder J, Kahlke V, Book M, et al. Gender differences in sepsis: Genetically determined? Shock. 2000;14:307–310. [PubMed] [Google Scholar]
- 46.Spratt DI, Morton JR, Kramer RS, et al. Increases in serum estrogen levels during major illness are caused by increased peripheral aromatization. Am J Physiol Endocrinol Metab. 2006;291:E631–E638. doi: 10.1152/ajpendo.00467.2005. [DOI] [PubMed] [Google Scholar]
- 47.Brueggemeier RW, Hackett JC, Diaz-Cruz ES. Aromatase inhibitors in the treatment of breast cancer. Endocr Rev. 2005;26:331–345. doi: 10.1210/er.2004-0015. [DOI] [PubMed] [Google Scholar]
- 48.Wei J, Xu H, Davies JL, et al. Increase of plasma IL-6 concentration with age in healthy subjects. Life Sci. 1992;51:1953–1956. doi: 10.1016/0024-3205(92)90112-3. [DOI] [PubMed] [Google Scholar]
- 49.Ansar AS, Penhale WJ, Talal N. Sex hormones, immune responses, and autoimmune diseases: Mechanisms of sex hormone action. Am J Pathol. 1985;121:531–551. [PMC free article] [PubMed] [Google Scholar]
- 50.Bengtsson AK, Ryan EJ, Giordano D, et al. 17beta-Estradiol (E2) modulates cytokine and chemokine expression in human monocyte-derived dendritic cells. Blood. 2004;104:1404–1410. doi: 10.1182/blood-2003-10-3380. [DOI] [PubMed] [Google Scholar]
- 51.Kramer PR, Kramer SF, Guan G. 17 beta-Estradiol regulates cytokine release through modulation of CD16 expression in monocytes and monocyte-derived macrophages. Arthritis Rheum. 2004;50:1967–1975. doi: 10.1002/art.20309. [DOI] [PubMed] [Google Scholar]
- 52.Mikkola TS, Clair RW. Estradiol reduces basal and cytokine induced monocyte adhesion to endothelial cells. Maturitas. 2002;41:313–319. doi: 10.1016/s0378-5122(01)00301-2. [DOI] [PubMed] [Google Scholar]
- 53.Mor G, Sapi E, Abrahams VM, et al. Interaction of the estrogen receptors with the Fas ligand promoter in human monocytes. J Immunol. 2003;170:114–122. doi: 10.4049/jimmunol.170.1.114. [DOI] [PubMed] [Google Scholar]
- 54.Vegeto E, Belcredito S, Etteri S, et al. Estrogen receptor-alpha mediates the brain anti-inflammatory activity of estradiol. Proc Nat Acad Sci U S A. 2003;100:9614–9619. doi: 10.1073/pnas.1531957100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Polan ML, Daniele A, Kuo A. Gonadal steroids modulate human monocyte interleukin-1 (IL-1) activity. Fertil Steril. 1988;49:964–968. [PubMed] [Google Scholar]
- 56.Matalka KZ. The effect of estradiol, but not progesterone, on the production of cytokines in stimulated whole blood, is concentration-dependent. Neuro Endocrinol Lett. 2003;24:185–191. [PubMed] [Google Scholar]
- 57.Tyree CM, Zou A, Allegretto EA. 17beta-Estradiol inhibits cytokine induction of the human E-selectin promoter. J Steroid Biochem Mol Biol. 2002;80:291–297. doi: 10.1016/s0960-0760(02)00022-5. [DOI] [PubMed] [Google Scholar]
- 58.Novotny EA, Raveche ES, Sharrow S, et al. Analysis of thymocyte subpopulations following treatment with sex hormones. Clin Immunol Immunopathol. 1983;28:205–217. doi: 10.1016/0090-1229(83)90155-1. [DOI] [PubMed] [Google Scholar]
- 59.Paavonen T, Andersson LC, Adlercreutz H. Sex hormone regulation of in vitro immune response: Estradiol enhances human B cell maturation via inhibition of suppressor T cells in pokeweed mitogen-stimulated cultures. J Exp Med. 1981;154:1935–1945. doi: 10.1084/jem.154.6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Screpanti I, Morrone S, Meco D, et al. Steroid sensitivity of thymocyte subpopulations during intrathymic differentiation: Effects of 17 beta-estradiol and dexamethasone on subsets expressing T cell antigen receptor or IL-2 receptor. J Immunol. 1989;142:3378–3383. [PubMed] [Google Scholar]
- 61.Florian M, Lu Y, Angle M, et al. Estrogen induced changes in Akt-dependent activation of endothelial nitric oxide synthase and vasodilation. Steroids. 2004;69:637–645. doi: 10.1016/j.steroids.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 62.Klinge CM, Blankenship KA, Risinger KE, et al. Resveratrol and estradiol rapidly activate MAPK signaling through estrogen receptors alpha and beta in endothelial cells. J Biol Chem. 2005;280:7460–7468. doi: 10.1074/jbc.M411565200. [DOI] [PubMed] [Google Scholar]
- 63.Christeff N, Auclair MC, Thobie N, et al. Effect of estradiol on endotoxin-induced changes in steroid hormone levels and lethality in male rats. Circ Shock. 1994;44:154–159. [PubMed] [Google Scholar]
- 64.Christeff N, Auclair MC, Dehennin L, et al. Effect of the aromatase inhibitor, 4 hydroxyandrostenedione, on the endotoxin-induced changes in steroid hormones in male rats. Life Sci. 1992;50:1459–1468. doi: 10.1016/0024-3205(92)90265-q. [DOI] [PubMed] [Google Scholar]