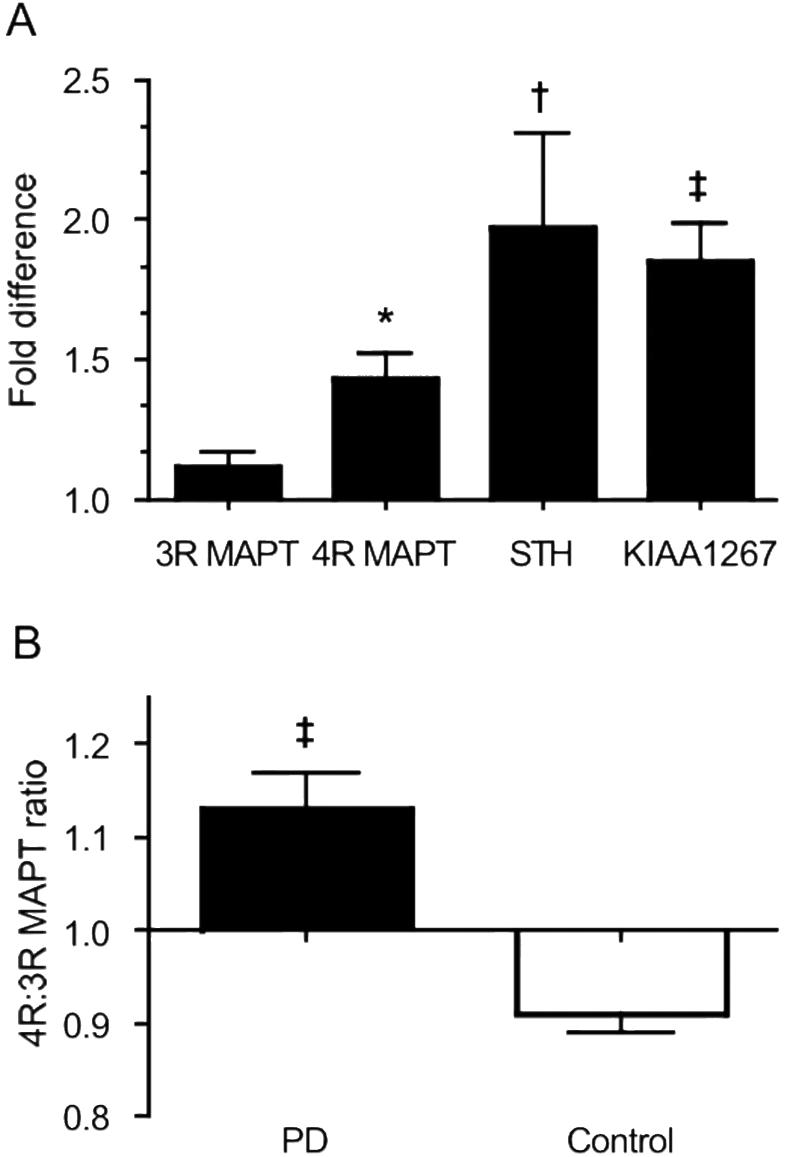
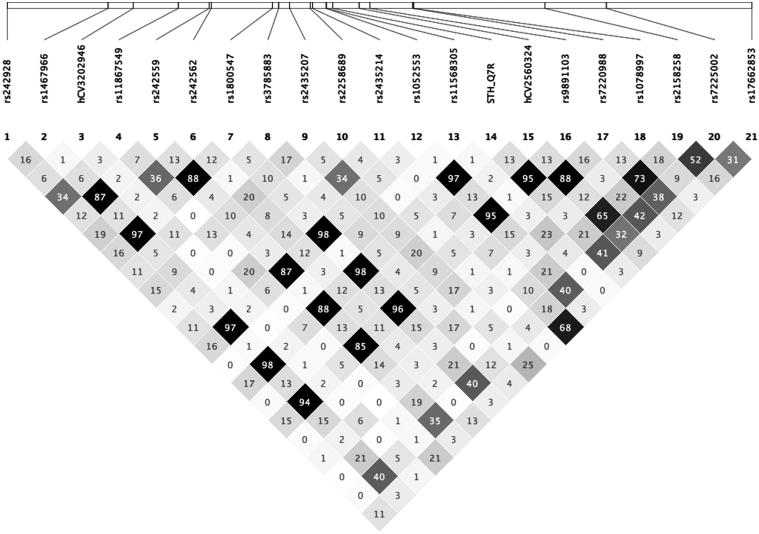
JE Tobin
JE Tobin, PhD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
JC Latourelle
JC Latourelle, MS
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
MF Lew
MF Lew, MD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
C Klein
C Klein, MD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
O Suchowersky
O Suchowersky, MD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
HA Shill
HA Shill, MD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
LI Golbe
LI Golbe, MD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
MH Mark
MH Mark, MD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
JH Growdon
JH Growdon, MD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
GF Wooten
GF Wooten, MD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
BA Racette
BA Racette, MD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
JS Perlmutter
JS Perlmutter, MD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
R Watts
R Watts, MD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
M Guttman
M Guttman, MD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
KB Baker
KB Baker, PhD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
S Goldwurm
S Goldwurm, MD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
G Pezzoli
G Pezzoli, MD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
C Singer
C Singer, MD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
MH Saint-Hilaire
MH Saint-Hilaire, MD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
AE Hendricks
AE Hendricks, BA
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
S Williamson
S Williamson, BS
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
MW Nagle
MW Nagle, BA
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
JB Wilk
JB Wilk, DSc
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
T Massood
T Massood, BS
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
JM Laramie
JM Laramie, PhD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
AL DeStefano
AL DeStefano, PhD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
I Litvan
I Litvan, MD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
G Nicholson
G Nicholson, MD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
A Corbett
A Corbett, MD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
S Isaacson
S Isaacson, MD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
DJ Burn
DJ Burn, MD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
PF Chinnery
PF Chinnery, MD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
PP Pramstaller
PP Pramstaller, MD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
S Sherman
S Sherman, MD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
J Al-hinti
J Al-hinti, MD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
E Drasby
E Drasby, MD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
M Nance
M Nance, MD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
AT Moller
AT Moller, MD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
K Ostergaard
K Ostergaard, MD, PhD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
R Roxburgh
R Roxburgh, PhD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
B Snow
B Snow, MD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
JT Slevin
JT Slevin, MD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
F Cambi
F Cambi, MD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
JF Gusella
JF Gusella, PhD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1,
RH Myers
RH Myers, PhD
1Departments of Neurology (J.E.T., J.C.L., M.H.S.-H., A.E.H., S.W., M.W.N., J.B.W., T.M., J.M.L., A.L.D., R.H.M.) and Anatomy and Neurobiology (J.E.T.), Boston University School of Medicine; Department of Biostatistics (A.E.H., A.L.D.), Boston University School of Public Health; Department of Bioinformatics (J.M.L.), Boston University; Department of Neurology (M.F.L.), University of Southern California, Los Angeles; Department of Neurology (C.K.), Medical University of Lübeck, Germany; Departments of Clinical Neurosciences and Medical Genetics (O.S.), University of Calgary, Alberta, Canada; Barrow Neurological Institute (H.A.S.), Phoenix, AZ; Department of Neurology (L.I.G., M.H.M.), University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School, New Brunswick; Department of Neurology (J.H.G.), Molecular Neurogenetics Unit (J.F.G.), Center for Human Genetic Research, Massachusetts General Hospital, Harvard Medical School, Boston; Department of Neurology (G.F.W.), University of Virginia Health System, Charlottesville; Department of Neurology (B.A.R., J.S.P.), Washington University School of Medicine, St. Louis, MO; Department of Neurology (R.W.), University of Alabama at Birmingham; Department of Medicine (M.G.), University of Toronto, Canada; Departments of Neurology and Neuroscience (K.B.B.), Cleveland Clinic Foundation; Parkinson Institute (S.G., G.P.), Istituti Clinici di Perfezionamento, Milan, Italy; Department of Neurology (C.S.), University of Miami, FL; Department of Neurology (I.L.), University of Louisville School of Medicine, KY; Neurology Department (G.N., A.C.), University of Sydney ANZAC Research Institute, Concord Hospital, Australia; Parkinson’s Disease and Movement Disorder Center of Boca Raton (S.I.), FL; Regional Neurosciences Centre (D.J.B., P.F.C.), Newcastle University, Newcastle upon Tyne, UK; Department of Neurology (P.P.P.), General Regional Hospital Bolzano, Italy; Department of Neurology (S.S.), University of Arizona, Tucson; Department of Neurology (J.A.), University of Arkansas for Medical Sciences; Port City Neurology (E.D.), Scarborough, ME; Park Nicollet Clinic (M.N.), St. Louis Park, MN; Department of Neurology (A.T.M., K.O.), Aarhus University Hospital, Denmark; Department of Neurology (R.R., B.S.), Auckland City Hospital, New Zealand; Department of Neurology (J.T.S., F.C.), University of Kentucky College of Medicine, Lexington
1