Abstract
Multidrug resistance (MDR) of breast cancer cells still represents an unmet medical need in chemotherapy. To this end, the purpose of this study was to determine efficacy of paclitaxel loaded in sterically stabilized, biocompatible and biodegradable sterically stabilized mixed phospholipid nanomicelles (SSMM; size, ~15 nm) and actively targeted vasoactive intestinal peptide-grafted SSMM (SSMM-VIP) in circumventing P-gp-mediated paclitaxel resistance in BC19/3 cells, a human breast cancer cell line that expresses >10-fold higher P-gp than its parental sensitive cell line, MCF-7. We found that in drug sensitive MCF-7 cells, paclitaxel loaded in SSMM (P-SSMM) and SSMM-VIP (P-SSMM-VIP) significantly inhibited cell growth in dose-dependent fashion (p<0.05). Both formulations were ~7-fold more potent than paclitaxel dissolved in DMSO (P-DMSO). Efficacy of P-SSMM and P-SSMM-VIP was similar (p>0.5). By contrast, in drug resistant BC19/3 cells, P-SSMM-VIP was significantly more effective than either P-SSMM or P-DMSO (~2- and 5-fold, respectively; p<0.05). Collectively, these data indicate that actively targeted paclitaxel-loaded SSMM-VIP overcomes multiple drug resistance of BC19/3 cells. We suggest this formulation should be further developed to treat MDR breast cancer.
Keywords: P-glycoprotein, nanomedicine, phospholipids, targeted drug delivery, VIP
INTRODUCTION
Despite recent advances in chemotherapy of malignant, multidrug resistance (MDR) still represents an unmet medical need in breast cancer chemotherapy [1,2].
Although the mechanisms underlying MDR are complex and multifactorial, resistance to cytotoxic drugs is most often linked to overexpression of P-glycoprotein (Pgp) [1,2]. Given Pgp is also expressed in normal tissues, mitigating MDR of breast cancer cells by systemic, non-selective administration of drugs could lead to serious adverse events [3-4]. Accordingly, passive and active targeting of chemotherapeutic drugs to overcome MDR of breast cancer cells using various carriers and targeting ligands selectively overexpressed in cancer cells has been advocated [5-11]. This innovative approach increases local and intracellular anti-cancer drug concentration that overwhelms resistance pathways in cancer cells while at the same time decreases systemic toxicity.
To this end, we developed sterically stabilized, biocompatible and biodegradable sterically stabilized mixed phospholipid nanomicelles (SSMM; size ~15 nm) grafted with vasoactive intestinal peptide (SSMM-VIP), a pleiotropic 28-amino acid mammalian peptide overexpressed in breast cancer cells but not in endothelial cells, to solubilize water-insoluble anticancer drugs, such as paclitaxel, and actively target them to breast cancer [12-18]. This long circulating, biocompatible and biodegradable nanocarrier composed of poly(ethylene glycol; mol. mass, 2000)-grafted disteraroyl-phosphatidylethanolamine] (DSPE-PEG2000) and egg phosphatidylcholine (PC) is simple to prepare and exhibits a low critical micellar concentration (<1 μM) which, in turn, contributes to its stability upon dilution in vivo. In addition, steric hindrance coupled with small size and active targeting with VIP moiety decreases clearance from the circulation and lowers systemic toxicity.
The purpose of this study was to evaluate efficacy of paclitaxel-loaded SSMM and SSMM-VIP in circumventing P-gp-mediated paclitaxel resistance in BC19/3 cells, a human breast cancer cell line that expresses >10-fold higher P-gp than its parental sensitive cell line, MCF-7 [18,19].
METHODS
Chemicals
Paclitaxel (>99% pure), dimethyl sulfoxide (DMSO) and (N-[2-Hydreoxyethyl] piperazine-N’-[2-ethanesulfonic acid]) (HEPES) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). Poly(ethylene glycol; mol. mass, 2000)-conjugated distearoyl phosphatidylethanolamine was obtained from Northern Lipids Inc. (Vancouver, BC, Canada). DSPE-PEG3400-succinimidyl propionate (SPA) was purchased from Nektar (Huntsville, AL). Egg-phosphatidylcholine was obtained from Lipoid GmbH (Ludwigshafen, Germany). Vasoactive intestinal peptide (VIP) was synthesized at the Protein Research Laboratory, University of Illinois at Chicago (Chicago, IL). All other reagents were purchased from Fisher Scientific (Ithasca, IL).
Breast cancer cells
BC19/3 MDR human breast cancer cell line and its parental sensitive MCF-7 cell line were kindly provided by Dr. Julie Horton (National Institute of Environmental Health Sciences, Research Triangle Park, NC) [19,20]. BC19/3 cells are MCF-7 cells transfected with human mdr1 (PGY1) cDNA isolated from doxorubicin-resistant MCF-7 cells as previously described by Fairchild et al [19]. MCF-7 cells were maintained in RPMI 1640 containing 10% fetal bovine serum at 37°C in a humidified 5% CO2 atmosphere. BC19/3 cells were grown in RPMI 1640 containing 10% fetal bovine serum and 10 nM doxorubicin under identical conditions.
Preparation of P-SSMM and P-SSMM-VIP
Paclitaxel solubilized in sterically stabilized, biocompatible and biodegradable sterically stabilized mixed phospholipid nanomicelles (P-SSMM) and SSMM-VIP (P-SSMM-VIP) were prepared as previously described in our laboratory [12-18]. Briefly, P-SSMM, 500 μg of paclitaxel and mixture of DSPE-PEG2000 and egg PC (90:10 ratio) were dissolved in methanol. Solvent was removed by vacuum rotary evaporation under stream of argon to form a dry film that was further dried under vacuum overnight to remove traces of methanol. Film was then rehydrated with 0.01 mM HEPES buffer (pH 7.4) to yield 5 mM lipid concentration by vortexing and sonicating for 5 min each. Resulting solution was then flushed with argon, sealed and equilibrated overnight at room temperature. Unsolubilized excess paclitaxel was removed by centrifugation at 13,000 g for 5 min to obtain a clear dispersion. For P-SSMM-VIP, conjugation of VIP (0.3 mM) to DSPE-PEG3400-SPA was prepared as previously described [14, 18] and then mixed with P-SSMM and left to equilibrate at room temperature for 30 min.
Particle size was determined by quasi-elastic light scattering using NICOMP 380 particle-size analyzer (Santa Barbara, CA).
Western blot analysis of VPAC1 receptors in MCF-7 and BC19/3 cells
MCF-7 and BC19/3 cells were washed twice with PBS containing 1 mM phenylmethylsulphonyl fluoride, scraped off culture dishes and pelleted at 1000 × g at 4 °C for 5 min. Cell pellets were then lysed in cold buffer [1M Tris-HCl (pH 6.8), 4% SDS, 20% glycerol, 0.2% 2-mercaptoethanol] for 20 min with occasional sonication. Protein concentration of cell homogenates was determined with Bio-Rad protein assay kit (Bio-Rad Laboratories, Richmond, CA). Twenty micrograms of protein were resolved by 12% SDS-PAGE and transferred to nitrocellulose membrane. Membrane was blocked with 5% nonfat dry milk in PBS/Tween 20 (0.05%) followed by incubation with VIP receptor antibody (VPAC1)(1:1000 dilution in 1% milk/PBS/Tween 20, Calbiochem, San Diego, CA) overnight [21]. Detection was accomplished using anti-mouse immunoglobulin G-horseradish peroxidase conjugate and enhanced chemiluminescence detection reagent (Pierce, Rockford, IL).
In vitro cytotoxicity
In vitro cytotoxicity was assessed by sulforhodamine B assay as previously described in our laboratory [12,14, 21]. Briefly, 5×103 MCF-7 and BC19/3 cells were plated in 96-well culture plates and cultured for a day to allow reattachment. Then, they were exposed to various concentrations of paclitaxel solubilized in 10% DMSO, SSMM and SSMM-VIP (0.128 ng/ml~2000ng/ml) for 72 hr. For serial dilution of P-SSMM and P-SSMM-VIP, 1 μM SSM was used to minimize micelle breakage leading to drug release from nanomicelles upon dilution. Drug-free 10% DMSO, SSMM and SSMM-VIP were used as controls. At the conclusion of drug exposure, cells were fixed to culture plates by adding 100 μl/well cold 20% trichloroacetic acid and incubating for 30 min at 4°C. Plates were then washed, air-dried and stained with 100 μl/well 0.4 % sulforhodamine B in 1% acetic acid for 30 min. Thereafter, plates were washed with 1% acetic acid twice and 200 μl/well 10 mM Tris buffer (pH 10.0) was added. Optical density was determined spectrophotometrically at 515 nm (SpectraMax Plus384, Molecular Devices, Sunnyvale, CA) and concentrations required to inhibit growth by 50% (IC50) were calculated using nonlinear regression analysis to determine cytotoxicity of paclitaxel in various formulations (Prism, GraphPad Software, San Diego, CA). Readings obtained for buffer controls were used to define 100% growth.
Data and statistical analyses
Data are reported as mean±standard deviation. Statistical analysis was performed using ANOVA followed by Tukey’s test. P<0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Expression of VPAC1 receptors using Western blot analysis revealed similar expression in MCF-7 and BC19/3 cells at ~50 kDa (Figure 1) [17, 21]. These data indicate that mdr-1 transfection and continuous exposure of doxorubicin to maintain drug resistance in BC19/3 cells does not alter VIP receptor expression in these cells [19, 20]. Accordingly, VIP receptor-targeted sterically stabilized phospholipid nanomicellar anticancer drug delivery to overcome MDR in B19/3 cells can be studied.
Figure 1.
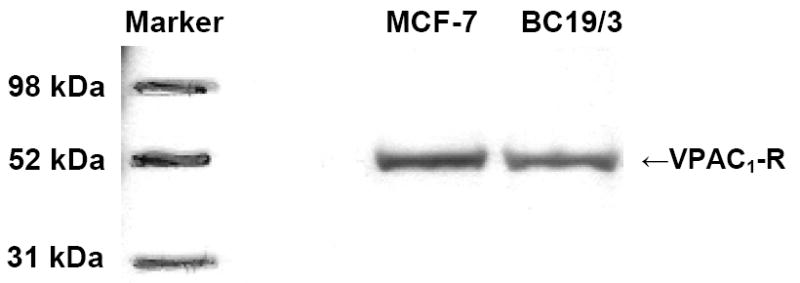
Representative Western blot of VPAC1 in MCF-7 and BC19/3 cells.
Cytotoxicity of paclitaxel dissolved in DMSO and loaded in SSMM and SSMM-VIP (size, ~15 nm) was determined in MCF-7 and BC19/3 cells (Figure 2&3). Paclitaxel loaded in SSMM and SSMM-VIP significantly inhibited MCF-7 cell growth in dose-dependent fashion with IC50 of 8.0±1.96 ng/ml and 7.1±2.04 ng/ml, respectively (Figures 2&3; each, n=3; p<0.05). IC50 of both formulation was ~7 fold higher than that of paclitaxel dissolved in DMSO (IC50 = 54.4±7.49 ng/ml; each, n=3; p<0.05; Figure 3A).
Figure 2.
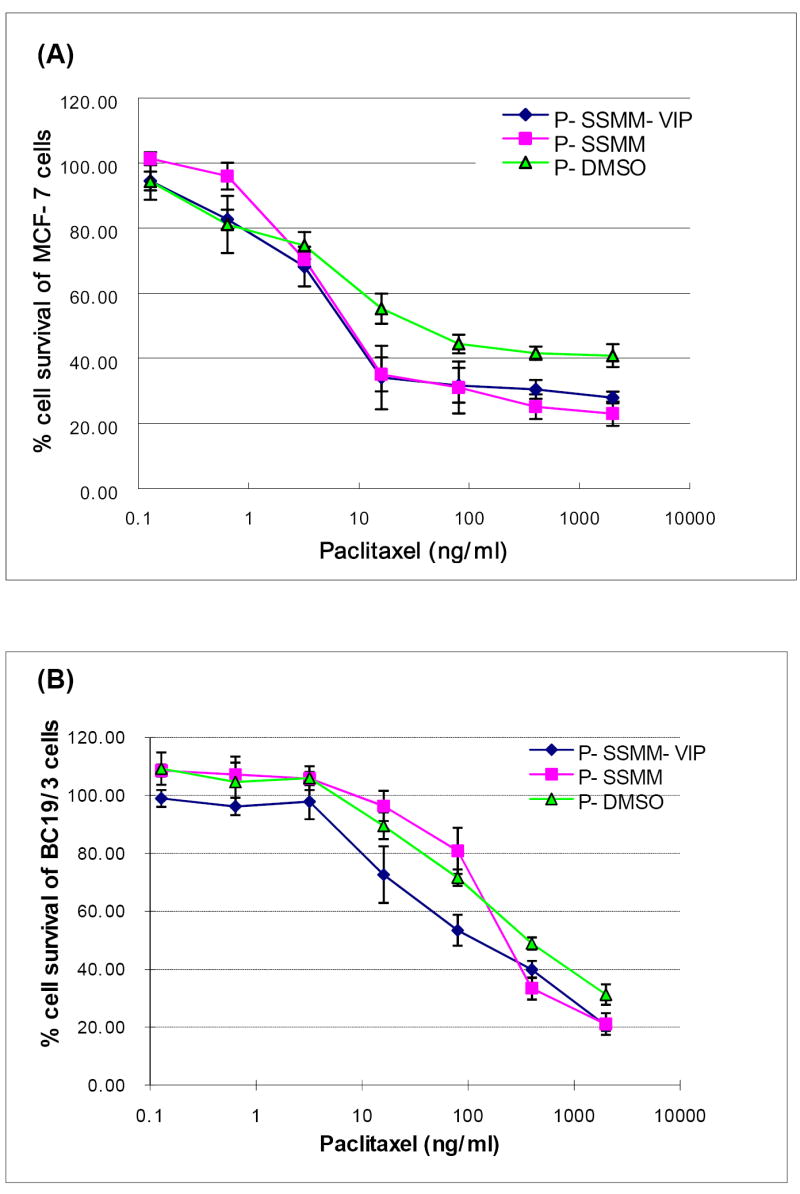
Cytotoxicity of the paclitaxel in DMSO( ), SSMM(□) and SSMM-VIP (○) in MCF-7 (A) and BC19/3 cells (B). Data are means±standard deviation of the mean of 3 separate experiments each conducted in triplicate. Significance markers were omitted for clarity (see Results and Discussion and Figure 3 for explanation).
Figure 3.
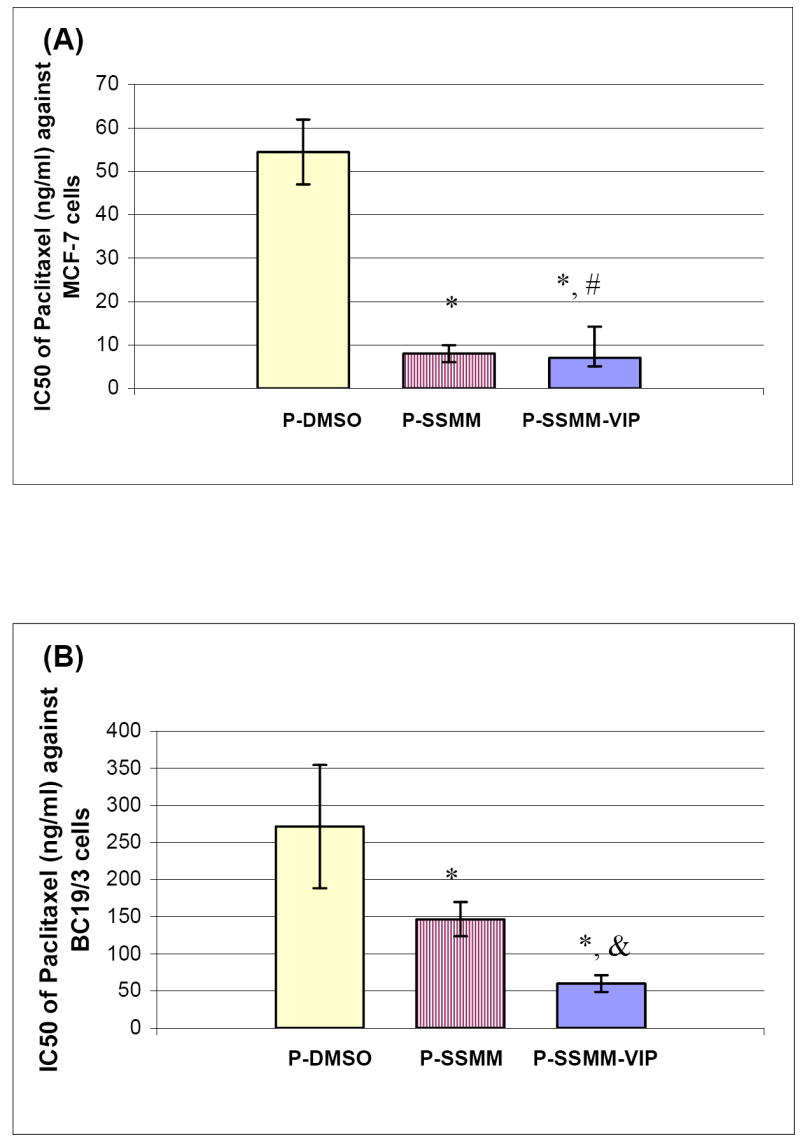
Comparison of IC50 of paclitaxel in DMSO, SSMM and SSMM-VIP for MCF-7 (A) and BC19/3 cells (B). Data are means±standard deviation of the mean of 3 separate experiments each conducted in triplicate; * and & p <0.05 in comparison to paclitaxel in DMSO and paclitaxel in SSMM, respectively; #p>0.5 in comparison to paclitaxel in SSMM.
This phenomenon may be related, in part, to active phagocytosis of nanoparticles by cancer cells due to rapid cell replication. However, IC50 of both P-SSMM and P-SSMM-VIP was similar due, most likely, to VIP receptor saturation leading to limited contribution of receptor-mediated particle internalization to overall nanomicellar paclitaxel uptake by cells. This notion is supported by previous studies from our laboratory on internalization of SSMM and SSMM-VIP loaded with quantum dots into MCF-7 cells [18]. We found that initial rate of SSMM-VIP internalization was significantly faster than that of SSMM but within few hours of incubation (less than that tested in the present study) both uptake rates were similar because of VIP receptor saturation.
Unlike the results of this study, we have previously shown that paclitaxel cytotoxicity to MCF-7 cells is similar when solubilized in SSMM and 10% DMSO [12]. This discrepancy could be attributed, in part, to differences in experimental design used in these studies. Conceivably, substantial dilution of P-SSMM and P-SSMM-VIP with buffer in absence of empty nanomicelles reported in our previous study could result in nanomicelle breakdown and release of paclitaxel which would then precipitate in vicinity of cancer cells. Accordingly, paclitaxel delivered to cells by this nanocarrier system now acts similarly to paclitaxel dissolved in 10% DMSO resulting in similar cytotoxicity. In contrast, in the present study we used excess empty SSMM (1 μM) during serial dilutions of P-SSMM and P-SSMM-VIP to ascertain whether sufficient monomers were available in solution to maintain a concentration above critical micellar concentration thereby minimizing breakage of paclitaxel-loaded nanomicelles upon dilution.
We found that IC50 of P-SSMM-VIP in BC19/3 cells (59.76±11.27 ng/ml) was significantly lower than that of P-SSMM and P-DMSO (136.7±23.0 ng/ml and 282.3±82.9 ng/ml, respectively; each, n=3; p<0.05; Figure 3B). Taken together, these data indicate that VIP receptor-mediated paclitaxel internalization elicits appreciable cytotoxicity in MDR breast cancer cells irrespective of Pgp efflux pump. Although the mechanism(s) underlying this phenomenon was not investigated in this study, several hypotheses could be advanced [19, 20, 22-26]. Firstly, DSPE-PEG2000 and egg PC upon interacting with BC19/3 cells may alter composition and fluidity of cell membrane thereby altering conformation and activity of membrane spanning proteins such as Pgp. In addition, both DSPE-PEG2000 and egg PC may themselves be substrates for Pgp thereby competing with paclitaxel for Pgp-mediated efflux from BC19/3 cells. This, in turn, increases intracellular drug concentration leading to cell death. While paclitaxel dissolved in DMSO enters cancer cells by passive diffusion, SSMM may transport paclitaxel into cancer cells by several mechanisms at any given time, such as diffusion and endocytosis, thereby further saturating Pgp-mediated efflux relative to paclitaxel alone. Lastly, both DSPE-PEG2000 and egg PC may alter Pgp activity in BC19/3 cells by activating intracellular signal transduction mechanism(s) that downregulates Pgp. Clearly, additional studies are indicated to support or refute this hypothesis.
Cytotoxicity of paclitaxel solubilized in SSMM-VIP to BC19/3 cells was significantly higher than that of paclitaxel solubilized in SSMM (Figures 2&3). We propose that multidrug resistant cancer cells express significantly greater quantities of the membrane efflux transporters and possess less nonspecific phagocytic particle activity because of increased membrane rigidity [24-26]. Hence, in resistant cancer cells particle uptake by nonspecific mechanism(s) is inefficient while receptor-mediated particle internalization predominates. In addition, receptor-mediated intracellular entry of paclitaxel-loaded SSMM-VIP followed by entrapment in endosome/lysosome renders the drug inaccessible for Pgp. Subsequent release of large concentrations of paclitaxel from endosome/lysosome then overwhelms Pgp efflux and leads to cell death as outlined above.
In summary, paclitaxel-loaded sterically stabilized, biocompatible and biodegradable phospholipid mixed nanomicelles markedly increased cytotoxicity of the drug in both sensitive and resistant human breast cancer cell lines. Importantly, VIP receptor-targeted delivery of paclitaxel-loaded nanomicelles amplified cytotoxicity toward MDR breast cancer cells even further. We suggest this formulation should be further developed to treat MDR breast cancer.
Acknowledgments
We thank Dr. Julie Horton for providing MCF-7 and B19/3 cells. This study was supported, in part, by VA Merit Review, Department of Defense grant BCRP DAMD 17-02-1-0415 and NIH grants R01 CA121797, R01 AG024026, R01 HL72323 and C06RR15482.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boumendjel A, Baubichon-Cortay H, Trompier D, Perrotton T, Di Pietro A. Anticancer multidrug resistance mediated by MRP1: recent advances in the discovery of reversal agents. Med Res Rev. 2005;25:453–472. doi: 10.1002/med.20032. [DOI] [PubMed] [Google Scholar]
- 2.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 3.Theis JG, Chan HS, Greenberg ML, Malkin D, Karaskov V, Moncica I, Koren G, Doyle J. Increased systemic toxicity of sarcoma chemotherapy due to combination with the P-glycoprotein inhibitor cyclosporine. Int J Clin Pharmacol Ther. 1998;36:61–64. [PubMed] [Google Scholar]
- 4.Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987;84:7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carrion C, de Madariaga MA, Domingo JC. In vitro cytotoxic study of immunoliposomal doxorubicin targeted to human CD34(+) leukemic cells. Life Sci. 2004;75:313–328. doi: 10.1016/j.lfs.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 6.Mamot C, Drummond DC, Hong K, Kirpotin DB, Park JW. Liposome-based approaches to overcome anticancer drug resistance. Drug Resist Updat. 2003;6:271–279. doi: 10.1016/s1368-7646(03)00082-7. [DOI] [PubMed] [Google Scholar]
- 7.Mayer LD, Shabbits JA. The role for liposomal drug delivery in molecular and pharmacological strategies to overcome multidrug resistance. Cancer Metastasis Rev. 2001;20:87–93. doi: 10.1023/a:1013108524062. [DOI] [PubMed] [Google Scholar]
- 8.Vauthier C, Dubernet C, Chauvierre C, Brigger I, Couvreur P. Drug delivery to resistant tumors: the potential of poly(alkyl cyanoacrylate) nanoparticles. J Control Release. 2003;93:151–160. doi: 10.1016/j.jconrel.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Alakhov VY, Moskaleva EY, Batrakova EV, Kabanov AV. Hypersensitization of multidrug resistant human ovarian carcinoma cells by pluronic P85 block copolymer. Bioconjug Chem. 1996;7:209–216. doi: 10.1021/bc950093n. [DOI] [PubMed] [Google Scholar]
- 10.Gaber MH. Modulation of doxorubicin resistance in multidrug-resistance cells by targeted liposomes combined with hyperthermia. J Biochem Mol Biol Biophys. 2002;6:309–314. doi: 10.1080/10258140290033066. [DOI] [PubMed] [Google Scholar]
- 11.St’astny M, Strohalm J, Plocova D, Ulbrich K, Rihova B. A possibility to overcome P-glycoprotein (PGP)-mediated multidrug resistance by antibody-targeted drugs conjugated to N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer carrier. Eur J Cancer. 1999;35:459–466. doi: 10.1016/s0959-8049(98)00373-6. [DOI] [PubMed] [Google Scholar]
- 12.Krishnadas A, Rubinstein I, Önyüksel H. Sterically stabilized phospholipid mixed micelles: in vitro evaluation as a novel carrier for water-insoluble drugs. Pharm Res. 2003;20:297–302. doi: 10.1023/a:1022243709003. [DOI] [PubMed] [Google Scholar]
- 13.Koo O, Rubinstein I, Önyüksel H. Role of nanotechnology in targeted drug delivery and imaging: a concise review. Nanomedicine. 2005;1:193–212. doi: 10.1016/j.nano.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Krishnadas A, Rubinstein I, Önyüksel H. Vasoactive intestinal peptide (VIP) conjugated phospholipid nanocarrier for active targeted delivery of paclitaxel to breast cancer. Proc Intl Symp Control Rel Bioact Mater. 2004:497. [Google Scholar]
- 15.Ashok B, Arleth L, Hjelm RP, Rubinstein I, Önyüksel H. In vitro characterization of PEGylated phospholipid micelles for improved drug solubilization: effects of PEG chain length and PC incorporation. J Pharm Sci. 2004;93:2476–2487. doi: 10.1002/jps.20150. [DOI] [PubMed] [Google Scholar]
- 16.Arleth L, Ashok B, Önyüksel H, Thiyagarajan P, Jacob J, Hjelm RP. Detailed structure of hairy mixed micelles formed by phosphatidylcholine and PEGylated phospholipids in aqueous media. Langmuir. 2005;21:3279–3290. doi: 10.1021/la047588y. [DOI] [PubMed] [Google Scholar]
- 17.Dagar S, Sekosan M, Lee BS, Rubinstein I, Önyüksel H. VIP receptors as molecular targets of breast cancer: implications for targeted imaging and drug delivery. J Control Release. 2001;74:129–134. doi: 10.1016/s0168-3659(01)00326-1. [DOI] [PubMed] [Google Scholar]
- 18.Rubinstein I, Soos I, Önyüksel H. Intracellular delivery of VIP-grafted sterically stabilized phospholipid mixed nanomicelles in human breast cancer cells. Chem Biol Interact. 2008;171:190–194. doi: 10.1016/j.cbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li D, Au JL. Mdr1 transfection causes enhanced apoptosis by paclitaxel: an effect independent of drug efflux function of P-glycoprotein. Pharm Res. 2001;18:907–913. doi: 10.1023/a:1010919823936. [DOI] [PubMed] [Google Scholar]
- 20.Fairchild CR, Moscow JA, O’Brien EE, Cowan KH. Multidrug resistance in cells transfected with human genes encoding a variant P-glycoprotein and glutathione S-transferase-pi. Mol Pharmacol. 1990;37:801–809. [PubMed] [Google Scholar]
- 21.Moody TW, Dudek J, Zakowicz H, Walters J, Jensen RT, Petricoin E, Couldrey C, Green JE. VIP receptor antagonists inhibit mammary carcinogenesis in C3(1)SV40T antigen mice. Life Sci. 2004;74:1345–1357. doi: 10.1016/j.lfs.2003.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Likhitwitayawuid K, Angerhofer CK, Cordell GA, Pezzuto JM, Ruangrungsi N. Cytotoxic and antimalarial bisbenzylisoquinoline alkaloids from Stephania erecta. J Nat Prod. 1993;56:30–38. doi: 10.1021/np50091a005. [DOI] [PubMed] [Google Scholar]
- 23.Bosch I, Dunussi-Joannopoulos K, Wu RL, Furlong ST, Croop J. Phosphatidylcholine and phosphatidylethanolamine behave as substrates of the human MDR1 P-glycoprotein. Biochemistry. 1997;36:5685–5694. doi: 10.1021/bi962728r. [DOI] [PubMed] [Google Scholar]
- 24.Seydel JK. Drug-membrane interactions and pharmacodynamics. Wiley-VCH Verlag GmbH & Co KGaA; 2003. pp. 1–33. [Google Scholar]
- 25.Goren D, Horowitz AT, Tzemach D, Tarshish M, Zalipsky S, Gabizon A. Nuclear delivery of doxorubicin via folate-targeted liposomes with bypass of multidrug-resistance efflux pump. Clin Cancer Res. 2000;6:1949–1957. [PubMed] [Google Scholar]
- 26.Moskaleva EY, Posypanova GA, Shmyrev II, Rodina AV, Muizhnek EL, Severin ES, Katukov VY, Luzhkov YM, Severin SE. Alpha-fetoprotein-mediated targeting--a new strategy to overcome multidrug resistance of tumour cells in vitro. Cell Biol Int. 1997;21:793–799. doi: 10.1006/cbir.1998.0201. [DOI] [PubMed] [Google Scholar]


