Summary
A vaginal microbicide should prevent pathogen transmission without disrupting tissue barriers to infection. Ideally it would not need to be applied immediately before sexual intercourse, when compliance is a problem. Intravaginal administration of small interfering RNA (siRNA) lipoplexes targeting Herpes Simplex Virus Type 2 (HSV-2) genes protects mice from HSV-2. However, protection is short-lived and the transfection lipid on its own unacceptably enhances transmission. Here we show that cholesterol-conjugated (chol)-siRNAs without lipid silence gene expression in the vagina without causing inflammation or inducing interferons. A viral siRNA prevents transmission within a day of challenge, whereas an siRNA targeting nectin-1, an HSV-2 receptor, protects for a week, but protection is delayed for a few days until the receptor is down-modulated. Combining siRNAs targeting a viral and host gene protects mice from HSV-2 for a week, irrespective of the time of challenge. Therefore, intravaginal siRNAs could provide sustained protection against viral transmission.
Introduction
Genital Herpes Simplex Virus 2 (HSV-2) infection causes significant morbidity (Whitley, 2001) and is an important cofactor for HIV-1 infection (Wald et al., 2002, Serwadda et al. 2003, Corey et al., 2004). A vaginal microbicide able to protect against HSV-2 transmission could contribute significantly to controlling sexually transmitted diseases. Intravaginal (ivag) administration of small interfering RNAs (siRNA) targeting two HSV-2 genes (UL27 and UL29) when mixed with the transfection lipid Oligofectamine (OF) protected mice against ivag challenge with 2LD50 of HSV-2 (Palliser et al., 2006). Topical administration was well tolerated with no signs of inflammation or induction of interferon response genes. siRNAs were effective when administered around the time of viral challenge or within a few hours after exposure. Effective siRNA delivery to the cervicovaginal and anal epithelium using lipoplexed siRNAs was also shown by Ramratnam and colleagues (Zhang et al. 2006).
One of the major obstacles to developing an effective topical agent is compliance, since most topical agents being considered need to be applied just before sexual intercourse. We previously found that gene silencing is sustained for several weeks in vivo in slowly dividing cells, including the genital tract of progesterone-treated mice. This suggested that an siRNA-based vaginal microbicide might be able to create a sustained antiviral milieu in the genital tract that could circumvent the need for application around the time of exposure. However, when we lengthened the time between viral challenge and siRNA application, mice treated with lipoplexed viral siRNAs against HSV-2 were no longer protected. This prompted us to explore ways of improving and prolonging the antiviral effect.
In this study we looked more closely at the durability of protection and drug features of lipoplexed anti-herpes siRNAs. We found several undesirable features: the transfection lipid on its own enhanced viral infection, limiting the maximal effective drug dose, and protection did not last for more than a day. To bypass these problems we investigated topical administration of stabilized, cholesterol-conjugated siRNAs (chol-siRNA). Intravenously administered chol-siRNAs are taken up by liver and jejeunal cells and silence apolipoprotein B gene expression to reduce cholesterol levels in mice and monkeys (Soutschek et al. 2004, Zimmermann et al. 2006). However, the dose required for efficient silencing after systemic administration (~50 mg/kg) is generally considered too high to be therapeutically useful. However, for topical administration dosing might not be a problem. Here we find that chol-siRNAs, stabilized with phosphorothioate residues at their ends, silence gene expression in cervicovaginal epithelium in the absence of a transfection reagent, without triggering interferon or inflammatory response genes.
In a previous in vitro study we found that siRNAs silencing the HIV coreceptor CCR5 persisted for longer in nondividing HIV-uninfected macrophages than siRNAs targeting HIV gag, leading us to speculate that the expression of a target mRNA might lead to a longer siRNA intracellular half-life (Song et al. 2003). The cell surface receptor nectin-1 has been identified as a receptor for HSV-2 by binding to the viral envelope glycoprotein D (Krummenacher et al., 1998, Shukla et al., 2000). Nectin-1 is expressed on the human and mouse vaginal epithelium. Whereas nectin-1 is expressed throughout the menstrual cycle on human epithelium, its expression on murine epithelium is restricted to proestrus and diestrus, which correlates with susceptibility to HSV-2 infection (Shukla et al, 2000 and Linehan et al, 2004). During diestrus, nectin-1 is predominantly located at the luminal edge of the epithelial cells. Nectin-1 gene-targeted knockout mice are less susceptible to HSV-2 infection, whereas no difference in infectivity is observed in mice deficient for an alternative HSV-2-entry receptor, HVEM (Taylor et al, 2007). Furthermore, siRNA-mediated knockdown of nectin-1 expressed on the apical surface of human epithelial cell lines results in reduced HSV-2 infection (Galen et al, 2006). We therefore investigated whether knocking down nectin-1 might interfere with HSV-2 infection and provide more durable protection. Nectin-1 siRNAs inhibit HSV-2 infection in vitro and in vivo. Moreover, siRNAs against nectin-1 persist and durably silence nectin-1 gene expression for a week. While mice treated with an UL29 siRNA are protected from viral challenge only around the time of siRNA instillation, mice treated with siRNAs against nectin-1 are protected for a week beginning 2 days after siRNA treatment, the time required to down-modulate the receptor. By combining UL29 and nectin-1 siRNAs, administered at a total dose of 1 mg/kg on two consecutive days, mice were uniformly protected for a week, irrespective of when they were challenged.
Results
Intravaginal application of Oligofectamine enhances HSV-2 transmission
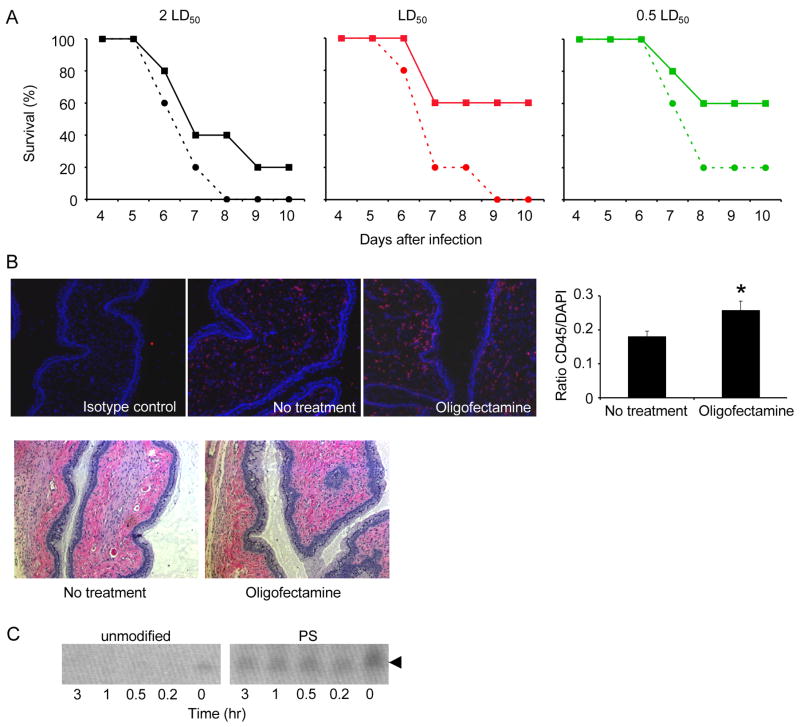
When we tried to prolong protection from HSV-2 challenge in mice treated with UL27 and UL29 siRNA by increasing the dose of OF-complexed siRNA, there was no survival advantage; instead paradoxically we observed increased lethality (data not shown). OF-complexed siRNAs did not disrupt the epithelium or cause detectable tissue cell death (Palliser et al., 2006) (and data not shown). Nonetheless we wondered whether increased concentrations of OF might enhance HSV-2 uptake across the epithelium, possibly by increasing the fusion of the viral envelope to cell membranes or mediating an inflammatory response. Therefore, we examined the effect of OF on its own on survival of mice challenged with HSV-2. Mice were treated with 4 μl OF, 2 hr prior and 4 hr after vaginal HSV-2 administration, a dosing schedule similar to that which conferred protection in (Palliser et al., 2006). At each dose of HSV-2 tested, mice treated with OF succumbed to infection faster than control mice and more mice died (comparison between treated and untreated mice by stratified log-rank test, P=0.04; Figure 1A). Although 20% and 60% of mock-treated mice survived when challenged with 2LD50 and 1LD50 virus, respectively, no OF-treated mice survived. Similarly, OF reduced survival of mice challenged with 0.5 LD50 from 60% to 20%. Therefore, OF treatment reduced the LD50 approximately 4-fold. Immunofluorescent staining of vaginal tissue sections isolated 24 hrs following administration of two doses of OF, 6 hrs apart, showed a slight, but significant (p<0.05) increase in CD45+ cells in the lamina propria (Figure 1B, top panel). Hematoxylin and eosin staining of vaginal tissue isolated at the same time point showed no other signs of inflammation or tissue disruption (Figure 1B, bottom panel). These results suggest that OF causes some inflammation that might enhance viral transmission.
Figure 1. Oligofectamine enhances HSV-2 transmission.
(A) Mice given Oligofectamine (OF, solid lines) ivag 2 hr before and 4 hr after challenge with different doses of HSV-2 were more susceptible to viral infection than control mice treated with PBS (dashed lines). (B) Increase in CD45+ cells in vaginal lamina propria of mice given OF twice, 6 hr apart. Twenty four hr after OF treatment, frozen sections of vaginal tissue were stained with antibody to CD45 or isotype control (red) and DAPI (blue) (top). Representative images (left) and mean + S.D. (right) of the ratio of CD45+ cells/total DAPI+ cells in the lamina propria in 10 fields of vision are shown. (*, p<0.05) Hematoxylin and eosin staining of paraffin-embedded vaginal sections, harvested 24 hrs following OF treatment (bottom), shows no gross signs of inflammation. (10x magnification) (C) When not complexed with a transfection lipid, unmodified siRNAs were rapidly degraded in vaginal wash fluid at 37oC compared to PS-modified siRNAs (PS). Arrow indicates siRNA.
Naked siRNAs have a short half-life in vaginal fluid
We therefore decided to investigate lipid-independent siRNA delivery. Our previous study was performed using chemically unmodified siRNAs. However, we were concerned that although complexation into lipoplexes might protect siRNAs from ambient RNases, unmodified uncomplexed siRNAs might be rapidly degraded in the genital tract. To evaluate the stability of uncomplexed and unmodified siRNA in cervicovaginal washes, we analyzed their stability by RNA gel electrophoresis after incubation in mouse genital wash fluid. Within 15 min of incubation at 37oC, no signal was detectable (Figure 1C). However, siRNAs modified by a single phosphorothioate (PS) substitution at each 3′-end were still detected after 3 hr incubation (Choung et al., 2006). Therefore RNases in cervicovaginal secretions could potentially interfere with effective silencing; all subsequent experiments were therefore performed using PS-modified siRNAs.
Knocking down nectin-1 reduces HSV-2 infection in vitro
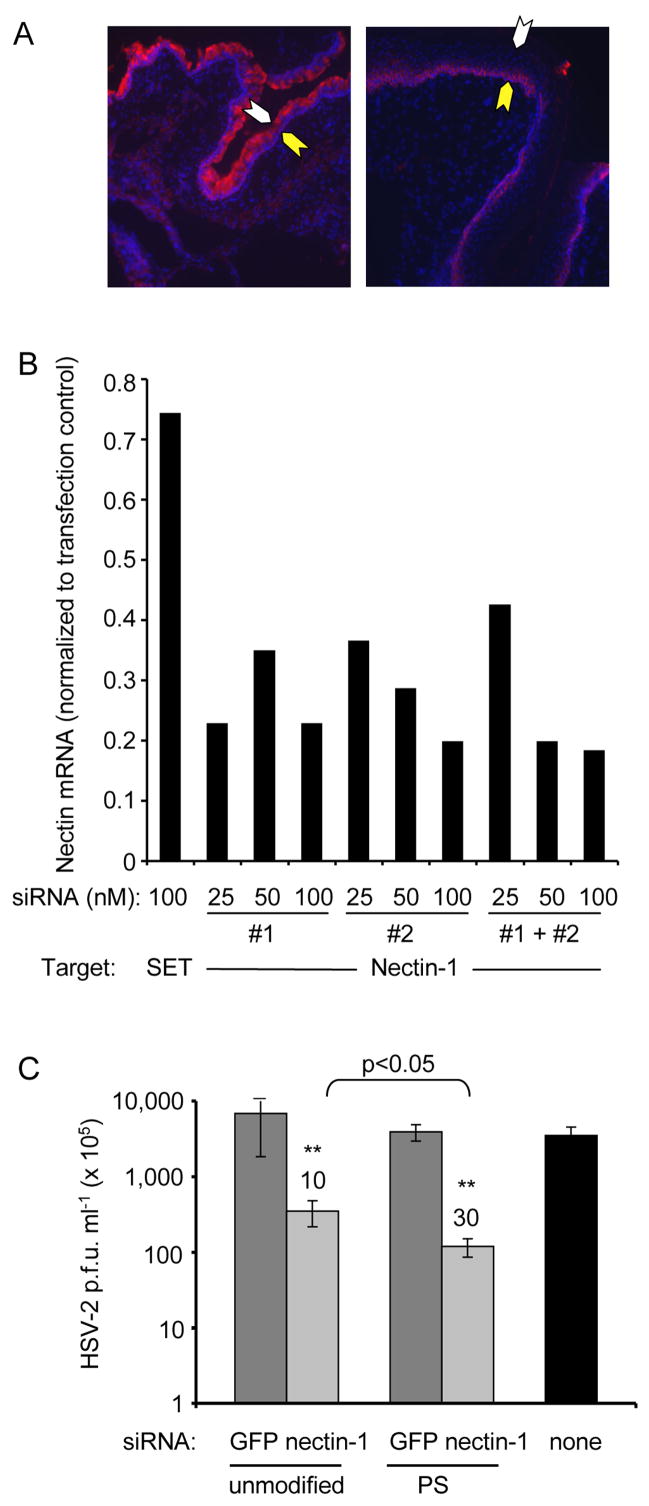
In the murine genital mucosa susceptibility to HSV-2 infection varies with the estrus cycle and is maximal at diestrus (Parr et al., 1994). As previously reported (Linehan et al, 2004), susceptibility coincides with nectin-1 expression - at diestrus the epithelial layer is maximally thin, and nectin-1 is highly expressed on the luminal side of the epithelium, while in proestrus the epithelial layer is thickened and nectin-1 is only expressed dimly near the basement membrane (Figure 2A). We therefore used the Dharmacon design program to design two nectin-1 siRNAs, which targeted the nectin sequence beginning at nt674 (siRNA#1) and nt1233 (siRNA #2) to investigate whether knocking down nectin-1 inhibits HSV-2 infection. Both siRNAs knocked down nectin-1 and inhibited HSV-2 infection, but siRNA #1 provided better knockdown and antiviral effect (Figures 2B, C; data not shown). Phosphorothioated (PS-) siRNA #1 was more effective than unmodified siRNA #1, even in vitro (Figure 2C; p<0.05). Therefore PS-siRNA #1 was used for all subsequent experiments.
Figure 2. Down-modulation of nectin-1, a putative HSV-2 receptor, which is upregulated in the vagina during diestrus when mice are susceptible to HSV-2, reduces HSV-2 replication in vitro.
(A) Nectin-1 (red) expression is enhanced at the luminal epithelial surface at diestrus (left) compared to proestrus (right). Nuclei are stained with DAPI (blue). The epithelial luminal surface is indicated with a white arrow and the basement membrane with a yellow arrow (x10). The epithelium is thinned during diestrus. (B) siRNAs #1 and #2 both down-modulate nectin-1 mRNA expression, assessed 24 hr after transfection relative to Gapdh by qRT-PCR in NIH3T3 cells relative to a control SET siRNA. (C) Knocking down nectin-1 inhibits HSV-2 replication in NIH3T3 cells. Cells were transfected on 2 consecutive days with either unmodified or PS-modified siRNA complexed with OF and then infected with HSV-2 the next day and harvested 20 hr later. Data show mean viral titer±S.D. **, p<0.001. PS-siRNAs afforded significantly more protection than unmodified siRNAs (p<0.05). Values above bars show fold reduction in viral plaques. Data are representative of at least two experiments.
More durable inhibition of HSV-2 replication in vitro by silencing nectin-1 than by silencing UL29
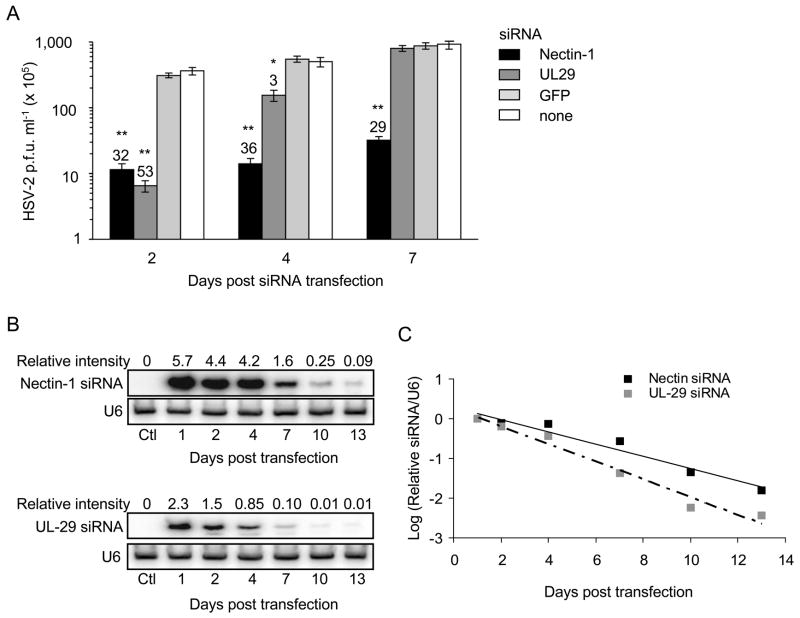
Our next goal was to determine whether silencing nectin-1 might have a more durable antiviral effect than silencing the viral DNA binding protein UL29. NIH3T3 cells transfected on 2 consecutive days with siRNAs targeting either nectin-1 or UL29 were infected with HSV-2 2, 4 or 7 days later. Viral replication, assessed by plaque assay, was significantly reduced by the nectin-1 siRNA ~30 fold at each time point (Figure 3A; p<0.001). UL29 siRNA reduced viral replication by 53-fold when NIH3T3 were infected with HSV-2 2 days after transfection (p<0.001) and by 3-fold after 4 days (p<0.05), but there was no protection when the challenge was deferred for 7 days. Therefore the nectin-1 siRNA had a sustained antiviral effect, while protection afforded by the UL-29 siRNA was short-lived. To evaluate whether the difference in durability might be due to a difference in siRNA half-life, we used Northern blot to quantify nectin-1 and UL29 siRNAs in NIH3T3 cells (Figures 3B and C). Both siRNAs declined with similar half-life (1.9 days for nectin-1 siRNA and 1.4 days for UL29 siRNA, p>0.05), although the nectin-1 siRNA signal on the blots was significantly more intense (2.5-fold on day 1). Both probes have calculated melting temperatures that differ by less than a degree (nectin-1 siRNA probe Tm = 52.1oC; UL29 siRNA Tm = 51.2 oC; hybridization T = 42 oC) suggesting that the difference in siRNA signal was not due to a difference in hybridization efficiency, but rather reflected a real difference in the intracellular level of nectin-1 siRNA compared to the UL29 siRNA. It is likely that maintaining higher intracellular levels of nectin-1 siRNA results in a more prolonged antiviral effect.
Figure 3. Nectin-1 siRNAs provide more lasting protection against HSV-2 than UL29 siRNAs in vitro.
(A) NIH3T3 cells were transfected on 2 consecutive days with nectin-1 (black), UL29 (dark grey) or GFP (light grey) siRNA or mock-transfected (white), then challenged with HSV-2 either 2, 4 or 7 days later. Viral titers assessed the following day by plaque assay show more prolonged protection from knocking down nectin-1 than with the antiviral siRNA. Values above bars show fold reduction in viral plaques. Data show mean±S.D. *, p<0.05; **, p<0.001, compared with control. (B and C) Nectin-1 and UL29 siRNA antisense strands were assessed at indicated days after transfection by Northern blot and quantified by densitometry relative to U6 RNA. Although the siRNA half-lives were similar, more nectin-1 than UL29 siRNA was present at all times. In (C) the relative expression of each siRNA was normalized to its value on day 1.
Durable nectin-1 silencing by chol-siRNA in vivo
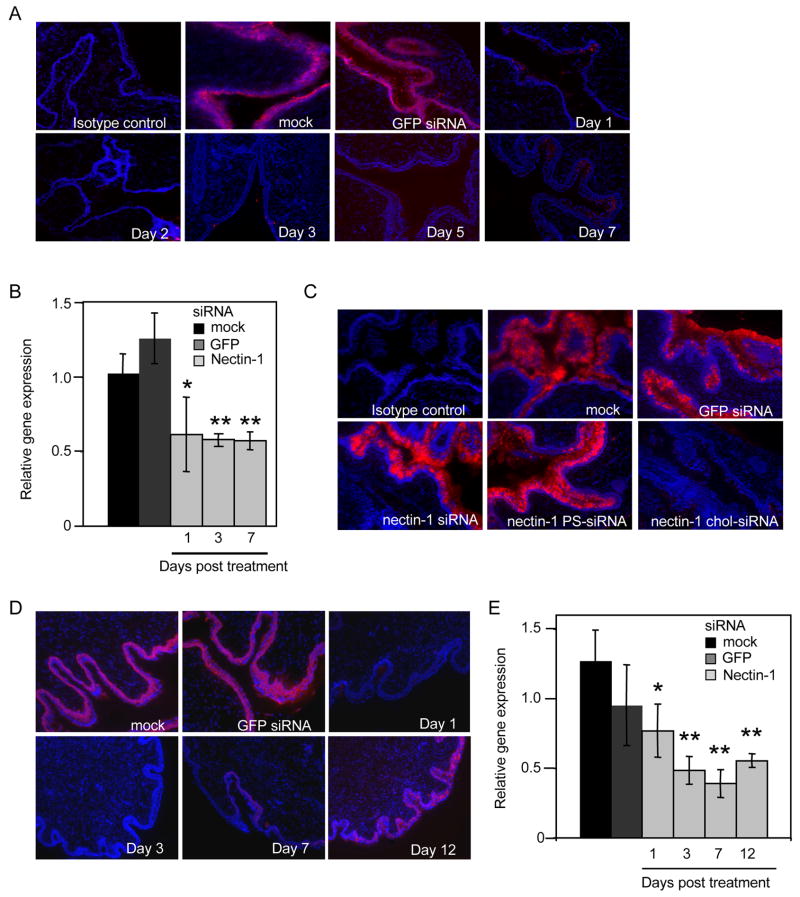
Since transfected nectin-1 siRNAs provided protection against HSV-2 replication in vitro for at least a week, even in dividing NIH3T3 cells, we next wanted to know whether we could achieve durable silencing in vivo. In an initial experiment we instilled 1 mg/kg lipoplexed siRNAs twice ivag into medroxyprogesterone-pretreated mice. Nectin-1 protein expression, evaluated by fluorescence microscopy was almost fully extinguished by day 1, was not detected on day 2, and remained undetectable on day 7 (Figure 4A). Nectin-1 mRNA quantified by qRT-PCR, was significantly decreased at all times evaluated (Figure 4B). Our next goal was to determine if we could obtain silencing without using a transfection lipid. siRNAs have been reported to be taken up by pulmonary epithelial cells, even without a transfection lipid (Bitko et al., 2005). However, when mice were treated on two consecutive days with either unmodified or PS-modified nectin-1 siRNAs (1 mg/kg) in the absence of OF, nectin-1 protein was not reduced when analyzed 1 day following the last dose, at a time that nectin-1 expression is decreased using OF-complexed siRNAs (Figure 4C). In the same experiment we also evaluated knock-down using the same dose of nectin-1 chol-siRNA without transfection lipid, which knocked down nectin-1 very effectively. We therefore investigated whether chol-siRNAs might efficiently and durably silence in the genital tract without requiring a transfection lipid (Figures 4D and E). Mice treated with either no siRNAs or chol-siRNAs targeting an irrelevant protein (GFP) expressed equivalent levels of nectin-1. Treatment with nectin-1 chol-siRNA (1 mg/kg x 2) down-modulated nectin-1 expression for at least 12 days. Although immunofluorescent staining for nectin-1 began to be detected on day 12 (still at reduced levels compared to control mice), nectin-1 mRNA expression remained low. Therefore chol-siRNA without OF can silence nectin-1 efficiently and durably in the genital tract.
Figure 4. Cholesterol-conjugated siRNAs down-modulate nectin-1 expression for a week in vivo without requiring a transfection lipid.
(A and B) Nectin-1 siRNAs (1 mg/kg) complexed with OF administered ivag for two consecutive days down-regulate nectin-1 within a day of the second instillation and down-regulation persists for at least 7 days. (A) shows immunofluorescence microscopy images of sections stained with nectin-1 or isotype control antibody (red) and DAPI (blue), while (B and E) provide quantification of nectin mRNA relative to Gapdh by qRT-PCR (OF only (black), GFP siRNA (dark grey), nectin-1 siRNA (light grey)). Controls were harvested 1 day after the second transfection. (C) Nectin-1 siRNAs (1 mg/kg x 2) administered ivag without a transfection reagent do not knock-down nectin-1 expression, but the same dose of chol-siRNAs do. Vaginal sections were harvested 2 days after the second treatment and stained for nectin-1 and DAPI as above. (D and E) Nectin-1 knock-down by chol-siRNAs is sustained for over a week. Data for (B) and (E) are mean±S.D. *, p<0.05, **, p<0.001. Control animals were given OF only (black) or GFP siRNA (dark grey). Images are magnified x10. Data shown are representative of two experiments.
A combination of nectin-1 and UL29 chol-siRNAs protects mice for 1 week
To test the effectiveness of nectin-1 chol-siRNAs at protecting against HSV-2 transmission, we first did a pilot experiment to define the dose response of chol-siRNAs using chol-conjugated UL-29 siRNA without OF and a dosing schedule that provided protection in the earlier study (Palliser et al., 2006). Mice were treated ivag with UL29 chol-siRNA or mock-treated 2 hr prior and 4 hr following HSV-2 challenge with 2LD50 of virus (Figure 5A). Twenty percent of control mice (1 of 5) survived this viral challenge. There was no increased survival in mice that received 0.25 mg/kg UL29 chol-siRNA, but 3 of 5 mice (60%) that received injections of 0.5 mg/kg UL29 chol-siRNA (1 nmol, twice the dose used in (Palliser et al., 2006) ) survived and 80% of mice given 1 or 2 mg/kg survived. However, a higher dose (5 mg/kg) did not provide protection for unclear reasons. We chose 1 mg/kg (2 nmol), the lowest dose that provided optimal protection, for subsequent challenge experiments.
Figure 5. Chol-siRNAs targeting nectin-1 and UL29 confer durable protection from lethal HSV-2 infection for a week.
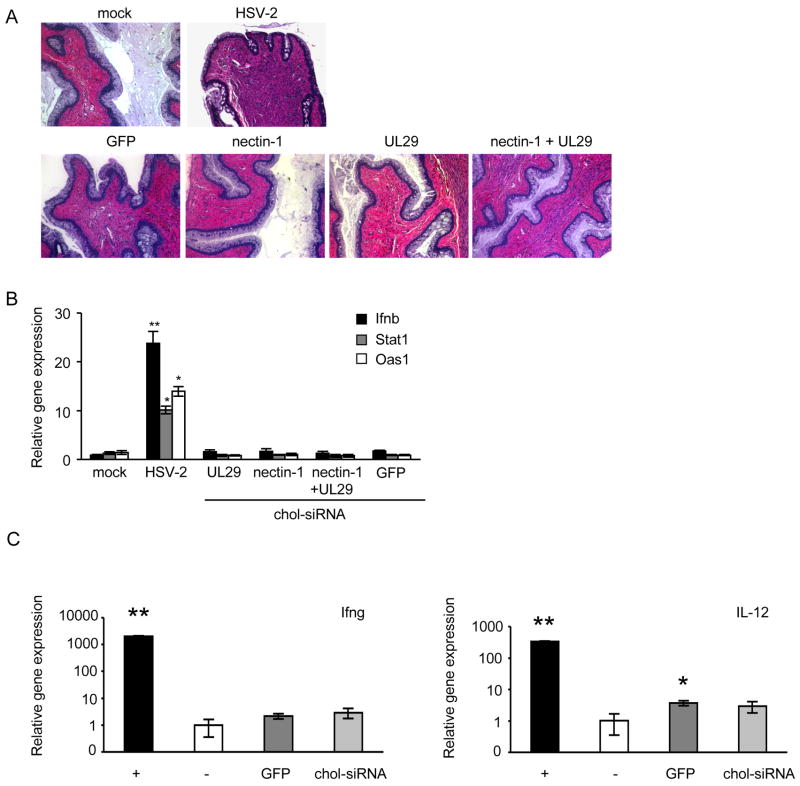
(A) Dose response for protection from challenge with 2LD50 HSV-2 by UL29 chol-siRNA. Groups of 5 mice were given the indicated dose of UL29 chol-siRNA 2 hr prior and 4 hr following viral challenge. Optimal protection was achieved with 1 and 2.5 mg/kg. (B-D) Groups of 5 mice received no siRNA (B), GFP chol-siRNA (administered 2 hr before and 4 hr after viral challenge) (C) or nectin-1 and/or UL29 chol-siRNA (D) at indicated times. The total siRNA dose for each administration was 1 mg/kg. Mice were scored for disease severity (purple, no signs of disease; blue, slight genital erythema and edema; green, moderate genital inflammation; yellow, purulent genital lesions; orange, hind limb paralysis; 5, death) over 14 days. The UL29 chol-siRNA protected around the time of viral challenge while the nectin-1 siRNA did not become effective until a few days later, but protection persisted for a week. Combining both siRNAs provided protection from challenge at any time during the week. (E) Vaginal histopathology (hematoxylin and eosin staining) 6 days following infection in mice treated as indicated. (Boxes indicate magnified area below). Data are representative of two experiments. Mice treated with a combination of nectin-1 and UL29 chol-siRNAs had essentially no signs of inflammation or cell death.
To investigate the onset and duration of protection, mice were treated at various times with nectin-1 and/or UL29 chol-siRNAs, given either before or after HSV-2 infection and followed for 14 days for clinical signs of disease, using a well-established scoring system, and survival. Twenty percent of mice that did not receive siRNAs or received GFP chol-siRNAs survived the 2LD50 HSV-2 challenge (Figures 5B and C). As previously shown with lipoplexed siRNAs (Palliser et al., 2006), mice that received UL29 chol-siRNAs either around the time of challenge or shortly thereafter, were protected (Figure 5D). However, mice treated with UL29-1 chol-siRNAs 1 and 2 days before viral challenge or before then were not significantly protected (log rank test for survival comparing pre and post infection treatment groups, P<0.01). Administering nectin-1 chol-siRNA 1 and 2 days or 3 and 4 days prior to HSV-2 challenge protected 60% of mice, while 80% of mice were protected when siRNAs were given 7 days before challenge. However, there was no benefit to mice injected with nectin-1 chol-siRNA immediately before or after HSV-2 (survival by log-rank test P=0.007, comparing pre- and post-challenge groups). Combining nectin and UL29 chol-siRNAs gave uniform and significant protection irrespective of the time of challenge (60–80% survival, P=0.007, log-rank test compared with the control). The disease course was consistent with histological examination of cervicovaginal tissue stained with hematoxylin and eosin isolated 6 days after viral challenge of mice given 1 mg/kg chol-siRNAs 1 and 2 days prior to the challenge. The vaginal tissue of mice that were mock-treated or given GFP chol-siRNA showed swelling, epithelial erosion, intense inflammatory infiltrates and apoptosis (Figure 5E). By contrast, tissues from challenged mice that received chol-siRNAs targeting nectin-1 and UL29 were virtually normal.
Nectin and UL29 Chol-siRNAs do not activate inflammation or interferons
As siRNA treatment can induce interferon and inflammatory responses which might have a nonspecific antiviral effect or alter normal tissue homeostasis (Schlee et al., 2006), we analyzed vaginal tissue from mice given chol-siRNA for inflammation and cytokine expression. Vaginas were harvested 1 day after mice were either mock-treated or given siRNAs that targeted GFP, nectin-1, UL29 or a mixture of nectin-1 and UL29 on 2 consecutive days. No inflammation was observed in hematoxylin and eosin stained tissue sections (Figure 6A). In comparison, cellular infiltrates were present in vaginal sections from a mouse infected with 50 LD50 HSV-2 for 24 hr as a positive control (Figure 6A). mRNAs for interferon and interferon-regulated genes Ifnb, Stat1 and Oas1, assessed by qRT-PCR on samples harvested 24 hr after the second dose, were also not up-regulated in mice given any of these siRNAs (Figure 6B). Vaginal tissue from mice treated with 1mg/kg chol-siRNA targeting GFP or nectin-1 on two consecutive days was also analyzed for induction of IL12 and Ifng mRNAs 24 hr after the second instillation by qRT-PCR and compared with untreated mouse vaginal tissue (Figure 6C). These genes also were not significantly induced.
Figure 6. Chol-siRNAs do not cause inflammation or activate interferon-response genes.
Vaginal tissue was harvested from mice 1 day following the second of 2 treatments with either 2 nmol chol-siRNA (no HSV-2), with HSV-2 only as a positive control or with PBS (mock) and divided for analysis by hematoxylin and eosin staining (A, x10 magnification) or for RNA extraction for qRT-PCR analysis of interferon-responsive genes Ifnb (black), Stat1 (grey) and Oas1 (white) (B). The chol-siRNAs did not induce inflammation or interferon-responsive gene expression. (C) RNA was also extracted from vaginal tissue of mice treated with 2 nmol chol-siRNA twice (without OF) 1 day following the second treatment, and analyzed by qRT-PCR for Ifng (left) and IL12 (right). Positive controls (+, black) were anti-CD3/CD28 activated T cells (Ifng) and LPS-activated splenocytes (IL12). PBS-treated vaginal tissues served as negative control samples (−, white). siRNAs either targeted GFP (dark grey) or nectin-1 (light grey). Relative gene expression compared to gapdh (mean±S.D.) is shown. *, p<0.05; **, p<0.001.
Discussion
Topical administration of chol-siRNAs targeting an HSV-2 viral gene and a host receptor induced sustained viral resistance in the genital tract of female mice. Mice were protected against challenge with 2LD50 of HSV-2 for at least a week independently of whether the challenge was performed just before siRNA administration or delayed until a week later. The administered siRNAs were stabilized by a single PS linkage to protect them from degradation by genital fluid RNases and were cholesterol-conjugated to facilitate intracellular entry.
These results improve upon our earlier study that used lipoplexed unmodified antiviral siRNAs (Palliser et al., 2006). We found that we needed to modify the approach we had developed for a topical siRNA-based antiviral microbicide because the transfection lipid used to deliver the siRNAs into cells on its own facilitated viral infection and because protection was short-lived. Our results suggest that the lipid transfection reagent induced slight, but significant, inflammation as measured by an increase in infiltrating hematopoietic cells, and this may be sufficient to enhance viral uptake, resulting in a decrease in mouse survival. Alternatively, the transfection lipid might enhance viral entry by facilitating fusion of the viral envelope with the cell membrane. Therefore lipoplexed siRNAs should probably not be considered for topical delivery of siRNAs to any mucosal surface, since they might also increase transmission of other enveloped viruses. Although unmodified siRNAs can apparently be taken up and induce silencing in the pulmonary epithelium without the aid of a transfection reagent (Bitko et al., 2005), we did not find that to be the case in the genital tract of mice. It is possible that application of significantly higher siRNA concentrations could knock-down gene expression in the tissue, but we did not evaluate this since chol-conjugated siRNAs provided an efficient and apparently nontoxic method for genital tract delivery. However, our evaluation of toxicity was limited to gross assessments of epithelial cell integrity and inflammatory infiltrates and induction of inflammatory cytokines. Although mice treated with a 5-fold higher dose of siRNA did not appear to become sick, this dose did not afford any viral protection. This may be because of dose-dependent off-target effects or toxicity, which would need to be carefully evaluated for further preclinical development.
Ivag administration of chol-siRNAs did not induce substantial interferon or inflammatory responses. In contrast to two recent studies that reported induction of high levels of inflammatory chemokines, IFNβ, IFNγ and IL12 by some siRNA preparations (Kleinman et al., 2008, Robbins et al., 2008), we found no indication that these cytokines were significantly induced following siRNA treatment at the low dose we used (1 mg/kg, twice). In addition, no survival advantage was observed in mice given siRNAs targeting GFP compared with mice receiving no siRNAs. Therefore, the protective effect is likely a direct consequence of specific siRNA-mediated gene silencing.
HSV-2 infects epithelial cells and dorsal root ganglion neurons. Our results support efficient chol-siRNA delivery to epithelial cells, but this acute HSV-2 infection model does not address whether neuronal processes that extend into the tissue are effectively transduced. Topically applied siRNAs might be useful to treat and prevent reactivation and sexual transmission of clinically latent HSV-2 infection, which is not truly latent since chronically infected women without clinical symptoms of active disease are constantly shedding infectious virus (Wald, 2004). An ideal drug would also prevent viral replication in the dorsal root ganglion viral reservoir. Further studies are therefore needed to examine siRNA transduction of these cells. We would also like to apply the results of this study to developing a topical microbicide to prevent HIV-1 infection, an urgent global public health need given the failures of HIV vaccine development. Since HIV, like HSV-2 is an enveloped virus, transfection lipid-mediated delivery would likely be inadvisable. To prevent HIV transmission, we need to develop a way to transduce immune cells in the genital tract responsible for HIV transmission, especially Langerhans cells, macrophages and T cells. Because these cells are rare in the uninflamed genital tissue of mice maintained under pathogen-free conditions, we do not yet know whether chol-siRNAs will be suitable, but are evaluating this.
This study confirms that nectin-1, a cell adhesion molecule that localizes at the adherans junctions of epithelial cells, is an important HSV-2 receptor (Geraghty et al., 1998, Cocchi et al., 1998, Shukla et al., 2000). In addition to nectin-1, herpesvirus entry mediator (HVEM) has been reported as an efficient entry receptor for HSV (Montgomery et al., 1996). However, in mice genetically deleted for HVEM, little difference in HSV-2 ivag infectivity was observed. By contrast, mice lacking nectin-1 exhibited reduced infection levels in the vaginal epithelium and decreased viral spread to the nervous system. Death was delayed in nectin-1 knockout mice and approximately half the mice succumbed to disease (Taylor et al., 2007). The superior protection from HSV-2 infection afforded by nectin-1 knock-down, where 80% of mice were protected, compared to knockout mice could be due to differences in mouse or virus strains or potentially to compensatory changes in expression of members of the nectin and nectin-like family in knockout animals. The coordinated change during diestrus in nectin-1 expression and subcellular localization to the luminal surface with susceptibility to HSV-2 infection in mice further supports the importance of nectin-1 as a viral receptor, as previously reported (Linehan et al., 2004). Of note, nectin-1 is expressed at all stages of the menstrual cycle in humans (Linehan et al., 2004).
Therapeutically knocking down a host gene can be deleterious if the gene is required for normal cellular function. Because nectin-1 localizes to adherens junctions, it might be required to maintain the integrity of the epithelial barrier. However, we saw no changes in histology of the epithelial layer by hematoxylin and eosin staining. Nectin-1 has been identified as a gene mutated in cleft lip or cleft palate syndrome. The incidence of this mutation in some populations may be a result of selection due to a survival advantage conferred by relative resistance to HSV-1 and HSV-2 (Suzuki et al., 2000). However, whether this mutation confers a protective effect is, as yet, undetermined. Therefore, nectin-1 may be needed for development but may be a nonessential gene (and good microbicide target) after that. However, the use of nectin-1 siRNAs might not be without risk during pregnancy.
siRNAs targeting viral genes were effective over a limited time frame, but siRNAs targeting a putative host receptor provided more sustained protection against both in vitro and in vivo viral challenge. We previously found that siRNAs targeting CCR5, the host coreceptor for HIV protected macrophages from HIV-1 infection in vitro for weeks, whereas protection waned after a few days when uninfected cells were transduced with HIV-1 gag siRNAs (Song et al., 2003). These data, together with the observation in C. elegans that siRNAs are more stable when a target mRNA is expressed (Plasterk et al., 2002), suggested that siRNAs targeting host genes might persist longer and provide more sustained protection than siRNAs targeting viral genes. Although the outcome was as expected, namely silencing nectin-1 provided more durable protection than UL29 siRNAs, the explanation was not what we expected. The half-lives of nectin-1 and UL29 siRNAs in uninfected NIH3T3 cells are in fact comparable. Similarly we did not find a difference in half-life of GFP siRNAs in GFP-expressing and nonexpressing HeLa cells (data not shown). Therefore the stability of an siRNA does not appear to be affected by the expression of its target gene. However, there was significantly more intracellular nectin-1 siRNA compared to UL29 siRNA at all time points. This may be because the nectin-1 siRNA was more efficiently taken up into RNA-induced silencing complexes (RISC) and available for gene silencing. In fact, the level of intracellular nectin-1 siRNA 7 days after transfection was similar to the level of UL29 siRNA on the first day after transfection. What this suggests is that the difference in durable protection may not be due to targeting a host receptor vs a viral gene, but rather to the use of a more effective siRNA. Therefore identifying more effective siRNAs that are either more efficiently incorporated into RISC or more active at directing target mRNA degradation might not only generate siRNAs active at a lower dose, but that last longer. For this study and our previous study, we only tested a few siRNAs for each target gene; a systematic approach to identify host or viral gene siRNAs active at pM concentrations in vitro would be advisable for preclinical development. These could be directed against either viral or host genes. Use of optimized siRNA sequences or further chemical modification of siRNAs to enhance stability in the genital tract might lower the siRNA dose or extend durability of effective protection against sexual transmission.
Experimental Procedures
Mice
6 week old BALB/c mice (Taconic Farms) were injected subcutaneously with 2 mg medroxyprogesterone acetate (Sicor) 1 week prior to siRNA treatment or HSV-2 challenge. Mice were challenged ivag with 2x104 (~2LD50), 104 or 5x103 p.f.u. HSV-2 strain 186. siRNA formulations were administered ivag twice on consecutive days. The following siRNAs were used: 1 nmol PS-modified siRNA without transfection reagent, 1 nmol PS-modified siRNAs complexed with 2 μL Oligofectamine (Invitrogen) or chol-PS-siRNA (2 nmol unless otherwise specified) without transfection reagent. Following HSV-2 challenge, disease progression was graded according to a five-point scale: 0, no signs of infection (purple); 1, slight genital erythema and edema (blue); 2, moderate genital inflammation (green); 3, purulent genital lesions (yellow); 4, hind limb paralysis (orange); 5, death (red) (Morrison et al., 1998). Vaginal tissue was dissected at the times indicated, fixed in 10% formalin (Sigma), paraffin embedded, cut into 8 μm sections and stained with hematoxylin and eosin. For RNA isolation, vaginal tissue was stored in RNAlater (Qiagen) prior to RNA extraction using RNeasy (Qiagen).
Viruses and transfection assays
For in vitro transfection assays the TK-negative mutant, replication-competent HSV-2 186 kpn was used (Jones et al., 2000). Wild-type 186 syn+ HSV-2 was used for in vivo experiments (Spang et al., 1983). Viruses were grown and titered on Vero cells, as described (Gao et al., 1989). An aliquot of virus used for each mouse experiment was assayed by plaque assay to confirm viral titer. For transfection assays, NIH3T3 cells were plated (2x105 cells per well in 6-well plates in 1 ml of complete medium). The following day, cells were transfected with 100 pmol siRNA complexed with Transit-siQuest (Mirus), according to the manufacturer’s instructions. After 18 hr incubation at 37oC, complexes were removed and replaced with complete medium. The transfection was repeated, as above, 6 hr later and the following day complexes were removed and complete medium was added to the cells. HSV-2 186 kpn was added 6 hr later at an MOI of 1, incubated for 1 hr at 37oC and the medium was then replaced. Cells were harvested 24 hr later and viral titer determined by plaque assay on Vero cells. Cells used in transfection experiments to determine siRNA half-life were transfected as described, but were maintained in 1% FCS serum to minimize cell turnover.
siRNAs
Unmodified siRNAs (Dharmacon) were prepared according to the manufacturer’s instructions. PS-modified and chol-PS-modified siRNAs were synthesized as described (Soutschek et al., 2004). The sequence for HSV-2 UL29 (Palliser et al., 2006) (nt 60324–60342 GenBank accession number NC_001798) is, sense 5′-CUUUCGCAAUCAAUUCCAAdT*dT-3′, antisense 5′- UUGGAAUUGAUUGCGAAAGdT*dT*chol-3′; nectin-1 sequence #1 (nt 742–760 GenBank accession number NM_021424), sense 5′-CCUGCAUUGUCAACUAUCAdT*dT*chol-3′, antisense 5′-UGAUAGUUGACAAUGCAGGdT*dT-3′; nectin-1 sequence #2 (nt 1234-1252 GenBank accession number NM_021424), sense 5′-GCACCAAGAAGCACGUGUAdT*dT-3′, antisense 5′-UACACGUGCUUCUUGGUGCdT*dT-3′; GFP (Novina et al., 2002) sense 5′-GGCUACGUCCAGGAGCGCAdT*dT*chol-3′, antisense 5′-UGCGCUCCUGGACGUAGCCdT*dT-3′, * designates substitution of a phosphodiester bond with a phosphorothioate linkage, chol indicates cholesterol conjugation. The cholesterol-conjugated sequences were purified by high-performance liquid chromatography (HPLC) on a reverse-phase column. RNA oligonucleotides were purified by anion-exchange HPLC. Fractions containing full-length oligonucleotides were pooled, desalted and lyophilized and characterized by ES mass spectrometry and capillary gel electrophoresis (Soutschek et al., 2004). Purity was in the range of 90%. Unmodified siRNAs contained no phosphorothioate linkages or cholesterol.
Quantitative RT-PCR
Total RNA (1 μg) was isolated from mouse vaginal tissue using the RNeasy RNA isolation kit (Qiagen) and reverse transcribed using Superscript III (Invitrogen) and random hexamers (IDT), according to the manufacturer’s instructions. Real-time PCR was performed on 0.2 μl of complementary DNA using Platinum Taq polymerase (Invitrogen) and a Biorad iCycler. SYBR green (Molecular Probes) was used to detect PCR products. All reactions were done in 25 μl in triplicate. Primers were:
Gapdh forward 5′-TTCACCACCATGGAGAAGGC-3′
Gapdh reverse 5′-GGCATGGACTGTGGTCATGA-3′
Nectin-1 forward 5′-AGATGTGAAGCTCACGTGCAAAGC-3′
Nectin-1 reverse 5′-TTGGTGGCCTCACAGATGTAGGTT-3′
Stat1 forward 5′-TTTGCCCAGACTCGAGCTCCTG-3′
Stat1 reverse 5′-GGGTGCAGGTTCGGGATTCAAC-3′
Oas1 forward 5′-GGAGGTTGCAGTGCCAACGAAG-3′
Oas1 reverse 5′-TGGAAGGGAGGCAGGGCATAAC-3′
Ifnb forward 5′-CTGGAGCAGCTGAATGGAAAG-3′
Ifnb reverse 5′-CTTGAAGTCCGCCCTGTAGGT-3′
Il12 forward 5′- GAAGTCCAATGCAAAGGCGGGAAT-3′
Il12 reverse 5′- AAAGCCAACCAAGCAGAAGACAGC-3′
Ifng forward 5′ TCTTCCTCATGGCTGTTTCTGGCT 3′
Ifng reverse 5′ CGCTTATGTTGTTGCTGATGGCCT 3′
PCR parameters consisted of 5 min Taq activation at 95oC, followed by 40 cycles of 95oC X 20 s, 60oC X 30 s and 69oC X 20 s. Standard curves were generated and relative amount of mRNA was normalized to Gapdh mRNA. Specificity was verified by melt curve analysis and agarose gel electrophoresis.
Tissue sections and microscopy
Nectin-1 expression in vaginal tissue was assayed using a protocol adapted from (Zhao et al., 2003). Briefly, frozen 8 μm sections were acetone-fixed, blocked in TNB buffer (NEN Life Sciences Products) containing 5% normal donkey serum followed by blocking in avidin-biotin solution (Vector Laboratories). Endogenous peroxidase activity was quenched with 1% H2O2. Slides were incubated overnight at 4oC with either polyclonal nectin-1 antibody (Orbigen) or isotype-control IgG. Slides were washed and then incubated for 1 hr at room temperature with biotin goat anti-rabbit Ig (InnoGenex) followed by incubation for 40 min at room temperature with steptavidin-HRP (InnoGenex). The antigenic signal was amplified with tyramide amplification kit (Invitrogen) according to the manufacturer’s instructions. Slides were then washed and mounted with Vectashield Mounting Medium containing the nuclear dye DAPI (Vector Laboratories). For CD45 staining, frozen sections were fixed and blocked, as above. Primary antibodies were anti-mouse CD45 antibody or rat IgG2b isotype control (Pharmingen). Alexa Fluor 594 donkey anti-rat IgG (Molecular Probes) was used as the secondary antibody. Following staining, sections were washed and mounted with Vectashield Mounting Medium, as above. For hematoxylin-eosin staining, tissues were fixed in 10% formalin followed by paraffin embedding. Images were acquired and analyzed using Slidebook software (Intelligent Imaging) on a Zeiss Axiovert 200M microscope.
RNA extraction and Northern Blot analysis
Total RNA was isolated using Trizol (Invitrogen). RNA samples (20 μg) were resolved on 15% TBE-Urea acrylamide gels containing 20% formamide, and then either visualized with stains-all (Sigma) or transferred onto Nytran-SPC Nylon Membranes (Whatman) and UV-crosslinked. The membranes were prehybridized at 42oC in 7% SDS/0.2 M sodium phosphate buffer and hybridized overnight at 42oC with an α32P dATP 3′ end-labeled oligoprobe against the siRNA antisense strand. The sequences of the DNA oligoprobes were:
siRNA nectin: 5′ CCTGCATTGTCAACTATCA3′
siRNA UL29: 5′ CTTTCGCAATCAATTCCAA 3′
After washing twice at room temperature with 2X SSC/0.1% SDS, the membranes were developed and bands quantified using a PhosphorImager (Storm 860, GE Healthcare). Membranes were probed with an oligoprobe against U6 as a loading control, which was used to normalize the intensity of each band.
Statistical analysis
The main outcome was overall survival (OS), defined as the time from treatment to death. OS curves were obtained using the Kaplan-Meier method (Kaplan et al., 1958), and compared by the log rank test (Mantel, 1966). We also tested the effect of treatment while adjusting for the effect of time of viral challenge. The log-rank linear rank statistics were computed by pooling over the strata defined by the time of the challenge, thus adjusting for the effect of time of the challenge. All in vitro measurements were compared by two-sample t-test. The half-lives of the nectin-1 and UL29 siRNAs were calculated after logarithmic transformation of the relative siRNA/U6 values and measuring the slope of the linear regression versus time. All P-values reflect two-sided tests of significance.
Acknowledgments
This work was supported by NIH grant AI070302 (JL), an amfAR Scholar Award (DP) and a CFAR pilot project grant AECOM/MMC 2P30AI051519 (DP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bitko V, Musiyenko A, Shulyayeva O, Barik S. Inhibition of respiratory viruses by nasally administered siRNA. Nat Med. 2005;11:50–55. doi: 10.1038/nm1164. [DOI] [PubMed] [Google Scholar]
- Choung S, Kim YJ, Kim S, Park HO, Choi YC. Chemical modification of siRNAs to improve serum stability without loss of efficacy. Biochem Biophys Res Commun. 2006;342:919–927. doi: 10.1016/j.bbrc.2006.02.049. [DOI] [PubMed] [Google Scholar]
- Cocchi F, Menotti L, Mirandola P, Lopez M, Campadelli-Fiume G. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J Virol. 1998;72:9992–10002. doi: 10.1128/jvi.72.12.9992-10002.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey L, Wald A, Celum CL, Quinn TC. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J Acquir Immune Defic Syndr. 2004;35:435–445. doi: 10.1097/00126334-200404150-00001. [DOI] [PubMed] [Google Scholar]
- Galen B, Cheshenko N, Tuyama A, Ramratnam B, Herold BC. Access to nectin favors herpes simplex virus infection at the apical surface of polarized human epithelial cells. J Virol. 2006;80:12209–12218. doi: 10.1128/JVI.01503-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Knipe DM. Genetic evidence for multiple nuclear functions of the herpes simplex virus ICP8 DNA-binding protein. J Virol. 1989;63:5258–5267. doi: 10.1128/jvi.63.12.5258-5267.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraghty RJ, Krummenacher C, Cohen GH, Eisenberg RJ, Spear PG. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- Jones CA, Taylor TJ, Knipe DM. Biological properties of herpes simplex virus 2 replication-defective mutant strains in a murine nasal infection model. Virology. 2000;278:137–150. doi: 10.1006/viro.2000.0628. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Meier P. Nonparametric Estimation from Incomplete Observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ, Albuquerque RJ, Yamasaki S, Itaya M, Pan Y, et al. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452:591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummenacher C, Nicola AV, Whitbeck JC, Lou H, Hou W, Lambris JD, Geraghty RJ, Spear PG, Cohen GH, Eisenberg RJ. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J Virol. 1998;72:7064–7074. doi: 10.1128/jvi.72.9.7064-7074.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan MM, Richman S, Krummenacher C, Eisenberg RJ, Cohen GH, Iwasaki A. In vivo role of nectin-1 in entry of herpes simplex virus type 1 (HSV-1) and HSV-2 through the vaginal mucosa. J Virol. 2004;78:2530–2536. doi: 10.1128/JVI.78.5.2530-2536.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantel N. Evaluation of Survival Data and Two New Rank Order Statistics Arising in Its Consideration. Cancer Chemotherap Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- Montgomery RI, Warner MS, Lum BJ, Spear PG. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- Morrison LA, Da Costa XJ, Knipe DM. Influence of mucosal and parenteral immunization with a replication-defective mutant of HSV-2 on immune responses and protection from genital challenge. Virology. 1998;243:178–187. doi: 10.1006/viro.1998.9047. [DOI] [PubMed] [Google Scholar]
- Novina CD, Murray MF, Dykxhoorn DM, Beresford PJ, Riess J, Lee SK, Collman RG, Lieberman J, Shankar P, Sharp PA. siRNA-directed inhibition of HIV-1 infection. Nat Med. 2002;8:681–686. doi: 10.1038/nm725. [DOI] [PubMed] [Google Scholar]
- Palliser D, Chowdhury D, Wang QY, Lee SJ, Bronson RT, Knipe DM, Lieberman J. An siRNA-based microbicide protects mice from lethal herpes simplex virus 2 infection. Nature. 2006;439:89–94. doi: 10.1038/nature04263. [DOI] [PubMed] [Google Scholar]
- Parr MB, Kepple L, McDermott MR, Drew MD, Bozzola JJ, Parr EL. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab Invest. 1994;70:369–380. [PubMed] [Google Scholar]
- Plasterk RH. RNA silencing: the genome’s immune system. Science. 2002;296:1263–1265. doi: 10.1126/science.1072148. [DOI] [PubMed] [Google Scholar]
- Robbins M, Judge A, Ambegia E, Choi C, Yaworski E, Palmer L, McClintock K, Maclachlan I. Misinterpreting the therapeutic effects of siRNA caused by immune stimulation. Hum Gene Ther. 2008 doi: 10.1089/hum.2008.131. e publication. [DOI] [PubMed] [Google Scholar]
- Schlee M, Hornung V, Hartmann G. siRNA and isRNA: two edges of one sword. Mol Ther. 2006;14:463–470. doi: 10.1016/j.ymthe.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Serwadda D, Gray RH, Sewankambo NK, Wabwire-Mangen F, Chen MZ, Quinn TC, Lutalo T, Kiwanuka N, Kigozi G, Nalugoda F, et al. Human immunodeficiency virus acquisition associated with genital ulcer disease and herpes simplex virus type 2 infection: a nested case-control study in Rakai, Uganda. J Infect Dis. 2003;188:1492–1497. doi: 10.1086/379333. [DOI] [PubMed] [Google Scholar]
- Shukla D, Dal Canto MC, Rowe CL, Spear PG. Striking similarity of murine nectin-1alpha to human nectin-1alpha (HveC) in sequence and activity as a glycoprotein D receptor for alphaherpesvirus entry. J Virol. 2000;74:11773–11781. doi: 10.1128/jvi.74.24.11773-11781.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E, Lee SK, Dykxhoorn DM, Novina C, Zhang D, Crawford K, Cerny J, Sharp PA, Lieberman J, Manjunath N, et al. Sustained small interfering RNA-mediated human immunodeficiency virus type 1 inhibition in primary macrophages. J Virol. 2003;77:7174–181. doi: 10.1128/JVI.77.13.7174-7181.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, Geick A, Hadwiger P, Harborth J, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- Spang AE, Godowski PJ, Knipe DM. Characterization of herpes simplex virus 2 temperature-sensitive mutants whose lesions map in or near the coding sequences for the major DNA-binding protein. J Virol. 1983;45:332–342. doi: 10.1128/jvi.45.1.332-342.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Hu D, Bustos T, Zlotogora J, Richieri-Costa A, Helms JA, Spritz RA. Mutations of PVRL1, encoding a cell-cell adhesion molecule/herpesvirus receptor, in cleft lip/palate-ectodermal dysplasia. Nat Genet. 2000;25:427–430. doi: 10.1038/78119. [DOI] [PubMed] [Google Scholar]
- Taylor JM, Lin E, Susmarski N, Yoon M, Zago A, Ware CF, Pfeffer K, Miyoshi J, Takai Y, Spear PG. Alternative entry receptors for herpes simplex virus and their roles in disease. Cell Host Microbe. 2007;2:19–28. doi: 10.1016/j.chom.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald A. Herpes simplex virus type 2 transmission: risk factors and virus shedding. Herpes. 2004;11(Suppl 3):130A–137A. [PubMed] [Google Scholar]
- Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis. 2002;185:45–52. doi: 10.1086/338231. [DOI] [PubMed] [Google Scholar]
- Whitley RJ. In: Field’s Virology. Knipe DM, Howley PM, editors. Philadelphia: Lippincott, Williams and Wilkins; 2001. pp. 2461–2510. [Google Scholar]
- Zhang Y, Cristofaro P, Silbermann R, Pusch O, Boden D, Konkin T, Hovanesian V, Monfils PR, Resnick M, Moss SF, et al. Engineering mucosal RNA interference in vivo. Mol Ther. 2006;14:336–342. doi: 10.1016/j.ymthe.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, Knipe DM, Iwasaki A. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J Exp Med. 2003;197:153–162. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, Harborth J, Heyes JA, Jeffs LB, John M, et al. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111–114. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]