Abstract
Drug-inducible lentiviruses encoding Oct4, Sox2, Klf4 and c-Myc were used to derive “primary” iPS cells and segregated through germline transmission generating mice and fibroblasts carrying subsets of the reprogramming factors. Drug treatment of the cells resulted in “secondary” iPS cell derivation only when the missing factor was introduced. This creates a defined platform for studying reprogramming mechanisms and allows screening of genetically homogenous cells for compounds that replace any transcription factor required for iPS cell derivation.
The generation of induced pluripotent stem (iPS) cells from mouse and human somatic cells through the forced expression of defined transcription factors1–4 constitutes a major breakthrough in regenerative biology5. However, current reprogramming strategies require viral transduction with potentially oncogenic transcription factors. Understanding the molecular changes underlying iPS cell derivation will be required to devise alternative and safer strategies for reprogramming e.g., by replacing the viral transduced factors with small molecules6–8.
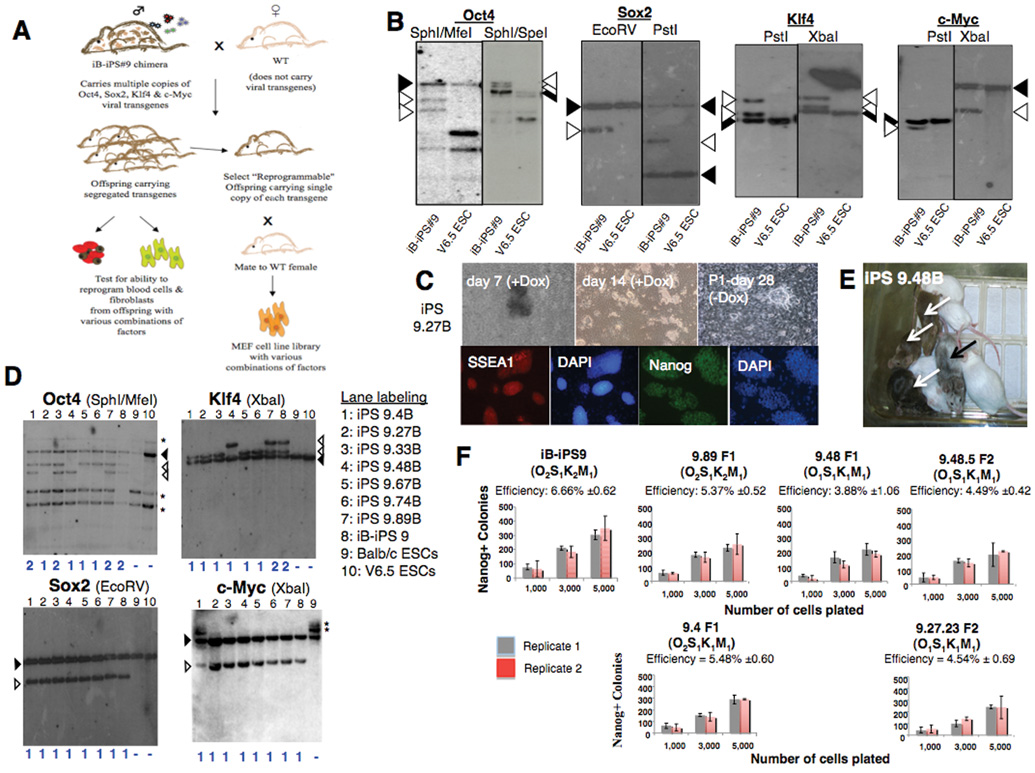
Screening approaches using infected cells are hampered by the genetic variability caused by the random integrations of multiple proviral copies9,10. Recently, we generated a “secondary” transgenic system that eliminates such heterogeneity9,10. In this approach, mouse embryonic fibroblasts (MEFs) heterozygous for the ROSA26-M2 reverse tetracycline transactivator (M2-rtTA) were infected with Doxycycline (Dox)-inducible lentiviruses carrying the four reprogramming factors (Oct4, Sox2, Klf4 and c-Myc) and induced to generate “primary” iPS cells by addition of Dox. These cells were used to obtain chimeric mice with genetically identical somatic cells that can be isolated and reprogrammed in vitro by addition of Dox. However, such “secondary” somatic cells require isolation from chimeric mice and contain copies of all four factors required for reprogramming, thus impeding their use in drug screens aimed at identifying components that can substitute for a given transcription factor. Here we describe the generation of genetically homogeneous mice and MEF lines containing different combinations of a defined set of Dox-inducible proviral genomes. This was achieved through random segregation of the integrated lentiviruses after germline transmission from “primary” iPS-derived chimeras (Fig.1A). We used the previously described Pro B cell-derived iB-iPS#9 cell line10 which carried a single c-Myc and Sox2 and two Klf4 and Oct4 proviral copies, respectively (O2S1K2M1) (Fig.1B, Supplementary Fig.1 online). To produce transgenic offspring an iB-iPS#9 chimera that transmitted the transgenes through the germline in 100% of the offspring was crossed to wild-type females (Fig.1A) and 91 individual offspring were genotyped. This analysis indentified mice carrying all possible combinations of one, two, three or all four vectors (supplementary Fig.2 online).
Figure 1. “Reprogrammable mice” carrying single copies of reprogramming factors.
A) Experimental outline. iB-iPS#9 chimera10 is mated to generate offspring with different transgene copy number. Blood and tail fibroblasts were collected from adult offspring and MEF cultures were established from day E13.5 embryos. B) Southern analysis of iBiPS#9 line and V6.5 ESCs as controls. Filled arrowheads: endogenous bands; open arrowheads: proviral integrations. C) Top Panels: iPS colony formation from F1 offspring 9.27 (O1S1K1M1). Immuno-fluorescent analysis of the same iPS cell line that grew independently of Dox is shown in the lower panel. D) Southern analysis of F1 progeny blood derived iPS lines *: non-specific background bands. E) iPS cells contribute to chimeras (black arrow) that exhibit germline transmission (transgenic offspring: white arrows). F) Reprogramming efficiency of CD11b+ cells, 28 days after Dox induction. Efficiencies calculated as the fraction of Nanog positive colonies to cells seeded. Error bars: SD in duplicate wells. The generation (F1 or F2) and transgene copy number (subscript) are shown. “B” indicates iPS-line derived from peripheral blood.
We determined whether germline transmission of the inducible transgenes would interfere with their ability to reprogram secondary somatic cells upon exposure to Dox. Peripheral blood samples were collected from 90 adult progeny obtained from the iB-iPS#9 chimera and cultured in the presence of Dox. Initial colonies (Fig.1C) appeared after 7–16 days of Dox induction in all seven samples derived from mice positive for M2-rtTA and all four factors (Supplementary Tables 1–2 online). All lines were expanded without Dox, had an ES cell-like morphology and expressed SSEA-1 and Nanog (Fig.1C). Four lines (iPS 9.27B, 9.48B, 9.67B and 9.74B) carried a single copy each of Oct4, Sox2, Klf4 and c-Myc (O1S1K1M1) (Fig.1D). Several iPS lines were injected into blastocysts (Supplementary Table 3 online) and produced chimeras with germline contribution (Fig.1E). To determine whether the copy number of Oct4 and/or Klf4 affected the reprogramming process, we analyzed reprogramming efficiency and kinetics of CD11b+ cells. No major differences were observed between cells carrying multiple or single copies of the reprogramming factors from F1 and F2 donor mice (Fig.1F and Supplementary Fig.3 online). These results together with the derivation of iPS lines from all M2Rtta+/− mice that carried at least one copy of each vector (Supplementary Tables 1–2 online) demonstrate that the lentiviral transgenes are not silenced following transmission through the germline. Also, multiple somatic cell types (tail-tip-derived fibroblasts, keratinocytes, liver cells and lymphocytes) from mice carrying single copies of each of the reprogramming factors were efficiently reprogrammed (Supplementary Fig.4–5 online).
We generated somatic cell lines with different combinations of factors by crossing transgenic male 9.27 (O1S1K1M1; Fig.1D) with wild-type females. MEF cultures were established from individual embryos and genotyped for the segregated transgenes (Fig.2A). “Single-copy four-factor” (O1S1K1M1) MEF lines reproducibly generated iPS cells with approximately 1% efficiency (Fig.2B, Supplementary Fig.6 online). In contrast, no iPS colony formation was observed with “three-factor” lines i.e., OSK (n=3), OSM (n=2), SKM (n=1), OKM (n=3) (Fig.2B). However, when these MEF lines were transduced with the missing factor and grown in the presence of Dox, iPS colonies appeared within 14–21 days (Fig.2B and Supplementary Fig.S7 online) at efficiencies comparable to the highest reported for fibroblasts9. All lines grew Dox-independently, expressed pluripotency markers and induced teratomas in vivo (Fig.2B, Supplementary Fig.S8 online).
Figure 2. MEF line library carrying different combinations of reprogramming factors.
A) PCR genotyping of select independent M2-rtTa+ MEF lines from mating offspring 9.27 (O1S1K1M1) to wild-type females. Genotype is indicated at the bottom. B) iPS cell derivation from MEF lines carrying three or more factor combinations. Missing factor was introduced by infection with TetO-FUW lentivirus (FUW) carrying the missing transcription factor. NA: not applicable, ND: not determined. The efficiencies reported are based on Nanog+ colonies fixed 30 days after plating 10,000 cells and addition of Dox. C) iPS cells from three factor MEF lines lacking c-Myc after transduction with Klf4. 200,000 O1S1K1 MEFs were infected with the indicated control virus and cultured in the present of Dox without passaging. Image of primary colony on Day 42 of Dox induction after infection with FUW-Klf4. Primary colonies were picked and passaged without Dox and expressed Nanog. Nine independent lines derived from two experiments. D) Kinetics of Nanog-GFP knock-in allele expression in two-factor lines, pre-treated or not with Dox, after transduction of the missing factors. 20,000 infected cells were seeded per well. Two wells were harvested every 48 hours for detection of Nanog-GFP by FACS. Nanog-GFP was defined by achieving >0.8% GFP positive cells. Blue dashed line: day of infection (d0). Pretreatment with Dox was done for 16 days. Two independent experimental sets are shown. Efficiency was determined after 28 days of Dox treatment as number of Nanog-GFP+ colonies per 10,000 cells initially seeded.
In contrast to previous reports11,12, reprogramming of tail-derived or embryonic fibroblasts (similar to peripheral blood cells) was not possible from three factor lines lacking c-Myc (Fig.2B) possibly because of suboptimal stoichiometry of the 3 factors. Indeed, infection of O1S1K1 (no c-Myc) fibroblasts with lentivirus expressing Klf4, but not Oct4 or Sox2 or GFP (control) viruses, allowed derivation of iPS lines (Fig.2C), suggesting that higher levels of Klf4 can substitute for the action of c-Myc. When M1K1 MEFs were pretreated with Dox prior to transduction with Sox2 and Oct4, we observed enhanced reprogramming efficiency and obtained Nanog-GFP positive iPS cells already after 12–14 days instead of 22–24 days (Fig.2D). In contrast, Dox pretreatment of O1S1 MEFs prior to re-infection with c-Myc and Klf4 lentiviruses did not alter reprogramming kinetics or efficiency (Fig.2D). This indicates that early induction of c-Myc and Klf4 sensitizes fibroblasts for the ectopic expression of Oct4 and Sox2 and enhances their reprogramming speed and efficiency. These results are consistent with the hypothesis that c-Myc and/or Klf4 might induce epigenetic changes that facilitate the interaction of Oct4 and Sox2 with their targets resulting in more rapid repgoramming1.
About 12% of the mice developed skin epithelial tumors, even though they were not treated with Dox, suggesting leaky transgene expression in our system. Tumors were only observed in mice carrying all three M2rTta, c-Myc and Oct-4 alleles (Supplementary Fig.9 online), indicating that Oct4 reactivation can also act in concert with c-Myc in tumor formation.
In addition to their potential use in high-throughput drug screens, somatic cell lines and mouse strains that are genetically identical and possess different combinations of minimal copy number of drug-inducible reprogramming factors will be useful for the study of reprogramming mechanisms and unraveling the mechanism of action of certain chemical compounds that modulate iPS generation, which remain largely unknown1. Such studies will enhance our understanding of how each of the reprogramming factors contributes to the rewiring of the transcriptional network and epigenetic state in differentiated somatic cells during the reprogramming process1.
Supplementary Material
Acknowledgements
We thank G. Bell, R. Flannery and members of the Jaenisch lab for assistance and comments. R.J. is supported by grants from the NIH:5-RO1-HDO45022, 5-R37-CA084198, and 5-RO1-CA087869. J.H. is a Novartis Fellow by the Helen Hay Whitney Foundation.
Footnotes
Conflict of interest: R.J. is an advisor to Stemgent.
Statement: The mice carrying the inducible reprogramming factors will be deposited at the Jackson Laboratory for distribution as soon as animals that are homozygous for a given transgene combination have been obtained.
References
- 1.Jaenisch R, Young R. Cell. 2008;132:567–582. doi: 10.1016/j.cell.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahashi K, Yamanaka S. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Wernig M, et al. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K, et al. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Hanna J, et al. Science. 2007;318:1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 6.Shi Y, et al. Cell Stem Cell. 2008;3:568–574. doi: 10.1016/j.stem.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Marson A, et al. Cell Stem Cell. 2008;3:132–135. doi: 10.1016/j.stem.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huangfu D, et al. Nature biotechnology. 2008;26:1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 9.Wernig M, et al. Nature biotechnology. 2008;26:916–924. doi: 10.1038/nbt1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanna J, et al. Cell. 2008;133:250–264. doi: 10.1016/j.cell.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wernig M, et al. Cell Stem Cell. 2008;2:10–12. doi: 10.1016/j.stem.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa M, et al. Nature biotechnology. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.